Abstract
The ventrolateral periaqueductal gray (vlPAG) is a key structure in the descending pain modulatory circuit. Activation of the circuit occurs via disinhibition of GABAergic inputs onto vlPAG output neurons. In these studies, we tested the hypothesis that GABAergic inhibition is increased during persistent inflammation, dampening activation of the descending circuit from the vlPAG. Our results indicate that persistent inflammation induced by Complete Freund's adjuvant (CFA) modulates GABA signaling differently in male and female rats. CFA treatment results in increased presynaptic GABA release but decreased high-affinity tonic GABAA currents in female vlPAG neurons. These effects are not observed in males. The tonic currents in the vlPAG are dependent on GABA transporter activity and are modulated by agonists that activate GABAA receptors containing the δ subunit. The GABAA δ agonist THIP (gaboxadol) induced similar amplitude currents in naive and CFA-treated rats. In addition, a positive allosteric modulator of the GABAA δ subunit, DS2 (4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl]benzamide), increased tonic currents. These results indicate that GABAA δ receptors remain on the cell surface but are less active in CFA-treated female rats. In vivo behavior studies showed that morphine induced greater antinociception in CFA-treated females that was reversed with microinjections of DS2 directly into the vlPAG. DS2 did not affect morphine antinociception in naive or CFA-treated male rats. Together, these data indicate that sex-specific adaptations in GABAA receptor signaling modulate opioid analgesia in persistent inflammation. Antagonists of GABAA δ receptors may be a viable strategy for reducing pain associated with persistent inflammation, particularly in females.
SIGNIFICANCE STATEMENT These studies demonstrate that GABA signaling is modulated in the ventrolateral periaqueductal gray by persistent inflammation differently in female and male rats. Our results indicate that antagonists or negative allosteric modulators of GABAA δ receptors may be an effective strategy to alleviate chronic inflammatory pain and promote opioid antinociception, especially in females.
Keywords: chronic pain, descending pain control, GABAA, opioid, sex difference, tonic current
Introduction
Chronic pain is a debilitating disease that affects >100 million Americans and has an annual cost in the United States of >500 billion dollars (Gaskin and Richard, 2012). The cellular mechanisms underlying chronic pain states are not understood, but it is generally thought that chronic pain is produced by sensitization of neuronal circuits (Sandkühler, 2009). The primary supraspinal circuit that regulates the response to pain is the descending pain modulatory pathway that projects from the ventrolateral periaqueductal gray (vlPAG) to the rostral ventromedial medulla (RVM) and then to the spinal cord (Heinricher and Ingram, 2008). Activity of the descending pain modulatory pathway is under tonic inhibition by GABA acting on GABAA receptors in the vlPAG. Analgesia occurs through disinhibition (or removal of GABAA-mediated inhibition) of vlPAG output neurons that project to the RVM (Heinricher and Ingram, 2008; Lau and Vaughan, 2014). Directly modulating GABA in the vlPAG affects nociception: GABAA receptor inhibitors induce analgesia and GABAA receptor agonists diminish morphine antinociception when microinjected into the vlPAG (Depaulis et al., 1987; Bobeck et al., 2014). Opioids suppress GABA release onto vlPAG neurons in in vitro studies (Vaughan and Christie, 1997; Vaughan et al., 1997; Ingram et al., 1998), and microinjection of opioids into the vlPAG in vivo induces analgesia (Jacquet and Lajtha, 1976; Morgan et al., 1998; Bodnar, 2000; Macey et al., 2009, 2010, 2015; Bobeck et al., 2012). Increased presynaptic GABA release has been observed in dissociated vlPAG neurons from rats in neuropathic pain (Hahm et al., 2011), suggesting that changes in GABA signaling may be involved in hyperalgesia associated with chronic pain states. We hypothesized that chronic pain may alter GABAA receptor signaling, modulating the activation of the descending pain pathway.
GABAA receptors are pentomeric ion channels (Barnard and Seeburg, 1988; Farrant and Nusser, 2005). There are 19 cloned GABAA subunits that comprise low-affinity receptors that reside in the synaptic cleft and mediate fast, phasic GABAA signaling and high-affinity extrasynaptic receptors that mediate tonic signaling (Kasugai et al., 2010). Both synaptic and extrasynaptic receptors have important roles in modulating neuronal excitability (Mody et al., 1994). Phasic inhibition produced by synaptic receptors influences information processing and spike timing (Klausberger and Somogyi, 2008). Extrasynaptic receptors set the gain of input/output functions and firing thresholds and may also serve as pools of receptors ready to be trafficked into synaptic densities during synaptic plasticity (Mitchell and Silver, 2003; Semyanov et al., 2004). Tonic, extrasynaptic GABAA signaling is critical for modulating excitability at the cell and circuit levels in areas, such as the thalamus, cerebellum, and cortex and is primarily mediated by GABAA receptors containing the δ subunit (Brickley and Mody, 2012). In the vlPAG, phasic, synaptic GABAA signaling is well characterized (Vaughan and Christie, 1997; Vaughan et al., 1997; Ingram et al., 1998; Hack et al., 2003; Bobeck et al., 2014); however, it is not known whether it is altered in chronic pain states. GABAA-mediated tonic currents have only recently been described and are sensitive to menthol, a substance known to activate pain-producing receptors (Lau et al., 2014), but it is not known how these currents are altered in persistent inflammatory pain.
The vlPAG and its descending circuit are sexually dimorphic, providing a substrate for the differential responses to pain and opioid analgesia observed in males and females (Loyd and Murphy, 2014). In the following studies, Complete Freud's adjuvant (CFA) injections into the hindpaw of male and female rats were used as a model of persistent inflammation. Sex-specific differences in postsynaptic GABAA-mediated currents and presynaptic GABA release were observed. Our results indicate that activation of GABAA δ receptors modulates antinociception and that selective antagonists of GABAA receptor subtypes may have therapeutic potential in the treatment of chronic pain states.
Materials and Methods
Animals.
Female and male Sprague Dawley rats (Harlan Laboratories and bred in house; 25–60 d postnatal for electrophysiology and 200–340 g for behavioral studies) were used. Vaginal cytology in females was performed at the time of death. Lights were on a 12 h light and dark cycle, and food and water were provided ad libitum. All experiments were approved by the Animal Care and Use Committee at Oregon Health & Science University.
CFA treatment.
Rats were lightly anesthetized with isoflurane and administered 100 μl of CFA (Sigma) into the left or right hindpaw. Animals were allowed to recover for 4–7 d before experimentation. Paw inflammation was measured at the time of death in a subgroup of rats.
Electrophysiology.
Rats were anesthetized with isoflurane and decapitated. Brains were quickly removed and immersed in ice-cold sucrose aCSF containing the following (in mm): 75 NaCl, 2.5 KCl, 0.1 CaCl2, 6 MgSO4, 1.2 NaH2PO4, 25 NaHCO3, 2.5 dextrose, 50 sucrose. Coronal slices containing the vlPAG were cut 220 μm thick with a vibratome (Leica Microsystems). Slices were placed in a holding chamber oxygenated with aCSF containing the following (in mm): 126 NaCl, 21.4 NaHCO3, 11.1 dextrose, 2.5 KCl, 2.4 CaCl2, 1.2 MgCl2, and 1.2 NaH2PO4, and equilibrated with 95% O2/5% CO2 at 32°C until recording. Brain slices were placed into a recording chamber on an upright Zeiss Examiner Z1 and superfused with 32°C aCSF. Neurons were visualized with infrared Nomarski optics and a water-immersion 40× objective. Recordings were made with electrodes pulled to 2–5 MOhm. Voltage-clamp recordings were made using an internal solution containing the following (in mm): 130 CsCl, 10 HEPES, 1.1 EGTA, 10 KCl, 2 MgCl2, 0.1 CaCl2, 4 MgATP, and 1 NaGTP (pH 7.4 with CsOH). Determination of reversal potential was done using an internal solution containing the following (in mm): 128 K-gluconate, 10 HEPES, 1 EGTA, 10 KCl, 1 MgCl2, 0.3 CaCl2, 4 MgATP, and 1 NaGTP (pH 7.4 with KOH). Series resistance (<12 MOhm) was compensated by 80% and continuously monitored throughout the experiment. Liquid junction potentials of 5 mV (CsCl intracellular solution) or 15 mV (K-gluconate intracellular solution) were corrected.
Spontaneous and evoked IPSCs were isolated in the presence of non-NMDA glutamate receptor antagonists DNQX (10–20 μm) or NBQX (5–10 μm) and the glycine receptor antagonist strychnine (5 μm). Miniature IPSCs (mIPSCs) were recorded in the presence of TTX (500 nm). Recordings were sampled at 20 kHz and filtered at 2 kHz (low-pass filter) for online and offline analyses (Axograph X; Axograph Scientific). Average holding current, variance, and mIPSCs were analyzed using Axograph X. The mIPSCs were detected by selecting events that exceeded a preset threshold (set to 2.1 SD above baseline noise) and fit the criteria: 10%–90% rise time (0 and 2 ms) and half-width (>4 ms). Events were verified individually. All distributions were tested for normality using the Shapiro–Wilk normality test. Frequency was determined over 1 or 2 min epochs after equilibration of each drug. Tonic currents and variance (Ivar) of the holding current were measured as an average of three measurements in segments lacking postsynaptic phasic currents for each drug treatment.
qRT-PCR.
Following treatment, rats were anesthetized with isoflurane and rapidly decapitated. Sections containing the vlPAG were placed in a 1:1 solution of RNase-free water and RNA later (Invitrogen), and the vlPAG on both sides was dissected from each section. Tissue was stored for 24–48 h at 4°C until RNA isolation. RNA later was removed from Eppendorf tubes containing tissue and replaced with 1 ml Trizol (Invitrogen). A phenol-chloroform extraction was performed, and the resulting RNA was processed with the Ambion PureLink kit (Invitrogen). Isolated RNA was then analyzed using a NanoDrop (Thermo Scientific) for quantification and quality control. qRT-PCR primers for the δ subunit were designed to bridge introns using Primer3 (Koressaar and Remm, 2007) as follows: GABAA δ subunit (Accession number NM_017289.1: forward, TGGCCAGCTGATTTGAAGTTC; reverse, ACTGGCCCAGTTCACTATCACC 200 bp); and β-actin (Accession number NM_031144.3: forward, CAGCCTTCCTTCCTGGGTATG; reverse, TAGAGCCACCAATCCACACAG. 247 bp) (Yamamoto et al., 2005).
Standard curves were performed using serial dilutions of vlPAG tissue. Primer efficiency was 100% for GABAA δ (slope: −3.36, r2 = 0.99) and 97% for β-actin (slope: −3.39, r2 = 0.99). Melting curves were examined after each experiment to ensure only one product was formed, and the products were sequenced. Isolated RNA (2 μg) was converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (Invitrogen). Controls lacking reverse transcriptase (RT) were also created. Reactions were performed using SYBR Select Master Mix on an ABI 7000 and were analyzed using SDS software (Applied Biosystems). Negative controls were performed without template cDNA as well as RT samples. Samples were run in triplicate (GABAA δ) or duplicate (β-actin), and ΔCt values were calculated as the difference between Ct of GABAA δ and β-actin, using a threshold during the exponential phase of amplification. The ΔΔCt value was determined for each individual animal using the averaged ΔCt value of the male naive samples as the calibrator. Values are reported as fold change using 2−ΔΔCt values. A two-way ANOVA and Tukey post hoc test (JMP software) was used for statistical analysis.
Microinjections.
Adult rats (200–340 g) were anesthetized with a mixture of ketamine (37.5 mg/kg), xylazine (7.5 mg/kg), and acepromazine (1.5 mg/kg) and implanted with a guide cannula (23 gauge, 9 mm long) aimed at the vlPAG (anteroposterior 1.7 mm, mediolateral −0.6 mm, dorsoventral −4.6 mm from lambda) using stereotaxic techniques. The guide cannula was attached to two screws in the skull by dental cement. CFA injections were made into one hindpaw in half of the rats while under anesthesia. After surgery, a stylet was inserted into the cannula, and rats were maintained under a heat lamp until awake. Rats were housed singly for 2 d after surgery and were then rehoused two per cage. Rats were handled daily, and experiments were conducted 7 d after surgery. Female rats cycled normally for 1 week before cannula placement. After cannulation, all female rats transitioned to a prolonged diestrus state. Estrous state was confirmed by vaginal swabbing.
Drugs were administered directly into the vlPAG through a 31-gauge injection cannula (0.25 mm OD and 0.127 mm ID) inserted into and extending 2 mm beyond the tip of the guide cannula. One day before testing, all rats received a sham injection in which the injection cannula was inserted into the guide cannula, but no drug was administered. This procedure reduces responses resulting from mechanical stimulation of neurons during the experiment and habituates the animal to the microinjection procedure. Testing with drug administration began the next day. All drugs were injected at a rate of 0.1 μl per 10 s while the rat was gently restrained by hand. The injection cannula remained in place an additional 20 s to minimize backflow of drug back up the cannula track.
Testing of antinociceptive behavior.
A Plantar Analgesia Meter Model 390G from IITC equipped with a glass platform heated to 32°C was used to test hindpaw thermal withdrawal latency. Rats were placed on the heated glass platform within individual acrylic transparent enclosures (21 cm × 10 cm × 13 cm) to acclimatize to the environment 3 d for 1 h/d and 1 h before testing on day 4. On test day, after the hour acclimatization period, baseline thermal latency was tested. Latency to withdraw the hindpaw from a focused heat stimulus (20 s cutoff) was measured 2 or 3 times per paw with 2 min intervals between the same paw. Animals were then removed, microinjected with 3 μg/0.5 μl of DS2 or DMSO (vehicle) into the vlPAG, returned to the enclosure, and thermal latency was tested 8 min later. At 20 min after the DS2 microinjection, rats were injected with 10 mg/kg morphine (s.c.), and thermal latency was tested at 30 min intervals. Only rats with injection sites in or on the border of the vlPAG were included in data analyses (Paxinos and Watson, 2005).
Data analyses.
All data are expressed as mean ± SEM. Statistical significance was determined in two group comparisons by paired t tests or Mann–Whitney U tests and in more than two group comparisons by one or two-way ANOVA when appropriate (p < 0.05), followed by Tukey or Sidak's multiple-comparison test (GraphPad Prism 4).
Results
Characterization of GABAA-mediated tonic currents in vlPAG
Little is known about GABAA signaling in the vlPAG in chronic pain states. We were interested in determining whether phasic or tonic GABAA receptor signaling was modulated in chronic pain, using the CFA model of persistent inflammation. GABAA-mediated currents were isolated in the presence of glutamate receptor antagonists NBQX (5 μm) or DNQX (10–20 μm) and measured at −70 mV using whole-cell patch-clamp recordings from vlPAG neurons from male and female rats. Tonic currents were measured as outward deflections of the holding current in the presence of several different GABAA receptor antagonists. GABAergic currents are inward at a holding potential of −70 mV when using CsCl intracellular recording solutions because the equilibrium potential for chloride flux is ∼0 mV. The nonselective GABAA antagonist bicuculline (30 μm) consistently blocked a small current (6 ± 1 pA, n = 16) in males (n = 11) and females (n = 5). The amplitude of the bicuculline-induced current was not different between males and females (t(14) = 1.98, p = 0.07). Bicuculline is known to also block SK potassium channels (Seutin and Johnson, 1999), so we compared the reversal potential of the bicuculline-blocked tonic current using CsCl and K-gluconate intracellular solutions to determine whether the shift in reversal potential was predicted for a chloride ion flux. Using a CsCl internal solution ([Cl−]in 134.2 mm), the experimental reversal potential was −17 ± 7 mV (n = 5) and shifted to −67 ± 9 mV (n = 4) when recording with a potassium gluconate internal solution ([Cl−]in 12.6 mm). The shift indicates that the primary ion conducted by the bicuculline-sensitive current is chloride.
We also tested the selective GABAA inhibitors, picrotoxin (300 μm) and gabazine (1 μm). Picrotoxin blocked a tonic current in both males and females (Fig. 1A,C) that was associated with a decrease in the variance of the holding current (Ivar) of 0.8 ± 0.1 pA2 and 2.3 ± 0.4 pA2 in males and females, respectively. The GABAA receptor antagonist gabazine (SR 95531) is selective for phasic, synaptic GABAA receptors at low (1 μm) concentrations but inhibits all GABAA-mediated currents at higher concentrations (20 μm) (Park et al., 2007; Lau et al., 2014). In the vlPAG, gabazine (1 μm) decreased spontaneous synaptic GABAA currents without changing the baseline holding current in both male and female rats (data not shown). Addition of bicuculline or picrotoxin to gabazine (1 μm) produced an outward deflection of the holding current (6 ± 1 pA, n = 4), comparable with that of either bicuculline or picrotoxin alone. In addition, because picrotoxin is also an open channel blocker of homomeric glycine receptors (Pribilla et al., 1992), we tested whether strychnine (1 μm) blocked a tonic current in vlPAG neurons. There was no tonic current blocked by strychnine (0.2 ± 0.2 pA, n = 5). Together, these results support the existence of a tonic current mediated by GABAA receptors. A previous study showed that the tonic current in vlPAG neurons of male rats did not display benzodiazepine sensitivity (Lau et al., 2014). To determine whether the tonic current in female PAG neurons was sensitive to benzodiazepine modulation, we superfused the benzodiazepine binding site inhibitor, ZK93426 (ZK). ZK had no measurable effects on the holding current and the tonic currents in the presence of ZK (8 ± 2 pA, n = 4) were not different compared with currents in the absence of ZK.
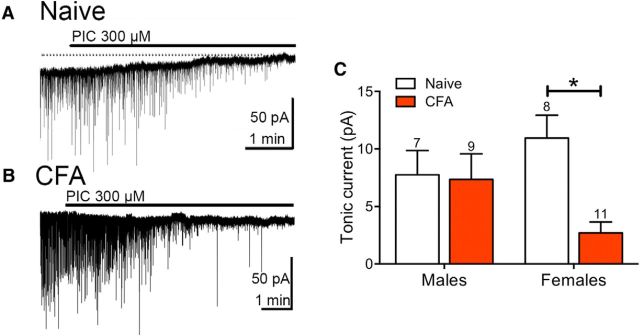
Figure 1.
GABAA-mediated tonic currents are reduced in CFA-treated female rats. A, The selective GABAA antagonist picrotoxin (PIC) induced outward deflection of the holding current at −70 mV and inhibited sIPSCs in naive rats. B, The deflection in holding current at −70 mV was reduced in a female rat pretreated with CFA 4–7 d before recording. C, Tonic currents were not affected in male rats but were reduced in female rats following CFA treatment (two-way ANOVA, effect of treatment; F(1,31) = 5.68, p = 0.024; Sidak's multiple-comparison test, *p < 0.05).
GABA regulates the output of the descending pain modulatory pathway from the vlPAG to the RVM such that disinhibition of vlPAG-RVM output neurons allows activation of descending antinociception. Thus, we were interested in determining whether GABA signaling is modulated in persistent inflammation. Male and female rats were injected with CFA (100 μl) into the hindpaw. CFA significantly increased paw diameter in males (158 ± 6%, n = 14) and females (165 ± 9%, n = 11), but the two groups were not significantly different (t(23) = 0.73, p = 0.47). Brain slices were removed for recordings 4–7 d later. Tonic currents were measured as outward deflections in the holding current produced by picrotoxin (300 μm), and these currents were not different in CFA-pretreated compared with naive male rats (Fig. 1C). However, picrotoxin-induced shifts in the holding current were smaller in CFA-treated female rats (Fig. 1B,C). This effect was also observed in the Ivar measurements (two-way ANOVA, effect of sex; F(1,31) = 14.15, p = 0.0007). In both current and variance measurements, there was a significant difference between the female naive and CFA groups (Sidak's multiple-comparison test, p < 0.05). These data suggest that GABAA-mediated tonic currents are regulated differently in male and female rats during persistent inflammation.
Presynaptic GABA release is increased in CFA female rats
To determine whether CFA treatment alters the release of GABA in the vlPAG, mIPSCs were recorded in the presence of glutamate receptor inhibitors and TTX (500 nm) (Fig. 2). In the vlPAG of female rats, mIPSC frequency was increased in CFA-treated rats compared with naive rats (Fig. 2B). The mean amplitude of mIPSCs was determined from a Gaussian fit to the histogram of individual event amplitudes. There was no difference in the mean amplitude of mIPSCs from CFA-treated rats compared with naive rats (Fig. 2C). In the absence of TTX, spontaneous IPSC (sIPSC) frequencies were similar in both naive (1.4 ± 0.2 Hz, n = 38) and CFA-treated female rats (1.8 ± 0.2 Hz, n = 37; Mann–Whitney U = 593, p = 0.25). Neither rise time or decay measurements of the mIPSCs or sIPSCs were significantly altered by CFA treatment in the female rats (data not shown).
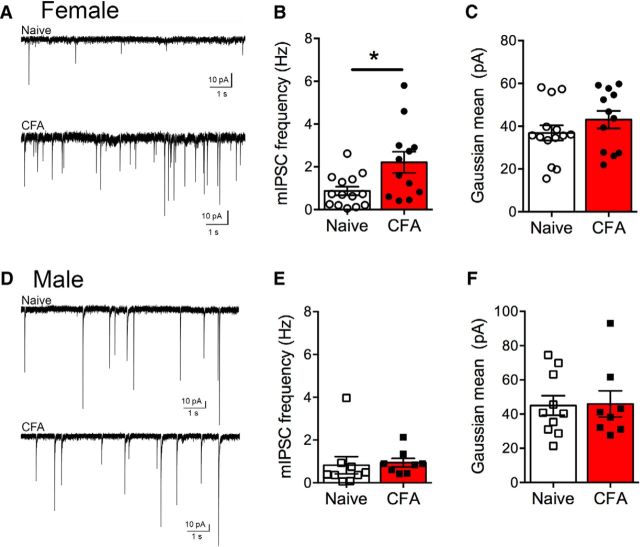
Figure 2.
Persistent inflammation increases GABA release in female rats. A, Representative traces of GABAergic mIPSCs in naive (top) and CFA-treated (bottom) female PAG neurons. B, The frequency of mIPSCs is increased in CFA-treated compared with female rats (Mann–Whitney U = 40, *p = 0.02). C, The mean amplitude of mIPSCs determined from a Gaussian fit to the amplitude histogram was not altered in CFA-treated female rats (t(24) = 1.15, p = 0.26). D, Representative traces of GABAergic mIPSCs in naive (top) and CFA-treated (bottom) male PAG neurons. E, The mIPSC frequency in male rats was not altered by CFA treatment (Mann–Whitney U = 19, p = 0.11). F, Mean amplitude of mIPSCs was also not altered in male rats pretreated with CFA (t(16) = 0.096, p = 0.92).
For comparison, we measured mIPSC frequency and amplitude in naive and CFA-treated male rats (Fig. 2D). There was no change in mIPSC frequency in CFA-treated male rats compared with naive rats (Fig. 2E). Similarly, there were no significant differences in mean amplitude of events (Fig. 2F).
GABAA δ subunit mRNA is lower in female rats
The GABAA δ subunit underlies the extrasynaptic GABAA current in a number of brain regions (Brickley and Mody, 2012). To determine whether the reduced tonic current in females treated with CFA is due to changes in GABAA δ subunit expression, we measured mRNA levels of GABAA δ subunits in naive and CFA-treated males and females. Primers were made against the rat GABAA δ subunit (Fig. 3A) and β-actin. The ΔΔCt analysis compared all animals with naive males. There was a significant difference between males and females in relative mRNA expression; females had lower levels of GABAA δ subunit transcripts (Fig. 3B). There were no significant differences in transcript levels following treatment with CFA in either males or females.
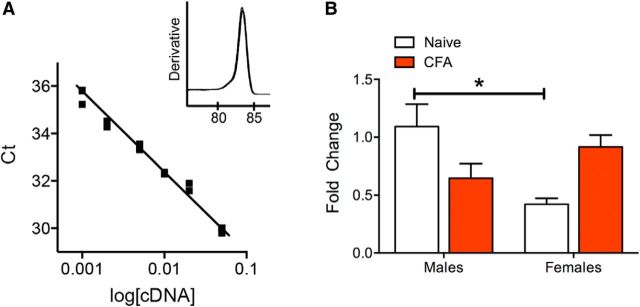
Figure 3.
GABAA δ mRNA expression from vlPAG are lower in female rats. A, The designed GABAA δ primer efficiency was 100% (slope: −3.36, r2 = 0.99) and produced a single product (inset). B, There was a significant interaction between treatment and sex (two-way ANOVA, F(3,17) = 3.38, p = 0.0093) when comparing relative GABAA δ mRNA expression. Naive female rats had lower levels than naive male rats (Tukey's post hoc, *p < 0.05). Although there was a strong trend toward an increase in expression with CFA treatment in female rats and a decrease in expression with CFA treatment in males, neither reached significance (N = 4–6 rats/group).
GABAA δ subunit contribution to evoked IPSCs is decreased following CFA treatment in female rats
A previous study determined that tonic currents do not contribute substantially to evoked GABAergic synaptic currents in the vlPAG of male rats (Lau et al., 2014). To further characterize whether a portion of evoked synaptic currents are mediated by GABAA receptors containing the δ subunit in female rats, we measured evoked synaptic currents elicited by bipolar stimulation of the vlPAG. There are no known specific inhibitors of GABAA δ subunits, so we took advantage of a positive allosteric modulator (PAM) of δ subunit-containing GABAA receptors, DS2 (4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl]benzamide) (Wafford et al., 2009; Herd et al., 2013; Jensen et al., 2013; Ye et al., 2013). The benefit of using a PAM for these studies is that it potentiates the effects of GABA but is ineffective in the absence of GABA. In naive female rats, DS2 (10 μm) increased the decay of IPSCs (Fig. 4A,B). The effect on the decay time constant was absent in CFA-treated female rats. These results suggest that GABA activates extrasynaptic GABAA receptors in naive vlPAG but, following CFA treatment, activation of these receptors is reduced, consistent with our findings in Figure 1. Notably, there was no effect of DS2 on mIPSC frequency, amplitude or kinetics (Fig. 4C) and no effect on sIPSCs (data not shown), indicating that potentiation of GABAA δ receptor-mediated currents does not affect presynaptic GABA release or GABAA receptors located within the synapse.
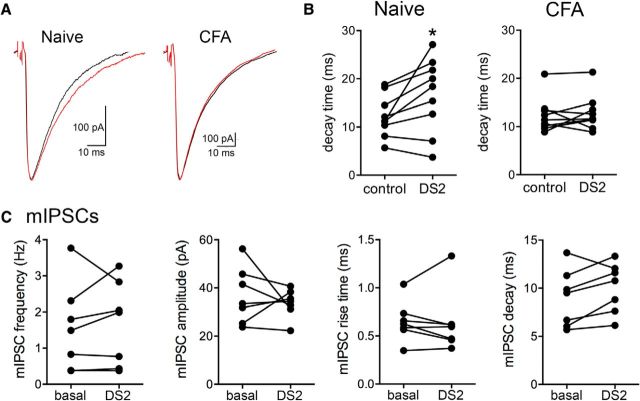
Figure 4.
GABAA δ receptors contribute to decay of evoked IPSCs in naive but not CFA-treated female PAG neurons. A, Representative traces of evoked IPSCs from neurons held at −70 mV from naive and CFA-treated rats. Black traces (control) and red traces (DS2). B, The GABAA δ subunit-positive allosteric modulator, DS2 (10 μm) increases the decay of evoked IPSCs in naive rats (paired t test; t(8) = 2.52, *p = 0.04). DS2 (10 μm) had no effect on the decay of evoked IPSCs in CFA-treated rats (paired t test; t(8) = 0.56, p = 0.59). C, There was no effect of DS2 on mIPSC frequency in females (Wilcoxon matched pairs, W = 8.0, p = 0.58), amplitude (paired t test, t(6) = 0.71, p = 0.50), rise time (paired t test, t(6) = 0.23, p = 0.82), and decay time (paired t test, t(6) = 2.0, p = 0.09).
GABAA δ subunits are available on the plasma membrane following CFA treatment
To determine whether the decrease in the tonic GABAA current is caused by a decrease in GABAA δ subunits at the plasma membrane following persistent inflammation, we applied THIP, a GABAA agonist that preferentially activates GABAA δ subunits at low concentration (Meera et al., 2011). THIP has been shown to be selective for GABAA δ subunits at submicromolar concentrations. THIP (1 μm) elicited a tonic current as measured by superfusion of GABAA antagonists (Fig. 5A–C) in both females and males that was accompanied by an increase in the variance of the holding current. There were no significant changes in the THIP responses following CFA treatment, suggesting that receptors activated by THIP were similar in both naive and CFA-treated vlPAG neurons.
Figure 5.
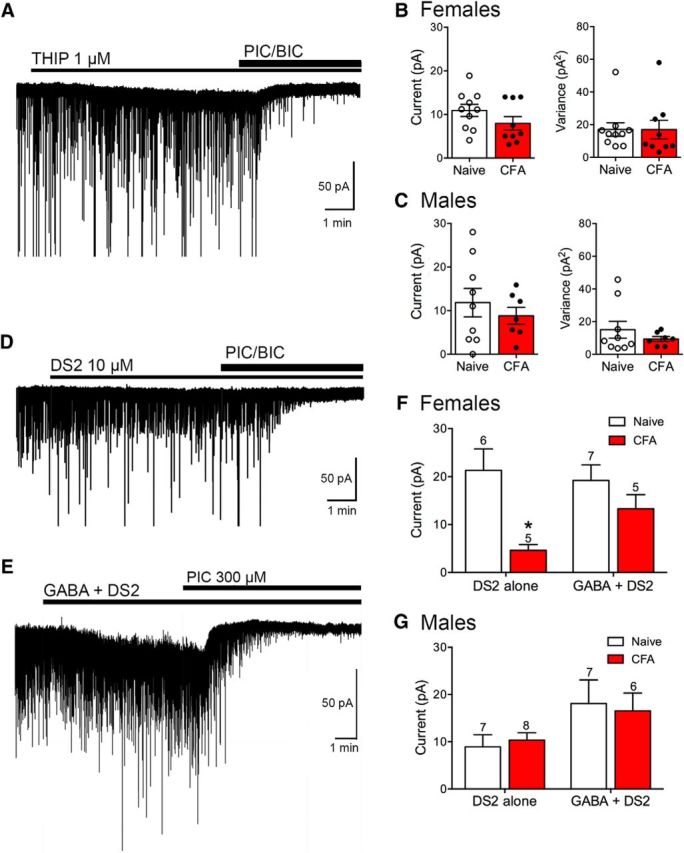
Agonists of GABAA δ subunits elicit a tonic current in vlPAG neurons. A, The GABAA δ subunit agonist THIP increases the holding current at −70 mV in a neuron from a naive female. The increase in holding current is reversed by the GABAA antagonists, picrotoxin (PIC; 100 μm) and bicuculline (BIC; 10 μm). B, Bar graphs represent compiled data from female slices for the THIP-mediated tonic currents. There were no differences in current amplitude (t(17) = 1.42, p = 0.17) or in variance of the holding current in the presence of THIP t(17) = 0.0048, p = 1.0) between naive and CFA-treated female rats. C, Bar graphs represent compiled data from male slices for the THIP-mediated tonic currents. There were no differences in current amplitude (t(14) = 0.74, p = 0.47) or in variance of the holding current in the presence of THIP (t(14) = 0.92, p = 0.37) between naive and CFA-treated male rats. D, Representative trace from a vlPAG neuron from a CFA-treated female rat held at −70 mV in the presence of DS2 (10 μm). Tonic currents were measured by the outward deflection in holding current produced by GABAA antagonists. E, Representative trace from a vlPAG neuron from a CFA-treated female rat held at −70 mV in the presence of GABA (10 μm) + DS2 (10 μm). F, Bar graph represents compiled data for DS2 alone versus GABA + DS2-mediated tonic currents in female rats. The DS2-mediated currents were smaller in CFA-treated rats (two-way repeated-measures ANOVA, effect of treatment, F(1,19) = 10.96, p = 0.004; Sidak's multiple-comparison test, *p < 0.05). DS2 currents were comparable with naive rats in the presence of GABA. G, Bar graph represents compiled data for DS2 alone versus GABA + DS2-mediated tonic currents in male rats (two-way repeated-measures ANOVA, effect of treatment, F(1,24) = 0.0003, p = 0.99). There was no difference in DS2-mediated currents in CFA-treated animals in the absence or presence of GABA.
To further test the role of GABAA δ subunits in the tonic current observed in the vlPAG, we tested the ability of the PAM DS2 to modulate the tonic current. In females, the outward shift in holding current produced by GABAA receptor antagonists in the presence of DS2 (10 μm) was smaller when recording from CFA-treated rats compared with naive rats (Fig. 5D,F). These results indicated that less GABA reached receptors containing GABAA δ subunits in CFA-treated female rats. To further test this hypothesis, we applied a low concentration of exogenous GABA (10 μm) and observed that DS2 had a larger effect in the CFA-treated female rats (Fig. 5E,F). This increase was accompanied by a significant increase in holding current variance in the presence of DS2 (data not shown). In males, the currents elicited in the presence of DS2 were not different in naive or CFA-treated rats in either the absence or presence of exogenous GABA (Fig. 5G). These results confirm the presence of GABAA receptors containing the δ subunit on the plasma membrane in both control animals and animals pretreated with CFA and provide further evidence that these receptors contribute to the tonic current measured in vlPAG neurons.
Based on these results, we hypothesized that CFA treatment may increase the activity of GABA transporters in females, effectively reducing the extracellular GABA concentration in the vicinity of extrasynaptic GABAA δ receptors. To test this hypothesis, we measured tonic currents in the presence of the GABA transporter (GAT)-1 inhibitor NO-711 (10 μm) and the nonselective GAT inhibitor SNAP-5114 (10 μm) in female rats in the absence of exogenous GABA. Interestingly, GAT inhibitors reduced the tonic current in all vlPAG neurons (Fig. 6A). Indeed, there was no measurable change in holding current in the presence of NO-711 + SNAP when GABAA receptor antagonists were applied: naive, 0.8 ± 0.5 pA (n = 10); CFA, 0 pA (n = 11). In the presence of GABA (10 μm), measurable currents were obtained in both groups (Fig. 6B–D). The tonic currents were significantly enhanced in both naive and CFA-treated female rats by addition of NO-711 + SNAP (Fig. 6E). Thus, in the presence of exogenous GABA, GAT inhibitors reversed the deficit in tonic currents in CFA-treated female rats, indicating that an increase in extracellular GABA concentrations can activate GABAA δ receptors similarly to naive animals.
Figure 6.
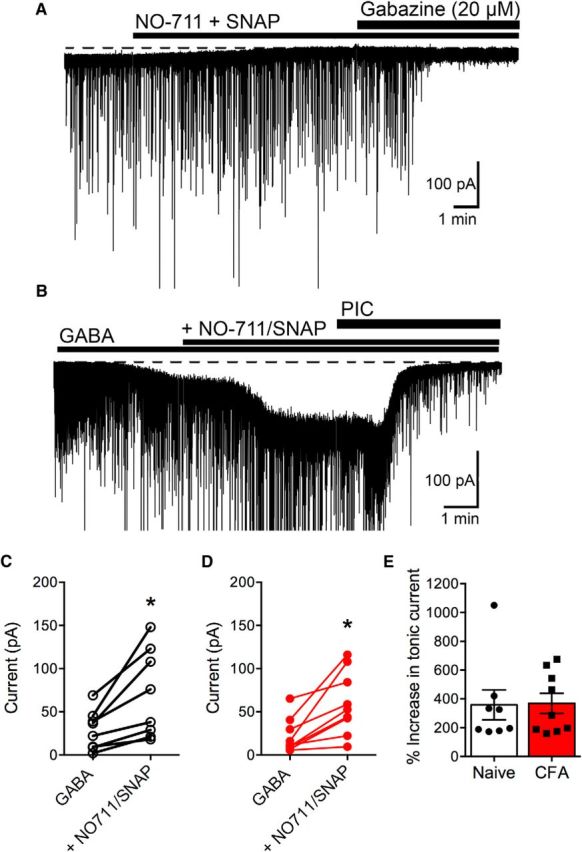
GAT inhibitors abolish the tonic current in the absence of exogenous GABA in vlPAG neurons from female rats. A, Whole-cell patch-clamp recording from a neuron from a CFA-treated female rat held at −70 mV in DNQX (10 μm). Superfusion of GAT inhibitors NO-711 (10 μm) and SNAP-5114 (SNAP; 10 μm) induces a change in the holding current. The GABAA inhibitor gabazine (20 μm) elicits no further effect on the holding current. B, Whole-cell patch-clamp recording from a neuron from a female CFA-treated rat held at −70 mV in DNQX (10 μm). sIPSCs are truncated for clarity of changes in membrane currents. Superfusion of GABA (10 μm) and GAT inhibitors NO-711 (10 μm) and SNAP (10 μm) increased the inward current. C, Compiled data showing the amplitude of the tonic current in individual cells in naive female rats in the presence of GABA and the increase induced by GAT inhibitors (paired t test, t(7) = 3.55, *p = 0.0093). D, Compiled data showing the amplitude of the tonic current in individual cells in CFA-treated female rats in the presence of GABA and the increase induced by GAT inhibitors (paired t test, t(8) = 3.95, *p = 0.0042). E, There was no significant difference in the increase in amplitude in the presence of GAT inhibitors in naive versus CFA-treated rats.
We also tested whether the GAT inhibitors affected mIPSCs. There was no effect of the GAT inhibitors on mIPSC frequency in naive (97 ± 20% of basal frequency, n = 5) compared with CFA-treated rats (100 ± 8% of basal frequency, n = 5). There was also no evidence of changes in mIPSC amplitude, rise time, or decay in the presence of GAT inhibitors (data not shown).
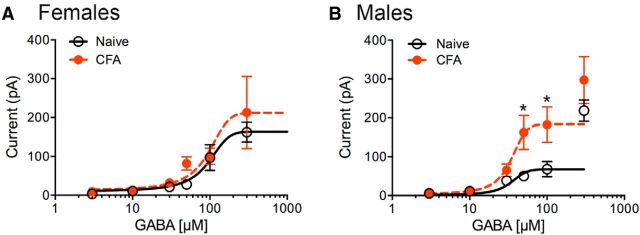
To further characterize GABAA-mediated currents in female versus male rats, concentration–response curves were generated with GABA (3–300 μm; Fig. 7). The dose–response curves reflected two different types of currents. GABA concentrations <100 μm elicited currents that were maintained with the drug administration, whereas higher concentrations elicited inward currents with two phases: an initial phase that reached a transient plateau followed by a fast inward current that often approached >1 nA and probably reflected a loss of space-clamp during the recording. The large currents also desensitized during the drug administration. We restricted our measurements to the initial plateau phase at GABA concentrations of 100 and 300 μm. In female rats, the GABA-induced currents were not altered by pretreatment with CFA (Fig. 7A); in males, currents elicited by high concentrations of GABA (low-affinity receptors) were increased by CFA pretreatment. These findings indicate that low- and high-affinity GABA-induced currents are modulated differently between sexes during persistent inflammation.
Figure 7.
GABA concentration–response curves differ in female and male rats following CFA treatment. A, In female rats, GABAA-mediated currents were similar in naive (black line) and CFA-treated (red dashed line) rats at all concentrations of GABA (two-way ANOVA, F(1,74) = 0.89, p = 0.35). N values at each concentration (μm, Naive/CFA) are 3(7/6); 10(9/11); 30(8/6); 50(5/5); 100(7/5); and 300(8/9). Prism curve fits were constrained by Bottom = 0 and Hill slope shared settings. B, In male rats, CFA pretreatment increases the GABA-mediated currents at higher concentrations (two-way ANOVA, F(1,58) = 13.29, p = 0.0006; Sidak's multiple-comparison test, *p < 0.05). N values at each concentration (μm, Naive/CFA) are 3(6/5); 10(7/6); 30(7/7); 50(6/5); 100(5/5); and 300(5/6). Prism curve fits were constrained by Bottom = 0 and Hill slope shared settings. The 300 μm points (male only) were not included in the fit.
Allosteric modulation of the δ subunit by DS2 decreases morphine antinociception in females in vivo
To determine whether the GABAA δ subunit is important for antinociception mediated by the vlPAG, we examined the effect of DS2 (3 μg/0.5 μl) microinjected into the vlPAG on systemic morphine-induced antinociception of naive and CFA-treated rats. On test day, baseline paw withdrawal latencies were measured in both paws in naive and CFA-treated female (Fig. 8A) and male (Fig. 8D) rats. In both sexes, the CFA-injected paw displayed lower thermal thresholds than the control paw (Sidak's multiple-comparison test, p < 0.05), consistent with the development of hyperalgesia. As expected, microinjection of DS2 alone into the vlPAG did not alter nociceptive thresholds in either paw in either sex. However, DS2 microinjections decreased systemic morphine (10 mg/kg, s.c.) antinociception in CFA-treated female rats (Fig. 8B,C). The time course of morphine antinociception was assessed every 15 min for 90 min, and the effect of morphine over time was calculated with area under the curve (AUC). In CFA-treated female rats, morphine induced greater antinociception than in naive rats. This effect in CFA-treated female rats was reversed in animals that received the DS2 microinjection (Fig. 8C; Dunnett's multiple-comparison test, p < 0.05). Although there was a trend toward a decrease in morphine antinociception in naive female rats, the effect did not reach significance (Tukey's multiple-comparison test, p > 0.05). In male rats, there were no significant differences in morphine antinociception or AUC measurements in any group (Fig. 8E,F).
Figure 8.
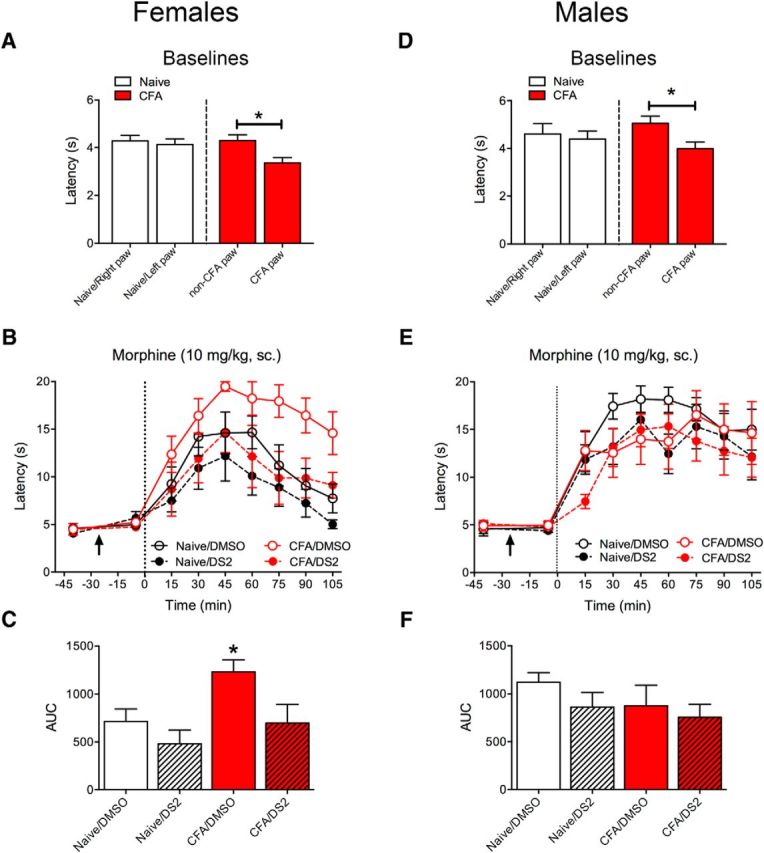
Systemic morphine antinociception in female rats is reversed with intra-PAG DS2. A–C, Female rats were cannulated into the vlPAG and half of the rats were injected with CFA (100 μl into one hindpaw). A, CFA injection into the hindpaw induces a decrease in thermal paw withdrawal latency (i.e., hyperalgesia) compared with the uninjected paw and paws from naive rats 7 d later (effect of paw, F(1,23) = 16.09, p = 0.0005; Sidak's multiple-comparison test, *p < 0.05). B, After baseline testing, rats were microinjected with either vehicle (DMSO) or DS2 (3 μg/0.5 μl; denoted by arrow) and tested 20 min later. All rats received a morphine injection (10 mg/kg, sc.) at time 0 (dotted line) and were tested every 15 min. Morphine increased thermal latencies (effect of time, F(8,384) = 45.56, p = 0.0001). Intra-PAG microinjections of DS2 reversed the increase in CFA-pretreated rats (effect of treatment, F(7,48) = 2.74, p = 0.019). Data from uninjected paws are shown in the graph. C, Time course of morphine effect was analyzed as AUC. Morphine produced significantly greater antinociception in the CFA/DMSO group (ANOVA, F(3,24) = 4.99, p = 0.008; Dunnett's multiple-comparison test, *p < 0.05 compared with naive/DMSO). D–F, Male rats were cannulated into the vlPAG and half of the rats were injected with CFA (100 μl into one hindpaw). D, Baseline latencies were decreased in the CFA injected paw compared with the uninjected paw (effect of paw, F(1,28) = 6.92, p = 0.014; Sidak's multiple comparison test, *p < 0.05). E, Male rats were treated identically to female rats in B. Morphine increased thermal latencies in male rats (effect of time, F(8,208) = 36.54, p = 0.0001). Intra-PAG microinjections of DS2 had no effect on morphine-induced response (effect of treatment, F(3,26) = 0.81, p = 0.50). Data from uninjected left paws are shown in graph. F, Time course of morphine effect was analyzed as AUC. DS2 did not significantly alter morphine-induced antinociception in either the naive or CFA-treated rats (ANOVA, F(3,26) = 1.16, p = 0.34).
Discussion
In this study, GABAA receptor signaling in vlPAG neurons was characterized in male and female rats during persistent inflammation. CFA treatment decreased a high-affinity tonic GABAA-mediated current in females. Interestingly, morphine-induced antinociception was increased in CFA-treated female rats and reversed with potentiation of GABAA δ receptor currents by DS2 in the vlPAG in vivo. In contrast, CFA treatment did not affect tonic currents in males but potentiated low-affinity GABAA currents. DS2 had no effect on morphine-induced antinociception in male rats. These results suggest that extrasynaptic GABAA receptors containing δ receptors represent a site of differential regulation between males and females following persistent inflammation.
Characterization of GABA signaling in the vlPAG
High-affinity GABAA receptors that mediate tonic currents typically contain δ subunits (Brickley and Mody, 2012). An immunocytochemical survey of GABAA subunits observed strong expression of α1, α3, and δ subunits, with low levels of α2 and α4 and no detection of α6 subunits in the PAG (Pirker et al., 2000), and another study confirmed the presence of GABAA receptors containing α4β1δ subunits in the PAG (Griffiths and Lovick, 2005; Lovick, 2006). We provide evidence for mRNA of the δ subunit in the vlPAG of males and females, although expression was lower in females. Functionally, the amplitude of the tonic current in males and females was approximately equal in naive animals, indicating that the difference in mRNA may not reflect differences in function. Consistent with this interpretation was the lack of detectable differences in total agonist-induced currents in either sex. Functional characterization of phasic GABAA signaling in the vlPAG has been studied in detail (Vaughan and Christie, 1997; Vaughan et al., 1997; Ingram et al., 1998; Hack et al., 2003; Bobeck et al., 2014), but tonic currents in the vlPAG have only been recently described (Lau et al., 2014). Lau and colleagues (2014) determined that modulators of GABAA δ receptors were effective in the vlPAG. However, the concentration of THIP that produced appreciable potentiation was ∼10-fold higher than expected based on studies in expression systems (Herd et al., 2009; Meera et al., 2011), so the authors speculated that the tonic current may also reflect activation of receptors containing subunits other than δ (Lau et al., 2014). We extended their studies by using the GABAA δ receptor-selective PAM DS2 (Wafford et al., 2009) and observed potentiation of tonic currents in the vlPAG. The DS2 results suggest that the difference in potentiation by low micromolar concentrations of THIP may reflect a decrease in the GABA concentration that a receptor is exposed to in the slice compared with heterologous or dispersed preparations (Meera et al., 2011), rather than a loss of selectivity. Consistent with this interpretation is the finding that currents mediated by THIP (1 μm) in cerebellar neurons (Meera et al., 2011) and even higher (30 μm) concentrations in ventrobasal thalamic neurons (Herd et al., 2009) are abolished in GABAA δ subunit knock-out mice.
DS2 is an important tool in these studies because it increases the maximal effect of GABA at GABAA δ receptors without affecting GABA potency and has no effect in the absence of GABA (Wafford et al., 2009). DS2 does not potentiate receptors containing γ subunits and has no effect in mice lacking δ subunits (Jensen et al., 2013). Because DS2 does not cross the blood–brain barrier (Ye et al., 2013), we microinjected DS2 directly into the vlPAG to determine its effects on pain behaviors. As expected, DS2 did not modulate baseline thermal thresholds but significantly decreased systemic morphine antinociception in the CFA-treated female rats. DS2 had a prominent effect in vivo, indicating that GABA concentrations in the vlPAG were sufficient to activate GABAA δ -containing receptors, an effect we did not observe in slice recordings from CFA-treated rats in the absence of exogenous GABA. This discrepancy revealed that changes in GABA clearance or GAT activity might be the underlying mechanism for the observed decreased tonic currents in vitro. Indeed, we found that the tonic currents in both naive and CFA-treated vlPAG neurons were abolished in the presence of GAT inhibitors, indicating that GAT activity is necessary for activation of the tonic currents. Tonic currents mediated by efflux of GABA through GAT have been reported in hippocampal neurons (Wu et al., 2007) and glia (Barakat and Bordey, 2002). GAT-induced excitatory currents have also been reported in the PAG (Bagley et al., 2005, 2011). However, the tonic currents measured in this study were blocked by GABAA receptor inhibitors, demonstrating that, although the tonic current is dependent on GAT activity, it is most likely mediated by efflux of GABA through the transporter rather than via an electrogenic cation current associated with GABA transport.
Although we observed an increase in mIPSC frequency in PAG neurons from CFA-treated rats, this increase was not a function of enhanced GAT-1-mediated currents because superfusion of GAT inhibitors did not alter sIPSC frequency in either naive or CFA-treated rats. Indeed, it is unlikely that spillover contributes to the tonic current because TTX does not alter the tonic current in the PAG (Lau et al., 2014). We also observed no modulation of mIPSC frequency, amplitude, or kinetics by DS2 and minimal effects of DS2 on evoked GABAergic IPSCs, indicating that the tonic currents do not significantly affect synaptic GABAergic responses.
Another interesting result from our behavioral study was the enhanced morphine antinociception in CFA-treated female rats. Enhanced opioid effects have been observed in the RVM in previous studies (Hurley and Hammond, 2000, 2001; Schepers et al., 2008), but the cellular mechanism for this change is unknown. DAMGO antinociception is increased in inflammation without affecting binding kinetics for DAMGO or stimulation of GTPyS (Sykes et al., 2007) so it is possible that opioid-induced antinociception reflects a synergy between opioids at their respective receptors, and the GABA tone. Microinjections of DS2 into the vlPAG decreased systemic morphine antinociception in females, alluding to the possibility that a negative allosteric agonist of GABAA δ receptors will promote the antinociceptive effects of opioids and may be a particularly attractive strategy for chronic pain in females.
Sex differences in the vlPAG
Studies by Murphy and colleagues have found evidence for anatomical and functional differences in activation of vlPAG neurons (Loyd and Murphy, 2006, 2014; Loyd et al., 2008b) in females and males. Activation of vlPAG to RVM output neurons is increased in males, even though females have twice as many projections (Loyd and Murphy, 2006), and mu opioid receptors and opioid analgesia are altered depending on the estrous state (Loyd et al., 2008a). These studies indicated a clear organizational difference in descending modulation at the level of the vlPAG-RVM between males and females (Bobeck et al., 2009). We observed an increase in the probability of GABA release in females as well as a decrease in the tonic current during persistent inflammation. We believe these differences are due to persistent inflammation and not the influence of ovarian hormones because CFA treatment disrupts the estrous cycle and produces a prolonged diestrus as observed in our study and by others (Wang et al., 2006). In cycling females, GABAA δ subunit expression in the vlPAG is highest in late diestrus (Griffiths and Lovick, 2005). The rats in our behavioral study were in persistent diestrus due to the cannulation and/or CFA treatment, so we expect that expression of the δ subunits was at their highest levels. Functionally, we do not find a difference in the response to exogenous GABA in males or females at low GABA concentrations with CFA treatment. Interestingly, CFA treatment potentiated responses to high GABA concentrations (>50 μm) selectively in males. This increase in low-affinity GABAA currents in CFA-pretreated rats may explain the lack of behavioral effect of DS2 in males.
Physiological consequences
The ability of the extrasynaptic GABAA current to attenuate morphine analgesia is of particular relevance to female physiology and the etiology of chronic pain in females. Human females report more chronic pain conditions (Berkley, 1997; Bartley and Fillingim, 2013) than men. There are conflicting reports on the effectiveness of opioid analgesics in women compared with men (Cepeda and Carr, 2003), even though animal studies generally show a decrease in opioid potency in female compared with male subjects (for review, see Mogil, 2012; Loyd and Murphy, 2014). The vlPAG has been postulated as a main site of action of this effect because of its significant role in opioid analgesia, sexually dimorphic behaviors, and mediation of opioid and sex steroid interaction (Krzanowska and Bodnar, 1999; Krzanowska et al., 2002). Thus, adaptations in GABA signaling may contribute to the sex differences observed in the prevalence of chronic pain syndromes. Our results indicate that selective antagonists of GABAA δ receptors may be useful therapeutics for chronic pain, especially in persistent inflammation.
Footnotes
This work was supported by National Institutes of Health Grants DA027625 and DA035316 to S.L.I. and Grant T32 AG023477 to K.J.T. We thank Dr. Julie Saugstad for advice and technical assistance and QiLiang Chen for comments on the manuscript.
The authors declare no competing financial interests.
References
- Bagley EE, Gerke MB, Vaughan CW, Hack SP, Christie MJ. GABA transporter currents activated by protein kinase A excite midbrain neurons during opioid withdrawal. Neuron. 2005;45:433–445. doi: 10.1016/j.neuron.2004.12.049. [DOI] [PubMed] [Google Scholar]
- Bagley EE, Hacker J, Chefer VI, Mallet C, McNally GP, Chieng BC, Perroud J, Shippenberg TS, Christie MJ. Drug-induced GABA transporter currents enhance GABA release to induce opioid withdrawal behaviors. Nat Neurosci. 2011;14:1548–1554. doi: 10.1038/nn.2940. [DOI] [PubMed] [Google Scholar]
- Barakat L, Bordey A. GAT-1 and reversible GABA transport in Bergmann glia in slices. J Neurophysiol. 2002;88:1407–1419. doi: 10.1152/jn.2002.88.3.1407. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Seeburg PH. Structural basis of the GABA-activated chloride channel: molecular biology and molecular electrophysiology. Adv Biochem Psychopharmacol. 1988;45:1–18. [PubMed] [Google Scholar]
- Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. 1997; discussion 435–513. [DOI] [PubMed] [Google Scholar]
- Bobeck EN, McNeal AL, Morgan MM. Drug dependent sex-differences in periaqueducatal gray mediated antinociception in the rat. Pain. 2009;147:210–216. doi: 10.1016/j.pain.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, Haseman RA, Hong D, Ingram SL, Morgan MM. Differential development of antinociceptive tolerance to morphine and fentanyl is not linked to efficacy in the ventrolateral periaqueductal gray of the rat. J Pain. 2012;13:799–807. doi: 10.1016/j.jpain.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, Chen Q, Morgan MM, Ingram SL. Contribution of adenylyl cyclase modulation of pre- and postsynaptic GABA neurotransmission to morphine antinociception and tolerance. Neuropsychopharmacology. 2014;39:2142–2152. doi: 10.1038/npp.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ. Supraspinal circuitry mediating opioid antinociception: antagonist and synergy studies in multiple sites. J Biomed Sci. 2000;7:181–194. doi: 10.1007/BF02255465. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg. 2003;97:1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Morgan MM, Liebeskind JC. GABAergic modulation of the analgesic effects of morphine microinjected in the ventral periaqueductal gray matter of the rat. Brain Res. 1987;436:223–228. doi: 10.1016/0006-8993(87)91665-9. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express alpha4, beta1 and delta GABAA receptor subunits: plasticity of expression during the estrous cycle. Neuroscience. 2005;136:457–466. doi: 10.1016/j.neuroscience.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Hack SP, Vaughan CW, Christie MJ. Modulation of GABA release during morphine withdrawal in midbrain neurons in vitro. Neuropharmacology. 2003;45:575–584. doi: 10.1016/S0028-3908(03)00205-3. [DOI] [PubMed] [Google Scholar]
- Hahm ET, Kim Y, Lee JJ, Cho YW. GABAergic synaptic response and its opioidergic modulation in periaqueductal gray neurons of rats with neuropathic pain. BMC Neurosci. 2011;12:41. doi: 10.1186/1471-2202-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Ingram SL. The senses: a comprehensive reference. San Diego: Academic; 2008. The brainstem and nociceptive modulation; pp. 593–626. [Google Scholar]
- Herd MB, Foister N, Chandra D, Peden DR, Homanics GE, Brown VJ, Balfour DJ, Lambert JJ, Belelli D. Inhibition of thalamic excitability by 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-ol: a selective role for delta-GABA(A) receptors. Eur J Neurosci. 2009;29:1177–1187. doi: 10.1111/j.1460-9568.2009.06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Brown AR, Lambert JJ, Belelli D. Extrasynaptic GABA(A) receptors couple presynaptic activity to postsynaptic inhibition in the somatosensory thalamus. J Neurosci. 2013;33:14850–14868. doi: 10.1523/JNEUROSCI.1174-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal mu opioid receptor agonists after inflammatory injury. J Neurosci. 2001;21:2536–2545. doi: 10.1523/JNEUROSCI.21-07-02536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci. 1998;18:10269–10276. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res. 1976;103:501–513. doi: 10.1016/0006-8993(76)90448-0. [DOI] [PubMed] [Google Scholar]
- Jensen ML, Wafford KA, Brown AR, Belelli D, Lambert JJ, Mirza NR. A study of subunit selectivity, mechanism and site of action of the delta selective compound 2 (DS2) at human recombinant and rodent native GABA(A) receptors. Br J Pharmacol. 2013;168:1118–1132. doi: 10.1111/bph.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Sieghart W, Shigemoto R, Somogyi P. Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling. Eur J Neurosci. 2010;32:1868–1888. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23:1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Bodnar RJ. Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821:224–230. doi: 10.1016/S0006-8993(98)01364-X. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res. 2002;929:1–9. doi: 10.1016/S0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- Lau BK, Vaughan CW. Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol. 2014;29:159–164. doi: 10.1016/j.conb.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Lau BK, Karim S, Goodchild AK, Vaughan CW, Drew GM. Menthol enhances phasic and tonic GABAA receptor-mediated currents in midbrain periaqueductal grey neurons. Br J Pharmacol. 2014;171:2803–2813. doi: 10.1111/bph.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA. Plasticity of GABAA receptor subunit expression during the oestrous cycle of the rat: implications for premenstrual syndrome in women. Exp Physiol. 2006;91:655–660. doi: 10.1113/expphysiol.2005.032342. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ. The neuroanatomy of sexual dimorphism in opioid analgesia. Exp Neurol. 2014;259:57–63. doi: 10.1016/j.expneurol.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008a;28:14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci. 2008b;27:1517–1524. doi: 10.1111/j.1460-9568.2008.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Bobeck EN, Hegarty DM, Aicher SA, Ingram SL, Morgan MM. Extracellular signal-regulated kinase 1/2 activation counteracts morphine tolerance in the periaqueductal gray of the rat. J Pharmacol Exp Ther. 2009;331:412–418. doi: 10.1124/jpet.109.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Ingram SL, Bobeck EN, Hegarty DM, Aicher SA, Arttamangkul S, Morgan MM. Opioid receptor internalization contributes to dermorphin-mediated antinociception. Neuroscience. 2010;168:543–550. doi: 10.1016/j.neuroscience.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Bobeck EN, Suchland KL, Morgan MM, Ingram SL. Change in functional selectivity of morphine with the development of antinociceptive tolerance. Br J Pharmacol. 2015;172:549–561. doi: 10.1111/bph.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA(A) receptors. J Neurophysiol. 2011;106:2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/S0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain Res. 1998;804:159–166. doi: 10.1016/S0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- Park JB, Skalska S, Son S, Stern JE. Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J Physiol. 2007;582:539–551. doi: 10.1113/jphysiol.2007.133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 5. Burlington, MA: Elsevier Academic; 2005. [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/S0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Zapata A, Chefer V, Shippenberg TS. The effects of local perfusion of DAMGO on extracellular GABA and glutamate concentrations in the rostral ventromedial medulla. J Neurochem. 2008;104:806–817. doi: 10.1111/j.1471-4159.2007.05017.x. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW. Recent advances in the pharmacology of quaternary salts of bicuculline. Trends Pharmacol Sci. 1999;20:268–270. doi: 10.1016/S0165-6147(99)01334-6. [DOI] [PubMed] [Google Scholar]
- Sykes KT, White SR, Hurley RW, Mizoguchi H, Tseng LF, Hammond DL. Mechanisms responsible for the enhanced antinociceptive effects of micro-opioid receptor agonists in the rostral ventromedial medulla of male rats with persistent inflammatory pain. J Pharmacol Exp Ther. 2007;322:813–821. doi: 10.1124/jpet.107.121954. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol. 1997;498:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance delta subunit containing GABA A receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol. 2006;291:R300–R306. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Díez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Narita A, Kagohata M, Shirai M, Akahori F, Arishima K. Effects of maternal exposure to 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) or 3,3′,4,4′,5,5′-hexachlorobiphenyl (PCB169) on testicular steroidogenesis and spermatogenesis in male offspring rats. J Androl. 2005;26:205–214. doi: 10.3389/fncir.2013.00203. [DOI] [PubMed] [Google Scholar]
- Ye Z, McGee TP, Houston CM, Brickley SG. The contribution of delta subunit-containing GABAA receptors to phasic and tonic conductance changes in cerebellum, thalamus and neocortex. Front Neural Circuits. 2013;7:203. doi: 10.1002/j.1939-4640.2005.tb01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]