Dear Editor:
Chronic pruritus is difficult to treat and severely affects patient's quality of life and psychological well-being. Substance P (SP) is an important mediator in the induction and maintenance of pruritus, and therefore represents a promising target for antipruritic treatment. Aprepitant is an oral neurokinin-1 receptor (NK-1R) antagonist, which acts by inhibiting the binding of the NK-1R with the SP ligand in the skin and central nervous system1,2.
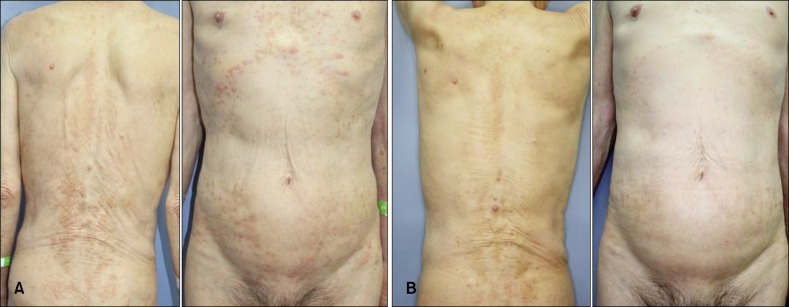
A 79-year-old man presented with refractory pruritus that had been resistant to local application of corticosteroid, standard systemic therapies such as antihistamine, cyclosporine, gabapentin and tricyclic antidepressant, and phototherapy. The origin of pruritus was unclear despite extensive laboratory and radiological investigation. Histopathological examination of a biopsy taken from the lower back showed superficial psoriasiform dermatitis with spongiosis and parakeratosis. He complained that he could not sleep for more than 1 hour because of the pruritus. After stopping antipruritic treatment for 3 weeks, we administered 125 mg of aprepitant on day 1 and 80 mg on day 2, 3 and 4 at the same time of day. Before treatment, visual analogue scale (VAS) score was 8/10 and Dermatologic Life Quality Index (DLQI) score was 24/30. After 24 hours from first administration, these values reduced to 4 and 16, respectively. He mentioned that pruritus was reduced significantly and he could consistently sleep for more than 4 hours. After 6 weeks, VAS and DLQI scores were 1 and 8 (Table 1), and his cutaneous lesions were much improved (Fig. 1). He was completely satisfied with deep sleep and no adverse effects were observed.
Table 1. Pruritus scores based on a visual analogue scale (VAS) and quality of life based on the Dermatology Life Quality Index (DLQI) before and after treatment with aprepitant.
| Before medication | After 24 hours (125 mg) |
After 48 hours (80 mg) |
After 72 hours (80 mg) |
After 96 hours (80 mg) |
After 6 weeks | |
|---|---|---|---|---|---|---|
| VAS | 8 | 4 | 4 | 4 | 3 | 1 |
| DLQI | 24 | 16 | 16 | 14 | 14 | 8 |
Fig. 1. (A) Multiple excoriated papules and linear excoriations on the trunk before treatment. (B) At the 6-week follow-up, the excoriated papules and linear excoriations appear to be significantly improved.
Aprepitant was developed and approved in 2003 for the prevention of chemotherapy-induced emesis and is usually administered for three days only (125 mg, 80 mg, 80 mg)1,2. Several studies have been performed to investigate the antipruritic effect of aprepitant. Although different regimens of aprepitant were used in previous studies, the results generally showed a significant reduction of pruritus without relevant side effects2,3,4,5. In this case, we administered 125 mg of aprepitant on day 1, and 80 mg on day 2, 3, and 4. Twenty-four hours after the first administration, the patient mentioned that his pruritus was significantly reduced (VAS 8 to 4). Other studies also demonstrated immediate antipruritic effect of aprepitant within 1 to 7 days after first administration3,4,5, and the median time to recurrence was 7 weeks from the first dose3. In our case, the antipruritic effect continued for 6 weeks. These results suggest that aprepitant has a long-lasting action. In vitro data have shown that aprepitant slowly dissociates from the human NK-1R and, as a competitive antagonist, it might also be a pseudo-irreversible antagonist3. Later, pruritus was aggravated temporarily, but it was easily relieved with standard antipruritic treatments. To date, he maintains improved state of pruritus.
To our knowledge, this is the first report of the antipruritic effect of aprepitant in Asia. This case supports previous reports of a significant antipruritic effect of aprepitant, especially in Asians, which is a good alternative treatment modality in chronic pruritus. Further studies are needed to determine a proper regimen and to prove the exact efficacy of aprepitant.
References
- 1.Lotts T, Ständer S. Research in practice: substance P antagonism in chronic pruritus. J Dtsch Dermatol Ges. 2014;12:557–559. doi: 10.1111/ddg.12364. [DOI] [PubMed] [Google Scholar]
- 2.Ständer S, Siepmann D, Herrgott I, Sunderkötter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One. 2010;5:e10968. doi: 10.1371/journal.pone.0010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santini D, Vincenzi B, Guida FM, Imperatori M, Schiavon G, Venditti O, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13:1020–1024. doi: 10.1016/S1470-2045(12)70373-X. [DOI] [PubMed] [Google Scholar]
- 4.Booken N, Heck M, Nicolay JP, Klemke CD, Goerdt S, Utikal J. Oral aprepitant in the therapy of refractory pruritus in erythrodermic cutaneous T-cell lymphoma. Br J Dermatol. 2011;164:665–667. doi: 10.1111/j.1365-2133.2010.10108.x. [DOI] [PubMed] [Google Scholar]
- 5.Vincenzi B, Tonini G, Santini D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010;363:397–398. doi: 10.1056/NEJMc1003937. [DOI] [PubMed] [Google Scholar]