Abstract
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome is an uncommon, life-threatening drug reaction. The basic findings are skin rash, multiorgan involvement, and eosinophilia. Most of the aromatic anticonvulsants, such as phenytoin, phenobarbital and carbamazepine can induce DRESS. Herein we report a 14-year-old patient with DRESS syndrome related to carbamazepine use. The patient presented with signs of involvement of the skin, lungs, liver, and microscopic hematuria. Carbamazepine treatment was discontinued; antihistamines and steroids were started. Hyperglycemia, commencing on the first dose of the steroid given, persisted even after the discontinuation of steroids and improvement of other signs. There were no signs of pancreatitis or type 1 diabetes clinically in laboratory tests. Her blood glucose levels were regulated at first with insulin and later with metformin. Within 1 year of follow-up, still regulated with oral antidiabetics, she has been diagnosed with type 2 diabetes. Formerly, long-term sequelae related to “drug rash with eosinophilia and systemic symptoms syndrome” such as hepatic and renal failure, type 1 diabetes mellitus, Grave's disease, autoimmune hemolytic anemia, and lupus have also been reported. However, up to date, no cases with type 2 diabetes have been reported as long-term sequelae. To our knowledge, this is the first case in the literature presenting with type 2 diabetes as long-term sequelae.
Keywords: DRESS syndrome, carbamazepine, pneumonia, type 2 diabetes
Introduction
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome was first described by Bocquet et al. in 1996. DRESS is a life-threatening and seldom-seen drug reaction. The incidence has reported as ranging from 1/1000 to 1/10 000 [1]. Reactions to numerous drugs leading to DRESS syndrome have been observed. However, the most frequent causes are anticonvulsants, sulfonamides, dapsone, allopurinol, minocycline, and gold salts [1–3]. In addition to hematologic, hepatic, cardiac, neurological, gastrointestinal, and endocrine abnormalities, pulmonary involvement is observed rarely [1]. In this study, DRESS syndrome was presented with multiorgan involvement based on carbamazepine use in a 14-year-old female patient. Pulmonary involvement presented in the form of pleurisy and atelectasis, different from the literature, and as a long-term sequela, type 2 diabetes was observed in our case.
Case report
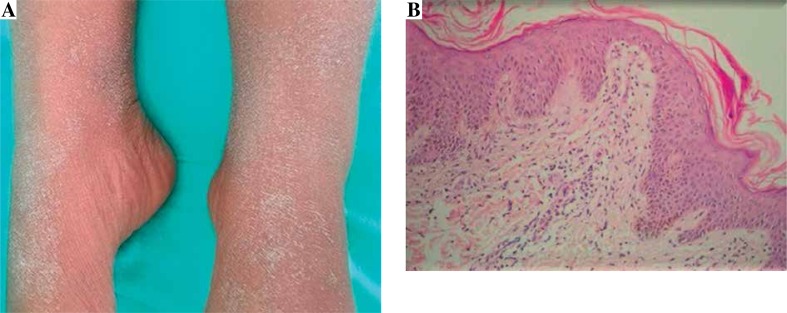
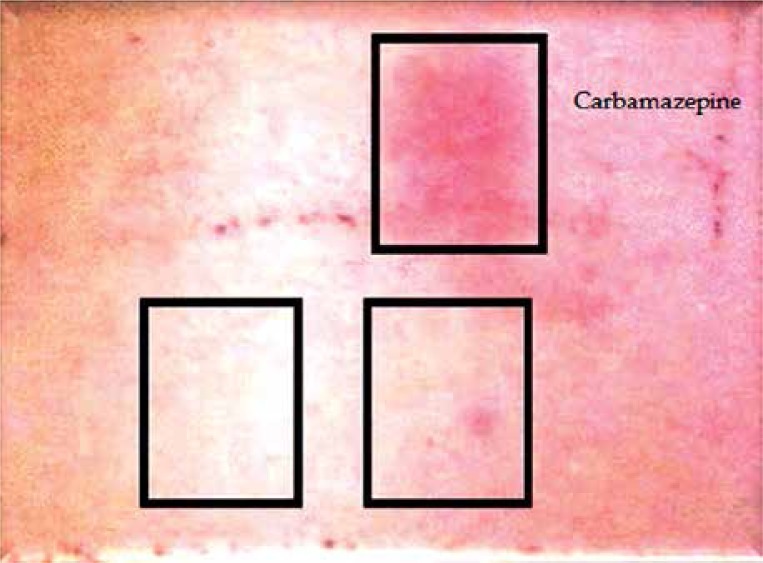
A 14-year-old female patient presented at our clinic due to skin rash and fever lasting for 1 week. It was found in her history that carbamazepine treatment had been initiated owing to epilepsy 15 days before her symptoms began. There had been no transmitted chronic disease history such as viral, bacterial, or parasitic infection before the patient's reaction in question. On the physical examination, she had 38.5°C core temperature, and there was a bilateral crepitant rale condition determined by listening on her respiratory system examination. No lymphadenopathy was determined at the organomegaly and pathologic level. In the dermatological examination, there were maculopapular rashes in the process of healing with desquamation that involved > 50% of the body (Fig. 1A). Her WBC was 24 000/mm3 (eosinophil 23%), ALT was 91 IU/L, and GGT was 123 IU/L. There was microscopic hematuria in her total urinalysis. In the viral serological evaluation of the case, Ebstein-Barr virus (EBV), herpes virus type 1-2, hepatitis A-B-C virus, HIV, and toxoplasma were established as negative. Anti-nuclear antibodies (ANA) and Anti ds-DNA were negative. Immunoglobulins, C3 and C4 levels, urinalysis were normal. In the punch biopsy obtained from the skin lesions, lymphocyte infiltration was identified in the papillary dermis (Fig. 1B). Due to the drug intake history and the clinical, laboratory, and histopathological findings, carbamazepine treatment was discontinued in our patient. Desloratadine antihistaminic 5 mg/day treatment and systemic steroid 1 mg/kg/day (40 mg/day) were added to the therapy. Owing to the chest pain and the reduction in lung sounds in basals and since the sinuses were monitored as closed in the chest radiography, thorax ultrasonography was carried out, and bilateral pleural effusion 6 mm thick was determined. In the thorax CT performed on the patient, a local consolidated area in the middle lobe segment of the right lung, atelectatic changes in the lower lobes of both lungs, and thickness in the left fissure were seen. Since there was symptomatic hyperglycemia on the second day of steroid treatment, the steroid was discontinued. The patient was found hyperglycemic (fasting blood glucose: 120-180 mg/dl, postprandial blood glucose:160-250 mg/dl). The 33-year-old mother was diagnosed with type 2 diabetes at the age of 26 years. Physical examination revealed a weight of 52.8 kg (64th percentile, 0.38 SDS), a height of 151 cm (8 percentile, –1.38 SDS), body mass index of 23.56 (1.52 SDS) and normal vital signs. Islet cells cytoplasmic autoantibodies (ICA) were negative as tested by indirect immunofluorescence and her glutamic acid decarboxylase autoantibodies (GADA), which was measured by radioimmunoassay, were also negative and fasting levels of C-peptide and insulin (Electrochemiluminescent immunoassay; Roche Diagnostics, Penzberg, Germany) remained detectable throughout the observation period (C-peptide 2.1-6.23 ng/ml). With an HbA1c value of 8.6%, she was diagnosed with diabetes mellitus. The combination of long-standing non-ketotic hyperglycemia, glycosuria, at a relatively high fasting and postprandial blood glucose, and negative pancreatic auto-antibodies in a child with a diabetic mother raised the possibility of MODY. Direct DNA sequencing of all exons and intron-exon bounders of the MODY genes revealed no mutation in our patient. Insulin treatment was carried out due to persisting hyperglycemia. Insulin treatment was discontinued as the hyperglycemia disappeared, and metformin treatment was initiated with the type 2 diabetes diagnosis. The patient was discharged from the hospital after the lung, skin, liver, and renal findings regressed. A patch test was performed with carbamazepine 10% concentration 6 weeks later. As a result of the evaluation carried out 48, 72, and 96 h later, +1 sensitivity was determined (Fig. 2). Although the skin, liver, renal and lung findings resolved during the 1-year follow-up period, regulation of type 2 diabetes with an oral antidiabetic continued.
Fig. 1.
A) Maculopapular lesion in the recovery process with desquamation on the erythematous base. B) Skin biopsy determined by lymphocytic infiltration in the papillary dermis
Fig. 2.
Epicutaneous patch test with carbamazepine
Discussion
DRESS syndrome, known as drug hypersensitivity syndrome, is a quite rare acute, idiosyncratic, and life-threatening drug reaction characterized by fever, skin rash, and single or multiple internal organ involvement [3, 4]. There is an average 3- to 9-week latent period (0.5-16 weeks) between drug use and the emergence of symptoms [5]. These findings can persist or exacerbate although the responsible drug is discontinued [1, 3, 5]. Fever, skin rash, liver involvement, hypereosinophilia, and lymphadenopathy can be seen in almost all patients. Symptoms had appeared 2 weeks after carbamazepine treatment was initiated in our patient. There was pulmonary involvement presented by pulmonary atelectasia and pleurisy as well as maculopapular rash, fever, and hypereosinophilia, liver involvement manifested with transaminase elevation, and renal involvement was characterized by microscopic hematuria in the case.
The pathophysiology of DRESS syndrome has not been entirely clarified yet. It has been suggested that there is a delayed hypersensitivity reaction related to T lymphocytes against toxic metabolites of drugs, and viral infections (EBV, human herpes virus, types 6 and 7) can also play a role in the etiology. It has been reported in the literature that there can be a genetic predisposition, and families of patients diagnosed with DRESS syndrome are also at risk in terms of this syndrome. It has been maintained in recent years that human herpes virus type 6 reactivation can also be used as a diagnostic marker [1, 3, 6, 7]. The viral serological study carried out in our patient was identified as negative. No similar history was established in her family. The positive result on the patch test with carbamazepine conducted 6 weeks later supports that there was a delayed hypersensitivity reaction.
The main principle in the treatment of DRESS syndrome is to discontinue the drug or drugs thought to be suspicious immediately and provide supportive care. Steroid and IVIG can be utilized in the treatment. Systemic steroid is particularly recommended in internal organ involvement [2, 3, 7]. It has also been stated in cases that parenteral pulse steroid treatment is beneficial [8]. Systemic steroid and antihistamine treatment were applied to our patient. Hyperglycemia thought to be dependent on steroid treatment in the first place preceded although steroid treatment was discontinued and insulin treatment had to be commenced. Type 1 diabetes and pancreatitis have been reported in patients with DRESS syndrome in the literature [6, 9]. However, our patient's lipase level was normal, and no laboratory finding supporting type 1 diabetes was diagnosed in our patient. Although the hyperglycemia finding in the patient was not linked to DRESS syndrome in the first place, it was evaluated as DRESS syndrome related to type 2 diabetes by the endocrine department since it had a clinical table regulated by oral antibiotic in 1-year follow-up period.
In the literature, various long-term sequelae concerning DRESS syndrome have been observed. In a study by Chen et al. [9], a long-term sequelae rate was reported as 11.5% in analyses of 52 patients of whom 9 were lost and 43 recovered. The researchers reported that a total of 4 patients had autoimmunity disease, including 2 with Grave's disease, 1 with type 1 diabetes, and 1 with autoimmune hemolytic anemia. They also observed that lifetime hemodialysis was necessary due to renal failure in 2 patients with organ involvement. In a study in which 34 patients were evaluated, however, the researchers reported that autoimmune disease developed in 2 patients, including 1 with lupus erythematosus and 1 with autoimmune thyroiditis [10]. Duboıs-Laforgue et al. [11] reported 11 patients with type 1 diabetes related to DRESS syndrome and observed that half of the patients developed non-autoimmune fulminant type 1 diabetes. However, no case of type 2 diabetes associated with DRESS syndrome has been reported thus far. To the best of our knowledge, this case is the first in whom DRESS syndrome initially developed followed by type 2 diabetes.
DRESS syndrome is an acute, life-threatening, and rarely seen drug reaction. It should be considered a definitive diagnosis in patients with fever, diffuse skin rash, and internal organ involvement. The drugs used by patients should be questioned. It should be kept in mind that a variety of organ involvement as well as long-term sequelae can also be seen in patients with DRESS syndrome. Our case showed that one of these sequelae could be type 2 diabetes.
Authors declare no conflict of interest.
References
- 1.Cacoub P, Musette P, Descamps, et al. The DRESS Syndrome: A Literatüre Review. Am J Med. 2011;124:588–597. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Park HK, Heo J, et al. Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) Syndrome Induced by Celecoxib and Anti-tuberculosis Drugs. J Korean Med Sci. 2008;23:521–525. doi: 10.3346/jkms.2008.23.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giri PP, Roy S, Bhattyacharya S, et al. DRESS Syndrome With Sepsıs, Acute Respıratory Dıstress Syndrome And Pneumomedıastınum. Indian J Dermatol. 2011;56:763–765. doi: 10.4103/0019-5154.91850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourgeois GP, Cafardi JA, Groysman V, Hugey LC. A review of DRESS-associated myocarditis. J Am Acad Dermatol. 2012;66:229–236. doi: 10.1016/j.jaad.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Alkhateeb H, Said S, Cooper CJ, et al. DRESS syndrome following ciprofloxacin exposure: An unusual association. Am J Case Rep. 2013;14:526–528. doi: 10.12659/AJCR.889703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou CC, Liang L, Fu JF. Type 1 diabetes mellitus in a child with phenobarbital hypersensitivity syndrome. J Endocrinol Invest. 2008;31:360–363. doi: 10.1007/BF03346371. [DOI] [PubMed] [Google Scholar]
- 7.Omairi NE, Abourazzak S, Chaouki S, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) induced by carbamazepine: a case report and literature review. Pan African Medical Journal. 2014;18:9. doi: 10.11604/pamj.2014.18.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natkunarajah J, Goolamali S, Craythorne E, et al. Ten cases of drug reaction with eosinophilia and systemic symptoms (DRESS) treated with pulsed intravenous methylprednisolone. Eur J Dermatol. 2011;21:385–391. doi: 10.1684/ejd.2011.1300. [DOI] [PubMed] [Google Scholar]
- 9.Chen YC, Chang CY, Cho YT, et al. Long-term sequelae of drug reaction with eosinophilia and systemis symptoms: a retrospective cohort study from Taiwan. J Am Acad Dermatol. 2013;68:459–465. doi: 10.1016/j.jaad.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Ushigome Y, Kano Y, Ishida T, et al. Short and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721–728. doi: 10.1016/j.jaad.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Dubois-Laforgue D, Moachon L, Laude H, Timsit J. Fulminant type 1 diabetes in the course of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome. Diabetes Care. 2013;36:68. doi: 10.2337/dc12-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]