Abstract
The aim of the study was to evaluate analgesic activity (“hot plate” test), anti-inflammatory activity (carrageenan-induced paw edema) and locomotor activity in rats under the influence of three fractions of Chelidonium majus herb extract: full water extract (FWE), protein enriched fraction (PEF), and non-protein fraction (NPF). Effects of the fractions on the level of chosen cytokines and their mRNA levels were also assessed using lipopolysaccharide (LPS) administration as a proinflammatory cue. All fractions and diclofenac did not affect the locomotor activity of rats in comparison with the control group. FWE and PEF three hours after administration showed statistically significant analgesic activities comparable to morphine (p < 0.05). A slight reduction in rat paw edema was observed after three (comparable with diclofenac) and six hours in the NPF group. FWE revealed a statistically significant pro-inflammatory effect after three hours in comparison with the control group. Peripheral IL-1 and IL-4 cytokine concentrations were reduced under FWE and NPF, PEF fractions. The combination of FWE, PEF and NPF together with LPS showed only the effects of LPS. We suggest that protein enriched fraction (PEF) produced centrally mediated (morphine-like) analgesic action, whereas the anti-inflammatory potential was shown only after LPS-induced inflammation. The precise mechanisms involved in the production of anti-nociceptive and anti-inflammatory responses of studied fractions are not completely understood, but they may be caused rather by the presence of protein more than alkaloids-enriched fraction. This fraction of the extract could be used as an alternative therapy for the prevention of inflammatory-related diseases in the future, but further studies are needed.
Keywords: LPS–induced rats, analgesic, anti-inflammatory, Chelidonium majus aqueous extract, non-protein fraction, protein enriched fraction, proinflammatory cytokines, mRNA
Introduction
Pain occurring in acute or chronic diseases is a very common challenge in medical care. Thus, pharmacotherapy of pain is one of the top priorities. This is even more important, because due to the fact that opioids and nonsteroidal anti-inflammatory drugs are among the most commonly used drugs in clinical practice, their use subsequently can induce unexpected drug interactions and/or several types of medicines often produce several adverse reactions [1, 2]. A very interesting option in this field may be a plant extract from Chelidonium majus (CM) which has been traditionally used in the treatment of skin diseases such as eczema, ringworm, oral infection, pains and nervous disorders and gastrointestinal diseases [3, 4]. It has been shown that CM extracts have anti-inflammatory, choleretic, antimicrobial, antiviral, antitumor, analgesic, anti-spasmodic and hepatoprotective properties [5–7]. Chelidonium majus (greater celandine) belongs to Papaveraceae and it is distributed throughout the world, including Europe, Asia, Northwest Africa and North America [5]. The aerial part of this plant contains isoquinoline alkaloids, such as chelidonine, chelerythrine, sanguinarine, berberine, coptisine and stylopine [4, 7, 8]. Moreover, this herb includes organic acids, carotenoids, flavonoids and proteins [9–11]. Studies showed that methanolic extract from the herb of CM significantly suppressed the progression of collagen-induced arthritis (mice model) and that this action was characterized by the decreased production of tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), interferon γ (IFN-γ), B cells, γδ T cells (in spleen) and increased proportion of CD4+CD25+ regulatory T cells in vivo. Moreover, it was observed that in the serum of mice, the levels of IgG and IgM RA factor were decreased [12]. Furthermore, recent studies showed that hydroalcoholic extracts and its alkaloids, especially berberine, chelidonine and sanguinarine have a significant hERG potassium channel blocking effect [5]. Other results revealed that the aqueous extract of Ch. majus suppressed glycine and gamma-aminobutyric acid (GABA), activated ion currents and elevated glutamate-activated ion currents in rat periaqueductal gray neurons, which represent a key structure of the descending pain control system [13, 14]. Other studies showed that berberine, one of isoquinoline alkaloids from CM, possesses neuroprotective action in an animal model of various CNS diseases [3, 15] and showed an antinociceptive effect on visceral hypersensitivity in rats [16]. Berberine was found to completely block both morphine-induced locomotor sensitization and analgesic tolerance, and reduce D(1) and NMDA receptor bindings in the cortex of mice [17]. According to Yoo et al. [17], berberine should be viewed as a potential novel means of attenuating morphine-induced sensitization and analgesic tolerance. However there are no data on the analgesic effect of fractions of CM extract. Thus, the aim of the present study was to evaluate: (1) analgesic activity using “hot plate” test, (2) anti-inflammatory activity in a model of carrageenan-induced paw edema, (3) locomotor activity of three fractions of the extract: full water extract (FWE), protein enriched fraction (PEF), and non-protein fraction (NPF) in rats. Moreover, effects of the fractions on level of some cytokines (IL-1, IL-4, IL-6) and their mRNA levels were also assessed.
Material and methods
Plant material
Plant material, aerial parts of Ch. majus L. cvar. Cynober, were collected from a controlled cultivation at the Institute of Natural Fibers and Medicinal Plants in Poznan, Poland. The field cultivation was established in spring by sowing seeds directly into ground (lessivé soil with granulometric composition of light loamy sands). The herb was harvested during beginning of flowering of plants. The raw material was dried at 55-60°C. The material was identified at the Department of Medicinal and Cosmetic Natural Products (Faculty of Pharmacy, Poznan University of Medical Sciences).
Phytochemical study
Extract preparation
Full water extract (FWE)
10 kg of raw plant material was extracted with water by percolation (3 h, 25 l/min). The extract was concentrated under vacuum to 1/3 volume and fractionated with acetone (4: 1).
Non-protein fraction (NPF) and protein enriched fraction (PEF)
10 kg of raw plant material was powdered and extracted with water (90 l, 90°C) by liquid-solid extraction (FT-29, Armfield, England). The extract was concentrated under vacuum to 1/3 volume. In the concentrated and clear extract, proteins were precipitated with cold acetone (4: 1; –20°C; 30 min). The protein suspension was centrifuged and the purified protein fraction was suspended in distilled water and then it was concentrated under vacuum. The supernatant, which was obtained after precipitation of proteins, followed the same procedure. The fractions were freeze-dried and frozen at –50°C (36 hours).
Determination of alkaloids by HPLC-DAD
Alkaloids were analyzed by means of modified methods: European Pharmacopea 6 (monography of greater celandine) and Sárközi et al. [18]. Sample: 0.5-1.0 g of dry greater celandine was weighted in a 250 ml round-bottomed flask and heated under reflux condenser for 30 min in 150 ml of 12% V/V glacial acetic acid. The sample was cooled down and diluted with the same solution to 200 ml. 6.0 ml of concentrated ammonium and 200.0 ml of methylene chloride was added to 60.0 ml of filtrate. The solution was shaken for 30 min. The organic fraction was evaporated to dryness in vacuum at a temperature not exceeding 40°C. The residue was dissolved in 5.0 ml of methanol. HPLC-DAD analysis was performed on an Agilent 1100. Separation of the methanolic sample was prepared on ZORBAX Poroshell 120 SB-C18 (Agilent) 3 × 100 nm (2.7 µm). The column temperature was: 40°C. The volume of injection was 50 µl. A gradient mixture of: phase A: 30 mM ammonium formate (pH = 2.8) and phase B: acetonitrile: methanol 14.7: 18.0 (V: V) were used as eluent, starting from 20% phase B to 60% phase B in 16 min. The flow-rate was: 0.50 ml/min. Peaks were identified by the addition of standard solutions and by UV-VIS spectra. Quantitative determination for coptisine and chelidonine was performed at 240 nm, for sanguinarine and chelerythrine – 280 nm and for berberine – 345 nm by an external standard method. Standard solutions were prepared in methanol.
Pharmacological study
Rats
Experiments with rats were performed in accordance with the Polish governmental regulations (Journal of Laws of 2005, no. 33, item 289). The study was conducted in accordance with the ethical guidelines for investigations in conscious animals and the study protocol was approved by the Local Ethics Committee (61/2010). The experiments were performed on six week-old male Wistar rats, housed in controlled room temperature (20 ±0.2°C) and humidity (65-75%) under a 12 h: 12 h light-dark cycle (lights on: 7 a.m.). Animals were kept in groups (8-10 rats/group) in light plastic cages (60 × 40 × 40 cm) and given ad libitum access to standard laboratory chow (pellets-Labofeed B) and tap water.
Treatments and groups
Experiments were performed on male Wistar rats housed in controlled conditions. The extracts were administered intragastrically (p.o.) at a dose of 200 mg/kg b.w. (groups FEW, PEF, NPF); morphine (5 mg/kg b.w., s.c.) and diclofenac (50 mg/kg b.w., i.p.) were used as standard analgesic drugs, control animals were treated with vehiculum (water) (p.o.). In order to measure locomotor activity (n = 53) and to perform the hot plate test (n = 40) 6 groups of rats were used (FEW, PEF, NPF, morphine, diclofenac 50, vehiculum (water) (p.o.). Anti-inflammatory activity was measured in a model of carrageenan-induced raw paw edema using 5 groups of rats (FWE, PEF, NPF, diclofenac, vehiculum (water)). Lipopolysaccharide (LPS) from Escherichia coli serotype 026:B6, (Sigma Chemicals Co.) was dissolved in an aqua pro injectione and administered in a single dose of 100 µg/kg intraperitoneally (i.p.) according to Manikowska et al. [19].
Measurement of locomotor activity
Locomotor activity assessment was performed with a licensed activity meter (Activity Cage, Ugo Basile, Italy) by placing the animals in the center of the apparatus and recording their horizontal activity. The data obtained were expressed as signals corresponding to animal movements for 5 min. The locomotor activity was measured 60 min after the administration of a single dose of extracts, or reference drugs or vehiculum. Any distracting factors were reduced to the minimum (noise, presence of people, presence of other rats).
Hot plate test
Rats were brought to the testing room and allowed to acclimate for 10 minutes before the test. Pain reflexes in response to a thermal stimulus were measured using a Hot-Plate Analgesia Meter. Each animal was tested only once and was not habituated to the apparatus prior to testing. The hot plate test was carried out in groups of rats using a hot plate apparatus (20 × 20 cm surrounded by a clear acrylic cage) maintained at 48 ±2°C. The extracts or reference drugs were given and the effects were measured at 3.0 and 6.0 h following the application. Using timer, the latency to respond was observed with a hind paw lick, hind paw flick or jump which was measured to the nearest 0.1 second. Subsequently, the mouse was immediately removed from the hot plate and returned to its home cage. The maximal time of the response was established as 30 seconds. Morphine (30 min before the test) treated animal group was included as positive controls. The cut-off time was 60 s in order to minimize skin damage.
Carrageenan-induced paw edema
Skin inflammation was induced in the right hind paw of rats by the topical application of 2 mg/paw of carrageenan dissolved in 0.2 ml of 0.9% saline solution. The rear left paw of the rats, which was used as the control, received the same volume of 0.9% saline solution. Single doses of the extract (dissolved in water) 60 min after carrageenan injection. For comparison (positive control), one group of rats was treated with the acute diclofenac injection 60 min after carrageenan administration. The rate of edema of the two paws was measured at 3.0, 6.0 h after carrageenan injection using a plethysmometer (Hugo Sachs Electronic, Germany).
Change of rat's paw thickness was evaluated using the following equation:
ΔG = (Lc – Lw) – (Rc – Rw),
ΔG – value expressing the change in paw's thickness against baseline (before inflammation),
Lw – left paw's thickness before carrageenan injection,
Rw – right paw's thickness before carrageenan injection,
Lc – left paw's thickness 3.0 or 6.0 h after carrageenan injection,
Rc – right paw's thickness 3.0 or 6.0 h after carrageenan injection.
Measurement of cytokine levels
On the next day after analgesic and anti-inflammatory activity tests, the extracts were given again and 60 min later, each group (treated with extracts or diclofenac) was divided into two subgroups, where one subgroup was administered a single dose of LPS (100 µg/kg, i.p.) and the remaining rats were injected with vehicle (aqua pro injectione). After next 60 min rats were sacrificed, the blood was collected and centrifuged at 4000 rpm for 15 minutes and the serum was separated and stored at –80°C for further measurements. Biochemical measurements were done by means of the ELISA method using commercially available kits (RayBiotech Inc.). These tests comprised recombinant cytokines from Escherichia coli and antibodies against rats IL-1, IL-4, and IL-6. The results were calculated based on the absorbance of complex cytokines-antibodies and concentrations were obtained from model curves.
Influence of protein and non-protein fraction extracts from Chelidonium majus L. on mRNA levels of studied genes
From the peripheral blood of rats, mononuclear cells (MNCs) were isolated via a gradient centrifugation in Ficoll. From the resulting cell pellets a total RNA was isolated using TriPure Isolation Reagent (Roche) according to the manufacturer's protocol. The integrity of RNA was visually assessed electrophoretically and spectrophotometrically (BioPhotometer Eppendorf). 1 µg of total RNA from all samples was used for reverse transcription into cDNA using Transcriptor First Strand Synthesis Kit (Roche), according to the manufacturer's protocol, then they were stored at –20°C or used directly for quantitative real-time PCR (qRT-PCR).
The resulting cDNA will be a template for PCR reaction in real time, to be conducted in the capillaries in the LightCycler® using the reagent kit of the LightCycler® FastStart DNA Master SYBR Green I (Roche). IL-1, IL-6, TLR-1 mRNAs levels were analyzed by two-step quantitative real-time PCR (qRT-PCR), in a volume of 10 µl reaction mixture, using relative quantification methodology with a LightCycler TM Instrument (Roche, Germany) and a LightCycler Fast Start DNA Master SYBR Green I kit (Roche Applied Science), according to the manufacturer's instructions. All primer sequences were self-designed using Oligo 6.0 software (National Biosciences) and verified by the electrophoretic assessment and by a single temperature dissociation peak (melting curve analysis) of each cDNA amplification product. A GAPDH gene was used as a housekeeping gene (endogenous internal standard). Standard curves were prepared from dilution of cDNA and generated from a minimum of four data points for each quantified gene. All quantitative PCR reactions were repeated twice. Data were evaluated using LightCycler Run 4.5 software (Roche Applied Science). Each PCR run included a non-template control to detect potential contamination of reagents.
Statistical analysis
All values were expressed as means ± SEM. Statistical comparison of the results was carried out using one-way analysis of variance (ANOVA), followed by Fisher's least significant difference post hoc test for detailed data analysis. A p value of < 0.05 was considered as statistically significant.
Results
Phytochemical study
Phytochemical analysis showed coptisine, chelidonine, berberine and chelerythrine in all fractions, but sanguinarine was observed only in PEF (Table 1). The HPLC analysis revealed that the highest concentration of berberine (23.4 mg/100 g) and coptisine (628.9 mg/100 g) was identified in FWE, chelidonine (134.3 mg/100 g) and chelerythrine (1.16 mg/100 g) – in NPF.
Table 2.
Analgesic and anti-inflammatory activities of fractions of water extract of Ch. majus
| Group | Locomotor activity test | Hot plate test | Carrageenan-induced paw edema | ||
|---|---|---|---|---|---|
| [number of impulses/5 min] | time [s] | ΔG [ml] | |||
| t = 3 h | t = 6 h | t = 3 h | t = 6 h | ||
| control | 646 ±92 | 31.9 ±2.3 | 39.9 ±2.9 | 1.62 ±0.09 | 1.51 ±0.10 |
| morphine | 325 ±63** | 44.2 ±1.2** | 53.2 ±2.6** | – | – |
| diclofenac | 703 ±64 | 39.7 ±6.4 | 47.4 ±2.3 | 1.25 ±0.10 | 0.75 ±0.10** |
| FWE | 499 ±63 | 51.7 ±2.7** | 48.9 ±1.5* | 2.15 ±0.08** | 1.73 ±0.08 |
| PEF | 662 ±66 | 50.1 ±3.1** | 42.8 ±3.0 | 1.94 ±0.13 | 1.90 ±0.10 |
| NPF | 714 ±82 | 40.7 ±6.1* | 45.2 ±5.7 | 1.50 ±0.19 | 1.21 ±0.18 |
values are means ± SEM; n = 6-7 in each group
FWE – full water extract; PEF – protein enriched fraction; NPF – non-protein fraction; t – time after administration of the substance (3 h and 6 h); ΔG – value expressing change in paw's thickness against baseline (before inflammation) after 3/6 h
significant difference vs. control group; p < 0.05 or p < 0.1, respectively
Measurement of locomotor activity
A one-way ANOVA (F(5,47) = 4.51; p < 0.002) analysis revealed that only morphine resulted in a significant, inhibitory effect against locomotor activity of rats expressed as their horizontal spontaneous activity (p < 0.05). All fractions (FWE, PEF, NPF) and diclofenac did not affect the locomotor activity of rats in comparison with the control group (Table 2). Therefore, it can be stated that they exclude the effect of sedation to the results obtained in further studies. Sedative effects were observed only for morphine, which is consistent with previous knowledge.
Table 1.
HPLC quantification of alkaloids in extracts of Ch. majus
| Rf | Compound | FWE (mg/100 g) |
NPF (mg/100 g) |
PEF (mg/100 g) |
|---|---|---|---|---|
| 8.8 (9) | coptisine | 628.9 | 509.2 | 404.3 |
| 8.4 | chelidonine | 109.7 | 59.3 | 134.3 |
| 10.4 | sanguinarine | – | – | 19.0 |
| 10.7 | berberine | 23.4 | 17.1 | 17.7 |
| 11.1 | chelerythrine | 0.67 | 1.16 | 0.29 |
FWE – full water extract; PEF – protein enriched fraction; NPF – non-protein fraction
Hot plate test
A one-way ANOVA revealed significant differences in analgesic activity of all substances (F(5,31) = 3.62; p < 0.01). However, no effect of time on the analgesic effects was noted (F(1,31) = 2.20; p < 0.16). At the same time, analysis of the interaction of both factors in this test indicated the significance of the action of both (F(5,31) = 3.00; p < 0.02). Detailed post-hoc analysis of the time of the pain response in animals (licking hind paw) after application of each substance compared to the control group shows that morphine has a statistically significant analgesic effect (p < 0.05), both after 3 and 6 hours after application. However, diclofenac did not show significant analgesic activity in any of the tested time intervals. It was found out that three hours after administration of the FWE and PEF, a statistically significant analgesic effect was observed (p < 0.05), whereas the NPF showed only a tendency to the analgesic effect (p < 0.1). It was also noted that these substances showed activity comparable to morphine, because there were no specific differences in relation to the opioid. After 6 hours, the PEF and the NPF, in addition to a low tendency of the FWE (p < 0.1), did not show significant analgesic activity compared to the control (Table 2).
Carrageenan-induced paw edema
A one-way ANOVA revealed significant differences in anti-inflammatory activity of all substances (F(4,55) = 11.6; p < 0.001). The influence of time on the anti-inflammatory activity (F(1,55) = 72.9; p < 0.001) was also observed. At the same time, analysis of the interaction of both factors in this test indicated the significance of the action of both F(4,55) = 7.94; p < 0.001). The detailed post-hoc analysis revealed a statistically significant anti-inflammatory activity of diclofenac after six hours, because a statistically significant diminishing of the values compared to control (p < 0.05) was shown. The reduction of rat paw edema was observed after three hours for diclofenac, and after three and six hours for NPF, but differences were statistically non-significant (p > 0.05). Moreover, it was noted that FWE after three hours resulted in a statistically significant pro-inflammatory effect in comparison with the control group (p < 0.05). Other substances did not show a significantly differences when compared with the control group (Table 2).
Peripheral cytokine concentration changes
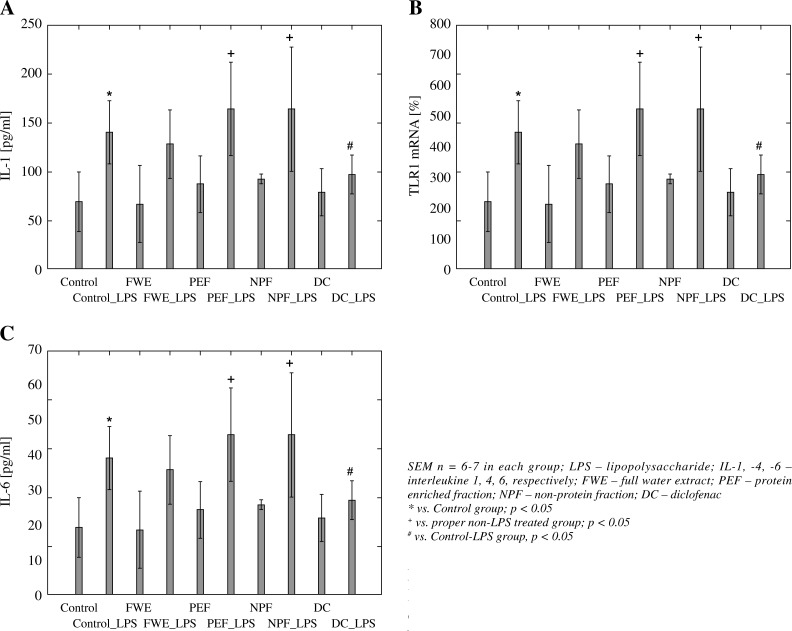
A one-way ANOVA revealed significant differences between the groups in the levels of IL-1 (F(9,50) = = 7.01, p < 0.001) (Fig. 1, Panel A). LPS treatment led to a significant increase in the cytokine concentration in rats when compared with the control group (p < 0.05). Diclofenac (DC) showed a significant reduction in IL-1 concentration only in LPS-induced rats in comparison to control-LPS-treated animals (p < 0.05). Moreover, the combination of FWE, PEF and NPF together with LPS showed only the effects of LPS since the differences were significant only when compared with the proper extract of non-LPS treated group (p < 0.05), whereas there were no statistical significant differences in IL-1 level in comparison to control LPS-treated animals (p > 0.05).
Fig. 1.
LPS-induced IL-1 (panel A), Il-4 (Panel B) and IL-6 (Panel C) serum levels of rats treated with fractions of water extract of Ch. majus. Data are expressed as means
Similarly, the one-way ANOVA revealed significant differences between the groups in the levels of IL-4 (F(9,50) = 3.01, p < 0.01) (Fig. 1, Panel B). All the effects were the same as observed for the above mentioned concentrations of IL-1.
On the contrary, the effects of extracts and DC administration on IL-6 level differed when compared with IL-1 and IL-4 concentrations. It was found that a one-way ANOVA revealed significant differences between the groups in the levels of IL-6 (F(9,50) = 4.05, p < 0.001) (Fig. 1, Panel C). However, detailed statistical analysis showed that both FWE and PEF produced anti-inflammatory activities similarly as DC, since FWE-LPS and PEF-LPS groups had lower IL-6 levels when compared with control-LPS group (p < 0.05). The effect is probably produced mainly by proteins presented in the extracts, because NPF-LPS did not show such activity when combined with LPS-treated rats (p > 0.05).
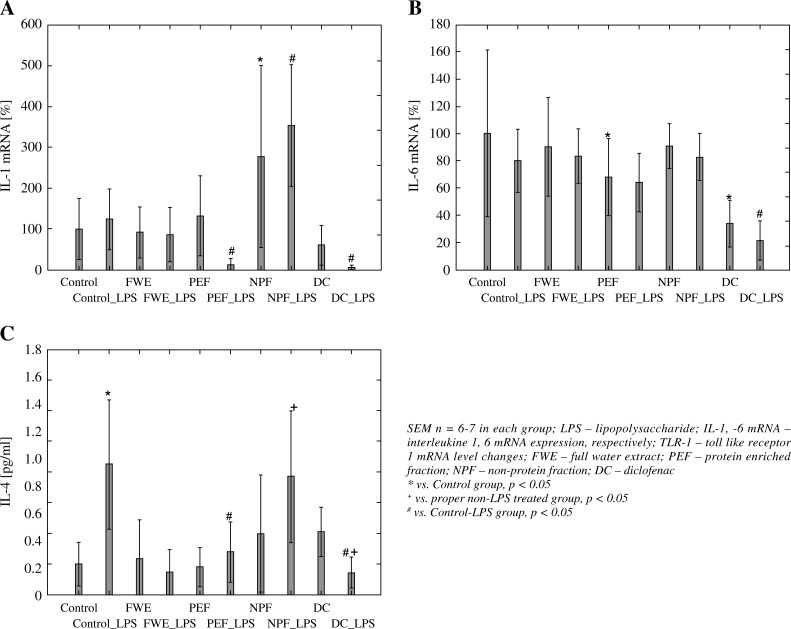
Cytokine (IL1B, IL6, TLR1) mRNA level changes
A one-way ANOVA revealed significant differences between the groups in the expression of interleukin 1B (IL1B) mRNA (F(9,48) = 7.40, p < 0.001) (Fig. 2, Panel A). In control rats the LPS administration (Control-LPS group) caused a slight, insignificant elevation of IL-1B mRNA level (p > 0.05) vs. non-LPS treated control rats (Control). Diclofenac (DC) showed a significant reduction in IL-1B mRNA expression only in LPS-induced rats in comparison to control LPS-treated animals (Control-LPS) (p < 0.05). The whole aqueous extract of Ch. majus herb (FWE) did not cause any statistically significant changes in the level of IL1B transcripts both in LPS-treated and LPS-non-treated rats On the contrary, PEF combined with LPS significantly lowered (by 86%) transcription of IL1B encoding gene (p < 0.05), whereas in non-LPS-treated rats, no such effect was found. However, both a combination of NPF with or without LPS produced a very strong, statistically significant induction of IL-1B mRNA transcription (by 250 and 178%, respectively) when compared with proper control groups (p < 0.05).
Fig. 2.
LPS-induced IL-1 mRNA (panel A), IL-6 mRNA (Panel B) and TLR1 mRNA (Panel C) expression of rats treated with fractions of water extract of Ch. majus. Data are expressed as means
A one-way ANOVA revealed significant differences between the groups in the expression of interleukin 6 (IL6) mRNA (F(9,50) = 4.68, p < 0.001) (Fig. 2, Panel B). Detailed post-hoc analysis showed only PEF a statistically significant decrease of IL 6 mRNA expression when compared with the control group (p < 0.05). Moreover, DC both in LPs-treated and non-LPS-treated rats lowered significantly the transcription of IL6 encoding gene (p < 0.05). The rest of the group did not produce statistically significant effects (p > 0.05).
A one-way ANOVA revealed significant differences between the groups in the expression of toll-like receptor 1 (TLR-1) mRNA (F(9,50) = 5.66, p < 0.0001) (Fig. 2, Panel C). It was found that LPS induction produced a very strong and statistically significant elevation of TLR1 mRNA (by 338%) when compared with control rats (p < 0.05). On the contrary, PEF and DC administration in LPS-treated animals showed a strong decreasing effect in comparison to Control-LPS group (p < 0.05). Moreover, the administration of NPF in LPS receiving rats caused a very strong elevation of TLR1 mRNA level (by 300%) when compared with the Control-LPS group (p < 0.05).
Discussion
Pain and inflammation occur in many diseases at different stages and are among the most important therapeutic goals of modern medicine. In addition to medications used for a long time, we are still looking for new drugs that are more effective in use, but also exhibit insignificant side effects. Several models of nociception and inflammation induced in animals are known and can be used to evaluate the analgesic and anti-inflammatory activities of new compounds and crude extracts from plants [20–22]. Thus, the aim of the present study was to evaluate analgesic and anti-inflammatory activities in various rat's models and assessment of the impact of full water extract (FWE), protein enriched fraction (PEF), non-protein fraction (NPF) of Ch. majus on the level and gene expression for selected mediators of inflammation.
In the present study, it was observed that single application of FEW, NPF and PEF did not affect the locomotor activity of rats, similarly as diclofenac, only morphine resulted in a significant, inhibitory effect against locomotor activity of rats expressed as their horizontal spontaneous activity. The sedative effect of morphine is consistent with the pharmacological profile of this opioid drug [23, 24].
In our study, to investigate the pain response the hot plate test was used. This test is a good model for the study of drugs acting through the central and supramedullary mechanisms of nociception [25, 26]. It was observed that after three hours the FEW and PEF exerted a pronounced analgesic effect, which strength was comparable to that of morphine. We found that the FEW contains many alkaloids, among which berberine is the major component (23.4 mg/100 g). Recent studies have shown that water extract of Ch. majus at 200 mg/kg dose, produced higher analgesic activity than aspirin at 90th minute (tail-flick test on mice) [27]. Moreover, it was observed that berberine have antiallodynic effects after streptozotocin (STZ)-induced diabetes using a rat model [28], and could mitigate allodynia induced by chronic constriction injury of the sciatic nerve in rats (a neuropathic pain model) [29]. Furthermore, Citoglu et al. [27] showed that chelidonine (other major alkaloid in CM) produced higher analgesic activity than aspirin (tail-flick test on mice). Thus, it can be concluded that berberine and chelidonine may be responsible for the analgesic effect in our study. On the other hand, it was observed that PEF also showed an analgesic effect, although the mechanism of action of these proteins is not known.
Most authors using different experimental models of inflammation in vitro, demonstrated anti-inflammatory activity of Ch. majus extracts [12, 30]. It was found that mainly alkaloids contained in extracts may be responsible for these anti-inflammatory effects [31, 32].
In our study, in a carrageenan-induced rat paw edema model, it was demonstrated that only NPF fraction caused a reduction in rat paw edema after three and six hours of the experiment duration (and after three hours for diclofenac), but differences were statistically non-significant. Moreover, FEW revealed some statistically significant pro-inflammatory effect after three hours (Table 2). Analysis of changes in concentrations of peripheral cytokines indicates some anti-inflammatory properties of FWE and NPF, PEF fractions in the case of IL-1 and IL-4 concentrations. We have observed that the combination of FWE, PEF and NPF together with LPS showed only the effects of LPS, since the differences were significant only when compared with the proper extract of the non-LPS treated group (Fig. 1, Panel A, B). In the case of IL-6 the anti-inflammatory potential was especially visible in both, FWE-LPS and PEF-LPS groups of animals resulting in its level reduction when compared with the Control-LPS group, similar to the effect of DC shown in LPS-treated rats. We assume that this effect could probably be produced mainly by proteins presented in the extracts, because NPF-LPS did not show such activity when combined with LPS-treated rats (Fig. 1, Panel C). It should be mentioned that the standard drug – diclofenac showed a strong anti-inflammatory effect lowering the cytokine levels after previous stimulation inflammation using LPS (Fig. 1, Panel A-C).
A transcription profile of genes encoding studied cytokines shows variabilities in the mRNA levels between particular LPS or non-LPS treated groups, regardless of whether the animals received FWE or its fractions (PEF, NPF) (Fig. 2, Panel A, B, C). For example, a statistically insignificant influence of FEW on the level of IL1B transcripts in both, LPS-treated and LPS-non-treated rats, a very strong statistically significant induction of transcription in the NPF group with or without LPS was observed, in contrast to the decreasing effect of PEF when combined with LPS. On the contrary, in the case of IL-6 mRNA a slight and generally insignificant reduction of transcription in FEW, PEF or NPF groups treated with or without LPS was observed. It is difficult to clearly explain the modifications in the cytokine mRNA expression profile in the fraction of lymphocytes and changes in their peripheral concentrations. Also an evident correlation between the degree of change in the TLR1 gene expression profile and the all studied genes encoding cytokines cannot be clearly seen. For example, a similar degree of induction of TLR mRNA transcription can also be seen in the case of a FEW in non-LPS animals for IL-1B encoding gene, while suppression of IL-1 and IL-6 mRNA levels occurred in the case of FEW-LPS animals and of IL-6 mRNA itself in the PEF-non-LPS-treated group. We have observed that many of analyzed groups of animals had, however, an altered state of TLR1 gene mRNA transcription in relation to other cytokines depending on whether the animals receiving the extract fractions were stimulated with LPS or not (Fig. 2). This may prove a diversified affinity of individual metabolites in studied fractions of the plant extract on the enzymatic machinery regulating transcription of the gene encoding the TLR1 receptor and above mentioned cytokines.
The observed changes in the transcriptional profile in non-LPS-treated and LPS-treated groups for each fraction of the extract may be the cause, at least in part, of different affinity components of studied plant extract fractions to the cellular machinery regulating the transcription of examined genes and the process of secretion of cytokines. In addition, it should be noted that molecular studies presented in this paper were conducted on the material being a fraction of various types of rats’ white cell.
Only a few in vivo experiments were carried out attempting to determine anti-inflammatory potential of the extract from Ch. majus [12, 33] or several isochinoline alkaloids [34–37], but they were made in a different experimental scheme. For example, in a study performed by Lenfeld et al., sanguinarine revealed a higher anti-inflammatory activity than chelerythrine, which was explained by the authors with the different oxygen electron donating substituents [34]. Chung et al. revealed that a water extract from Ch. majus significantly induced the production of TNF-α and NO in peritoneal macrophages in mice via nuclear factor-κB (NF-κB) dependent pathway. This effect was more significant when co-administered this extract (1 mg/ml) with recombinant IFN-γ (rIFN-γ, 10 U/ml). The increased production of NO and TNF-α was almost completely inhibited by pyrrolidone dithiocarbamate (100 µm) – a NF-κB inhibitor, which suggests that NF-κB may play a crucial role in the anti-inflammatory action of this Ch. majus extract [33]. Le et al. reported that the methanolic extract from Ch. majus dose dependently [4, 40, 400 mg/kg/day] suppressed the progression of collagen-induced arthritis in mice causing the reduction in secretion of TNF-α, IL-6, IFN-γ, B cells, γδ T cells (in spleen), increased proportion of CD4+CD25+ regulatory T cells in vivo as well as a decrease in the immunoglobulins IgG and IgM (rheumatoid arthritis factors) in the serum [12]. Results from these studies were, at least, partially different than ours.
The reason for this discrepancy in above-mentioned results and results from our study may be due to different experimental conditions, applied in our research, different doses and the nature of the extract of Ch. majus. Furthermore, the above-mentioned studies have not evaluated the changes in the peripheral concentrations of the cytokines.
Several studies showed that berberine, one of the most studied alkaloid occurring in Chelidonium majus and many other plants (i.e. Berberis vulgaris, Hydrastis canadensis, Coptis chinensis), exerted blocking the induction of edema on mouse ear, inhibition of cyclooxygenase-2 (COX-2) transcriptional activity, immunomodulation of adjuvant-induced arthritis [38]. Previous studies have shown that berberine reduced prostaglandin E2 production in vitro (in the oral cancer cell line and KB cells) and in vivo (in carrageenan induced air pouch in rats), moreover berberine inhibited activator protein 1, a key transcriptional factor in inflammation and carcinogenesis [39]. It was shown that a stylopine concentration-dependently reduced, among others, IL-6 production as well as IL-1B, prostaglandin E2 (PGE2) and TNF-α cytokine concentrations in LPS stimulated RAW 264.7 cells (macrophages) [40]. It was observed that chelerythrine showed pronounced inhibition of the acetic acid-induced writhing response and has a significant anti-inflammatory action, which may be relevant to the inhibition of the release/production of exudates and prostaglandin E(2) mediated through cyclooxygenase-2 regulation [36]. Li et al. observed that this alkaloid inhibits LPS-induced TNF-α level and NO production in LPS-induced murine peritoneal macrophages through selective inhibition of p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) activation [35]. Another alkaloid, sanguinarine, occurring in Ch. majus, greatly inhibited the production of PGE2, and also decreased COX-2 protein expression, without affecting COX-1 expression, in LPS-stimulated peritoneal macrophages [37]. However, in none of the above experiments the gene expression profile of selected pro- and anti-inflammatory cytokines under specific alkaloids has not been examined. A screening analysis was also conducted in LPS stimulated RAW 264.7 macrophages for the anti-inflammatory potential of several alkaloids, such as stylopine, methyl 2’-(7,8-dihydrosanguinarine-8-yl)acetate, protopine, norchelidonine, chelidonine, berberine and 8-hydroxydihydrosanguinarine, isolated from Ch. majus in which chelidonine and 8-hydroxydihydrosanguinarine showed strong inhibitory activities toward the LPS-induced NO production in RAW264.7 cells with IC(50) values of 7.3 and 4.5 µM, respectively. A transcription profile of COX-2 and iNOS mRNA in dose-dependent manner under these two compounds was also declined, indicating that these compounds attenuated the syntheses of these transcripts at the transcriptional level [32].
On the other hand, there were also many reports on the immunomodulating activity of milk proteins (caseins), and whey proteins (i.e. α-lactalbumin, β-lactoglobulin, lactoferrin, osteopontin, immunoglobulins) [41, 42]. A recent study showed that protein hydrolysate from Pisum sativum (seeds) showed significant inhibition of NO production by activated macrophages, significantly inhibited their secretion of pro-inflammatory cytokines. Moreover, oral administration of pea protein hydrolysate enhanced the phagocytic activity of their peritoneal macrophages and stimulated the gut mucosa immune response [43]. In other study, it was shown that albumin fraction from Pisum sativum seed extract ameliorated the colonic mRNA expression of different proinflammatory markers: cytokines, inducible enzymes, metalloproteinases, adhesion molecules, and toll-like receptors, as well as proteins involved in maintaining the epithelial barrier functioning [44]. Also, soy proteins showed immunomodulating activity on proliferation of murine splenic lymphocytes and phagocytic effect of peritoneal macrophages [45].
According to our conviction presented in this paper study is the first attempt to clarify the molecular mechanism of action (analysis of changes in mRNA transcription profile of selected cytokines, assessment of changes in the concentration of cytokines in peripheral blood of animals) of different fractions from the Chelidonium majus aqueous extract in experimental animals undergoing induction of LPS.
Conclusions
In conclusion, the results presented in this study suggest that protein enriched fraction produced centrally mediated (morphine-like) analgesic action and anti-inflammatory activity in LPS-induced inflammation. The precise mechanisms involved in the production of the anti-nociceptive and anti-inflammatory responses of fractions of Ch. majus extract are not completely understood, but they may be caused by the presence of protein more than alkaloids. Thus, this fraction of extract could be used as an alternative therapy for the prevention of inflammatory-related diseases in the future, but further studies are needed.
The research project financed by the Ministry of Science and Higher Education grant No. N-405 677740. Authors declare no conflict of interest with any financial organization regarding the material discussed in the manuscript.
The authors declare no conflict of interest.
References
- 1.Woroń J, Filipczak-Bryniarska I, Dorazil-Dudzik M, Wordliczek J. Bezpieczeństwo pacjenta w farmakoterapii bólu. Ból. 2009;10(3):47–72. [Google Scholar]
- 2.Ożarowski M, Mikolajczak PŁ, Bogacz A, et al. Progress in study of Cannabis sativa leaves extracts without psychotropic cannabinoids in animal model of neuropathic pain. J Med Sci. 2014;4:282–289. [Google Scholar]
- 3.Arora D, Sharma A. A review on phytochemical and pharmacological potential of genus Chelidonium. Pharmacogn J. 2013;5:184–190. [Google Scholar]
- 4.EMA. European Medicines Agency. Committee on Herbal Medicinal Products (HMPC) Assessment report on Chelidonium majus L., herba; 2011. EMA/HMPC/369801/2009. [Google Scholar]
- 5.Orvos P, Virág L, Tálosi L, et al. Effects of Chelidonium majus extracts and major alkaloids on hERG potassium channels and on dog cardiac action potential – a safety approach. Fitoterapia. 2015;100:156–165. doi: 10.1016/j.fitote.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Gilca M, Gaman L, Panait E, et al. Chelidonium majus – an integrative review: traditional knowledge versus modern findings. Forsch Komplementmed. 2010;17:241–248. doi: 10.1159/000321397. [DOI] [PubMed] [Google Scholar]
- 7.Colombo ML, Bosisio E. Pharmacological activities of Chelidonium majus L. (Papaveraceae) Pharmacol Res. 1996;33:127–134. doi: 10.1006/phrs.1996.0019. [DOI] [PubMed] [Google Scholar]
- 8.Kędzia B, Łożykowska K, Gryszczyńska A. Skład chemiczny i zawartość substancji biologicznie aktywnych w Chelidonium majus L. Postępy Fitoterapii. 2013;3:174–181. [Google Scholar]
- 9.Nawrot R, Zauber H, Schulze WX. Global proteomic analysis of Chelidonium majus and Corydalis cava (Papaveraceae) extracts revealed similar defense-related protein compositions. Fitoterapia. 2014;94:77–87. doi: 10.1016/j.fitote.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Horvath G, Molnar P, Farkas A, et al. Separation and Identification of Carotenoids in Flowers of Chelidonium majus L. and Inflorescences of Solidago canadensis L. Chromatographia. 2010. pp. 1–6. [Google Scholar]
- 11.Stancic-Rotaru M, Mititelu M, Crasmaru M, Balaban D. Spectroanalytical Profile of Flavonoids from Chelidonium majus L. Roumanian Biotechnological Letters. 2003;8:1093–1100. [Google Scholar]
- 12.Lee YC, Kim SH, Roh SS, et al. Suppressive effects of Chelidonium majus methanol extract in knee joint, regional lymph nodes, and spleen on collagen-induced arthritis in mice. J Ethnopharmacol. 2007;112:40–48. doi: 10.1016/j.jep.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Shin MC, Jang MH, Chang HK, et al. Modulation of Chelidonii herba on glycine-activated and glutamate-activated ion currents in rat periaqueductal gray neurons. Clin Chim Acta. 2003;337:93–101. doi: 10.1016/j.cccn.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Shin M, Chung J, et al. Modulation of Chelidonii herba on GABA activated chloride current in rat PAG neurons. Am J Chin Med. 2001;29:265–279. doi: 10.1142/S0192415X01000290. [DOI] [PubMed] [Google Scholar]
- 15.Kim M, Cho KH, Shin MS. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson's disease. Int J Mol Med. 2014;33:870–878. doi: 10.3892/ijmm.2014.1656. [DOI] [PubMed] [Google Scholar]
- 16.Tang QL, Lai ML, Zhong YF, et al. Antinociceptive effect of berberine on visceral hypersensitivity in rats. World J Gastroenterol. 2013;19:4582–4589. doi: 10.3748/wjg.v19.i28.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo JH, Yang EM, Cho JH, et al. Inhibitory effects of berberine against morphine-induced locomotor sensitization and analgesic tolerance in mice. Neuroscience. 2006;142:953–961. doi: 10.1016/j.neuroscience.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Sárközi A, Janicsák G, Kursinszki L, Kéry Á. Chromatographia Suppl. 2006;63:S81–86. [Google Scholar]
- 19.Manikowska K, Mikołajczyk M, Mikołajczak PŁ, Bobkiewicz-Kozłowska T. The influence of mianserin on TNF-a, IL-6 and IL-10 serum levels 4 in rats under chronic mild stress. Pharmacol Rep. 2014;66:22–27. doi: 10.1016/j.pharep.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Abdala S, Dévora S, Martín-Herrera D. Antinociceptive and anti-inflammatory activity of Sambucus palmensis link, anendemic Canary Island species. J Ethnopharmacol. 2014;155:626–632. doi: 10.1016/j.jep.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Couto VM, Vilela FC, Dias DF, et al. Antinociceptive effect of extract of Emilia sonchifolia in mice. J Ethnopharmacol. 2011;134:348–353. doi: 10.1016/j.jep.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Shi GB, Zhao MH, Zhao QC, et al. Mechanisms involved in the antinociception of petroleum ether fraction from the EtOH extract of Chrysanthemum indicum in mice. Phytomedicine. 2011;18:609–616. doi: 10.1016/j.phymed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Penson R, Joel P, Bakhshi K, Clark S, et al. Randomized placebo-controlled trial of the activity of the morphine glucuronides. Clin Pharmacol Ther. 2000;68:667–676. doi: 10.1067/mcp.2000.111934. [DOI] [PubMed] [Google Scholar]
- 24.Granmo M, Jensen T, Schouenborg J. Nociceptive transmission to rat primary somatosensory cortex – comparison of sedative and analgesic effects. PLoS One. 2013;8:e53966. doi: 10.1371/journal.pone.0053966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthes H, Maldonado R, Simonin F, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 26.Milano J, Oliveira S, Rossato M, et al. Antinociceptive effect of novel trihalomethyl-substituted pyrazoline methyl esters in formalin and hot-plate tests in mice. Eur J Pharmacol. 2008;581:86–96. doi: 10.1016/j.ejphar.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 27.Çitoglu GS, Özbek H, Acikara ÖB, Gacs EB. Isolation of chelidonine as an analgesic compound from Chelidonium majus L. J Fac Pharm (Ankara) 2009;38:9–16. [Google Scholar]
- 28.Kim SO, Kim HJ. Berberine Ameliorates Cold and Mechanical Allodynia in a Rat Model of Diabetic Neuropathy. J Med Food. 2013;16:511–517. doi: 10.1089/jmf.2012.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HJ. Berberine Ameliorates Allodynia Induced by Chronic Constriction Injury of the Sciatic Nerve in Rats. J Med Food. 2015;18:909–915. doi: 10.1089/jmf.2014.3346. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Lee K, Lee MH, et al. Inhibitory effects of Chelidonium majus extract on atopic dermatitis-like skin lesions in NC/Nga mice. J Ethnopharmacol. 2011;138:398–403. doi: 10.1016/j.jep.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Saeed S, Gilani A, Majoo R, Shah B. Anti-thrombotic and anti-inflammatory activities of protopine. Pharmacol Res. 1997;36:1–7. doi: 10.1006/phrs.1997.0195. [DOI] [PubMed] [Google Scholar]
- 32.Park JE, Cuong TD, Hung TM, et al. Alkaloids from Chelidonium majus and their inhibitory effects on LPS-induced NO production in RAW264.7 cells. Bioorg Med Chem Lett. 2011;21:6960–6963. doi: 10.1016/j.bmcl.2011.09.128. [DOI] [PubMed] [Google Scholar]
- 33.Chung HS, An HJ, Jeong HJ, et al. Water extract isolated from Chelidonium majus enhances nitric oxide and tumour necrosis factor-alpha production via nuclear factor-kappaB activation in mouse peritoneal macrophages. J Pharm Pharmacol. 2004;56:129–134. doi: 10.1211/0022357022467. [DOI] [PubMed] [Google Scholar]
- 34.Lenfeld J, Kroutil M, Maršálek E, et al. Antiinflammatory activity of quaternary benzophenanthridine alkaloids from Chelidonoum majus. Planta Med. 1981;43:161–165. doi: 10.1055/s-2007-971493. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Fan T, Zhang Y, et al. Effect of chelerythrine against endotoxic shock in mice and its modulation of inflammatory mediators in peritoneal macrophages through the modulation of mitogen-activated protein kinase (MAPK) pathway. Inflammation. 2012;35:1814–24. doi: 10.1007/s10753-012-9502-1. [DOI] [PubMed] [Google Scholar]
- 36.Niu XF, Zhou P, Li WF, Xu HB. Effects of chelerythrine, a specific inhibitor of cyclooxygenase-2, on acute inflammation in mice. Fitoterapia. 2011;82:620–625. doi: 10.1016/j.fitote.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Li H, Mu Q, et al. Protective effect of sanguinarine on LPS-induced endotoxic shock in mice and its effect on LPS-induced COX-2 expression and COX-2 associated PGE2 release from peritoneal macrophages. Int Immunopharmacol. 2014;22:311–317. doi: 10.1016/j.intimp.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 39.Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Jang S, Kim BH, Lee WY, et al. Stylopine from Chelidonium majus inhibits LPS-induced inflammatory mediators in RAW 264.7 cells. Arch Pharm Res. 2004;27:923–929. doi: 10.1007/BF02975845. [DOI] [PubMed] [Google Scholar]
- 41.Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol. 2013;45:1730–1747. doi: 10.1016/j.biocel.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 42.Malinowski J, Klempt M, Clawin-Rädecker I, et al. Identification of a NFκB inhibitory peptide from tryptic β-casein hydrolysate. Food Chem. 2014;165:129–133. doi: 10.1016/j.foodchem.2014.05.075. [DOI] [PubMed] [Google Scholar]
- 43.Ndiaye F, Vuong T, Duarte J, et al. Anti-oxidant, anti-inflammatory and immunomodulating properties of an enzymatic protein hydrolysate from yellow field pea seeds. Eur J Nutr. 2012;51:29–37. doi: 10.1007/s00394-011-0186-3. [DOI] [PubMed] [Google Scholar]
- 44.Utrilla MP, Peinado MJ, Ruiz R, et al. Pea (Pisum sativum L.) seed albumin extracts show anti-inflammatory effect in the DSS model of mouse colitis. Mol Nutr Food Res. 2015;59:807–819. doi: 10.1002/mnfr.201400630. [DOI] [PubMed] [Google Scholar]
- 45.Kong X, Guo M, Hua Y, et al. Enzymatic preparation of immunomodulating hydrolysates from soy proteins. Bioresour Technol. 2008;99:8873–8879. doi: 10.1016/j.biortech.2008.04.056. [DOI] [PubMed] [Google Scholar]