Abstract
Purpose
Regorafenib is a standard-care option for treatment-refractory metastatic colorectal cancer that increases median overall survival by 6 weeks compared with placebo. Given this small incremental clinical benefit, we evaluated the cost-effectiveness of regorafenib in the third-line setting for patients with metastatic colorectal cancer from the US payer perspective.
Methods
We developed a Markov model to compare the cost and effectiveness of regorafenib with those of placebo in the third-line treatment of metastatic colorectal cancer. Health outcomes were measured in life-years and quality-adjusted life-years (QALYs). Drug costs were based on Medicare reimbursement rates in 2014. Model robustness was addressed in univariable and probabilistic sensitivity analyses.
Results
Regorafenib provided an additional 0.04 QALYs (0.13 life-years) at a cost of $40,000, resulting in an incremental cost-effectiveness ratio of $900,000 per QALY. The incremental cost-effectiveness ratio for regorafenib was > $550,000 per QALY in all of our univariable and probabilistic sensitivity analyses.
Conclusion
Regorafenib provides minimal incremental benefit at high incremental cost per QALY in the third-line management of metastatic colorectal cancer. The cost-effectiveness of regorafenib could be improved by the use of value-based pricing.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer death in men and women in the United States.1 In 2010, $14 billion was spent in the United States on management of CRC.2 Multiple drug regimens are available for the treatment of metastatic CRC (mCRC), including combination therapies with fluorouracil, oxaliplatin, irinotecan, bevacizumab, cetuximab, and panitumumab. Before 2012, there was no approved treatment available for patients who had experienced progression after these standard regimens.
Regorafenib is an oral multikinase inhibitor that targets angiogenic, stromal, and oncogenic receptor tyrosine kinases.3 The CORRECT (Colorectal Cancer Treated With Regorafenib or Placebo After Failure of Standard Therapy) trial compared the effects of regorafenib with those of placebo in patients who experienced progression after standard regimens.4 The trial demonstrated a median overall survival (OS) benefit of 1.4 months for regorafenib when compared with placebo. Grade 3 to 4 treatment-related adverse events (AEs) occurred in 54% of patients assigned to treatment with regorafenib and 14% of patients assigned to placebo. The most frequent grade 3 to 4 AEs occurring more commonly with regorafenib than placebo were hand-foot skin reaction (17% v 1%), fatigue (10% v 6%), diarrhea (7% v 1%), hypertension (7% v 1%), and rash or desquamation (6% v 0%). Regorafenib was subsequently approved by the US Food and Drug Administration in September 2012 and has become a standard-care option for mCRC refractory to standard regimens.
Given that regorafenib has a significant AE profile, provides a small incremental benefit, and is associated with a high cost, the value of this treatment relative to its benefit remains unclear. To address this issue, we developed a Markov model to evaluate the cost-effectiveness of regorafenib as third-line therapy in patients with mCRC from the perspective of the US payer.
METHODS
The structure of the Markov model consisted of an initial decision regarding treatment with regorafenib or best supportive care. Patients who initially received regorafenib could end therapy because of disease progression or intolerance of grade 3 to 4 AEs. Patients who experienced progression after regorafenib could receive best supportive care. All patients in each health state could experience progression to death (Fig 1).
Fig 1.
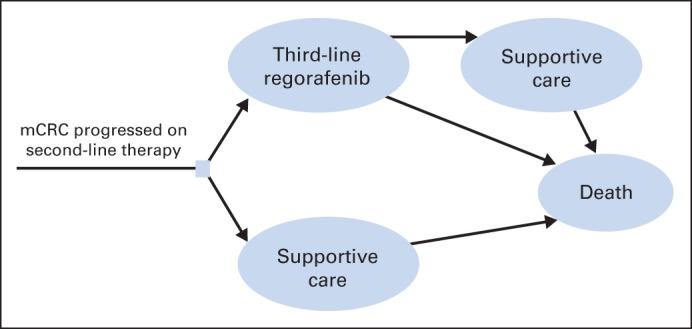
Markov model. mCRC, metastatic colorectal cancer.
Each model cycle represented 4 weeks, because in clinical practice, patients receive regorafenib daily for 3 weeks followed by a 1-week break. The primary outputs of the model included cost, life-years (LYs), and quality-adjusted LYs (QALYs), which were used to calculate the incremental cost-effectiveness ratio (ICER). The Markov model was implemented in TreeAge Pro 2013 software (https://www.treeage.com),5 and statistical analyses were performed in R software (http://www.r-project.org).
Model Survival Estimates
We based our assumption describing the survival benefits associated with regorafenib on the results of the CORRECT trial.4 The overall mortality rate, which corresponded to the probability of death, was derived from the OS curves for treatment with regorafenib and placebo published in the CORRECT trial. Engauge Digitizer software (version 4.1; http://digitizer.sourceforge.net) was used to extract the data points from the OS curves, and these data points were then used to fit parametric survival models.6 We found that Weibull and log-logistic models provided a good fit for all curves according to the Akaike information criterion and the Schwarz–Bayesian criterion.7 We used a Weibull distribution to model survival because it can have an increasing hazard rate and is suitable for modeling the events occurring early during follow-up periods. On the basis of the fitted Weibull OS model, denoted as S(t), we computed the cause-specific mortality M at cycle t as: M = (S[t] − S[t + 1])/S(t).
Progression Risk
In the regorafenib treatment group, treatment discontinuation due to AEs or progression on therapy was estimated assuming an exponential distribution based on the median treatment duration published in the CORRECT trial. Estimates of mortality and progression risk beyond the follow-up time in the clinical trials were extrapolated based on the fitted survival models.
Utility Estimates
Each health state was assigned a health utility score based on quality-of-life data collected in the CORRECT trial. In the trial, EQ-5D8 index scores were 0.73 in the regorafenib group and 0.74 in the placebo group at baseline. At the end of treatment, both groups had a score of 0.59. In the model, we assigned all patients a utility of 0.66, which is the mean of these values. We used 0.59 and 0.735 as the boundaries of the range in sensitivity analyses. To compute the total QALYs in the Markov model, survival time was adjusted by the utility. We included grade 3 to 4 AEs in the model that had significantly different rates between the arms of the CORRECT trial, which were hand-foot syndrome, hypertension, diarrhea, and fatigue. Disutilities associated with AEs were estimated based on established values in the literature.9 For the temporary health states associated with AEs modeled in this study (fatigue, hand-foot syndrome, diarrhea, and hypertension), the measured decreases in utilities from the published literature and the unmeasured decreases in utilities for these same health states in the CORRECT study were expected to be similar. The duration of AEs was estimated based on clinical experience. Hand-foot syndrome was assumed to last for 14 days, with a disutility of −0.116. Hypertension was assumed to last for 5 days, with a disutility of zero. Diarrhea was assumed to last for 5 days, with a disutility of −0.103. Fatigue was assumed to last for 10 days, with a disutility of −0.115. The duration-adjusted disutility was subtracted from the baseline utility to calculate the overall utility of each health state.
Cost Estimates
Only direct medical costs were considered and stated in 2014 US dollars. To estimate the unit price of each drug, we used 2014 average wholesale price (AWP) data from the Centers for Medicare and Medicaid Services, as described by the Academy of Managed Care Pharmacy.10 Regorafenib is dosed in 40-mg tablets, and the recommended starting dose is 160 mg. The AWP is $147.26 per 40-mg tablet. The CORRECT trial states that the mean daily dose received was 147 mg. We performed analyses in the model with three different dosing strategies: 120, 160, and 147 mg daily. Although 147 mg is not a realistic dose in clinical practice, it provides an average value for assessing the expected cost of regorafenib in a patient cohort.
Assumptions for management of AEs were based on recently published guidelines.11 Hand-foot syndrome was assumed to be managed with 0.05% clobetasol cream and 4% lidocaine cream. Hypertension was assumed to be managed with amlodipine 5 mg daily. Diarrhea was assumed to be managed by a physician visit and lomotil and loperamide. Fatigue was assumed to have no specific medical management. AE costs were calculated according to the Medicare physician fee schedule for 2014. The fees for outpatient physician visits were based on current procedural terminology codes.12 The methods used for these cost calculations were previously described by Tumeh et al.13 We did not perform annual discounting of the costs and benefits in this analysis, because the OS rate was 24% at 1 year for both groups.
Sensitivity Analysis
We performed internal model validations demonstrating that the OS curves generated by the Markov model simulation closely approximated those presented in the CORRECT trial (Appendix Fig A1, online only). A series of sensitivity analyses were performed to evaluate the robustness of the model and address uncertainty in the estimation of model parameters. Utilities were varied over their 95% CIs. Drug costs were varied within ± 20% of their baseline values, in accordance with established approaches.14,15 In univariable sensitivity analyses, we varied the value of one parameter at a time over its defined range and examined the effect on the ICER. We used the lower boundary for 120-mg dosing ($7,422 for one cycle of therapy) and the upper boundary for 160-mg dosing ($14,843 for one cycle of therapy) to provide the range of costs of regorafenib. In probabilistic sensitivity analyses, we performed 10,000 Monte Carlo simulations, each time randomly sampling from the distributions for all parameters simultaneously. We used gamma distribution for the cost parameters and beta distribution for utility and probability parameters. The baseline values, ranges, and distributions of model parameters are listed in Table 1.
Table 1.
Model Parameters: Baseline Values, Ranges, and Distributions for Sensitivity Analysis
| Variable | Value | Range | Reference | Distribution |
|---|---|---|---|---|
| AEs with regorafenib | ||||
| Hand-foot syndrome | 0.17 | 0.136 to 0.204 | CORRECT | Beta |
| Fatigue | 0.09 | 0.072 to 0.108 | CORRECT | Beta |
| Diarrhea | 0.07 | 0.056 to 0.084 | CORRECT | Beta |
| Hypertension | 0.07 | 0.056 to 0.084 | CORRECT | Beta |
| AEs with best supportive care | ||||
| Hand-foot syndrome | 0 | 0 to 0 | CORRECT | Beta |
| Fatigue | 0.05 | 0.04 to 0.06 | CORRECT | Beta |
| Diarrhea | 0.01 | 0.008 to 0.012 | CORRECT | Beta |
| Hypertension | 0.01 | 0.008 to 0.012 | CORRECT | Beta |
| AE disutilities | ||||
| Hand-foot syndrome | −0.116 | −0.093 to −0.139 | Lloyd et al9 | Beta |
| Fatigue | −0.115 | −0.093 to −0.139 | Lloyd et al9 | Beta |
| Diarrhea | −0.103 | −0.082 to −0.123 | Lloyd et al9 | Beta |
| Hypertension | 0 | 0 to 0 | ||
| AE duration, days | ||||
| Hand-foot syndrome | 14 | 11.2 to 16.8 | Estimated | Gamma |
| Fatigue | 10 | 8 to 12 | Estimated | Gamma |
| Diarrhea | 5 | 4 to 6 | Estimated | Gamma |
| Hypertension | 5 | 4 to 6 | Estimated | Gamma |
| AE cost, $ | ||||
| Hand-foot syndrome | 134.48 | 107.58 to 161.38 | Gamma | |
| Fatigue | 0 | 0 to 0 | Gamma | |
| Diarrhea | 81.60 | 65.28 to 97.92 | Gamma | |
| Hypertension | 59.10 | 47.28 to 70.92 | Gamma | |
| Other | ||||
| Cost of regorafenib dose, $ per 28-day cycle | ||||
| 120 mg | 9,277 | 7,422 to 11,132 | Gamma | |
| 147 mg | 11,364 | 9,091 to 13,639 | Gamma | |
| 160 mg | 12,369 | 9,896 to 14,843 | Gamma | |
| Utility | 0.66 | 0.59 to 0.735 | CORRECT | Beta |
Abbreviations: AE, adverse event; CORRECT, Colorectal Cancer Treated With Regorafenib or Placebo After Failure of Standard Therapy.
RESULTS
Base Case Results
The base case model results are listed in Table 2. The use of regorafenib compared with best supportive care produced a gain of 6 weeks of life (0.13 LYs). When adjusted for quality of life, use of regorafenib produced a gain of 2 quality-adjusted life-weeks (0.04 QALYs). On the basis of the dosing strategy used, the incremental cost of a course of treatment with regorafenib was $32,000 (120-mg dosing) to $43,000 (160-mg dosing). The ICER for regorafenib compared with best supportive care was between $730,000 and $980,000 per QALY.
Table 2.
Base Case Results
| Cost/Effect | Incremental Difference With Regorafenib | ||
|---|---|---|---|
| Dose, mg | 120 | 147 | 160 |
| Cost, $ | 32,141 | 39,391 | 42,838 |
| ICER, $ per LY | 253,663 | 310,881 | 338,090 |
| ICER, $ per QALY | 732,242 | 897,411 | 975,954 |
Abbreviations: ICER, incremental cost-effectiveness ratio; LY, life-year; QALY, quality-adjusted life-year.
Sensitivity Analyses
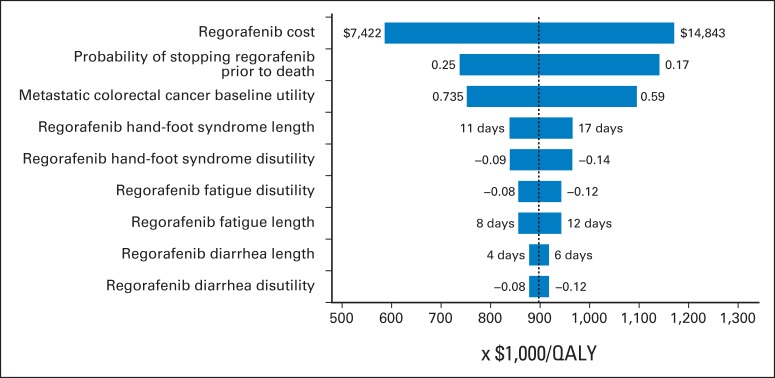
The results of univariable sensitivity analyses are presented in the tornado diagram (Fig 2). The parameters with the greatest influence on the ICER were cost of regorafenib, probability of stopping regorafenib before death because of an AE, and baseline utility value. Across broad variation in the ranges for each parameter, the ICER remained > $550,000 per QALY. The duration, cost, and disutility for AEs had a minor influence on the ICER.
Fig 2.
Univariable sensitivity analyses. QALY, quality-adjusted life-year.
The results of the probabilistic sensitivity analyses are shown in the cost-effectiveness acceptability curves in Figure 3. These curves show the probability that regorafenib is cost-effective across increasing willingness-to-pay (WTP) values. These results demonstrated nearly 0% probability that regorafenib is cost-effective at WTP values < $600,000 per QALY. There was a 50% chance that regorafenib is cost-effective at a WTP value of approximately $900,000 per QALY.
Fig 3.
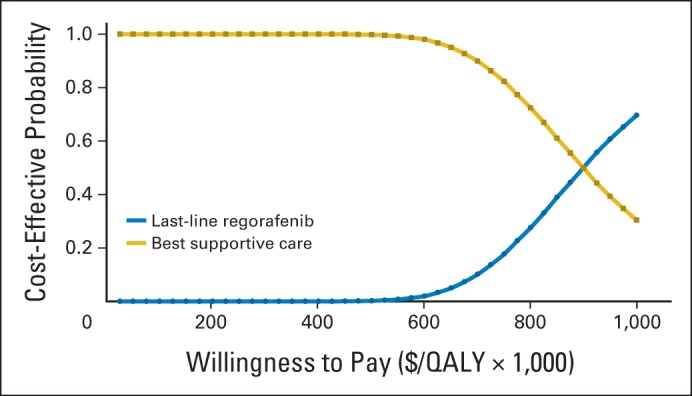
Cost-effectiveness acceptability curves. QALY, quality-adjusted life-year.
DISCUSSION
A fluoropyrimidine in combination with either oxaliplatin or irinotecan is an established treatment for mCRC, with good clinical effectiveness at a favorable cost.15 In the past decade, advances have been made by pairing chemotherapy with biologic agents. By targeting angiogenesis, efficacy has been demonstrated with the addition of bevacizumab in the first-line, second-line, and maintenance settings.16–20 Ziv-aflibercept, which also targets the angiogenesis pathway, produced a 1.4-month median OS benefit when added to FOLFIRI (fluorouracil, leucovorin, and irinotecan) compared with FOLFIRI alone in the second-line setting.21 The RAISE trial recently demonstrated a 1.6-month median OS benefit when ramucirumab was added to chemotherapy in the second-line setting.22 By targeting the epidermal growth factor receptor, alternative monoclonal antibodies have been paired with chemotherapy. Moreover, there are meaningful data to support the addition of either cetuximab (chimeric immunoglobulin G1 isotype antibody) or panitumumab (human immunoglobulin G1 isotype antibody) to chemotherapy in patients with RAS wild-type disease.23–32
These biologic agents, however, are not without significant cost, and their value remains under scrutiny.33,34 Cost-effectiveness analyses provide a standard methodology for examining the cost of a drug, in the context of the survival benefit, quality of life, costs of administration and AEs, and duration of therapy. Bevacizumab has recently been shown to have an ICER > $350,000 per QALY in both the first- and second-line settings.15 The value of ziv-aflibercept was evaluated from the perspective of a payer in the United Kingdom and found to have an ICER > £62,000 per QALY ($95,000 per QALY).35 In this evaluation, the authors reported that the cost estimate for ziv-aflibercept was based on a patient access schema but did not report the direct cost of the drug, which may be significantly lower in the United Kingdom than in the United States. There are multiple published estimates of the cost-effectiveness of EGFR-targeted therapies.36–43 These studies have involed a variety of methodologies, across multiple countries, with ICERs ranging from $20,000 to $3 million per LY.44 These data provide useful reference points for assessing the total cost of therapy and the value of regimens for mCRC, but it remains challenging to compare cost data from different health systems because of the wide variations among countries and the scope of costs considered in individual studies.45
The CORRECT trial demonstrated that by targeting angiogenic, stromal, and oncogenic receptor tyrosine kinases, survival could be prolonged for some patients with mCRC who had exhausted other systemic options. However, given the modest incremental benefit and significant AEs associated with this therapy, the role of regorafenib has been controversial. We performed the first study to our knowledge examining the cost-effectiveness of regorafenib in patients with mCRC who had experienced progression with standard regimens. On the basis of our model, regorafenib provides modest incremental benefit at high incremental cost per QALY. Even when using assumptions favorable to regorafenib in univariable sensitivity analyses, the ICER remained > $550,000 per QALY. The probabilistic sensitivity analyses, varying all model parameters in 10,000 Monte Carlo simulations, revealed that it was highly unlikely that regorafenib would be considered cost-effective at any WTP threshold < $600,000 per QALY and suggested that regorafenib exceeds the usually accepted values for cost-effective health care interventions.46–49
Moreover, patients who do not have supplemental insurance to cover Medicare Part D drugs are required to cover approximately 20% of drug costs. These patients would incur an average out-of-pocket expense of $7,000 for the total cost of regorafenib therapy. We recommend a careful discussion between physicians and patients regarding the additional benefit and potential total drug cost before starting regorafenib.
Our analysis was limited by data availability and our assumptions.50 Quality of life was reported at the beginning and end of treatment in the CORRECT trial. We used these values to estimate the quality of life during treatment. Although this estimation is not ideal, actual variations in health-related quality of life would be accounted for by the range of utility values used in the sensitivity analyses. It was challenging to simulate real-life dosages of regorafenib within the model, because the average dose was 147 mg, but the drug is available in 40-mg tablets. With an average dose of 147 mg, we feel that our alternative analyses using both 120 and 160 mg provide appropriate estimations for the average dosing that would occur in a population and the practical range of dosing that could occur in practice. We recognize that some clinicians initiate therapy with an 80-mg dose, despite the lack of efficacy data to support this dosing strategy. If we were to assume the same level of efficacy as with the standard dose, the ICER for regorafenib dosed at 80 mg per day would be $488,524 per QALY.
We used the AWP51 to ascertain drug cost; however, an alternative approach would have been to use the average sales price.52 Although there may be slight variations in cost when using the AWP as opposed to the average sales price as an estimate for drug cost, these variations in cost are subsumed in the ranges and probability distributions examined in the sensitivity analyses. In addition, we obtained the average sales price for regorafenib using the described method52 and found that the value fell within the range of values used in our sensitivity analyses. These variations in drug cost would not alter the general conclusions drawn from our model. Substantial variation in the cost of regorafenib would be necessary for it to be considered cost-effective by commonly applied thresholds.46–49
In addition, although the OS curves remained close together throughout the follow-up period, the progression-free survival curves for the CORRECT trial split after 50 days of therapy. This suggests the presence of a patient population that may benefit more from regorafenib. Currently, there is no biomarker available to identify patients who are most likely to benefit from regorafenib, which could improve the incremental cost-effectiveness of regorafenib. In the CORRECT trial, patients discontinued therapy because of either disease progression or AEs, with variation in the rate of discontinuation between groups. For example, the rate of discontinuation of therapy for AEs was 18% in the regorafenib group and 13% in the group receiving placebo. Irrespective of the reason for discontinuing treatment, the drug and AE costs incurred in the model would not change, and as a result, the ICER also would not change. For this reason, we did not explicitly use the reason for stopping therapy as a parameter in the model. Treatment discontinuation because of AEs or disease progression during therapy was estimated assuming an exponential distribution based on the median treatment duration published in the CORRECT trial. The published median treatment duration was used to estimate the time on treatment until discontinuation. Estimates of mortality and progression risk beyond the follow-up time in the clinical trials were extrapolated based on the fitted survival models.
Our study demonstrates the high incremental cost with low incremental benefit of regorafenib in mCRC over wide variations in the assumptions incorporated into the models. On the basis of our analysis, regorafenib provides low value at its current cost. New pricing and payment systems are needed to support delivery of cost-effective care, and many have been suggested. These include value-based pricing,53,54 third-party buy and bill,55 indication-specific pricing,56 bundled payment,57 pathway adherence,55 payment by results,58 and inclusive shared savings.59 With increasing deductibles and copays, our patients are now bearing a significant burden of the cost of drugs. Novel approaches to drug discovery and development have dramatically increased the number of targets for cancer therapies. The advent of these agents presents a challenge in terms of timeline for drug development and health care costs. New enrichment strategies are needed in patient selection for clinical trials, guideline development, and payer coverage determination that address clinical value in addition to statistically significant clinical benefit. These strategies are necessary to guide delivery of high-value interventions to our patients.
Appendix
Fig A1.
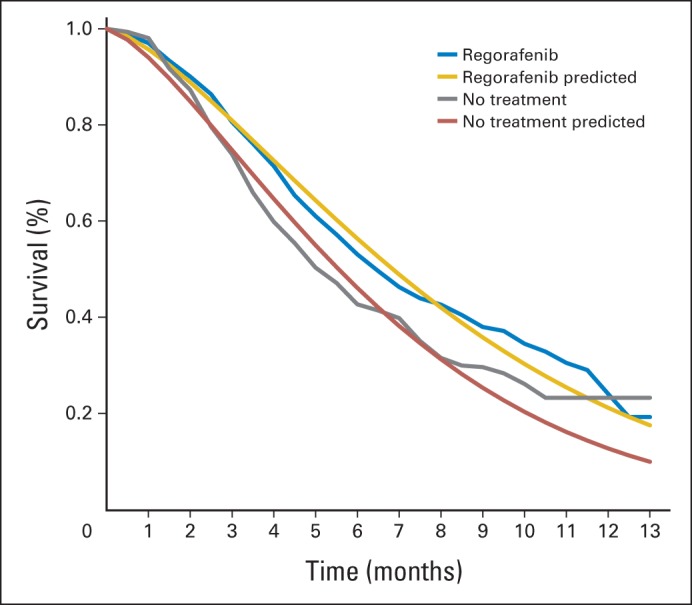
Internal validation of overall survival model.
Footnotes
Supported by an endowment from the Chester P. Rochfort Fellowship and National Institutes of Health Grant No. T32CA160040.
Presented as a poster at the American Society of Clinical Oncology GI Cancers Symposium, San Francisco, CA, January 15-17, 2015.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel A. Goldstein, Qiushi Chen, David H. Howard, Joseph Lipscomb, Bassel F. El-Rayes, Christopher R. Flowers
Financial support: Christopher R. Flowers
Administrative support: Christopher R. Flowers
Provision of study materials or patients: Christopher R. Flowers
Collection and assembly of data: Daniel A. Goldstein, Bilal B. Ahmad, Qiushi Chen, Christopher R. Flowers
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cost-Effectiveness Analysis of Regorafenib for Metastatic Colorectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Daniel A. Goldstein
No relationship to disclose
Bilal B. Ahmad
No relationship to disclose
Qiushi Chen
No relationship to disclose
Turgay Ayer
No relationship to disclose
David H. Howard
Honoraria: Onyx Pharmaceuticals
Research Funding: Pfizer
Travel, Accommodations, Expenses: Onyx Pharmaceuticals, Pfizer, Bayer
Joseph Lipscomb
No relationship to disclose
Bassel F. El-Rayes
Consulting or Advisory Role: Genentech/Roche, Merrimack
Research Funding: Taiho Pharmaceutical (Inst), Bristol-Myers Squibb (Inst), Boston Biomedical (Inst), Cleave Biosciences (Inst), Genentech (Inst), AVEO Pharmaceuticals (Inst), Pfizer (Inst)
Christopher R. Flowers
Consulting or Advisory Role: OptumRx, Algeta, Seattle Genetics
Research Funding: Acerta (Inst), Infinity (Inst), Onyx Pharmaceuticals (Inst), Janssen Oncology (Inst), Gilead Sciences (Inst), Spectrum (Inst), Celgene (Inst), TG Therapeutics (Inst), Genentech/Roche (Inst), Pharmacyclics (Inst), Abbvie
Other Relationship: Celgene
REFERENCES
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 4.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 5.Toffoli G, Cecchin E, Corona G, et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 2006;24:3061–3068. doi: 10.1200/JCO.2005.05.5400. [DOI] [PubMed] [Google Scholar]
- 6.Hoyle MW, Henley W. Improved curve fits to summary survival data: Application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139. doi: 10.1186/1471-2288-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auget J-L, Balakrishnan N, Mesbah M, et al. Advances in Statistical Methods for the Health Sciences. New York, NY: Springer; 2007. [Google Scholar]
- 8.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95:683–690. doi: 10.1038/sj.bjc.6603326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Academy of Managed Care Pharmacy. AMCP Guide to Pharmaceutical Payment Methods (version 3.0) http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=16476. [PubMed]
- 11.Grothey A, George S, van Cutsem E, et al. Optimizing treatment outcomes with regorafenib: Personalized dosing and other strategies to support patient care. Oncologist. 2014;19:669–680. doi: 10.1634/theoncologist.2013-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services. Medicare physician fee schedule, 2013. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html.
- 13.Tumeh JW, Moore SG, Shapiro R, et al. Practical approach for using Medicare data to estimate costs for cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. 2005;5:153–162. doi: 10.1586/14737167.5.2.153. [DOI] [PubMed] [Google Scholar]
- 14.Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health. 2011;14:836–845. doi: 10.1016/j.jval.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: A United States–based cost-effectiveness analysis. J Clin Oncol. 2015;33:1112–1118. doi: 10.1200/JCO.2014.58.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 17.Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 18.Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 19.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 20.Koopman M, Simkens L, May A, et al. Final results and subgroup analyses of the phase 3 CAIRO3 study: Maintenance treatment with capecitabine and bevacizumab versus observation after induction treatment with chemotherapy and bevacizumab in metastatic colorectal cancer (mCRC) J Clin Oncol. 2014;(suppl 3):32. abstr LBA388. [Google Scholar]
- 21.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 22.Tabernero J, Cohn AL, Obermannova R, et al. RAISE: A randomized, double-blind, multicenter phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab (RAM) or placebo (PBO) in patients (pts) with metastatic colorectal carcinoma (CRC) progressive during or following first-line combination therapy with bevacizumab (bev), oxaliplatin (ox), and a fluoropyrimidine (fp) J Clin Oncol. 2015;(suppl 3):33. abstr 512. [Google Scholar]
- 23.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 25.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 26.De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]
- 27.Peeters M, Douillard JY, Van Cutsem E, et al. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: Assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol. 2013;31:759–765. doi: 10.1200/JCO.2012.45.1492. [DOI] [PubMed] [Google Scholar]
- 28.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 29.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 30.Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240–2247. doi: 10.1200/JCO.2013.53.2473. [DOI] [PubMed] [Google Scholar]
- 31.Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): A prospectively stratified randomised trial. Lancet Oncol. 2013;14:749–759. doi: 10.1016/S1470-2045(13)70163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 33.Bach PB, Saltz L, Wittes R. In cancer care, cost matters. New York Times. 2015 Oct 15;:A25. [Google Scholar]
- 34.Saltz LB. Can money really be no object when cancer care is the subject? J Clin Oncol. 2015;3:1093–1094. doi: 10.1200/JCO.2014.60.1401. [DOI] [PubMed] [Google Scholar]
- 35.Wade R, Duarte A, Simmonds M, et al. The clinical and cost effectiveness of aflibercept in combination with irinotecan and fluorouracil-based therapy (FOLFIRI) for the treatment of metastatic colorectal cancer which has progressed following prior oxaliplatin-based chemotherapy: A critique of the evidence. Pharmacoeconomics. 2015;33:457–466. doi: 10.1007/s40273-015-0257-z. [DOI] [PubMed] [Google Scholar]
- 36.Starling N, Tilden D, White J, et al. Cost-effectiveness analysis of cetuximab/irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer. 2007;96:206–212. doi: 10.1038/sj.bjc.6603561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiroiwa T, Motoo Y, Tsutani K. Cost-effectiveness analysis of KRAS testing and cetuximab as last-line therapy for colorectal cancer. Mol Diagn Ther. 2010;14:375–384. doi: 10.1007/BF03256395. [DOI] [PubMed] [Google Scholar]
- 38.Mittmann N, Au HJ, Tu D, et al. Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: Evaluation of National Cancer Institute of Canada Clinical Trials Group CO.17 trial. J Natl Cancer Inst. 2009;101:1182–1192. doi: 10.1093/jnci/djp232. [DOI] [PubMed] [Google Scholar]
- 39.Blank PR, Moch H, Szucs TD, et al. KRAS and BRAF mutation analysis in metastatic colorectal cancer: A cost-effectiveness analysis from a Swiss perspective. Clin Cancer Res. 2011;17:6338–6346. doi: 10.1158/1078-0432.CCR-10-2267. [DOI] [PubMed] [Google Scholar]
- 40.Behl AS, Goddard KA, Flottemesch TJ, et al. Cost-effectiveness analysis of screening for KRAS and BRAF mutations in metastatic colorectal cancer. J Natl Cancer Inst. 2012;104:1785–1795. doi: 10.1093/jnci/djs433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Annemans L, Van Cutsem E, Humblet Y, et al. Cost-effectiveness of cetuximab in combination with irinotecan compared with current care in metastatic colorectal cancer after failure on irinotecan: A Belgian analysis. Acta Clin Belg. 2007;62:419–425. doi: 10.1179/acb.2007.061. [DOI] [PubMed] [Google Scholar]
- 42.Norum J. Cetuximab in the treatment of metastatic colorectal cancer: A model-based cost-effectiveness analysis. J Chemother. 2006;18:532–537. doi: 10.1179/joc.2006.18.5.532. [DOI] [PubMed] [Google Scholar]
- 43.Vijayaraghavan A, Efrusy MB, Göke B, et al. Cost-effectiveness of KRAS testing in metastatic colorectal cancer patients in the United States and Germany. Int J Cancer. 2012;131:438–445. doi: 10.1002/ijc.26400. [DOI] [PubMed] [Google Scholar]
- 44.Lange A, Prenzler A, Frank M, et al. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2014;50:40–49. doi: 10.1016/j.ejca.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Yabroff KR, Borowski L, Lipscomb J. Economic studies in colorectal cancer: Challenges in measuring and comparing costs. J Natl Cancer Inst Monogr. 2013;2013:62–78. doi: 10.1093/jncimonographs/lgt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkelmayer WC, Weinstein MC, Mittleman MA, et al. Health economic evaluations: The special case of end-stage renal disease treatment. Med Decis Making. 2002;22:417–430. doi: 10.1177/027298902236927. [DOI] [PubMed] [Google Scholar]
- 47.Lee CP, Chertow GM, Zenios SA. An empiric estimate of the value of life: Updating the renal dialysis cost-effectiveness standard. Value Health. 2009;12:80–87. doi: 10.1111/j.1524-4733.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- 48.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: The curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 49.Hirth RA, Chernew ME, Miller E, et al. Willingness to pay for a quality-adjusted life year: In search of a standard. Med Decis Making. 2000;20:332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 50.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS): Explanation and elaboration—A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Curtiss FR, Lettrich P, Fairman KA. What is the price benchmark to replace average wholesale price (AWP)? J Manag Care Pharm. 2010;16:492–501. doi: 10.18553/jmcp.2010.16.7.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360:626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 53.Gandjour A. Convergence of decision rules for value-based pricing of new innovative drugs. Expert Rev Pharmacoecon Outcomes Res. 2015;15:209–213. doi: 10.1586/14737167.2015.972374. [DOI] [PubMed] [Google Scholar]
- 54.Danzon P, Towse A, Mestre-Ferrandiz J. Value-based differential pricing: Efficient prices for drugs in a global context. Health Econ. 2015;24:294–301. doi: 10.1002/hec.3021. [DOI] [PubMed] [Google Scholar]
- 55.Bach PB. Reforming the payment system for medical oncology. JAMA. 2013;310:261–262. doi: 10.1001/jama.2013.8127. [DOI] [PubMed] [Google Scholar]
- 56.Bach PB. Indication-specific pricing for cancer drugs. JAMA. 2014;312:1629–1630. doi: 10.1001/jama.2014.13235. [DOI] [PubMed] [Google Scholar]
- 57.Elkin EB, Bach PB. Cancer's next frontier: Addressing high and increasing costs. JAMA. 2010;303:1086–1087. doi: 10.1001/jama.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garber AM, McClellan MB. Satisfaction guaranteed: “Payment by results” for biologic agents. N Engl J Med. 2007;357:1575–1577. doi: 10.1056/NEJMp078204. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt H, Emanuel EJ. Lowering medical costs through the sharing of savings by physicians and patients: Inclusive shared savings. JAMA Intern Med. 2014;174:2009–2013. doi: 10.1001/jamainternmed.2014.5367. [DOI] [PubMed] [Google Scholar]