Abstract
There is an increasing evidence suggesting the role of fork head boxP3 (FoxP3) in the development and the regulation of CD4+CD25+ Treg cells. T-cell regulatory mechanisms in rheumatoid arthritis patients were evaluated by the contributing factors such as pro-inflammatory cytokines, circulating immune complexes, HLA DR expression, ligand binding biomarkers, FoxP3 expression in paired samples of peripheral blood (PB) and synovial fluid (SF). These cellular responses were further correlated with the humoral immune responses such as anti-cyclic citrullinated peptides IgG (CCP), circulating immune complex-c1q IgG (CIC), immunoglobulin G (IgG) and immunoglobulin M (IgM) of the rheumatoid arthritis factor (RAF). The results suggest a definitive role of Tregs in the homeostatic control because there is an increase in FoxP3 (37%) and HLA-DR (45%) expression in the synovial fluid as compared to PB. Furthermore, humoral responses as a downstream effector mechanism are positively correlated with the pathogenesis of rheumatoid arthritis (RA). A positive relationship exists between quantitative anti-CCP production and the expression of HLA-DR. The study relates an increased and pivotal role of B cell activation in the synovial fluid thereby permitting the need to ablate the targeted B cell immune responses.
Keywords: rheumatoid arthritis, FoxP3, regulatory T cell, circulating immune complex-c1q IgG, anti-cyclic citrullinated peptides IgG, rheumatoid factor IgG, rheumatoid factor IgM
Introduction
Rheumatoid arthritis (RA) is a systemic chronic immuno-inflammatory disease of unclear aetiology involving progressive and destructive polyarthritis in association with serological evidence of auto-reactivity leading to persistent and progressive synovitis. This progressive autoimmune response mediates mononuclear cell infiltration in the sub-intimal layer of the synovium [1, 2]. The debilitating factor in severe disease progression of RA is Tregs cells and their precise mechanism of regulation by which they mediate activated T cells and B cells is yet unknown. Modification of antigen presenting cells (APC) and cytokines in both peripheral blood and synovium is an attempt to look beyond the elicited conventional cellular and humoral immune responses. In severe acute inflammation, synovium accumulates a repertoire of inflammatory cytokines, dysregulated Tregs with FoxP3 expression, leading to a corrosive form of RA. Synovial Tregs express an increased regulatory capacity in comparison with Tregs derived from the peripheral blood, as demonstrated by several in vitro assays. In synovium, Tregs might be inhibited by different mechanisms such as inflammatory cytokines, including tumour necrosis factor α (TNF-α) or stimulation by APC, which in concert might allow synovial inflammation to evolve and persist despite the enhanced frequencies of synovial Tregs. Synovial Tregs alone cannot ameliorate disease activity completely but are involved in regulating synovial inflammation in vivo. Future treatment strategies of autoimmune diseases can be envisaged in which Tregs generated and/or expanded in vitro will be employed to control local and systemic autoimmune inflammation in vivo [8].
Approximately, 75% of RA patients have anti-cyclic citrullinated peptide antibodies (ACPA) compared to 1% of healthy individuals [3]. Isotype IgM RA factor is the most common, while antibodies against cyclic citrullinated peptides (CCP) are a predictor for increased joint destruction. These auto-antibodies form immune complexes contributing to persistent inflammation and complement system activation [4]. Furthermore, an increased HLA-DR expression in synovium implicates an active elicitation of T cell responses. B cells generally influence the T cell differentiation and its activity in disease progression. But it is also evidenced that in RA, B cells directly contribute to the synthesis of cytokines locally at the site [5]. Typically the impaired Tregs are regulated by cytokine TNF-α in maintaining the T helper cell (Th) 17 and Th1 balance [6]. Recent studies have now emphasized a pivotal role for B cells in the pathogenesis of RA. The clinical efficacy of B cell-depleting biologic treatments highlights a key role for auto reactive B cell activation in the pathogenesis of RA [7]. It appears logical that novel therapies should aim to target the inter-cellular communications in the synovium rather than ablate a single cell population.
Material and methods
Patient classification criteria and clinical manifestations
Prior written informed consent was obtained from individual participants of the study cohort. The experimental protocol was carried out in accordance with the guidelines of the Helsinki declaration and approved by the Institutional Ethics Committee of Global Hospitals, Hyderabad with ref #.GMERF/BS/SAC/IEC/IC_SCR 2014/02R3.
A total of 120 subjects were included in the study comprising 84 clinically diagnosed patients with rheumatoid arthritis and categorized as per the ACR and EULAR classification (2010). The control group included patients with meniscal tear (n = 36). No samples from the healthy control were compared in this study. Paired samples of SF and PB were collected from RA patients from Aware Global Hospitals. The classification parameters were joint involvement, serology (rheumatoid factor and anti-cyclic citrullinated peptide – anti-CCP), levels of acute phase reactants and the duration of the symptoms [9]. Individuals were excluded if the history of another autoimmune antibody was recorded or received immune suppressive or glucocorticoid therapies within the past 6 months. Patients were on anti-inflammatory drugs like indomethacin (75 mg daily) or ibuprofen (400 mg thrice daily). Paired samples of SF and PB were collected from RA patients at Aware Global Hospitals, Hyderabad. The average duration of symptoms up to the day of synovial fluid aspiration was 12 weeks in the case of patients with RA. The demographic and clinical data including age, sex and diagnostic parameters of individual subjects are summarized in Table 1.
Table 1.
Demographic features, ACR/EULAR classification and seropositive markers of patient and control groups
| Male/Female | 15/69 | 7/29 |
| Duration of symptoms | 10-24 weeks | < 6 weeks |
| CRP (mean) | 28.5 mg/dl | 4.2 mg/dl |
| ESR(mean) | 60 mM/first hour | 10 mM/first hour |
| *Anti-CCP (+), PB/SF | 3.72/4.19 | 0.56/0.6 |
| *CIC-c1q (+), PB/SF | 1.08/0.71 | 0.59/0.54 |
| *IgG RF (+), PB/SF | 2.42/1.57 | 0.55/0.52 |
| *IgM RF (+), PB/SF | 7.32/8.63 | 0.52/0.56 |
| *Anti dsDNA(+), PB/SF | 0.6/0.72 | 0.1/0.14 |
| #Score based algorithm | > 6/10 | < 6/10 |
Data are shown as median (range) of each group of subjects. 82.14% of the patient group are females. All 84 RA patients were positive for anti-CCP antibody as well as for IgM RF. A significant correlation exists in the RA patients between anti-CCP and IgM Rheumatoid Factor positivity (p < 0.01). ESR: erythrocyte sedimentation rate (normal range: men, 0-15 mM/h; women, 0-20 mM/h).
Values were expressed as mean for PB/SF;
Score-based algorithm is evaluated as per the ACR and EULAR classification.
Flow cytometry analysis of HLA DR and FoxP3/CD4/CD25 expression
The FoxP3 expressing CD4+CD25+Tregs, HLA-DR cells in the synovial fluid and peripheral blood were investigated by flow cytometry using surface and intra cellular staining. Paired peripheral blood mononuclear cells (PBMC) and synovial fluid mononuclear cells (SFMC) of RA and control groups were prepared by using ficoll gradient centrifugation (Histopaque, Sigma). Cell viability was 95% using the trypan blue exclusion method. Briefly, the cell suspension was stained primarily by CD4-FITC (10 µl) and CD25-APC (10 µl) (eBiosciences, USA) for 30 min in the dark at 4°C. Further intracellular staining was done immediately for FoxP3 by adding 2 ml of cold 1X permeabilization buffer for 5 minutes at 4°C. Anti-FoxP3 Antibody (10 µl) (eBiosciences, USA) was added, mixed thoroughly and incubated for 30-60 minutes in the dark at 4°C. Cell pellet was re-suspended in a suitable amount of flow cytometry staining buffer for analysis. For HLA-DR, the cells were re-suspended with 195 µl of PBS and 5 µl of HLA-DR (PE) (BD Biosciences, USA) and incubated for 30 minutes in the dark at 4°C. After staining, cell pellet was re-suspended in 500 µl of flow cytometry staining buffer. The same procedure was repeated for the cells isolated from PB and SF of the control group. The cells were analysed with FACS Calibur (Becton Dickinson) using Cell Quest Software.
Seropositivity of predictive immune markers by ELISA and IFA
The semi-quantitative in vitro assays for predictive immune markers of RA – anti cyclic citrullinated peptides IgG (CCP), circulating immune complex-c1q IgG (CIC), rheumatoid factor IgG and rheumatoid factor IgM antibodies against particular antigens in serum of all the samples were quantified by using ELISA (Euroimmune, Germany). Polystyrene microplate strips coated with purified, biochemically characterized antigens were used as a solid phase containing bound antigens. The micro titer wells pre-coated with specific antigens were incubated with 100 µl of controls, calibrators and diluted sera, for 30 min at 23°C. Autoantibody recognizing the particular antigen binds during the first incubation, and is followed by the anti-human IgG (γ chain specific) conjugate reaction. Subsequently, the immune complex formation was observed by the chromogen substrate reaction. The absorbance values observed at 450 nm demonstrate the amount of the autoantibody present in the individual patient. The semi quantitative method is deemed positive when the ratio is above 1.0 on the calibrator 2 value.
An indirect immunofluorescence assay (IFA) was used to detect RA-associated autoantibodies. A diluted serum sample (1: 40) was incubated with the antigen-coated HEp-2 cells (HEp-20-10; Euroimmun, Lübeck, Germany) tissue substrate. The fluorescein isothiocyanate (FITC) IgG conjugate step was followed to identify the antigen antibody complex in the form of pattern observation under the fluorescence microscopy. A nuclear homogeneous pattern apart from nuclear speckled has a clinical significance to substantiate RA and was recorded as per grading based on the fluorescence intensity. IFA titre greater than 1: 40 dilution was considered positive and the specific fluorescence pattern was recorded.
Cytokine analysis
TNF-α, IFN-γ and IL-10 in serum and SF of all the samples were quantified by ELISA as per the manufacturer's recommendations (Biolegend ELISA MAX™). Control samples (n = 36) were run in parallel experiments for tests of significance. Quantifications were mentioned as pg/µl.
Statistical analysis
Statistical significance was tested using chi-square and student's t-test for paired samples. The co-efficient of correlation (r value) for cellular vs. humoral responses is calculated using Pearson's correlation co-efficient. All p values were determined by 2 tailed t test and were deemed significant at p < 0.05.
Results
Increased frequency of CD4+CD25+ FoxP3/HLA-DR expression in synovial fluid as compared to peripheral blood in RA patients
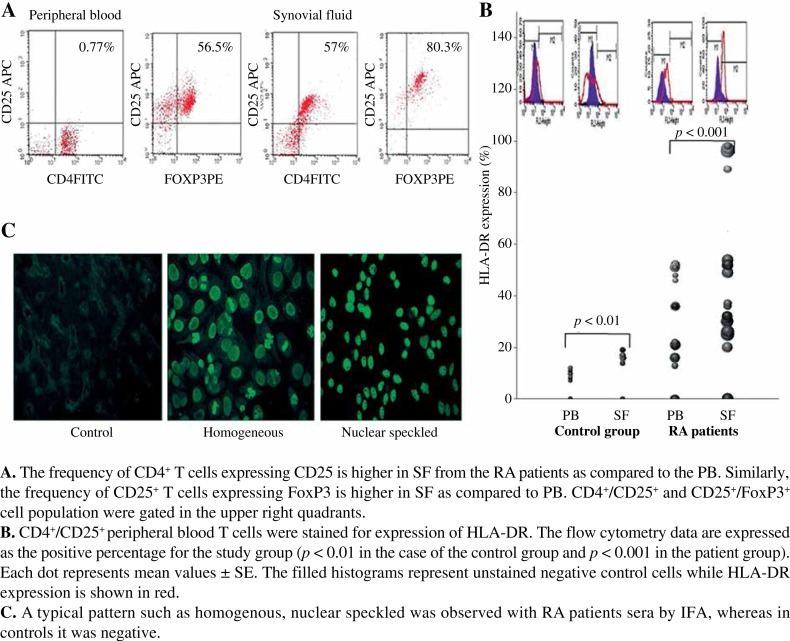
There is a higher expression of CD25 on CD4+ and FoxP3+ cells as well as HLA-DR expression on CD4+ CD25+ from SF than from PB in RA patients. There is an associated significant increase in the FoxP3 expression (Fig. 1A). Further, CD4+/CD25+ peripheral blood T cells of paired PB & SF samples were stained for the expression of HLA-DR. The flow cytometry data are expressed as a positive percentage for both control and RA groups. A greater shift towards positivity of HLA-DR expression (45%) was observed in the RA group (p < 0.001) which was relatively high in SF when compared with PB of the RA group (Fig. 1B). An increased expression of HLA-DR on CD4+CD25+ T cells associated with disease progression in rheumatoid arthritis suggests that the CD4+CD25+ Treg cells may undergo maturation in the joint.
Fig. 1.
A) Expression of CD4+/CD25+/FoxP3+ in RA patients. B) HLA-DR expression in the study cohort. C) IFA patterns observed in control and RA patients
Elevated humoral and cellular responses in the synovial fluid as compared to peripheral blood
The patients in the experimental group (n = 84) had RA confirmed based on > 6 on a scale of 10 as per the ACR & EULAR classification criteria. The humoral responses were evaluated with the early markers of RA i.e. anti-cyclic citrullinated peptides IgG (CCP), circulating immune complexes (CIC) and rheumatoid factor IgM. Severe RA was predominant in females (82.14%) with mean age of 54 years. Anti-CCP antibodies and IgM RF were positive in 90% of the patients showing a significant association with severe RA disease when compared with IgG RF and CIC. A significant correlation exists in the RA patients between anti-CCP and IgM RF positivity (χ2 = 6.212, p < 0.01). Anti-ds DNA antibodies were observed in 1.68% of subjects in the experimental group, whereas the control group did not exhibit positive anti dsDNA antibody levels. All the diagnostic humoral markers of RA were found to be elevated in SF than the serum fraction.
Anti-nuclear antibodies patterns observed in rheumatoid arthritis
Out of 84 RA subjects, 9 were positive for the presence of anti-nuclear antibodies (ANA) in their serum with a titre greater than 1: 40, while 7 patients exhibited a nuclear homogenous pattern and other 2 demonstrated a nuclear speckled pattern (Fig. 1C). About 88.8% were negative for IgM RF and anti CCP. FoxP3 expression on CD4+ CD25+ cells is negatively correlated (p < 0.01) with the frequency of ANA showing that T-cell tolerance is often a more important mechanism for preventing autoantibody production. On the contrary, none of the control group was positive for ANA.
Positive correlation between cellular responses and humoral responses in patients with rheumatoid arthritis
The change in the distribution of the Treg subsets was correlated with the clinical and serologic features of RA. Cellular and humoral responses were correlated statistically using Pearson's correlation co-efficient (r) values. Pearson's correlation co-efficient (r) ranges between –1.0 and 1.0. A correlation is interpreted positive when ‘r’ value is close to +1.0 and negative when it is close to –1. Herein, we observed a positive correlation between the cellular and humoral responses. Phenotypic characteristics of Treg subsets and cytokine production were evaluated. Humoral responses included early markers i.e. anti-CCP, IgM & IgG RF, CIC, IgM RF scored a high r value in comparison to IgG RF. This demonstrates that in the clinically diagnosed RA patients, IgM RF is the earliest prognostic marker in the diagnosis of rheumatoid arthritis. The cellular responses are inversely correlated with anti-ds DNA antibody in cases of RA as evidenced by negative values. A positive correlation exists between HLA-DR expression and anti CCP levels (χ2 = 5.2, p < 0.01) suggesting that the HLA-DR expression increases with the disease progression (Table 2).
Table 2.
Pearson's correlation coefficient (r) values obtained by each cell marker correlated with each of the humoral marker and cytokines a
| Pearson's correlation co-efficient (r) | ||||
|---|---|---|---|---|
| CD4 | CD25 | FoxP3 | HLA-DR | |
| CIC-c1q | 0.04 | 0.04 | 0.12 | 0.06 |
| Anti CCP | 0.42 | 0.48 | 0.62 | 0.41 |
| IgG RF | 0.07 | 0.156 | 0.25 | 0.1 |
| IgM RF | 0.53 | 0.59 | 0.73 | 0.52 |
| Anti-ds DNA | –0.12 | –0.18 | –0.21 | –0.142 |
| TNF-α | –0.23 | –0.242 | –0.23 | 0.216 |
| IFN-γ | 0.32 | 0.345 | 0.46 | 0.25 |
| IL-10 | 0.39 | 0.463 | 0.59 | 0.28 |
The change in the distribution of the Treg subsets was correlated with serologic features of RA. Pearson's correlation coefficient (r) between 0 and 1 demonstrates a positive correlation between the cellular and humoral responses. In overview, data (Table 2) suggest a positive correlation between cellular responses from synovium and early humoral markers. It was observed that a positive correlation exists between IgM RF and expression of all the cellular markers, while a negative correlation existed between anti-ds DNA and expression of investigated molecules. TNF-α shows a negative correlation with FoxP3 expression whereas IL-10 and IFN-γ levels were positively correlated with FoxP3 expression.
Cytokine levels and their relationship with CD4+ CD25+ FoxP3+
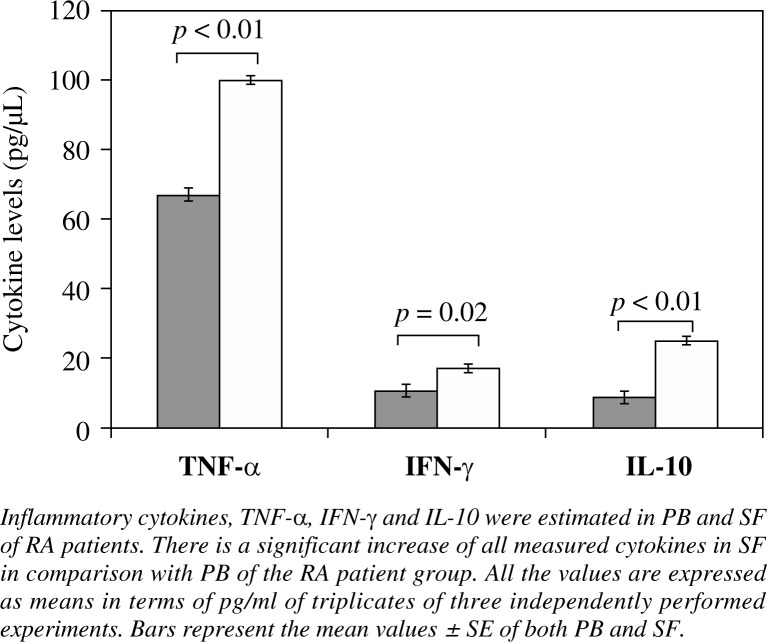
Since both Tregs and cytokines seem to be involved in the pathogenesis of RA, we tried to investigate the possible relationship between different cytokines, related to the generation or the effector functions of Treg cells, and the extent of FoxP3 expression. To this end, we measured IFN-γ, TNF-α and IL-10 in the serum and SF of RA patients (Fig. 2).
Fig. 2.
Cytokine levels in control and RA groups
In the case of cellular responses, it was found that an increased levels of IFN-γ, TNF-α and IL-10 were present in SF as compared to PB of the patient group (p = 0.02; p < 0.01; p < 0.01, respectively) (Fig. 2). These data clearly demonstrate higher production of pro-inflammatory cytokines by T cells of synovial tissue than PB in RA patients, indicating that activated T cells play a role in the pathophysiological events of RA. Interestingly, also IL-10 concentration is higher in SF than PB of RA patients.
In patients with high levels of TNF-α, a negative correlation (r = –0.23) was observed with FoxP3 expression showing that it disrupts regulatory T cells by affecting the phosphorylation and function of FoxP3 expressed in these cells. Interleukin 10 and IFN-γ levels with FoxP3 expression appear to be positively correlated as mentioned in Table 2. Also, CD25+ cells showed an altered balance in the production of these cytokines. Serum levels of IL-10 were shown to correlate significantly with serum IgM RF (p < 0.05) suggesting that there is an increased production of IL-10 by non-T cells in patients with RA. This may contribute to the diminished T cell function and increased antibody and RF production in these patients.
Discussion
Activated B cells and plasma cells secrete antibodies giving rise to immune complex formation and activation of the inflammatory cascade [10]. Circulating immune complex levels were elevated in the majority of RA patients early in the disease, suggesting a correlation between the disease activity and the level of circulating immune complexes.
We analysed possible associations of both the positivity and serum concentrations of IgM RF, anti-CCP antibody and the expression of HLA-DR. Our results indicate an association between anti-CCP and RF positivity as reflected in the findings published by other researchers [11, 12]. In the present study, 90% of the patients were both IgM RF and anti-CCP positive, while neither IgM RF nor anti-CCP could be detected as is the case in 5% of the patients. In contrast to anti CCP and IgM RF, we established an association between the anti-CCP positivity and HLA-DR expression in RA patients. These correlations were published previously by other groups [13, 14]. In the present study, not only the presence of anti-CCP antibodies but also the serum levels of this antibody could be significantly associated with the expression of HLA-DR. RF still remains an imperative marker in RA diagnosis. However, the association of highly specific anti-CCP autoantibody levels and HLA DR could provide a specific disease marker combination in evaluating future disease progression. Since activation of T cells is important in B cell responses, an association exists between HLA-DR expression and anti-CCP antibodies along with the ability of citrulline-containing peptides to bind to certain HLA class II molecules [15]. Quantitative anti-CCP production is positively correlated with HLA-DR expression and the presence of these two factors is indicative of a severe disease course.
In the present study, 9 subjects developed ANA amongst which 2 subjects (1.68%) developed anti-ds DNA antibodies in the patient group. This rare occurrence of anti-ds DNA antibodies has been reported in healthy individuals, relatives of patients with autoimmune diseases, and with an increasing frequency in the elderly population without any obvious clinical manifestations or effects [16]. The development of SLE-like symptoms and SLE-related autoantibody production was observed with an increased risk in patients with SLE-related HLA haplotypes and increased serum IL-10 levels with ANA nuclear speckled patterns [17].
The present study explored the phenotypical characteristics of CD4+CD25+ Treg cells in patients with RA. CD4+CD25+ cells from SF showed the phenotype of Treg cells and expressed high levels (80%) of FoxP3 as well as HLA-DR. The high number of CD4+CD25+ Treg cells in SF is a defined response to inflammatory synovial micro-environment as well as due to recruitment of Tregs [18]. Thus, the data demonstrate CD4+CD25+HLA-DR+ expression on Treg cells, with the potential to regulate the function of effector T cells in synovium of RA patients. Further, all the humoral markers of RA were found to be elevated in SF in comparison to the serum fraction due to localized response to synovial environment as well as due to recruitment of B cells.
The recruitment, activation, and effector function as a part of cellular immune response is directed primarily by a network of cytokines. In this study, levels of TNF-α is negatively correlated with FoxP3 expression probably as a result of TNF-α affecting the phosphorylation and function of FoxP3 expressed in these cells. Results of the current study support the findings of other research groups that FoxP3 activity and suppressive function of Tregs is regulated by the phosphorylation at Ser418 in C-terminal DNA-binding domain. Also the expression and enzymatic activity of protein phosphatase 1 (PP1) on Tregs were induced in the inflamed synovium by TNF-α, leading to an impaired Treg function. Furthermore, IL-10 and IFN-γ levels with FoxP3 expression appear to be moderately significant and perhaps require a genetic predisposition factor for activation within the inflamed synovium in rheumatoid arthritis. Thus, TNF-α controls the balance between regulatory T cells and pathogenic Th17 and Th1 cells in the synovium of individuals with rheumatoid arthritis through FoxP3 dephosphorylation [19, 20].
In conclusion, all these findings suggest that a negative feedback system exists which is active at the site of inflammation. The analysis of immune markers with that of CD4+CD25+ and FoxP3 expression indicates that the cellular responses are directly proportional to the humoral responses. This provides an insight into the dysregulation of T-cell homeostasis in the synovium of RA patients. The results define the role of Tregs in the pathogenesis, their function and accumulation in synovium as well as the degree of FoxP3 expression at the site of inflammation. Thus, further studies at a translational level are warranted to optimize novel therapies based on the expansion of Tregs in RA patients.
The authors declare no conflict of interests.
References
- 1.Kourilovitch M, Maldonado CG, Prado EO. Diagnosis and classification of rheumatoid arthritis. J Autoimmun. 2014;48:26–30. doi: 10.1016/j.jaut.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Boissier MC, Semerano L, Challal S, et al. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun. 2012;39:222–228. doi: 10.1016/j.jaut.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Van Venrooij WJ, Van Beers JJ, Pruijn GJ. Anti-CCP antibody a marker for the early detection of rheumatoid arthritis. Ann N Y Acad Sci. 2008;1143:268–285. doi: 10.1196/annals.1443.013. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal S, Misra R, Agarwal A. Autoantibodies in rheumatoid arthritis: association with severity of disease in established RA. Clin Rheum. 2007;26:201–204. doi: 10.1007/s10067-006-0275-5. [DOI] [PubMed] [Google Scholar]
- 5.Yeo L, Toellner KM, Salmon M, et al. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2011;70:2022–2028. doi: 10.1136/ard.2011.153312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong N, Yingxia Z, Runsheng L, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19:322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 7.Bugatti S, Vitolo B, Caporali R. B Cells in Rheumatoid Arthritis: From Pathogenic Players to Disease Biomarkers. BioMed Research International. 2014;681678:14. doi: 10.1155/2014/681678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leipe J, Skapenko A, Lipsky PE, Koops HS. Regulatory T cells in rheumatoid arthritis. Arthritis Res Ther. 2005;7:93–99. doi: 10.1186/ar1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 10.De Clerck LS. B lymphocytes and humoral immune responses in rheumatoid arthritis. Clin Rheumatol Suppl. 1995;14:14–18. doi: 10.1007/BF02215852. [DOI] [PubMed] [Google Scholar]
- 11.Kapitany A, Szabo Z, Lakos G, et al. Associations between serum anti-CCP antibody, rheumatoid factor levels and HLA-DR4 expression in Hungarian patients with rheumatoid arthritis. Isr Med Assoc J 2008. 2008;10:32–36. [PubMed] [Google Scholar]
- 12.Forslind K, Ahlmén M, Eberhardt K, et al. Prediction of radiological outcome in early rheumatoid arthritis in a clinical practice: role of antibodies to citrullinated peptides (anti-CCP) Ann Rheum Dis. 2004;63:1090–1095. doi: 10.1136/ard.2003.014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Gaalen FA, Aken JV, Huizinga TW, et al. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum. 2004;50:2113–2121. doi: 10.1002/art.20316. [DOI] [PubMed] [Google Scholar]
- 14.Irigoyen P, Lee AT, Wener MH, et al. Regulation of anti-cyclic citrullinated peptide antibodies in rheumatoid arthritis: contrasting effects of HLA-DR3 and the shared epitope alleles. Arthritis Rheum. 2005;52:3813–3818. doi: 10.1002/art.21419. [DOI] [PubMed] [Google Scholar]
- 15.Hill JA, Southwood S, Sette A, Jevnikar AM, et al. The conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLADRB1* 0401 MHC class II molecule. J Immunol. 2003;171:538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 16.Shoenfeld Y, Isenberg DA. The mosaic of autoimmunity. Immunol Today. 1989;10:123–126. doi: 10.1016/0167-5699(89)90245-4. [DOI] [PubMed] [Google Scholar]
- 17.Worrall JG, Snaith ML, Batchelor JR, Isenberg DA. SLE: a rheumatological view analysis of the clinical features, serology and immunogenetics of 100 SLE patients during long-term follow-up. Q J Med. 1990;74:319–330. [PubMed] [Google Scholar]
- 18.Cao D, Malmström V, Allan CB, et al. Isolation and functional characterization of regulatory CD25 bright CD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–223. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 19.Bromberg J. TNF-α trips up Treg cells in rheumatoid arthritis. Nat Med. 2013;19:269–270. doi: 10.1038/nm.3124. [DOI] [PubMed] [Google Scholar]
- 20.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]