Abstract
Background
Lung transplantation, in patients with end-stage lung disease, is limited by chronic rejection which occurs with an incidence and severity exceeding most other transplanted organs. Alloimmune responses play an important role in progression to chronic rejection that manifests as bronchiolitis obliterans syndrome (BOS), but no biomarker can currently predict the progression to BOS. Studies in animal models suggest that intra-graft T regulatory cells (Tregs) are important in maintaining transplantation tolerance and FoxP3 is the protoypic Treg marker.
Methods
Leukocytes in blood and bronchoalveolar lavage fluid (BAL) were compared for expression of FoxP3 by flow cytometry in 14 stable lung transplant recipients and 6 lung transplant recipients who eventually developed BOS.
Results
Stable patients had a significantly increased percentage of FoxP3+ cells among CD4+ cells in BAL and greater levels of the Treg-attracting chemokine CCL22, than patients who subsequently developed BOS. At the time of acute rejection (AR), limited sequential analyses revealed a higher percentage of BAL CD4+FoxP3+ cells in patients who did not progress to BOS. In this pilot study, a threshold of 3.2% CD4+/FoxP3+ cells in the BAL distinguished stable recipients from those developing BOS subsequently within the first two years post transplantation.
Conclusion
Thus, the proportion of FoxP3+ cells among CD4+ cells in BAL may help predict lung allograft outcome and guide therapeutic immunosuppression in lung transplant recipients.
Keywords: Regulatory T cells, FoxP3, tolerance, lung transplantation, BOS
INTRODUCTION
Long term outcome after lung transplantation remains limited due to chronic rejection. (1). In lung transplantation, chronic rejection manifests as obliterative bronchiolitis (OB), a process of fibro-obliterative occlusion of the small airways, and is diagnosed functionally as Bronchiolitis Obliterans Syndrome (BOS). Greater than 50% of lung transplant recipients are reported to develop terminal BOS in 5 years (2). Acute Rejection (AR) remains the strongest predisposing factor in the development of BOS (2). Because AR is T cell-dependent, it is hypothesized that T cells directly or indirectly contribute to the etiology of chronic rejection (3-6).
Several subsets of T cells can exert suppressor function, but particular attention has been devoted to CD4+FoxP3+ T cells (Tregs), because they are essential for maintenance of T cell homeostasis and prevention of autoimmunity (7-10). Expression of the transcription factor FoxP3 confers a suppressive phenotype; however the presence of FoxP3 does not preclude the expression of other effector functions and human conventional T cells transiently express FoxP3 upon activation (11,12). In a mouse model of cardiac transplantation, we have previously shown that allograft acceptance correlated with an increase in the ratio of Tregs to conventional CD4+ T cells in the allograft but not in secondary lymphoid organs. In parallel, allograft acceptance was associated with greater intra-graft expression levels of the Treg-attracting chemokines CCL17 and CCL22 (11, 12). These results suggest that a critical element determining allograft outcome may be the graft microenvironment and its suppression of adaptive alloimmune responses by local Tregs. The goal of this study was to investigate the percentage of CD4+FoxP3+ T cells in lung recipients over time to evaluate whether FoxP3 may be used as a biomarker to predict graft stability versus chronic rejection.
RESULTS
Clinical characteristics of the Study Cohort
The demographic data of the transplant recipients is shown in Table 1. There was no significant difference in the clinical characteristics of patients between the two groups (Table 1 and Supplemental Figure 1). There were no BAL samples with clinically significant infection (ie bronchitis or pneumonia) during the course of this study.
Table 1.
Demographic data and clinical variables of the patient population
| Stable (14) | BOS (6) | |
|---|---|---|
| Age (median, IQR) | 59.5 (43.5, 63.5) | 56.0 (52.5, 64.5) |
| Gender (female %) | 3 (21%) | 2 (33%) |
| Initial disease: | ||
| COPD | 3 | 2 |
| IPF | 9 | 2 |
| Other | 2 | 2 |
| SLTx | 7 | 4 |
| BLTx | 7 | 2 |
| Follow up time (median, IQR) | 20(11, 27) | -- |
| Time to BOS (median, IQR) | -- | 12 (11,16) |
| Immunosuppression: | ||
| Tac/Aza/Pred | 8 | 1 |
| Tac/MMF/Pred | 4 | 1 |
| Tac/Sir/Pred | 2 | 3 |
| CsA/Sir/Pred | 1 | |
| Average number of BAL specimens (per lung transplant recipient) | 3.1 ± 1.1 | 3.2 ± 1.2 |
| Mean time from transplant to BAL specimens (months) | 7.3 ± 6.9 | 7.9 ± 4.6 |
| # patients with AR | 10 (71%) | 5 (83%) |
| # episodes of AR | 13 | 8 |
| Grade of AR: | ||
| Grade A1 | 6 | 5 |
| Grade A2 or higher | 4 | 2 |
| Grade B | 3 | 1 |
COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; SLTx, single lung transplant; BLTx, bilateral lung transplant; BOS, Bronchiolitis Obliterans Syndrome; Tac, tacrolimus; Aza, azathioprine; Pred, prednisone; MMF, mycophenolate mofetil; Sir, sirolimus; CsA, cyclosporine A; BAL, bronchoalveolar lavage; AR, acute rejection
Similar proportion of T cells in stable lung transplant recipients and patients with BOS
Animal studies suggest that T cell profiles at the graft site may be more revealing of the relevant alloimmune response than those at distant locations. To characterize the distribution profile of T cell subsets in lung transplant recipients, mononuclear cells from blood and BAL obtained prior to development of BOS were analyzed. A representative example of the flow cytometry results is shown in Figures 1A and 1B. Both stable and BOS patients had a greater percentage of CD3+ T cells in their peripheral blood than in BAL (Figure 1A and 1C). There was no difference between the two patient groups with respect to the percentage of CD3+, CD4+ or CD8+ T cells in either compartment (Figure 1B and 1C). Both groups had a greater proportion of CD4+ than CD8+ T cells in their peripheral blood. In contrast, this ratio was reduced in the BAL from both groups (Figure 1C) (13).
Figure 1. Similar proportion of T cells in stable lung transplant recipients and patients with BOS.
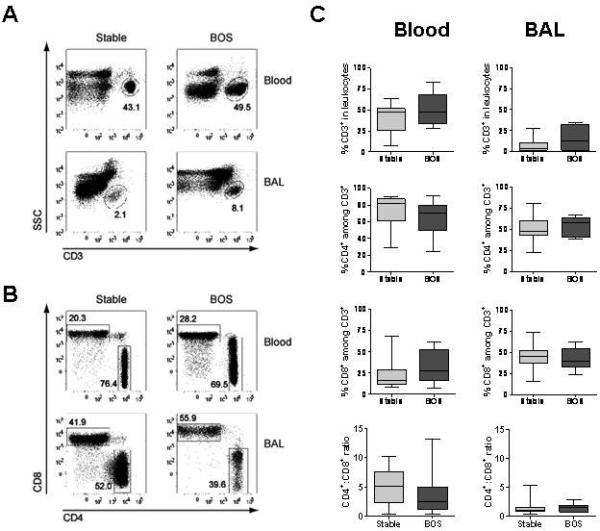
Leukocytes isolated from peripheral blood and BAL were analyzed by flow cytometry for expression of CD3, CD4 and CD8. Numbers in the plots represent the percentage of boxed events within each plot. A. Representative example of flow cytometry identifying the percentage of CD3+ T cells among mononuclear cells following gating of live events. B. Representative example of CD4 and CD8 staining among CD3-gated events. C. Sequential samples for all stable and BOS patients (prior to BOS development) were averaged. Box plots show the median (horizontal line) and the 25th to 75th percentile range. The error bar shows the total range of values.
Increased percentage of CD4+FoxP3+ cells in the BAL, but not blood, of stable patients versus patients who later developed BOS
To determine whether expression of FoxP3 at the protein level correlated with persistent stability or subsequent development of BOS, blood and BAL mononuclear cells were analyzed by flow cytometry for intracellular expression of FoxP3 relative to staining with a control isotype. Expression of FoxP3 was only detected in CD4+ cells and FoxP3+ cells were uniformly CD25int or CD25high. As all activated conventional T cells express CD25, we did not deem CD25 to be a reliable marker of Tregs and simplified our staining strategy to identify FoxP3+ cells among CD4+ cells. A representative example of the flow cytometry is shown in Figure 2A. A statistically significant (p<0.001) increase in the percentage of FoxP3+ cells among CD4+ was observed in the BAL of patients who remained stable when compared with patients who later developed BOS, but there were no observed differences in the peripheral blood (Figure 2B). To minimize potential confounding effects of time variability in sample collection, we used a single time point for each patient as close to one year following transplantation as possible (12 + 2 months). As shown in Figure 2C, the percentage of CD4+FoxP3+ BAL cells at 1 year remained statistically significantly higher in patients who would remain stable versus those who developed BOS after the one year mark (p=0.017). Furthermore, the proportion of CD4+FoxP3+ cells in the BAL from stable patients at one year post-transplantation was significantly higher than in their blood, suggesting preferential accumulation of FoxP3+ cells in the lung of stable patients rather than reduced retention of FoxP3+ cells in the lung of patients who will develop BOS (Figure 2C).
Figure 2. Increased percentage of CD4+FoxP3+ cells and protein level of CCL 22 in the BAL, but not blood, of stable patients versus patients with BOS.
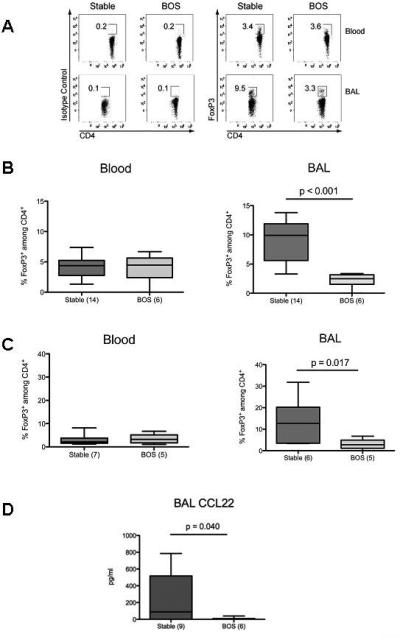
Mononuclear cells from blood and BAL were analyzed by flow cytometry for percentage of FoxP3-expressing cells within CD4+ cells. A. Representative flow cytometry for CD4 versus Foxp3 or its control isotype in blood and BAL samples from stable and BOS patients. The numbers in the plots represent the percentage of events in the boxed areas within that plot. B. The percentages of FoxP3-expressing cells among CD4+ cells in blood and BAL from all samples from each stable or BOS patient (before development of BOS) were averaged and a single value was used per patient. Results represent the median and interquartile ranges of this value in all stable versus all BOS patients. C. The percentages of FoxP3-expressing cells among CD4+ cells in blood and BAL obtained at approximately 1 year post-transplantation in stable patients versus BOS patients (before development of BOS) were averaged in each group and displayed as median and interquartile ranges. D. The protein level of CCL22 from the all BAL samples from each stable or BOS patient prior to development of BOS were averaged and a single value was used per patient. Results represent the median and the interquartile ranges of this value in all stable versus all BOS patients.
Increased percentage of stable lung transplant recipients had elevated CCL22 protein levels in BAL
The increased proportion of FoxP3+ cells in the BAL of stable patients may result from greater intra-graft recruitment of CD4+FoxP3+ cells. To determine if expression of CCL17 and CCL22 could explain the accumulation of FoxP3+ cells in stable lung allografts, BAL protein levels of CCL17 and CCL22 were analyzed. Six of nine (67%) stable lung transplant recipients had detectable levels of CCL22 compared to only one of six (17%) BOS patients (p =0.04) (Figure 2D). Higher levels of CCL22 were detected whether results were normalized to total protein levels in the BAL or not (data not shown). In comparison, 3/9 (33%) stable recipients and 1/6 (17%) BOS recipients had detectable levels of CCL17 (p = ns).
Low percentage (< 3.2 %) of BAL CD4+ FoxP3+ cells is a predictor for development of BOS
No biological predictors have been identified to anticipate the onset of BOS in lung transplant recipients. Given the association of reduced percentage of BAL CD4+FoxP3+ cells with the subsequent development of BOS, a threshold value of Fox P3 expression was identified based upon the 25th percentile of the percent FoxP3+ cells among all 20 patients. In this pilot study, this threshold level of 3.2% BAL CD4+ FoxP3+ cells was able to distinguish stable recipients from those who developed BOS within the first two years after lung transplantation by Kaplan Meier method (Figure 3).
Figure 3. A threshold value of 3.2% for BAL FoxP3+ cells among CD4+ cells is associated with the development of, versus freedom from, BOS.
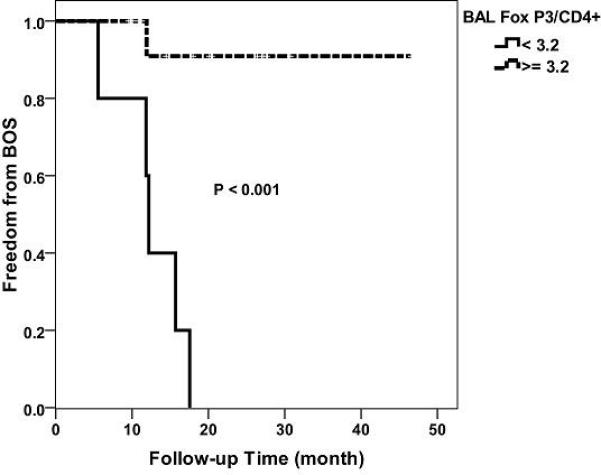
The composite level of BAL CD4+FoxP3+ percentages was assessed for both the stable patients and the BOS patients prior to BOS development. A threshold level of 3.2% was identified to distinguish the two groups with respect to the subsequent development of BOS.
Increased proportion of CD4+FoxP3+ cells during AR correlates with lower incidence of BOS
Most lung transplant recipients experience episodes of AR after transplantation. However, some patients revert to a stable condition whereas others progress to BOS. To determine whether the proportion of CD4+FoxP3+ cells during AR episodes may predict development of BOS, BAL and blood of patients were obtained during each episode of AR (see Supplemental Figure 1). There was no significant difference in the percentage of BAL or blood FoxP3+ cells during AR when compared to patients who never developed AR episodes (Figure 4A). However, when the proportion of FoxP3+ cells among CD4+ cells during the AR episodes was compared between patients who reverted to a stable status and patients who later developed BOS, an increased percentage of Foxp3+ cells during AR episodes was observed in patients whose lung function stabilized. (Figure 4B, p=0.002). Our data are consistent with a hypothesis that intra-graft recruitment of CD4+FoxP3+ cells during AR episodes may protect the transplanted lung from chronic rejection.
Figure 4. Increased proportion of CD4+FoxP3+ cells during AR correlates with lower incidence of BOS.
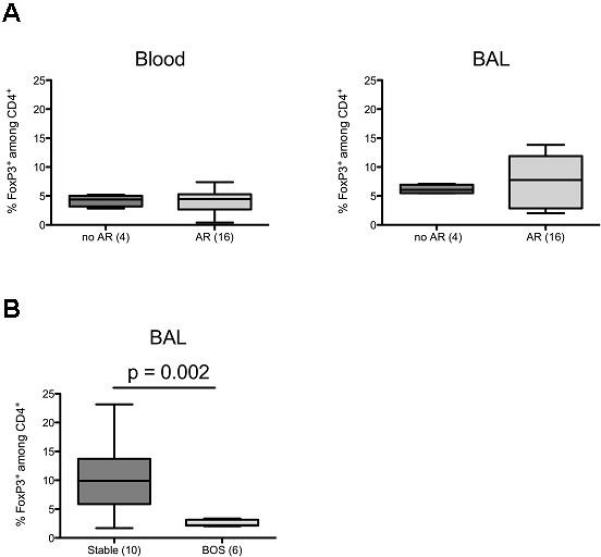
A. The percentages of FoxP3-expressing cells among CD4+ cells were assessed in blood and BAL obtained from patients at the time of AR episodes and compared to samples obtained from patients who did not develop AR episodes. B. The percentages of FoxP3-expressing cells among CD4+ cells in BAL during episodes of AR were compared in patients who resolved the AR and reverted to a stable phenotype versus those who developed BOS.
DISCUSSION
To our knowledge, this is the first study demonstrating a lower percentage of CD4+FoxP3+ cells in the BAL fluid of lung transplant recipients who eventually develop BOS compared to those who remain stable. In addition, CCL22, a chemokine involved in the recruitment of Tregs, was also increased in the BAL of the majority of stable patients, suggesting a potential mechanism by which these cells selectively traffic to the lung allograft. During episodes of AR, a significantly decreased percentage of BAL CD4+FoxP3+ cells was found in patients who eventually developed BOS compared to those who returned to stable lung function, suggesting that the CD4+FoxP3+ cells in the lung during AR may contribute to determining ensuing graft function. Interestingly, no differences were observed in peripheral blood CD4+Foxp3+ cells between stable and BOS patients. Together, these findings suggest that the phenotype and function of CD4+ T cells at the graft site may constitute a valuable biomarker to predict the development of BOS after lung transplantation.
A handful of studies have attempted to characterize the percentage of Tregs in lung transplant recipients. Those investigations have mostly focused on blood samples and expression of CD4 and CD25. However, all activated T cells upregulate CD25, the IL-2Rα chain, making this marker less than optimal to distinguish Tregs from activated effector T cells. Furthermore, circulating T cells may not reflect the local immune response at the graft site, whereas T cells in the lung may have a more critical role in determining graft outcome. In contrast to the BAL, our cohort of BOS patients did not display reduced proportion of CD4+FoxP3+ cells in their peripheral blood. Previous studies in lung transplant recipients have suggested a decrease in regulatory T cells identified by expression of CD4 and CD25 in the peripheral blood of patients with BOS compared to those who remain stable (14, 15). Whether differences between these studies and ours are solely due to the different markers used to identify putative Tregs, remains to be established. Nonetheless our results suggest that it may be preferable to analyze cells at the graft site than in the circulation.
A subset of activated Tregs expresses CCR4 that binds CCL17 and CCL22, but CCR4 is also found in other cell types including Th2 cells, mast cells, basophils and eosinophils (16). In mouse models of cardiac and renal allografts, we and others have found that expression CCL22 and CCL17 in the graft correlates with enhanced intra-graft recruitment of Tregs and graft survival (11, 17, 18). The importance of CCR4 for graft acceptance is further supported by observations in CCR4-deficient mice, which are resistant to the induction of transplantation tolerance (17, 19). Genetic ablation of CCR4 in Tregs alone results in lymphocytic infiltration and severe inflammation of the lungs and skin (20), underscoring the role of Treg CCR4 in lung homeostasis. CCL22 has been shown to recruit human Tregs to the tumor microenvironment in cancer patients (21, 22). In mice, NK cells can produce CCL22 in the lung, resulting in recruitment of Tregs in a model of metastatic lung carcinoma (23). NK cells, macrophages and dendritic cells in human BAL can theoretically produce CCL22 (24). Although the source of CCL22 in lung transplant recipients remains to be established, our data suggest that CCL22 may attract Tregs to the graft after lung transplantation in patients.
Our data suggest that higher percentage of FoxP3+ cells among CD4+ cells in the BAL fluid during AR episodes may protect against development of BOS or promote the return of allograft stability. This is conceptually attractive as FoxP3-expressing Tregs can effectively reduce inflammation by suppressing the function of many cells types that contribute to inflammation (25). Neujhar and colleagues have demonstrated increased frequency of BAL CD4+FoxP3+ cells during acute lung allograft rejection, but whether higher levels protected against BOS was not examined (26). Enhanced expression of urinary FoxP3 at the mRNA level or by immunohistochemistry in renal transplantation has also been reported during AR of kidney allografts, although the association of Fox P3 expression with outcome of the allograft was inconsistent among the different studies (27, 28). Thus, FoxP3-expressing cells may not be associated with immunological quiescence but instead be actively recruited to the allograft (or FoxP3 upregulated within the allograft) as a response to allograft inflammation.
In addition, we have ascertained in this pilot study that percentages lower than 3.2% FoxP3+ among BAL CD4+ cells within the first year after lung transplantation were associated with the early development of BOS. Percent BAL FoxP3+ cells may serve as a biomarker to identify patients at risk for early development of BOS, enabling physicians to intervene sooner.
It is important to acknowledge a few caveats to the current investigation. As is often true with the collection of clinical biological samples, data was not available at each pre-specified time point due to the clinical condition of the patient or logistics of specimen collection and processing. Nevertheless, the data remains robust despite these missing samples. Additionally, we cannot exclude that different patient phenotypes, the variety of clinical events and different types of immunosuppression may bias our results. However, our analysis shows that there were no statistically different clinical variables (patient characteristics, infections, AR episodes, immunosuppression and timing of sample collection) between these two patient groups (see Table 1 and Supplemental Figure 1). Further analysis of a larger, prospective cohort of patients will help validate these observations.
In conclusion, we have shown that increased percentage of BAL FoxP3+ cells among CD4+ cells in lung transplant recipients is associated with stability of early lung function. Cox regression with a time-dependent covariate in a prospective larger cohort will further test the association between the development of BOS and BAL CD4+FoxP3+ cells. Our data suggest the possibility that BAL CD4+FoxP3+ cells may serve as a potential biomarker for the early development of BOS and that therapeutic interventions centered on augmenting lung recruitment of FoxP3+ cells may be beneficial for the long-term survival of lung allografts.
MATERIALS AND METHODS
Study cohort
This study was approved by the University of Chicago Institutional Review Board and informed consent was obtained from all patients included in this analysis. All lung transplant recipients at our transplant center were approached for inclusion in this study between February 2007 and January 2009, within the first three months after transplantation. The average time to enrollment was 3.1 ± 4.1 months in the stable group and 4.4 ± 3.0 months in the BOS group (p = ns). There were a total of 29 lung transplants performed during this time period. Nine patients did not participate due to the following reasons: language barrier to consent (3), mortality < 30 day (3), patient was too sick to undergo regular bronchoscopies (1) and refusal (2). Twenty lung transplant recipients underwent 62 bronchoscopies and had simultaneous analysis of their peripheral blood. Six (30%) patients developed BOS per criteria specified by the International Society of Heart and Lung Transplantation (29) (ISHLT). Samples collected after development of BOS were not included in this study.
Immunosuppression
Baseline immunosuppression for all patients included tacrolimus (target trough level: 10ng/ml), azathioprine (2mg/kg/day) and prednisone (tapered to 5mg/day by three months post transplantation). Daclizumab induction therapy was administered to all patients per the manufacturer's instructions. Immunosuppression was changed due to declining pulmonary function per the discretion of the physician.
Acute rejection
AR was determined by histological analysis of transbronchial biopsies in accordance with International Society of Heart and Lung Transplant (ISHLT) Guidelines (18). All analyses included both Grade A and Grade B AR episodes.
Bronchoscopy samples
Bronchoscopy specimens were collected during surveillance bronchoscopies at 1, 3, 6, 9 and 12 months post transplantation and during clinical bronchoscopies as deemed necessary by the pulmonary physician (see Supplemental Figure 1). All bronchoscopies were performed by one of two physicians and the bronchoscopic technique was standardized between the two physicians. One 60ml and one 30ml aliquot were instilled into the distal airways and aspirated. In general, 40-50ml of BAL fluid was recovered. BAL samples were pooled and ½ of the BAL sample was used for research purposes and ½ was sent to the microbiology laboratory. All samples are expressed per milliliter as per the recommendation of the ERS task force. BAL samples were centrifuged at 300g and 4°C for 10 minutes. The supernatant was removed, passed through a 1.2μm filter and flash frozen at −80°C for later use. BAL cells were subsequently analyzed by flow cytometry.
Flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by Ficoll-Hypaque density gradient centrifugation (Pharmacia, Sweden). The cell yield was approximately 106 PBMC per milliliter of whole blood.
Surface cell phenotypes from both peripheral blood and BAL were analyzed by three color flow cytometry using monoclonal antibodies to CD3 (APC), CD4 (PE) and CD8 (FITC) and monoclonal antibodies to CD4 (FITC), CD25 (APC) and FoxP3 (PE) (BD Biosciences, San Jose, CA). After surface staining, some samples were fixed, permeabilized, stained with antibodies to FoxP3 or control isotype. Acquisition was performed using an LSR II cytometer equipped with a blue laser (488nm) a red laser (640nm), a violet laser (405nm) and a UV light (355nm). Compensation was performed using samples stained with single fluorochromes. The percentage of FoxP3+ cells among CD4-gated events was determined using the fluorescence minus one (FMO) standard approach.
Enzyme-linked immunosorbent assay analysis
Levels of CCL17 and CCL22 were analyzed using ELISA (R and D Systems, Minneapolis, MN) as per the manufacturer's protocols. Sensitivity threshold was 7pg/ml for CCL17 and 62.5pg/ml for CCL22.
Statistical analysis
Data were expressed as the median with interquartile ranges. Box plots were created to visually display the median (horizontal line within box), the 25th to 75th percentile (box) and 10th to 90th percentile (whiskers). Multiple samples from each patient were averaged and used as a representative value of stability for that patient. Likewise, all episodes of AR were averaged for a given patient and used as a representative value for the patient. Samples from BOS patients were averaged prior to the development of BOS. Data analyses were performed using Mann-Whitney tests due to the limited sample size. Categorical variables were compared using the Fisher exact test or chi square analysis. A threshold value based on the 25th percentile of the percent FoxP3+ cells among all 20 patients was identified. Kaplan Meier analysis was performed using the log rank test to determine if this threshold value would be able to distinguish patients who remained stable from those who developed BOS The P value based on the log-rank test was used to assess the strength of association between BAL CD4+FoxP3+ and BOS status. All data were analyzed by IBM Statistics SPSS version 17.
Supplementary Material
Abbreviations
- AR
acute rejection
- BAL
bronchoalveolar lavage
- BOS
Bronchiolitis obliterans syndrome
- Treg
T regulatory cells
Footnotes
SMB participated in the research design, in the writing of the manuscript and in the data analysis. SMB received Department of Medicine funds from the University of Chicago. SMB has no conflict of interest.
HC participated in the performance of the research. HC has no conflict of interest.
LM provided expert guidance in designing and interpreting the flow cytometry experiments. LM has no conflict of interest.
CL participated in the data analysis. CL has no conflict of interest.
ERG provided clinical samples and reviewed the manuscipt. ERG has no conflict of interest.
WTV provided clinical samples and reviewed the manuscript. WTV has no conflict of interest.
RS and AS provided intellectual input and helped in study design and editing of the manuscript. RS and AS have no conflict of interest.
AC participated in the research design, data analysis and manuscript writing. AC has no conflict of interest.
MLA participated in the research design, in the writing of the manuscript and in the data analysis. MLA has no conflict of interest.
REFERENCES
- 1.Tilney NL, Whitley WD, Diamond JR, Kupiec-Weglinski JW, Adams DH. Chronic rejection--an undefined conundrum. Transplantation. 1991;52(3):389–398. doi: 10.1097/00007890-199109000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166(4):440–444. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 3.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6(8):1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 5.Bobadilla JL, Love RB, Jankowska-Gan E, Xu Q, Haynes LD, Braun RK, et al. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am J Respir Crit Care Med. 2008;177(6):660–668. doi: 10.1164/rccm.200612-1901OC. Epub 2008 Jan 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol. 2006;177(8):5631–5638. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- 7.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 8.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 10.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6(10):2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Ahmed EM, Wang T, Ochando JC, Chong AS, Alegre ML. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182:6217–6225. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slebos DJ, Kauffman HF, Koeter GH, Verschuuren EA, Bij W, Postma DS. Airway cellular response to two different immunosuppressive regimens in lung transplant recipients. Clin Transplant. 2005;19(2):243–249. doi: 10.1111/j.1399-0012.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 14.Meloni F, Vitulo P, Bianco AM, Paschetto E, Morosini M, Cascina A, et al. Regulatory CD4+CD25+ T cells in the peripheral blood of lung transplant recipients: correlation with transplant outcome. Transplantation. 2004;77(5):762–766. doi: 10.1097/01.tp.0000116565.86752.6b. [DOI] [PubMed] [Google Scholar]
- 15.Mamessier E, Lorec AM, Thomas P, Badier M, Magnan A, Reynaud-Gaubert M. T regulatory cells in stable posttransplant bronchiolitis obliterans syndrome. Transplantation. 2007;84(7):908–916. doi: 10.1097/01.tp.0000281408.20686.cb. [DOI] [PubMed] [Google Scholar]
- 16.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2002;38(12-13):881–885. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201(7):1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng L, Wu Z, Wang Y, Lassman C, Busuttil RW, Zhai Y, et al. Differential impact of CD154 costimulation blockade on alloreactive effector and regulatory T cells in murine renal transplant recipients. Transplantation. 2008;85(9):1332–1338. doi: 10.1097/TP.0b013e31816c4f2b. [DOI] [PubMed] [Google Scholar]
- 19.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652–662. doi: 10.1038/ni1333. Epub 2006 Apr 2023. [DOI] [PubMed] [Google Scholar]
- 20.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204(6):1335–1347. doi: 10.1084/jem.20070081. Epub 2007 Jun 1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. Epub 2004 Aug 2022. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006;97(11):1139–1146. doi: 10.1111/j.1349-7006.2006.00307.x. Epub 2006 Sep 1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mailloux AW, Young MR. NK-dependent increases in CCL22 secretion selectively recruits regulatory T cells to the tumor microenvironment. J Immunol. 2009;182(5):2753–2765. doi: 10.4049/jimmunol.0801124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita U, Kuroda E. Regulation of macrophage-derived chemokine (MDC, CCL22) production. Crit Rev Immunol. 2002;22(2):105–114. [PubMed] [Google Scholar]
- 25.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;24:24. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neujahr DC, Cardona AC, Ulukpo O, Rigby M, Pelaez A, Ramirez A, et al. Dynamics of human regulatory T cells in lung lavages of lung transplant recipients. Transplantation. 2009;88(4):521–527. doi: 10.1097/TP.0b013e3181b0e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown K, Wong W. Diagnostic value of regulatory T cells: a new facet of a much studied cell population. Transplantation. 2008;86(11):1485–1491. doi: 10.1097/TP.0b013e31818f3d2a. [DOI] [PubMed] [Google Scholar]
- 28.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353(22):2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 29.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


