Summary
Background
Mutations in the gene encoding the bone morphogenetic protein receptor type II (BMPR2) are the commonest genetic cause of pulmonary arterial hypertension (PAH). However, the effect of BMPR2 mutations on clinical phenotype and outcomes remains uncertain.
Methods
We analysed individual participant data of 1550 patients with idiopathic, heritable, and anorexigen-associated PAH from eight cohorts that had been systematically tested for BMPR2 mutations. The primary outcome was the composite of death or lung transplantation. All-cause mortality was the secondary outcome. Hazard ratios (HRs) for death or transplantation and all-cause mortality associated with the presence of BMPR2 mutation were calculated using Cox proportional hazards models stratified by cohort.
Findings
Overall, 448 (29%) of 1550 patients had a BMPR2 mutation. Mutation carriers were younger at diagnosis (mean age 35·4 [SD 14·8] vs 42·0 [17·8] years), had a higher mean pulmonary artery pressure (60·5 [13·8] vs 56·4 [15·3] mm Hg) and pulmonary vascular resistance (16·6 [8·3] vs 12·9 [8·3] Wood units), and lower cardiac index (2·11 [0·69] vs 2·51 [0·92] L/min per m2; all p<0·0001). Patients with BMPR2 mutations were less likely to respond to acute vasodilator testing (3% [10 of 380] vs 16% [147 of 907]; p<0·0001). Among the 1164 individuals with available survival data, age-adjusted and sex-adjusted HRs comparing BMPR2 mutation carriers with non-carriers were 1·42 (95% CI 1·15–1·75; p=0·0011) for the composite of death or lung transplantation and 1·27 (1·00–1·60; p=0·046) for all-cause mortality. These HRs were attenuated after adjustment for potential mediators including pulmonary vascular resistance, cardiac index, and vasoreactivity. HRs for death or transplantation and all-cause mortality associated with BMPR2 mutation were similar in men and women, but higher in patients with a younger age at diagnosis (p=0·0030 for death or transplantation, p=0·011 for all-cause mortality).
Interpretation
Patients with PAH and BMPR2 mutations present at a younger age with more severe disease, and are at increased risk of death, and death or transplantation, compared with those without BMPR2 mutations.
Funding
Cambridge NIHR Biomedical Research Centre, Medical Research Council, British Heart Foundation, Assistance Publique-Hôpitaux de Paris, INSERM, Université Paris-Sud, Intermountain Research and Medical Foundation, Vanderbilt University, National Center for Advancing Translational Sciences, National Institutes of Health, National Natural Science Foundation of China, and Beijing Natural Science Foundation.
Introduction
Pulmonary arterial hypertension (PAH) is a rare disorder characterised by progressive remodelling of the small pulmonary arteries resulting in increased pulmonary vascular resistance and ultimately right ventricular failure and death.1, 2 The diagnosis of PAH requires a mean pulmonary artery pressure of 25 mm Hg or more with a pulmonary artery wedge pressure of 15 mm Hg or less at right heart catheterisation in the absence of chronic thromboembolic, left heart, or respiratory disease.3 The classification of PAH includes idiopathic and heritable forms. Additionally, PAH might occur after drug or toxin exposure (eg, anorexigens) or in association with other conditions such as congenital heart disease, connective tissue disease, liver disease, or HIV infection.4
In 2000, heterozygous germline mutations in the gene encoding the bone morphogenetic protein receptor type II (BMPR2) were identified as the main genetic cause of familial PAH.5, 6 Over 300 different BMPR2 mutations have been identified with a prevalence of greater than 75% in families with PAH.7, 8 BMPR-II is a receptor for the bone morphogenetic proteins (members of the transforming growth factor-β superfamily). Mutations in the BMPR2 gene cause loss-of-function and reduced signalling downstream of the receptor. Subsequently, BMPR2 mutations have been identified in apparently sporadic cases of idiopathic PAH with a frequency ranging from 11%9 to 40%.10 The occurrence of BMPR2 mutations in sporadic PAH cases in the absence of a family history can be accounted for by the relatively low penetrance of BMPR2 mutations (20–30%) and the occurrence of de novo mutations.11
Research in context.
Evidence before this study
In the year 2000, mutations in the BMPR2 gene were identified as the major genetic cause of pulmonary arterial hypertension (PAH). Some small studies have examined the effect of BMPR2 mutations on the presentation, haemodynamic profile, and outcomes in patients with PAH. These studies suggested that those with BMPR2 mutations present at a younger age with more severe derangements of cardiopulmonary haemodynamics. Due to a lack of statistical power, lack of adjustment for important factors such as age and sex, and confounding from inclusion of prevalent cases without necessary adjustments in many of these studies, the effect of BMPR2 mutations on long-term outcomes has not been reliably established.
Added value of this study
By harmonising individual participant data from 1550 patients in eight published and unpublished studies, with updated follow-up, this study provides the most definitive assessment of the effect of BMPR2 mutations on the haemodynamic profile at diagnosis and long-term outcomes in patients with PAH. This study has shown that possession of a BMPR2 mutation is associated with an increased risk of death or transplantation and all-cause mortality. This association appears to be mediated by a more severe haemodynamic profile measured at diagnosis with the greatest proportion of the risk accounted for by the lower cardiac index in BMPR2 mutation carriers. There was a strong interaction between the effect of a BMPR2 mutation and age at diagnosis, such that the increased risk of death or transplantation and all-cause mortality associated with possession of a BMPR2 mutation was greater in younger patients.
Implications of all the available evidence
Patients with PAH with underlying BMPR2 mutations are younger at diagnosis, have more severe disease, and have a worse prognosis than patients without BMPR2 mutations. The role of routine genetic testing for BMPR2 mutations on the management of patients with PAH deserves further study.
Recent European guidelines for the management of PAH recommend offering genetic counselling and screening for BMPR2 mutations to patients diagnosed with idiopathic, heritable, and anorexigen-associated PAH, mainly to enable predictive genetic testing of relatives.12 Studies have suggested that patients with PAH carrying causal BMPR2 mutations present at an earlier age with more severe haemodynamic compromise.13, 14, 15, 16, 17 Although this might be expected to confer a worse survival, robust evidence describing the effect of BMPR2 mutations on long-term outcomes in these patients is lacking, primarily due to the limited power of individual studies and survival bias.18, 19
We established the BMPR2 Studies Collaboration to investigate the effect of BMPR2 mutations on clinical phenotypes and long-term outcomes in patients with PAH. This international consortium has allowed central collation and harmonisation of participant data on 1550 patients with PAH from eight cohorts based in six different countries.
Methods
Data sources
We sought individual participant data from studies identified through systematic searches of the published literature using MEDLINE and Embase (search terms “BMPR2” and “pulmonary hypertension”, up to March 18, 2014), searches of conference proceedings (restricted to the English language), and discussions with investigators. Cohort studies were eligible for inclusion if they met the following criteria: included patients with idiopathic PAH, heritable PAH, or anorexigen-associated PAH; sequenced patients for BMPR2 mutations; recorded baseline information on demographic and haemodynamic data at diagnosis, and for analysis of survival, recorded information on outcomes (death or transplantation). Inclusion of patients recruited to cohorts and registries since publication of original manuscripts was provided where available, including updated survival data. Details of contributing cohorts are presented in the appendix. Data from each study were obtained using a standardised spreadsheet; raw data were examined, and inconsistencies or irregularities were clarified with the relevant investigators.
Ethical approval was obtained for each of the individual studies included in this analysis and all participants provided informed written consent. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Individual Participants Data (PRISMA-IPD) guidelines (checklist available in the appendix).20
Study participants
All contributing studies used international criteria for the diagnosis of PAH.21 For the purpose of this study, the expert physician diagnosis of idiopathic PAH, heritable PAH, or anorexigen-associated PAH in a specialist centre was sufficient and data pertaining to the full range of investigations at diagnosis were not collected.
Vasodilator responsiveness was defined as a reduction in mean pulmonary arterial pressure of at least 10 mm Hg to a level below 40 mm Hg with no reduction in cardiac output after administration of inhaled nitric oxide, although some centres have historically used inhaled prostacyclin or intravenous prostacyclin, according to local practice. Treatment for PAH was prescribed consistent with prevailing international guidelines at the time of recruitment, at the discretion of the clinical team in each institution. Data regarding initial and subsequent PAH targeted therapy were not available for this analysis. Cohorts comprised a combination of incident patients, defined for the purposes of this study as those who were enrolled in their respective study and thus committed to genotyping within 6 months of PAH diagnosis, and prevalent patients who were enrolled more than 6 months after PAH diagnosis.
Patients were excluded from the analysis if they had PAH associated with conditions such as connective tissue disease, HIV, congenital heart disease, or portal hypertension. Furthermore, to avoid potential confounding from mutations in other genes or undetected BMPR2 mutations, patients with a family history of PAH but with no identifiable BMPR2 mutation were also excluded. Patients with a history of anorexigen exposure were included since BMPR2 mutations have been recorded in these patients, and the disease is indistinguishable from idiopathic PAH.4, 22
Outcomes
The primary outcome was the composite of death or lung transplantation. All-cause mortality was the secondary outcome. Patients contributed only the first outcome recorded during follow-up (ie, deaths preceded by transplantation were not included) because data regarding post-transplant survival were not available. Outcomes were censored if a patient was lost to follow-up or reached the end of the follow-up period. In analysis of all-cause mortality, patients were censored at the time of transplantation. Date of PAH diagnosis was defined as the date of diagnostic right heart catheterisation.
Statistical analysis
Baseline characteristics of patients according to BMPR2 mutation status were compared using t test for continuous variables and χ2 test for categorical variables. Associations of BMPR2 mutation status with risk of death or transplantation and all-cause mortality recorded during follow-up were assessed using Cox proportional hazards regression models stratified by cohort and timing of study entry (ie, incident or prevalent). We used a one-stage stratified model rather than two-stage random effects model for our primary analysis because of the small number of participants in some studies. As a sensitivity analysis, we pooled data using a two-stage random effects model and assessed for heterogeneity between studies by calculating the I2 statistic and assessed statistical significance based on Cochran's Q test p value. Unless stated otherwise, hazard ratios (HRs) were adjusted for age at diagnosis and sex. The proportional hazards assumption was satisfied for the composite of death or transplantation and all-cause mortality. Survival curves comparing patients with and without BMPR2 mutations were calculated using unadjusted Kaplan-Meier estimates and compared using the log-rank test.
To restrict the scope for potential bias due to inclusion of prevalent patients (ie, those diagnosed with PAH more than 6 months before study enrolment), Cox proportional hazards regression models and survival curves were fitted allowing for left truncation arising from the interval between diagnosis and enrolment. These patients were only included in the risk set from the time of study entry and were excluded if they entered the study more than 10 years after diagnosis. In patients for whom the date of enrolment in the study was not available, patients entered the risk set on the date they were genotyped for BMPR2 mutations. Given that worse survival has been reported in incident patients compared with prevalent patients in observational studies,18 and a higher risk of PAH-related death or admission to hospital was reported in incident patients in a clinical trial population,23 Cox models were also stratified by timing of study entry. Data pertaining to familial clustering of individuals and mutations were not available; however, to account for this, survival models were adjusted for clustering by sets of individuals with the same mutation from the same cohort. Two studies were not included in the survival analysis (due to insufficient survival data) but were included in the description of demographic and haemodynamic characteristics.
We explored interactions between the effect of BMPR2 mutation with age at diagnosis and sex within the one-stage stratified Cox models. The interaction with age at diagnosis was assessed with age as a continuous variable, with cases separated into three post-hoc groups according to age at diagnosis for illustrative purposes (<30 years, 30–50 years, and >50 years).
To assess the degree to which the association of BMPR2 mutations with outcome was mediated by haemodynamic characteristics at diagnosis, we calculated the percentage of excess risk mediated (PERM) by three mediators thought likely to be in the causal pathway: pulmonary vascular resistance, cardiac index, and vasodilator responsiveness. Each of these mediators was added to the age-adjusted and sex-adjusted Cox proportional hazards models individually, and then all three simultaneously. The PERM, that is the degree to which the HR is attenuated by addition of the mediator in question, was calculated using the equation:
For this analysis, missing covariate data were imputed using multiple imputation by chained equations in those individuals included in the survival analysis, to generate ten datasets with complete covariates. Cox proportional hazards models were fitted within each imputed dataset and combined using Rubin's rules. This analysis was repeated using only cases that had complete data for all three mediators as a sensitivity analysis.
In an exploratory analysis, we compared haemodynamic parameters at presentation (compared using one-way ANOVA) and survival in patients by BMPR2 mutation type. Mutations were assigned to one of five categories (frameshift, stop-gained, splice site or intronic, large deletions or exonal duplications, missense).
A two-sided p value less than 0·05 was considered statistically significant throughout. Statistical analyses were done using Stata (version 14; StataCorp, College Station, TX, USA).
Role of the funding source
No funding bodies had any role in the design, conduct, analysis of this study, or writing of the manuscript. The corresponding author had full access to the data and had the final responsibility for the decision to submit this manuscript with the permission of all coauthors.
Results
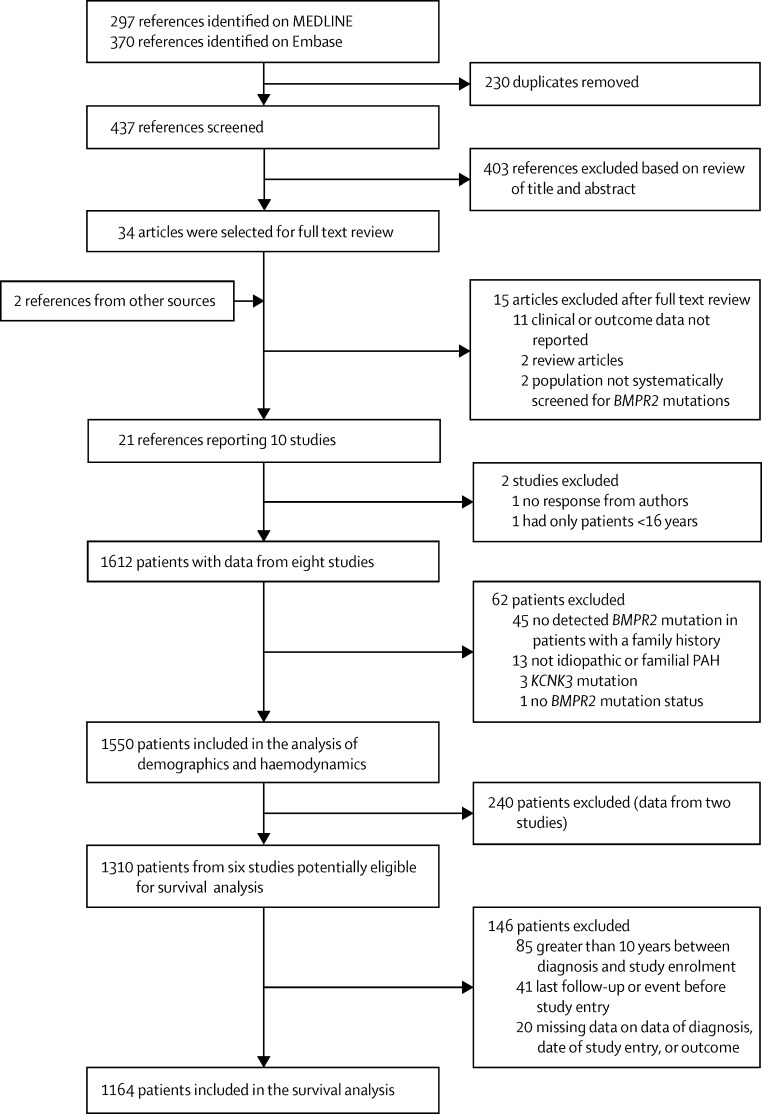
Figure 1 shows the inclusion and exclusion of studies and patients. Of ten studies identified, one eligible cohort (that involved 61 patients and was available only in abstract form)24 did not contribute data to the current analysis, and one cohort was excluded because it exclusively included 47 patients younger than 16 years.25 We analysed data from a total of 1550 patients with idiopathic, heritable, and anoroxigen-associated PAH from eight cohorts (appendix).13, 14, 15, 16, 17, 26, 27, 28 The mean age at diagnosis was 40·1 (SD 17·2) years, 72% (1105/1545 [data for five patients were unavailable]) were women, 60% (931/1550) were from western Europe, 18% (276/1550) from North America, and 22% (343/1550) from east Asia. Overall, 448 (29%) of 1550 patients had an identified BMPR2 mutation and 86 (6%) of 1550 patients had a recorded history of anorexigen exposure. Histograms of the dates during which patients included in the survival analyses were diagnosed and recruited into these studies are shown in the appendix.
Figure 1.
Study and patient selection
The proportion of patients with BMPR2 mutations varied between studies (appendix). In patients with no recorded family history of PAH, a BMPR2 mutation was identified in 17% (200/1174), whereas in patients with a family history of PAH a mutation was identified in 82% (202/247). Patients with a BMPR2 mutation were younger at diagnosis (mean age 35·4 years vs 42·0 years, p<0·0001) and the proportion of patients with a BMPR2 mutation was greater in those diagnosed at a younger age (37% [162/434] in those aged <30 years, 33% [187/562] in those aged 30–50 years, and 17% [75/451] in those aged >50 years at diagnosis; p<0·0001). A comparison of haemodynamic and functional parameters measured at the time of diagnosis between carriers and non-carriers of a BMPR2 mutation is shown in table 1. Those carrying a BMPR2 mutation had a higher mean pulmonary artery pressure and pulmonary vascular resistance, and lower cardiac index. No difference was recorded in the severity of symptoms assessed by New York Heart Association functional class or exercise capacity assessed by 6 min walk distance. Patients with a BMPR2 mutation were less likely to respond to acute vasodilator testing (table 1).
Table 1.
Demographics and clinical measurements at diagnosis
| All patients |
BMPR2 mutation status |
||||
|---|---|---|---|---|---|
| Non-carriers (N=1102) | Carriers (N=448) | p value | |||
| Age at diagnosis (N=1447), years | 40·1 (17·2) | 42·0 (17·8) | 35·4 (14·8) | <0·0001 | |
| Male sex | 440/1545 (28%) | 302/1097 (28%) | 138/448 (31%) | 0·20 | |
| Family history of PAH | 202/1376 (15%) | .. | 202/402 (50%) | .. | |
| Body-mass index (N=1206), kg/m2 | 24·9 (9·1) | 24·9 (10·6) | 24·9 (5·9) | 0·99 | |
| 6-min walk distance (N=1072), m | 378 (124) | 374 (128) | 388 (113) | 0·088 | |
| NYHA functional class | 0·38 | ||||
| I–II | 423/1426 (30%) | 313/1031 (30%) | 110/394 (28%) | ||
| III | 896/1426 (63%) | 647/1031 (63%) | 249/394 (63%) | ||
| IV | 107/1426 (8%) | 72/1031 (7%) | 35/394 (9%) | ||
| Mean pulmonary artery pressure (N=1503), mm Hg | 57·6 (15·0) | 56·4 (15·3) | 60·5 (13·8) | <0·0001 | |
| Pulmonary vascular resistance (N=1300), Wood units | 14·0 (8·4) | 12·9 (8·3) | 16·6 (8·3) | <0·0001 | |
| Right atrial pressure (N=1253), mm Hg | 8·2 (5·5) | 8·0 (5·7) | 8·6 (5·2) | 0·065 | |
| Cardiac output (N=1202), L/min | 3·98 (1·44) | 4·20 (1·50) | 3·50 (1·17) | <0·0001 | |
| Cardiac index (N=1358), L/min per m2 | 2·40 (0·88) | 2·51 (0·92) | 2·11 (0·69) | <0·0001 | |
| Vasodilator responder | 157/1287 (12%) | 147/907 (16%) | 10/380 (3%) | <0·0001 | |
Data are n/N (%) or mean (SD), unless otherwise stated. PAH=pulmonary arterial hypertension. NYHA=New York Heart Association.
Characteristics of the 1164 patients from the six studies that contributed to the survival analysis are shown in the appendix. Survival curves by BMPR2 mutation status in the combined dataset are shown in figure 2. Of the 1164 patients, 723 (62%) were incident cases and 441 (38%) were prevalent cases. The median interval between diagnosis and study entry in the prevalent patients was 1·8 years (IQR 1·1–4·5). During 5870 person-years at risk (median duration of follow-up from diagnosis 5·4 years [IQR 3·0–8·2]), there were 354 deaths and 74 patients underwent lung transplantation. Age-adjusted and sex-adjusted HRs comparing BMPR2 mutation carriers with non-carriers were 1·42 (95% CI 1·15–1·75; p=0·0011) for the composite of death or lung transplantation and 1·27 (1·00–1·60; p=0·046) for all-cause mortality (table 2). HRs were unchanged after adjusting for previous exposure to anorexigens. Addition of each of the three mediators assessed to the age-adjusted and sex-adjusted models attenuated the HRs associated with BMPR2 mutation for both death or transplantation and all-cause mortality (table 2). Cardiac index mediated the greatest proportion of excess risk, accounting for 65% and 79% of the increased HR for death or transplantation and all-cause mortality, respectively. The PERM by the combination of pulmonary vascular resistance, cardiac index, and vasodilator responsiveness was 71% for death or transplantation and 100% for all-cause mortality. In the complete case sensitivity analysis (913 patients; appendix) the PERM by each mediator was similar.
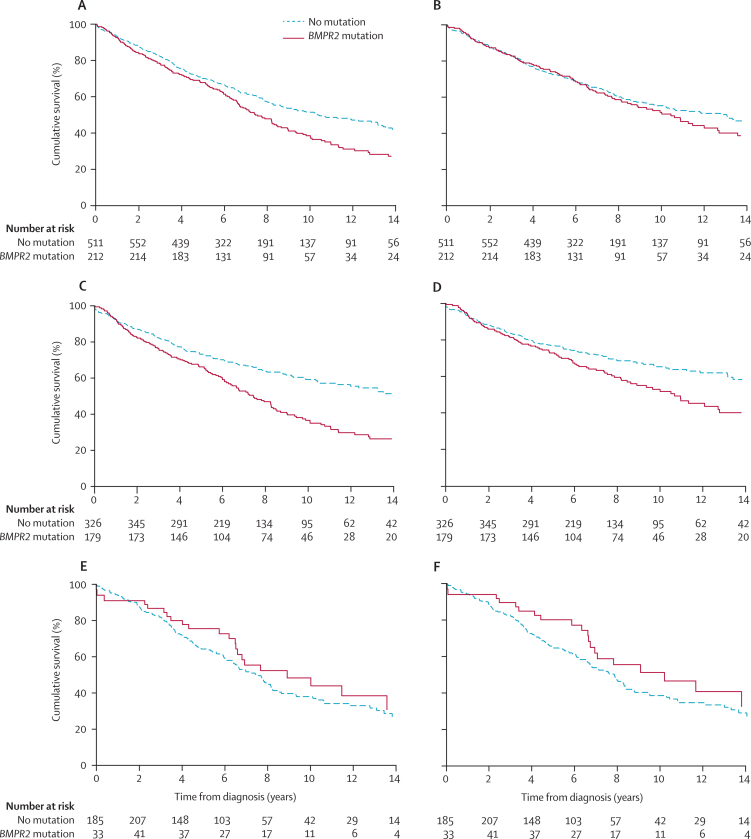
Figure 2.
Kaplan-Meier survival curves according to BMPR2 mutation status
(A) Transplant-free survival, all patients (p=0·0016). (B) Overall survival, all patients (p=0·32). (C) Transplant-free survival, younger than 50 years at diagnosis (p<0·0001). (D) Overall survival, younger than 50 years at diagnosis (p=0·0032). (E) Transplant-free survival, older than 50 years at diagnosis (p=0·27). (F) Overall survival, 50 years or older at diagnosis (p=0·16). Survival curves are not adjusted for age at diagnosis or sex and are not stratified by study cohort.
Table 2.
Proportion of excess risk mediated by haemodynamic variables at diagnosis
| HR (95% CI) for BMPR2 mutation | p value | PERM | ||
|---|---|---|---|---|
| Death or transplantation | ||||
| Pulmonary vascular resistance | ||||
| Adjusted for age and sex | 1·42 (1·15–1·75) | 0·0011 | ||
| Adjusted for age, sex, and pulmonary vascular resistance | 1·28 (1·03–1·58) | 0·024 | 34% | |
| Cardiac index | ||||
| Adjusted for age and sex | 1·42 (1·15–1·75) | 0·0011 | ||
| Adjusted for age, sex, and cardiac index | 1·18 (0·95–1·47) | 0·14 | 65% | |
| Vasoreactivity | ||||
| Adjusted for age and sex | 1·42 (1·15–1·75) | 0·0011 | ||
| Adjusted for age, sex, and vasoreactivity | 1·26 (1·02–1·57) | 0·036 | 37% | |
| Combined model | ||||
| Adjusted for age and sex | 1·42 (1·15–1·75) | 0·0011 | ||
| Adjusted for age, sex, pulmonary vascular resistance, cardiac index, and vasoreactivity | 1·12 (0·89–1·41) | 0·33 | 71% | |
| All-cause mortality | ||||
| Pulmonary vascular resistance | ||||
| Adjusted for age and sex | 1·27 (1·00–1·60) | 0·046 | ||
| Adjusted for age, sex, and pulmonary vascular resistance | 1·13 (0·89–1·43) | 0·33 | 53% | |
| Cardiac index | ||||
| Adjusted for age and sex | 1·27 (1·00–1·60) | 0·046 | ||
| Adjusted for age, sex, and cardiac index | 1·06 (0·83–1·35) | 0·67 | 79% | |
| Vasoreactivity | ||||
| Adjusted for age and sex | 1·27 (1·00–1·60) | 0·046 | ||
| Adjusted for age, sex, and vasoreactivity | 1·14 (0·89–1·45) | 0·29 | 49% | |
| Combined model | ||||
| Adjusted for age and sex | 1·27 (1·00–1·60) | 0·046 | ||
| Adjusted for age, sex, pulmonary vascular resistance, cardiac index, and vasoreactivity | 1·00 (0·77–1·29) | 0·98 | 100% | |
Hazard ratios (HRs) associated with possession of a BMPR2 mutation after addition of each mediator individually to age-adjusted and sex-adjusted Cox proportional hazards models with the percentage of excess risk mediated (PERM) by each mediator. Missing data for mediators generated by multiple imputation.
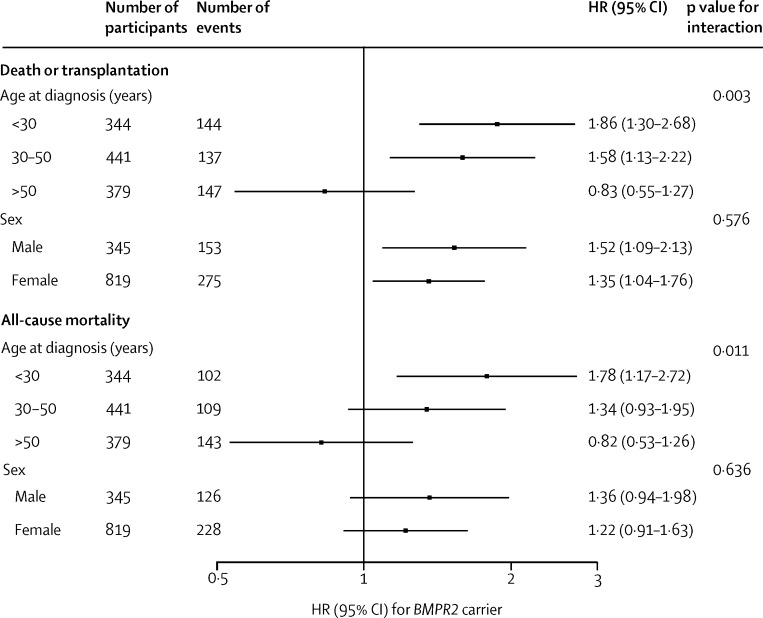
HRs associated with possession of a BMPR2 mutation were similar in men and women (p=0·576 for death or transplantation, p=0·636 for all-cause mortality), but higher in patients with a younger age at diagnosis (p=0·0030 for death or transplantation, p=0·011 for all-cause mortality; figure 3, appendix). The interaction of BMPR2 and age at diagnosis persisted after adjustment for potential mediators (appendix).
Figure 3.
Hazard ratios (HRs) for the effect of a BMPR2 mutation on death or transplantation and all-cause mortality by age at diagnosis and sex
p value for interaction of BMPR2 and age at diagnosis calculated with age at diagnosis as a continuous variable.
Similar results were recorded with meta-analysis using a two-stage approach with random effects (appendix). Between-study heterogeneity was modest, both for death or transplantation (I2=36·9% [95% CI 0–70]; p=0·16) and all-cause mortality (I2=20·1% [0–65]; p=0·28).
There were no significant differences in haemodynamic parameters at diagnosis between those with different mutation subtypes (appendix). Patients with missense mutations were slightly younger at diagnosis. No significant difference was recorded in the risk of death or transplantation or all-cause mortality among carriers of different types of BMPR2 mutations (appendix).
Discussion
To our knowledge, this meta-analysis of individual participant data from published and unpublished studies provides the most comprehensive standardised assessment of associations of BMPR2 mutations with long-term outcomes in patients with idiopathic, heritable, and anorexigen-associated PAH.
We have shown that patients diagnosed with PAH have an increased risk of death or transplantation and all-cause mortality if they possess a mutation in the BMPR2 gene. HRs associated with possession of a BMPR2 mutation were similar in males and females, but greater with younger age at diagnosis. Furthermore, we have confirmed in this analysis the previously reported observations that patients with BMPR2 mutations present at a younger age, have more severe haemodynamic compromise at diagnosis with higher mean pulmonary artery pressure and pulmonary vascular resistance and lower cardiac index, and are less likely to respond to acute vasodilator testing and more likely to undergo lung transplantation.13, 14, 15, 16, 17, 26
The precise mechanisms underlying the difference in survival in those with a BMPR2 mutation remain unclear. We have found that after adjusting for pulmonary vascular resistance, cardiac index, and vasodilator responsiveness, HRs for death or lung transplantation and all-cause mortality were attenuated. The low number of BMPR2 mutation carriers having vasodilator responsiveness, a phenotype associated with a good prognosis when treated with calcium-channel blocker therapy,29, 30 is consistent with a predominance of extensive vascular remodelling rather than vasoconstriction. Given that the greatest percentage of the excess risk associated with a BMPR2 mutation was accounted for by reduced cardiac index at diagnosis, impaired right ventricular adaptation to increased afterload in those with BMPR2 mutations could be an important factor, as has been suggested in preclinical studies.31
Our finding of a greater proportion of BMPR2 mutations in younger age groups is consistent with the common observation that diseases with a major genetic contribution tend to present with an earlier onset. The strong interaction between BMPR2 mutation status and age at diagnosis is of great interest and has not been reported before. Patients carrying a BMPR2 mutation in which PAH manifests at a younger age might have a more severe mutation that not only results in more extensive pulmonary vascular remodelling or impaired right ventricular adaptation at diagnosis, but also results in more rapid progression of the disease process. This hypothesis is supported by the observation that the worse prognosis associated with a BMPR2 mutation in patients diagnosed before the age of 30 years in this study was not completely attenuated after adjustment for pulmonary vascular resistance, cardiac index, and vasoreactivity.
Data from the UK PAH registry32 suggest that patients with PAH diagnosed after the age of 50 years are phenotypically distinct compared with younger patients. Older patients have a higher prevalence of cardiovascular comorbidities including systemic hypertension, atrial fibrillation, and diabetes. In the present study, although we did not collect data on comorbidities, we found that the proportion of patients with a BMPR2 mutation is lower in those diagnosed after the age of 50 years than in younger patients. Additionally, we show that after adjusting for age and sex, mutations do not affect survival in these older patients, and prognosis might even be better in those with mutations. BMPR2 mutations present in those who do not develop PAH until later in life might be less deleterious. Alternatively, in the older age group, comorbidities might outweigh the effect of BMPR2 mutations on survival.
Our study confirms the relatively high frequency of BMPR2 mutations in idiopathic and heritable PAH, and supports a central role for the BMPR2 pathway in the initiation of this disease. Moreover, the effect of BMPR2 mutation on survival suggests a role for BMPR-II dysfunction in the clinical progression of the disease. Both of these observations support further investigations into the potential targeting of the BMPR-II pathway for therapeutic intervention in PAH.33, 34
The main reason to test for the presence or absence of a BMPR2 mutation in a patient with PAH is to guide predictive genetic testing in unaffected relatives. Although our findings show that BMPR2 mutations are associated with a worse survival, the usefulness of this result for prognostic purposes might be restricted in the clinic, since the majority of this risk appears to be accounted for by the known haemodynamic predictors of mortality measured during the diagnostic assessment during right heart catheterisation. Despite this, in younger patients, in which the increased risk appears to persist after adjustment for these factors, albeit only in subgroup analyses, screening for mutations might add value, and this warrants further investigation.
Our analysis has major strengths. We had access to data for more than 95% of participants from eligible cohorts. We analysed individual participant data to avoid limitations of literature-based reviews. We had information on both all-cause mortality and death or transplantation. We studied clinically relevant subpopulations (such as by age and sex) reliably, exploiting the study's considerable statistical power. We avoided potential over-adjustment in the primary analysis by not adjusting for variables (eg, pulmonary vascular resistance, cardiac index, and vasoreactivity) that could mediate associations between BMPR2 and death or transplantation and all-cause mortality. We ensured generalisability by studying cohorts located across east Asia, Europe, and North America.
Our analysis has some limitations. Studies included differed in their methods of recruitment and data collection, and in the proportion of familial cases and individuals with BMPR2 mutations, which might explain the heterogeneity recorded between studies. Nevertheless, we obtained similar results to those in our primary analysis based on a stratified Cox proportional hazards model when we used a two-stage random effects meta-analysis model in sensitivity analysis. Additionally, given the evidence for interaction recorded between mutation status and age, the differences in age at diagnosis in different studies could partly explain the heterogeneity recorded in two-stage meta-analyses. The inclusion of prevalent patients in survival analyses can introduce bias; however, we addressed this in the Cox proportional hazards model by allowing for left truncation arising from the interval between diagnosis and study entry and also stratifying by timing of enrolment. Additionally, we observed no interaction between BMPR2 mutation status and timing of enrolment. Finally, the lack of data regarding the timing and use of PAH-directed therapies might introduce some bias, although we believe any effect is likely to be very small. Indeed, if patients with BMPR2 mutations were treated more aggressively due to their more severe haemodynamic derangements at diagnosis, this could have resulted in an attenuation of the association we have recorded.
By harnessing data from observational studies done worldwide, we have shown that in patients with idiopathic, familial, and anorexigen-associated PAH, the presence of a mutation in the BMPR2 gene is associated with an increased risk of death or lung transplantation and all-cause mortality, particularly in those diagnosed at a younger age. Our analysis suggests that this association is largely mediated by the more severe haemodynamic derangements and low frequency of vasodilator responsiveness at diagnosis seen in those with BMPR2 mutations.
Acknowledgments
Acknowledgments
The authors would like to acknowledge Laurence Rottat (APHP, Centre de Référence de l'Hypertension Pulmonaire Sévère, Service de Pneumologie, Hôpital de Bicêtre, Le Kremlin Bicêtre, France), Chiara Barozzi and Luciana Tomasi (University of Bologna, Bologna, Italy), Hiroki Kabata (Keio University Hospital, Tokyo, Japan), Melanie Eyries (Université Pierre et Marie Curie-Paris, Paris, France), Robyn Barst and Jane Morse (Columbia University, New York, NY, USA). We acknowledge funding from Cambridge NIHR Biomedical Research Centre, Medical Research Council (MR/K020919/1), British Heart Foundation (CH/09/001/25945), Assistance Publique-Hôpitaux de Paris, INSERM, Université Paris-Sud, National Natural Science Foundation of China (81320108005), Beijing Natural Science Foundation (7141009), Intermountain Research and Medical Foundation (1007044), National Center for Advancing Translational Sciences (UL1TR000445), and National Institutes of Health (R01 HL060056, P01 HL108800, and K23 HL098743). The contents of this paper are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Contributors
JDWE and NWM conceived the study. JDWE, EDAn, MH, and NWM designed the study. BG, DM, X-JW, NG, EDAu, GE, KA, EG, YY, Z-CJ, AM, MP, LAW, IN, TS, CE, KH, MW, EBR, WKC, FS, GS, OS, and MH collected the data. JDWE and NWM coordinated the study. JDWE, SG, and SK analysed the data. All authors interpreted the results. JDWE, EDAn, MH, and NWM drafted the manuscript with critical revisions for important intellectual content from all authors. All authors approved the final version.
Declaration of interests
BG reports personal fees from Actelion, GlaxoSmithKline, Bayer, and Pfizer, outside of the submitted work. DM reports grants and personal fees from Actelion and Bayer, and personal fees from GlaxoSmithKline, Pfizer, Novartis, and BMS, outside of the submitted work. GE reports grants from NIH, during the conduct of the study; personal fees from Bellerophon, Actelion, and Bayer, and grants from Actelion, Gilead, and United Therapeutics, outside of the submitted work. GS reports grants and personal fees from Actelion, GlaxoSmithKline, and Bayer, and personal fees from Pfizer, outside of the submitted work. OS reports grants, personal fees, and non-financial support from Actelion, GlaxoSmithKline, and Bayer, grants and personal fees from Pfizer, and personal fees and non-financial support from United Therapeutics, outside of the submitted work. EDAn reports grants from The British Heart Foundation, Medical Research Council, NHS Blood and Transplant, National Institute for Health Research, and European Union, and personal fees from Elsevier (France), outside of the submitted work. MH reports personal fees and non-financial support from Actelion and Pfizer, and grants, personal fees, and non-financial support from Bayer and GlaxoSmithKline, outside of the submitted work. JDWE, X-JW, NG, EDAu, KA, EG, YY, Z-CJ, AM, MP, LAW, IN, TS, CE, KH, MW, EBR, WKC, FS, SG, SK, and NWM declare no competing interests.
Supplementary Material
References
- 1.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Morrell NW, Archer SL. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:S13–S24. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Badesch DB, Champion HC, Sanchez MA. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Gatzoulis MA, Adatia I. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:S34–S41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Deng Z, Morse JH, Slager SL. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International PPHC, Lane KB, Machado RD. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 7.Soubrier F, Chung WK, Machado R. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:S13–S21. doi: 10.1016/j.jacc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Machado RD, Southgate L, Eichstaedt CA. Pulmonary arterial hypertension: a current perspective on established and emerging molecular genetic defects. Hum Mutat. 2015;36:1113–1127. doi: 10.1002/humu.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koehler R, Grunig E, Pauciulo MW. Low frequency of BMPR2 mutations in a German cohort of patients with sporadic idiopathic pulmonary arterial hypertension. J Med Genet. 2004;41:e127. doi: 10.1136/jmg.2004.023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morisaki H, Nakanishi N, Kyotani S, Takashima A, Tomoike H, Morisaki T. BMPR2 mutations found in Japanese patients with familial and sporadic primary pulmonary hypertension. Hum Mutat. 2004;23:632. doi: 10.1002/humu.9251. [DOI] [PubMed] [Google Scholar]
- 11.Larkin EK, Newman JH, Austin ED. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:892–896. doi: 10.1164/rccm.201205-0886OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galie N, Humbert M, Vachiery JL. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 13.Sztrymf B, Coulet F, Girerd B. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med. 2008;177:1377–1383. doi: 10.1164/rccm.200712-1807OC. [DOI] [PubMed] [Google Scholar]
- 14.Liu D, Wu WH, Mao YM. BMPR2 mutations influence phenotype more obviously in male patients with pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5:511–518. doi: 10.1161/CIRCGENETICS.111.962209. [DOI] [PubMed] [Google Scholar]
- 15.Austin ED, Phillips JA, Cogan JD. Truncating and missense BMPR2 mutations differentially affect the severity of heritable pulmonary arterial hypertension. Respir Res. 2009;10:87. doi: 10.1186/1465-9921-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girerd B, Montani D, Eyries M. Absence of influence of gender and BMPR2 mutation type on clinical phenotypes of pulmonary arterial hypertension. Respir Res. 2010;11:73. doi: 10.1186/1465-9921-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfarr N, Szamalek-Hoegel J, Fischer C. Hemodynamic and clinical onset in patients with hereditary pulmonary arterial hypertension and BMPR2 mutations. Respir Res. 2011;12:99. doi: 10.1186/1465-9921-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humbert M, Sitbon O, Yaici A. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36:549–555. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 19.Miller DP, Gomberg-Maitland M, Humbert M. Survivor bias and risk assessment. Eur Respir J. 2012;40:530–532. doi: 10.1183/09031936.00094112. [DOI] [PubMed] [Google Scholar]
- 20.Stewart LA, Clarke M, Rovers M. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313:1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 21.Galie N, Hoeper MM, Humbert M. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 22.Humbert M, Deng Z, Simonneau G. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J. 2002;20:518–523. doi: 10.1183/09031936.02.01762002. [DOI] [PubMed] [Google Scholar]
- 23.Simonneau G, Channick RN, Delcroix M. Incident and prevalent cohorts with pulmonary arterial hypertension: insight from SERAPHIN. Eur Respir J. 2015;46:1711–1720. doi: 10.1183/13993003.00364-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrios E, Lapuniza P, Vincente Mm. Preliminary results of the determination of BMPR2 mutations and clinical implications in a Spanish population of patients with pulmonary arterial hypertension. Eur Heart J. 2013;34:S306. (abstract). [Google Scholar]
- 25.Chida A, Shintani M, Yagi H. Outcomes of childhood pulmonary arterial hypertension in BMPR2 and ALK1 mutation carriers. Am J Cardiol. 2012;110:586–593. doi: 10.1016/j.amjcard.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Elliott CG, Glissmeyer EW, Havlena GT. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113:2509–2515. doi: 10.1161/CIRCULATIONAHA.105.601930. [DOI] [PubMed] [Google Scholar]
- 27.Kabata H, Satoh T, Kataoka M. Bone morphogenetic protein receptor type 2 mutations, clinical phenotypes and outcomes of Japanese patients with sporadic or familial pulmonary hypertension. Respirology. 2013;18:1076–1082. doi: 10.1111/resp.12117. [DOI] [PubMed] [Google Scholar]
- 28.Rosenzweig EB, Morse JH, Knowles JA. Clinical implications of determining BMPR2 mutation status in a large cohort of children and adults with pulmonary arterial hypertension. J Heart Lung Transplant. 2008;27:668–674. doi: 10.1016/j.healun.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 30.Sitbon O, Humbert M, Jaïs X. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 31.Hemnes AR, Brittain EL, Trammell AW. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:325–334. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling Y, Johnson MK, Kiely DG. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 33.Spiekerkoetter E, Tian X, Cai J. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123:3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long L, Ormiston ML, Yang X. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21:777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.