Abstract
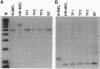
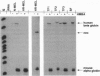
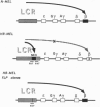
The human beta-globin locus control region (LCR) is a complex DNA regulatory element that controls the expression of the cis-linked beta-like globin genes located in the 55 kilobases 3' of the LCR. We have initiated the functional analysis of the LCR by homologous recombination in murine erythroleukemia cell somatic hybrids that carry a single copy of human chromosome 11 on which the beta-globin locus is situated. High-level expression of the human beta-globin gene normally occurs when these hybrid cells are induced to differentiate. We have reported that the insertion of an expressed selectable marker gene (driven by the Friend virus enhancer/promoter) into the LCR disrupts the LCR-mediated regulation of globin transcription. In these cells, beta-globin is no longer expressed when the cells differentiate; instead, expression of the selectable marker gene increases significantly after differentiation. Since present techniques for homologous recombination require the insertion of a selectable marker, further progress in using homologous recombination to analyze the LCR depends on deletion of the selectable marker and demonstration that the locus functions normally after the insertion, expression, and deletion of the selectable marker. Here we show that after precise deletion of the selectable marker by using the FLP recombinase/FRT (FLP recombinase target) system, the locus functions as it did before the homologous recombination event. These studies demonstrate the feasibility of using homologous recombination to analyze the LCR in particular, and other complex cis-regulatory DNA elements in general, in their normal chromosomal context.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron M. H., Maniatis T. Rapid reprogramming of globin gene expression in transient heterokaryons. Cell. 1986 Aug 15;46(4):591–602. doi: 10.1016/0092-8674(86)90885-8. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Ryan T. M., Palmiter R. D., Brinster R. L., Townes T. M. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990 Mar;4(3):380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. Developmental regulation of beta-globin gene switching. Cell. 1988 Oct 7;55(1):17–26. doi: 10.1016/0092-8674(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Clark S. P., Kaufhold R., Chan A., Mak T. W. Comparison of the transcriptional properties of the Friend and Moloney retrovirus long terminal repeats: importance of tandem duplications and of the core enhancer sequence. Virology. 1985 Jul 30;144(2):481–494. doi: 10.1016/0042-6822(85)90288-0. [DOI] [PubMed] [Google Scholar]
- Curtin P. T., Liu D. P., Liu W., Chang J. C., Kan Y. W. Human beta-globin gene expression in transgenic mice is enhanced by a distant DNase I hypersensitive site. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7082–7086. doi: 10.1073/pnas.86.18.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epner E., Kim C. G., Groudine M. What does the locus control region control? Curr Biol. 1992 May;2(5):262–264. doi: 10.1016/0960-9822(92)90379-o. [DOI] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G., Reitman M. Control of globin gene transcription. Annu Rev Cell Biol. 1990;6:95–124. doi: 10.1146/annurev.cb.06.110190.000523. [DOI] [PubMed] [Google Scholar]
- Fiering S. N., Roederer M., Nolan G. P., Micklem D. R., Parks D. R., Herzenberg L. A. Improved FACS-Gal: flow cytometric analysis and sorting of viable eukaryotic cells expressing reporter gene constructs. Cytometry. 1991;12(4):291–301. doi: 10.1002/cyto.990120402. [DOI] [PubMed] [Google Scholar]
- Fong T. C., Emerson B. M. The erythroid-specific protein cGATA-1 mediates distal enhancer activity through a specialized beta-globin TATA box. Genes Dev. 1992 Apr;6(4):521–532. doi: 10.1101/gad.6.4.521. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Epner E., Driscoll M. C., Enver T., Brice M., Papayannopoulou T., Groudine M. A deletion of the human beta-globin locus activation region causes a major alteration in chromatin structure and replication across the entire beta-globin locus. Genes Dev. 1990 Oct;4(10):1637–1649. doi: 10.1101/gad.4.10.1637. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Thompson C., Elder J. T., Groudine M. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1359–1363. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q., Dean A. Enhancer-dependent transcription of the epsilon-globin promoter requires promoter-bound GATA-1 and enhancer-bound AP-1/NF-E2. Mol Cell Biol. 1993 Feb;13(2):911–917. doi: 10.1128/mcb.13.2.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M., Sadowski P. D. Determination of DNA sequences essential for FLP-mediated recombination by a novel method. J Biol Chem. 1985 Oct 5;260(22):12320–12327. [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hasty P., Ramírez-Solis R., Krumlauf R., Bradley A. Introduction of a subtle mutation into the Hox-2.6 locus in embryonic stem cells. Nature. 1991 Mar 21;350(6315):243–246. doi: 10.1038/350243a0. [DOI] [PubMed] [Google Scholar]
- Jung S., Rajewsky K., Radbruch A. Shutdown of class switch recombination by deletion of a switch region control element. Science. 1993 Feb 12;259(5097):984–987. doi: 10.1126/science.8438159. [DOI] [PubMed] [Google Scholar]
- Kim C. G., Epner E. M., Forrester W. C., Groudine M. Inactivation of the human beta-globin gene by targeted insertion into the beta-globin locus control region. Genes Dev. 1992 Jun;6(6):928–938. doi: 10.1101/gad.6.6.928. [DOI] [PubMed] [Google Scholar]
- Kim C. G., Swendeman S. L., Barnhart K. M., Sheffery M. Promoter elements and erythroid cell nuclear factors that regulate alpha-globin gene transcription in vitro. Mol Cell Biol. 1990 Nov;10(11):5958–5966. doi: 10.1128/mcb.10.11.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. G., Kang H. M., Jang S. K., Shin H. S. Construction of a bifunctional mRNA in the mouse by using the internal ribosomal entry site of the encephalomyocarditis virus. Mol Cell Biol. 1992 Aug;12(8):3636–3643. doi: 10.1128/mcb.12.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R. Expression of the herpes thymidine kinase gene in Xenopus laevis oocytes: an assay for the study of deletion mutants constructed in vitro. Nucleic Acids Res. 1980 Dec 20;8(24):5931–5948. doi: 10.1093/nar/8.24.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon A. M., Ley T. J. Functional properties of the beta-globin locus control region in K562 erythroleukemia cells. Blood. 1991 May 15;77(10):2272–2284. [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman S., Fox D. T., Wahl G. M. Recombinase-mediated gene activation and site-specific integration in mammalian cells. Science. 1991 Mar 15;251(4999):1351–1355. doi: 10.1126/science.1900642. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Senecoff J. F., Bruckner R. C., Cox M. M. The FLP recombinase of the yeast 2-micron plasmid: characterization of its recombination site. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7270–7274. doi: 10.1073/pnas.82.21.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes T. M., Behringer R. R. Human globin locus activation region (LAR): role in temporal control. Trends Genet. 1990 Jul;6(7):219–223. doi: 10.1016/0168-9525(90)90182-6. [DOI] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valancius V., Smithies O. Testing an "in-out" targeting procedure for making subtle genomic modifications in mouse embryonic stem cells. Mol Cell Biol. 1991 Mar;11(3):1402–1408. doi: 10.1128/mcb.11.3.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M., Stamatoyannopoulos G., Papayannopoulou T. Monoclonal antibody 53.6 recognizes a novel proliferation-associated antigen encoded on human chromosome 11. J Immunol. 1987 Apr 1;138(7):2208–2212. [PubMed] [Google Scholar]