Abstract
Purpose
The impact of a personalized cancer treatment strategy (ie, matching patients with drugs based on specific biomarkers) is still a matter of debate.
Methods
We reviewed phase II single-agent studies (570 studies; 32,149 patients) published between January 1, 2010, and December 31, 2012 (PubMed search). Response rate (RR), progression-free survival (PFS), and overall survival (OS) were compared for arms that used a personalized strategy versus those that did not.
Results
Multivariable analysis (both weighted multiple linear regression and random effects meta-regression) demonstrated that the personalized approach, compared with a nonpersonalized approach, consistently and independently correlated with higher median RR (31% v 10.5%, respectively; P < .001) and prolonged median PFS (5.9 v 2.7 months, respectively; P < .001) and OS (13.7 v 8.9 months, respectively; P < .001). Nonpersonalized targeted arms had poorer outcomes compared with either personalized targeted therapy or cytotoxics, with median RR of 4%, 30%, and 11.9%, respectively; median PFS of 2.6, 6.9, and 3.3 months, respectively (all P < .001); and median OS of 8.7, 15.9, and 9.4 months, respectively (all P < .05). Personalized arms using a genomic biomarker had higher median RR and prolonged median PFS and OS (all P ≤ .05) compared with personalized arms using a protein biomarker. A personalized strategy was associated with a lower treatment-related death rate than a nonpersonalized strategy (median, 1.5% v 2.3%, respectively; P < .001).
Conclusion
Comprehensive analysis of phase II, single-agent arms revealed that, across malignancies, a personalized strategy was an independent predictor of better outcomes and fewer toxic deaths. In addition, nonpersonalized targeted therapies were associated with significantly poorer outcomes than cytotoxic agents, which in turn were worse than personalized targeted therapy.
INTRODUCTION
Many therapies for patients with cancer have a modest effect on survival that is often in the range of several months or less.1 It may be that the relatively small gains observed with therapy are a result of a subgroup of patients who respond well, while other patients gain no benefit or may even be harmed by the therapy. There is now a wealth of evidence that cancers are frequently driven by specific genomic abnormalities,2 and with the rapid introduction of potent targeted agents into the clinic, these aberrations have also become actionable. In parallel, technologic developments in genomic and biomarker testing are advancing at a startling pace. These tests are rapidly being made available in the clinic, potentially facilitating a personalized treatment strategy.3–5 However, to date, only a minority of trials implement this approach, and strategies to better select patient populations likely to respond to these new drugs to maximize benefits are under intensive discussion.
Examples already exist where treatment selection based on biomarkers that reflect the underlying cancer biology have brought remarkable advances in oncology. These include the use of the human epidermal growth factor receptor 2 antibody trastuzumab in breast cancer,6–8 the BCR-ABL inhibitor imatinib in chronic myelogenous leukemia,9–12 and the more recent experience with the BRAF inhibitors vemurafenib and dabrafenib in melanoma.13–15 As a result, the utility of using molecular diagnostics to select targeted treatment has been acknowledged, and many new drugs are being developed with companion diagnostic tests.16–22
The evidence supporting the salutary effects of a broad personalized approach, involving the use of a targeted drug in the specific population that harbors the cognate biomarker or pathway protagonist, is in development and its range of applicability is still a matter of debate. Herein, we performed a systematic review and meta-analysis of 570 phase II studies of single agents published in a 3-year period (between January 1, 2010, and December 31, 2012). Our objective was to compare the main outcome end points (response rate [RR]), progression-free survival [PFS]) and overall survival [OS]) between trials that adopted a personalized therapy strategy versus those that used an unselected population.
METHODS
Search Strategy and Study Selection
A search was conducted using PubMed (http://www.ncbi.nlm.nih.gov/pubmed), using the word “cancer” in the search toolbar. “Clinical Trials, phase II,” “Publication dates from 2010/01/01 to 2012/12/31,” and studies in “Humans” were selected as additional filters. Only single-agent arms were included in the analysis. Studies describing pediatric cancers, supportive care, locoregional treatments, hormonal therapies, and cellular or vaccine therapy were excluded. Adjuvant/neoadjuvant trials were excluded to eliminate trials involving patients with early-stage/nonadvanced malignancies. The study inclusion and exclusion steps and the full list of references can be found in the Data Supplement.
Data Extraction and Categorization
Data extraction was conducted independently by two investigators (M.S. and M.Z.). Any discrepancies were resolved by consensus in frequent meetings in the presence of the moderator (R.K.). To be included, the study had to describe a phase II trial published between January 1, 2010, and December 31, 2012, evaluate a drug as a single agent, and report adequate efficacy end points. If the trial reported one arm with a single agent and another arm with a combination, only the arm with the single agent was included in our analysis. All deaths reported by investigators as possibly, probably, or definitely related to treatment were considered toxicity-related deaths.
For the RRs, only complete and partial responses were considered. Median PFS (or time to treatment progression if PFS was not reported) and OS were extracted, as well as their corresponding 95% CIs when available. The number of deaths per arm was also recorded.
For the purpose of our analysis, we defined personalized therapy as when a treatment met one of the following criteria: cognate biomarker was used for treatment indication, or no cognate biomarker was used, but at least 50% of patients were known to harbor the cognate biomarker. Full details of the definitions for personalized versus nonpersonalized therapies can be found in the Data Supplement.
Statistical Analysis
We performed a multivariable pooled analysis of the RR, PFS, and OS using the weighted least squares method to account for the size effects. For the meta-analysis, the random effects model was used because this model takes into account within-study heterogeneity. Heterogeneity between studies was quantified by the between-study variance and the Cochran Q test and/or the I2 statistic, which describes the percentage of variation across studies that is a result of heterogeneity rather than chance.23 The DerSimonian-Laird24 method was used to calculate the relative risks and corresponding 95% CIs for the RR meta-analysis. For the RR meta-analysis, the number of responders and sample size were used. For the PFS and OS meta-analysis, medians and corresponding 95% CIs were used. Random effects meta-regression models (linear mixed model) were used to assess the relationship between the estimates and personalized therapy status, adjusted for other potential confounders/mediators, as appropriate. The data were stratified by personalized or nonpersonalized approach, study design (randomized or not), chemotherapy-naive or not (chemotherapy-naive patients had advanced/metastatic disease), 5-year impact factor of journal for the published studies (≤ or > 10), the number of patients per arm (≤ or > the median = 35 patients), the class of agent (cytotoxic or targeted), the administration route (oral or injection), approval status (US Food and Drug Administration [FDA] or European Medicines Agency [EMA]) at the date of analysis (yes or no), the tumor type (solid tumor or hematologic malignancy), and the number of treating centers for the trial (single center or multiple centers). Only the significant variables in univariable analysis were included in multivariable analysis (eg, meta-regressions). Medians for RR, PFS, and OS were calculated. Statistical dispersion was measured by the 95% CIs. Assessment of continuous variables between independent samples was done using a Wilcoxon rank sum test. Several confounding variables were assessed using random effects meta-regression or weighted (least squares method) multiple linear regression models. Two-sided P ≤ .05 was considered statistically significant. To rank small P values, t statistic values were presented for the weighted least squares analysis, and z values were presented for meta-regression analysis. The t statistic (pooled analysis) and z values (meta-analysis) correspond to the ratio of the coefficient divided by the SE. The higher the t statistic and z value, the smaller (and more significant) is the P value (and the greater is the weight of the variable to predict the model). Statistical analyses were performed and reviewed by M.S. and J.J.L. using SPSS (version 22; SPSS, Chicago, IL) and Comprehensive Meta-Analysis (Version 3; Biostat, Englewood, NJ) software.
RESULTS
Search Results and Clinical Trial Characteristics
We initially identified 3,536 results from the PubMed search, but only 763 studies met our inclusion criteria (described in the Methods section). Careful reading of these 763 studies resulted in exclusion of 193 more studies (see Data Supplement for reasons). Overall, 570 phase II studies published in the designated 3-year time period were included in our analysis.
In total, 641 single-agent arms were included, comprising 32,149 patients. There were 212 arms with cytotoxic agents. Among them, all were considered nonpersonalized except one. Conversely, 429 arms involved targeted agents with 111 personalized arms (25.9%) and 318 nonpersonalized arms (74.1%; Table 1). Personalized trials accrued a total number of 8,078 patients in the experimental arms (n = 112 arms) compared with 24,071 patients for nonpersonalized trials (n = 529 arms). Both the personalized and nonpersonalized arms had a comparable median number of patients per arm (36 v 35 patients, respectively). Most of the arms using a personalized strategy used oral agents (84% v 49% for nonpersonalized arms; P < .001) and were published in higher impact journals (23% had a 5-year impact factor > 10, compared with 15% when a nonpersonalized approach was applied; P = .035; Table 1). More details about the population characteristics and types of treatments (cytotoxic and targeted) can be found in Table 2.
Table 1.
Comparison of Study Characteristics Between the Personalized and Nonpersonalized Approach
| Characteristic | Personalized Strategy |
Nonpersonalized Strategy |
P* | ||
|---|---|---|---|---|---|
| No. of Arms (%) | No. of Patients | No. of Arms (%) | No. of Patients | ||
| All studies | 112 (100) | 8,078 | 529 (100) | 24,071 | |
| Study design† | .330 | ||||
| Randomized | 15 (14) | 1,300 | 92 (18) | 5,538 | |
| Nonrandomized | 95 (86) | 6,734 | 418 (82) | 17,829 | |
| Chemotherapy status | .151 | ||||
| Chemotherapy naïve | 28 (25) | 1,792 | 99 (19) | 4,944 | |
| Prior chemotherapy | 84 (75) | 6,286 | 430 (81) | 19,127 | |
| 5-Year impact factor of journal | .035 | ||||
| ≤ 10 | 86 (77) | 5,632 | 446 (85) | 18,639 | |
| > 10 | 26 (23) | 2,446 | 78 (15) | 4,923 | |
| No. of patients per arm‡ | .467 | ||||
| ≤ 35 | 54 (48) | 1,307 | 277 (52) | 6,249 | |
| > 35 | 58 (52) | 6,771 | 252 (48) | 17,822 | |
| Agent class | < .001 | ||||
| Cytotoxic | 1 (1) | 18 | 211 (40) | 9,647 | |
| Targeted | 111 (99) | 8,060 | 318 (60) | 14,424 | |
| Administration route | < .001 | ||||
| Oral | 94 (84) | 7,216 | 258 (49) | 11,059 | |
| Injection | 18 (16) | 862 | 271 (51) | 13,012 | |
| FDA or EMA approval | .149 | ||||
| No | 16 (14) | 934 | 110 (21) | 4,837 | |
| Yes | 96 (86) | 7,144 | 419 (79) | 19,234 | |
| Tumor type | .120 | ||||
| Solid | 88 (79) | 4,168 | 449 (85) | 20,505 | |
| Hematologic | 24 (21) | 3,910 | 80 (15) | 3,566 | |
| No. of treating centers§ | .079 | ||||
| Single center | 32 (29) | 1,259 | 110 (21) | 3,299 | |
| Multiple centers | 79 (71) | 6,798 | 415 (79) | 20,609 | |
Abbreviations: EMA, European Medicines Agency; FDA, US Food and Drug Administration.
P values are from Fisher's exact test and have been calculated using the number of arms.
Data not available for 21 arms.
The cutoff value of 35 patients represents the median of patients per arm in all 641 arms evaluated (range, four to 390 patients). The mean number of patients per arm was 47 patients (standard deviation, 46.6 patients).
Data not available for five arms.
Table 2.
Breakdown of Type of Drugs and Malignancies
| Drug and Malignancy Types | Arms |
|
|---|---|---|
| No. | % | |
| Drug | ||
| Targeted | 429 | 67 |
| Small molecules | 367 | 57 |
| Antibodies | 62 | 10 |
| Cytotoxic | 212 | 33 |
| Alkylating agents | 20 | 3 |
| Antimetabolites | 69 | 11 |
| Antitumor antibiotics | 18 | 3 |
| Topoisomerase inhibitors | 16 | 2.5 |
| Mitotic inhibitors | 60 | 9 |
| Proteasome inhibitors | 13 | 2 |
| Miscellaneous | 16 | 2.5 |
| Total | 641 | 100 |
| Malignancy | ||
| Lung | 133 | 21 |
| Hematologic | 104 | 16 |
| GI | 92 | 14 |
| Urinary | 73 | 11 |
| Breast | 54 | 8 |
| Gynecologic | 53 | 8 |
| Skin/melanoma | 38 | 6 |
| Brain | 36 | 6 |
| Head and neck | 24 | 4 |
| Other | 34 | 5 |
| Total | 641 | 100 |
In our data set, 163 (25.5%) of 638 arms that had a value for the RR had a 0% RR, and 126 (19.7%) of 641 total arms included drugs that were not FDA or EMA approved at the time of analysis. RRs of 0% correlated with trials with a nonpersonalized approach (29% v 10% with personalized approach; P < .001), lower impact (5-year impact factor ≤ 10) journals (28% v 11% with higher impact; P < .001), previously treated patients (28% v 14% with chemotherapy-naïve patients; P = .001), solid tumors (28% v 13% with hematologic tumors; P = .002), targeted therapy (29% v 19% with cytotoxic therapy; P = .007), arms testing drugs that were not approved by either the FDA or EMA at the time of analysis (40% v 22% with approved drugs; P < .001), and arms with a smaller number of patients (≤ v > 35 patients: 40% v 10%, respectively; P < .001).
Personalized Therapy Subanalysis
A subanalysis of the personalized arms, dichotomized into personalized-direct (alteration was the direct target of the drug tested or separated by only one intermediate effector) and personalized-indirect (drug affects a target at least two effectors removed from the molecular aberration) demonstrated that both significantly correlated with better outcomes, as reflected by higher RRs and longer PFS and OS. Results were similar if personalized-direct was defined as a drug that strictly impacts directly the product of the molecular alteration or a protein preferentially expressed on the tumor cells (not its first immediate downstream effector; Data Supplement).
We also attempted to determine whether there was a difference in outcome when distinct types of biomarkers used for treatment selection were compared. For the purpose of this analysis, biomarkers were classified into protein biomarkers (if the protein overexpression was used for classification into personalized) or genomic biomarkers (if a genomic alteration was used for classification into personalized). Across both a pooled analysis and meta-analysis, personalized arms using a genomic biomarker had higher RR and prolonged PFS and OS (all P < .05; Data Supplement).
Lastly, although most of the personalized arms (99%) used targeted agents, the majority of the targeted arms used a nonpersonalized approach (ie, did not select patients using a cognate biomarker [74%]). A subanalysis within targeted agents showed that across both a pooled analysis and meta-analysis, and for all the outcome parameters tested (RR, PFS, and OS), targeted arms using a personalized strategy had statistically improved outcomes compared with targeted arms that lacked a personalized approach (all P < .001; Data Supplement). Similarly, another subanalysis demonstrated that personalized arms using targeted agents had better outcomes than arms using cytotoxic agents (all P < .001). Lastly, nonpersonalized arms using targeted agents led to poorer outcomes compared with nonpersonalized arms using cytotoxic arms, with P < .001 for RR and PFS and a trend for OS (P = .054 in the pooled analysis and P = .048 in the meta-analysis; Data Supplement).
Personalized Therapy Is Associated With Higher RRs
We first performed a pooled analysis including all the arms that had an RR available. Six hundred thirty-eight arms (99.5%) of 641 total arms were included (31,994 patients). A univariable analysis demonstrated that most of the tested variables showed statistically significant differences (Table 3). Only the variables of study design (randomized v not) and number of treating centers (single center v multiple centers) variables were not significant. We then performed a weighted, multiple linear regression analysis (multivariable analysis) including all the variables that were significant in the univariable analysis. This analysis demonstrated that the variables that were most significantly associated with higher response rates were a personalized approach (median RR, 29.2% v 6.2% for not personalized; P < .001; t statistic = 13.4), hematologic malignancies (median RR, 27.6% v 6.2% for solid tumors; P < .001; t statistic = 14), and chemotherapy-naïve patients (median RR, 14.0% v 6.6% for patients who received prior therapy; P < .001; t statistic = 6.1; Table 3 and Fig 1).
Table 3.
Pooled Analysis and Meta-Analysis for the RR
| Parameter | Pooled Analysis (n = 638) |
Meta-Analysis (random effect; n = 638) |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||
| median RR (%; 95% CI) | P* | t | P† | Median RR (%; 95% CI) | P‡ | z | P§ | |
| Total studies | 8 (6.8 to 8.9) | 12.7 (11.6 to 13.9) | ||||||
| Personalized strategy | < .001 | 13.4 | < .001 | < .001 | 13.0 | < .001 | ||
| Yes | 29.2 (24 to 35) | 31 (26.8 to 35.6) | ||||||
| No | 6.2 (5 to 7.4) | 10.5 (9.6 to 1.5) | ||||||
| Chemotherapy status | < .001 | 6.1 | < .001 | < .001 | 4.6 | < .001 | ||
| Chemotherapy naïve | 14 (10 to 23) | 20 (16.6 to 24.1) | ||||||
| Prior chemotherapy | 6.6 (5 to 8) | 11.3 (10.2 to 12.5) | ||||||
| Tumor type | < .001 | 14.0 | < .001 | < .001 | 10.7 | < .001 | ||
| Solid | 6.2 (5 to 7) | 10.6 (9.6 to 11.6) | ||||||
| Hematologic | 27.6 (21.5 to 33) | 31.0 (26.8 to 35.4) | ||||||
| Agent class | < .001 | 4.6 | < .001 | < .001 | 7.5 | < .001 | ||
| Cytotoxic | 11.9 (9.3 to 14.5) | 16.1 (14.3 to 18) | ||||||
| Targeted | 5.8 (5 to 7.1) | 11.2 (9.9 to 12.7) | ||||||
| FDA/EMA approval | < .001 | 3.8 | < .001 | < .001 | 3.7 | < .001 | ||
| No | 3 (1.3 to 5) | 7.1 (5.6 to 8.9) | ||||||
| Yes | 9 (8 to 11) | 14.4 (13.1 to 15.9) | ||||||
| 5-Year impact factor‖ | < .001 | 2.3 | .021 | < .001 | 2.7 | .006 | ||
| ≤ 10 | 7 (5.8 to 8.2) | 12.2 (11.1 to 13.5) | ||||||
| > 10 | 13.8 (9.6 to 21) | 16 (12.8 to 19.9) | ||||||
| Study design | .523 | — | — | .032 | 1.9 | .058 | ||
| Randomized | 7 (5 to 8.9) | 10.2 (8.2 to 12.7) | ||||||
| Nonrandomized | 8 (6.6 to 9) | 13.2 (12 to 14.6) | ||||||
| No. of patients per arm¶ | < .001 | 1.0 | .308 | .258 | — | — | ||
| ≤ 35 | 4.6 (3.3 to 8) | 12.3 (10.8 to 13.9) | ||||||
| > 35 | 9 (8 to 11.6) | 13.6 (12 to 15.3) | ||||||
| Administration route | .041 | 0.8 | .406 | .159 | — | — | ||
| Oral | 8.9 (6.7 to 10) | 13.6 (12 to 15.3) | ||||||
| Injection | 7 (5.2 to 8.2) | 12.0 (10.5 to 13.6) | ||||||
| No. of treating centers | .356 | — | — | .017 | 2.3 | .024 | ||
| Single center | 9.8 (5.7 to 16) | 16.1 (13.4 to 19.2) | ||||||
| Multiple centers | 7.4 (6 to 8.6) | 11.9 (10.7 to 13.2) | ||||||
NOTE. Only variables that were significant in the univariable models were included in the multivariable analysis. The t and z values are used to compute the corresponding P values, and the higher they are, the more they contribute to the model. The pooled analysis and meta-analysis both included 638 arms.
Abbreviations: EMA, European Medicines Agency; FDA, US Food and Drug Administration; RR, response rate.
Wilcoxon test; the median in the univariable was not weighted.
Multiple linear regression model using a weighted least squares model.
Mixed effects analysis.
Random effects meta-regression model.
Cutoff value chosen to discriminate higher impact factor journals versus lower impact factor journals (sum of median and interquartile range).
Cutoff value used was the median of distribution.
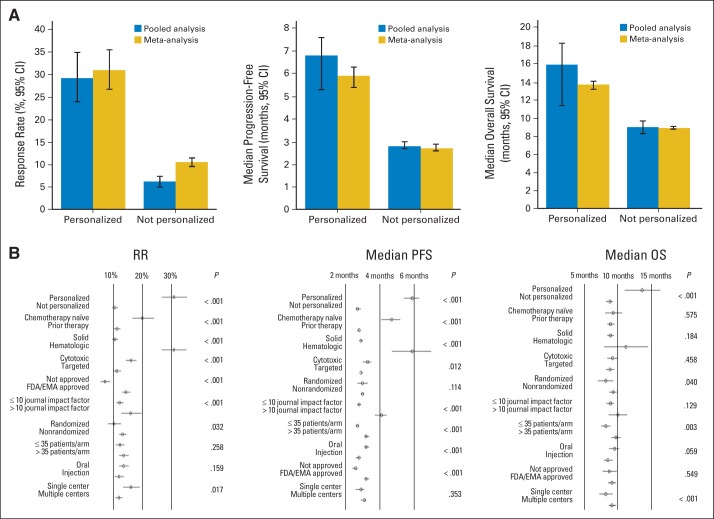
Fig 1.
Benefit of personalized therapy. (A) Results from the pooled and meta-analysis comparing the personalized strategy versus nonpersonalized strategy are represented for response rate (RR), progression-free survival (PFS), and overall survival (OS). All P < .001 comparing arms adopting a personalized approach versus a not personalized approach. Six hundred thirty-eight arms had values available for the RR analysis (pooled analysis and meta-analysis; 112 arms were personalized, and 526 were not). For the PFS analysis, 530 arms had values for the pooled analysis (personalized, n = 86; not personalized, n = 444), and 342 arms had median PFS values and their corresponding 95% CIs available for the meta-analysis (personalized, n = 59; not personalized, n = 283). For the OS analysis, 441 arms had values for the pooled analysis (personalized, n = 49; not personalized, n = 392), and 247 arms had median OS values and their corresponding 95% CIs available for the meta-analysis (personalized, n = 21; not personalized, n = 226). (B) Forest plots for RR, PFS, and OS (left to right). EMA, European Medicines Agency; FDA, US Food and Drug Administration.
Second, a meta-analysis that included the 638 arms demonstrated that all the variables (personalized strategy, chemotherapy status, tumor type, agent class, FDA/EMA approval, 5-year impact factor, study design, number of patients per arm, administration route, and number of treating centers) were significantly associated with the RR, except the number of patients per arm (≤ or > 35 patients) and the administration route (oral v injection). A meta-regression including all the significant variables was performed and demonstrated that variables that correlated independently with higher response rates were a personalized approach (median RR, 31% v 10.5% for not personalized; P < .001; z value = 13), hematologic tumors (median RR, 31% v 10.6% for solid tumors; P < .001; z value = 10.7), and chemotherapy-naïve patients (median RR, 20% v 11.3% for patients who received prior therapy; P < .001; t statistic = 4.6; Table 3 and Fig 1).
Personalized Therapy Is Associated With Longer PFS
Similarly, we performed a pooled analysis that included all the arms that had a median PFS reported (n = 530 arms; 24,489 patients). In the univariable analysis, all the variables were significantly associated with PFS except the agent class, the study design, and the number of treating centers and were consequently included in the multivariable analysis (weighted multiple linear regression) to account for possible confounding variables. The variables that correlated independently with a prolongation of median PFS by at least 2 months were the personalized approach (6.8 v 2.8 months for the nonpersonalized approach; P < .001; t statistic = 8.7) and hematologic malignancies (5.4 v 2.9 months for solid tumors; P < .001; t statistic = 7.4; Table 4 and Fig 1).
Table 4.
Pooled and Meta-Analysis for PFS
| Parameter | Pooled Analysis (n = 530) |
Meta-Analysis (random effect; n = 342) |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||
| Median (months; 95% CI) | P* | t | P† | Median (months; 95% CI) | P‡ | z | P§ | |
| Total studies | 3.0 (2.8 to 3.2) | 3.0 (2.9 to 3.1) | ||||||
| Personalized strategy | < .001 | 8.7 | < .001 | < .001 | 11.1 | < .001 | ||
| Yes | 6.8 (5.3 to 7.6) | 5.9 (5.4 to 6.3) | ||||||
| No | 2.8 (2.7 to 3.0) | 2.7 (2.6 to 2.9) | ||||||
| Chemotherapy status | < .001 | 5.6 | < .001 | < .001 | 5.3 | < .001 | ||
| Chemotherapy naïve | 3.0 (2.6 to 4.5) | 4.7 (4.1 to 5.2) | ||||||
| Prior chemotherapy | 2.8 (2.7 to 3.0) | 2.8 (2.7 to 2.9) | ||||||
| Tumor type | < .001 | 7.4 | < .001 | < .001 | 5.6 | < .001 | ||
| Solid | 2.9 (2.7 to 3.0) | 2.9 (2.8 to 3.0) | ||||||
| Hematologic | 5.4 (4.0 to 9.0) | 5.9 (4.7 to 7.0) | ||||||
| Agent class | .281 | — | — | .012 | 4.9 | < .001 | ||
| Cytotoxic | 3.3 (3.0 to 3.7) | 3.3 (3.0 to 3.5) | ||||||
| Targeted | 2.9 (2.7 to 3.0) | 2.9 (2.9 to 3.0) | ||||||
| Study design | .250 | — | — | .114 | — | — | ||
| Randomized | 2.8 (2.6 to 3.1) | 3.0 (2.7 to 3.3) | ||||||
| Nonrandomized | 3.0 (2.8 to 3.4) | 3.0 (2.9 to 3.1) | ||||||
| 5-Year impact factor‖ | .001 | 0.4 | .662 | < .001 | 4.4 | < .001 | ||
| ≤ 10 | 2.9 (2.7 to 3.0) | 2.8 (2.7 to 2.9) | ||||||
| > 10 | 3.8 (3.3 to 4.6) | 4.1 (3.7 to 4.4) | ||||||
| No. of patients per arm¶ | .021 | 1.0 | .334 | < .001 | 2.0 | .041 | ||
| ≤ 35 | 2.8 (2.5 to 3.0) | 2.7 (2.6 to 2.8) | ||||||
| > 35 | 3.3 (2.9 to 3.6) | 3.2 (3.1 to 3.4) | ||||||
| Administration route | < .001 | 0.1 | .908 | < .001 | 3.9 | < .001 | ||
| Oral | 3.5 (3.0 to 3.8) | 3.2 (3.1 to 3.4) | ||||||
| Injection | 2.8 (2.6 to 3.0) | 2.8 (2.6 to 2.9) | ||||||
| FDA/EMA approval | < .001 | 2.2 | .030 | < .001 | 2.0 | .050 | ||
| No | 2.7 (2.1 to 2.9) | 2.6 (2.4 to 2.7) | ||||||
| Yes | 3.1 (2.9 to 3.5) | 3.2 (3.1 to 3.4) | ||||||
| No. of treating centers | .357 | — | — | .353 | — | — | ||
| Single center | 2.9 (2.5 to 3.4) | 2.9 (2.6 to 3.1) | ||||||
| Multiple centers | 3.0 (2.8 to 3.3) | 3.1 (2.9 to 3.2) | ||||||
NOTE. Only variables that were significant in the univariable models were included in the multivariable analysis. The t and z values are used to compute the corresponding P values, and the higher they are, the more they contribute to the model. The pooled analysis included 530 arms, and the meta-analysis included 342 arms for which median PFS and the corresponding 95% CI values were available.
Abbreviations: EMA, European Medicines Agency; FDA, US Food and Drug Administration; PFS, progression-free survival.
Wilcoxon test; the median in the univariable was not weighted.
Multiple linear regression model using a weighted least squares model.
Mixed effects analysis.
Random effects meta-regression model.
Cutoff value chosen to discriminate higher impact factor journals versus lower impact factor journals (sum of median and interquartile range).
Cutoff value used was the median of distribution.
The meta-analysis included arms that reported a median PFS and their corresponding 95% CIs (n = 342 arms; 15,513 patients; Table 4). The results of the pooled analysis were confirmed by the random effects meta-regression model demonstrating that the two variables extending the median PFS most were the personalized approach (5.9 v 2.7 months for the nonpersonalized approach; P < .001; z value = 11.1) and hematologic malignancies (5.9 v 2.9 months for solid tumors; P < .001; z value = 5.6; Fig 1B).
Personalized Therapy Is Associated With Longer OS
We carried out a pooled analysis including 441 arms (21,817 patients) that reported a median OS (Table 5 and Fig 1). In the univariable analysis, the personalized strategy, chemotherapy-naïve patients, hematologic malignancies, arms with a smaller number of patients, oral administration, and trials implemented in multiple sites were associated with longer median OS and were thus included in the weighted multiple linear regression model (multivariable analysis). The only two variables that remained independent predictors of a prolonged median survival were the personalized strategy (15.9 v 9 months with nonpersonalized strategy; P < .001; t statistic = 6.4) and chemotherapy-naïve patients (10 v 9 months in patients who previously received chemotherapy; P < .001; t statistic = 3.8).
Table 5.
Pooled and Meta-Analysis for OS
| Parameter | Pooled Analysis (n = 441) |
Meta-Analysis (random effect; n = 247) |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||
| Median (months; 95% CI) | P* | t | P† | Median (months; 95% CI) | P‡ | z | P§ | |
| Total studies | 9.4 (8.8 to 10.0) | 9.1 (8.6 to 9.6) | ||||||
| Personalized strategy | < .001 | 6.4 | < .001 | < .001 | 3.8 | < .001 | ||
| Yes | 15.9 (11.4 to 18.3) | 13.7 (11.1 to 16.4) | ||||||
| No | 9.0 (8.3 to 9.7) | 8.9 (8.3 to 9.3) | ||||||
| Chemotherapy status | .012 | 3.8 | < .001 | .575 | — | — | ||
| Chemotherapy naïve | 10.0 (9.3 to 12.0) | 9.4 (8.2 to 10.7) | ||||||
| Prior chemotherapy | 9.0 (9.2 to 9.7) | 9.0 (8.5 to 9.6) | ||||||
| Tumor type | .022 | 1.2 | .213 | .184 | — | — | ||
| Solid | 9.2 (8.5 to 9.8) | 9.0 (8.5 to 9.5) | ||||||
| Hematologic | 12.9 (8.7 to 16.5) | 11.3 (8.0 to 14.6) | ||||||
| Agent class | .611 | — | — | .458 | — | — | ||
| Cytotoxic | 9.4 (8.5 to 10.4) | 9.3 (8.5 to 10.1) | ||||||
| Targeted | 9.4 (8.5 to 10.1) | 8.9 (8.3 to 9.6) | ||||||
| Study design | .388 | — | — | .040 | 2.1 | .040 | ||
| Randomized | 8.8 (7.3 to 9.7) | 8.3 (7.1 to 9.4) | ||||||
| Nonrandomized | 9.7 (8.8 to 10.2) | 9.4 (8.8 to 9.9) | ||||||
| 5-Year impact factor | .487 | — | — | .129 | — | — | ||
| ≤ 10 | 9.4 (8.5 to 10.0) | 9.0 (8.4 to 9.5) | ||||||
| > 10 | 9.2 (8.1 to 10.5) | 10.1 (8.7 to 11.5) | ||||||
| No. of patients per arm | .003 | 1.3 | .201 | .0027 | 2.8 | .005 | ||
| ≤ 35 | 8.5 (7.6 to 9.8) | 8.3 (7.6 to 9.0) | ||||||
| > 35 | 9.9 (9.2 to 10.5) | 9.8 (9.1 to 10.5) | ||||||
| Administration route | .049 | 1.0 | .325 | .059 | — | — | ||
| Oral | 9.7 (8.8 to 10.5) | 9.6 (8.8 to 10.3) | ||||||
| Injection | 9.0 (8.0 to 10.0) | 8.6 (8.0 to 9.3) | ||||||
| FDA/EMA approval | .361 | — | — | .549 | — | — | ||
| No | 9.5 (7.8 to 10.0) | 8.8 (7.8 to 9.9) | ||||||
| Yes | 9.4 (8.8 to 10.4) | 9.2 (8.6 to 9.8) | ||||||
| No. of treating centers | .021 | 1.6 | .103 | < .001 | 1.3 | .187 | ||
| Single center | 8.3 (7.0 to 10.3) | 8.3 (7.3 to 9.3) | ||||||
| Multiple centers | 9.7 (8.8 to 10.0) | 9.2 (8.7 to 9.8) | ||||||
NOTE. Only variables that were significant in the univariable models were included in the multivariable analysis. The t and z values are used to compute the corresponding P values, and the higher they are, the more important they are for the multivariable model. The pooled analysis included 441 arms, and the meta-analysis included 247 arms for which median OS and the 95% CI values were available.
Abbreviations: EMA, European Medicines Agency; FDA, US Food and Drug Administration; OS, overall survival.
Wilcoxon test; the median in the univariable was not weighted.
Multiple linear regression model using a weighted least squares model.
Mixed effects analysis.
Random effects meta-regression model.
Cutoff value chosen to discriminate higher impact factor journals versus lower impact factor journals (sum of median and interquartile range).
Cutoff value used was the median of distribution.
The meta-analysis included arms that reported median OS and their corresponding 95% CIs (n = 247 arms; 11,860 patients; Table 5). The results of the meta-regression analysis, adjusting for potential confounders, confirmed that the personalized approach correlated with prolonged survival (13.7 v 8.9 months with the nonpersonalized approach; P < .001; z value = 3.8; Fig 1B).
Safety of Personalized Therapy
Of the 641 arms included, 620 (97%) had information about treatment-related deaths. Results from a meta-analysis established that the median treatment-related mortality rate was 1.52% (95% CI, 1.23% to 1.87%) for arms that used a personalized strategy versus 2.26% (95% CI, 2.04% to 2.49%) for nonpersonalized arms, which was statistically significant (P < .001). Of note, a separate meta-analysis established that arms testing cytotoxic agents had higher treatment-related death rates (median, 2.42%; 95% CI, 2.08% to 2.83%) than arms testing targeted agents (median, 1.94%; 95% CI, 1.74% to 2.17%; P = .023).
DISCUSSION
We conducted a comprehensive meta-analysis and systematic review of single agents tested in phase II clinical trials (January 1, 2010, to December 31, 2012). Five hundred seventy studies comprising 32,149 patients were included. Although several variables were associated with higher RR and longer median PFS and OS, the use of a personalized strategy was the only factor that correlated strongly, consistently, and independently across the three end points (all P < .001; Tables 3 to 5). Personalized therapy arms were also statistically safer (P < .001, meta-analysis), with a median treatment-related mortality rate of 1.52% compared with 2.26% for nonpersonalized treatments.
The most appropriate method to analyze combined and heterogeneous results is to conduct a meta-analysis using the random effects model. Although this was possible for all of the arms reporting RR, it was not feasible for the arms reporting median PFS or OS without their 95% CIs. The meta-analysis for RR, PFS, and OS confirmed personalized therapy as an independent predictor of these outcome variables. To attenuate any bias in the meta-analysis that might have been introduced by excluding arms without 95% CIs (35% of the arms reported median PFS but not 95% CI, and 44% of arms reported median OS but not 95% CI), we also used a pooled analysis, which was weighted (by sample size, using the least squares method) for the multiple linear regressions models to account for effect size and potential confounders. Similar to the meta-analysis, the weighted linear regression analysis showed that personalized therapy was independently associated with prolonged PFS and OS.
In both our analyses, personalized therapy consistently stood out as the most significant contributor to higher RR (P < .001), prolonged PFS (P < .001), and prolonged OS (P < .001; Tables 3 to 5. Of note, however, arms that used cytotoxic drugs (v targeted agents) also correlated with higher RR and PFS but not with OS in multivariable analysis. A subanalysis showed that when cytotoxics (only one of 212 studies was personalized) were compared with personalized targeted agents (n = 111 studies), the RR, PFS, and OS were significantly better for the personalized targeted agents (all P < .001; Data Supplement). Further, when comparing targeted arms using a personalized strategy versus targeted arms not selecting patients, the former had statistically improved outcomes (RR, PFS, and OS all P < .001; Data Supplement). Indeed, nonpersonalized targeted arms had poor outcomes compared with either personalized targeted therapy arms or cytotoxic arms, with median RRs of 4%, 30%, and 11.9%; median PFS times of 2.6, 6.9, and 3.3 months; and median OS times of 8.7, 15.9, and 9.4 months, respectively (all P ≤ .05; Data Supplement). Taken together, the data suggest that the worst outcomes were associated with use of targeted agents in a nonpersonalized strategy. Targeted personalized therapy resulted in the best outcomes. The outcomes of personalized cytotoxic therapies were not assessable because there was only one such study.
As mentioned earlier, cytotoxics resulted in a better RR and PFS than targeted therapy (when personalized and nonpersonalized were considered together), but despite these salutary effects, OS was not improved. The reasons for this are unclear, but we did note higher drug-related mortality rates in cytotoxic versus targeted agent arms (median, 2.4% v 1.9%, respectively; P = .023), perhaps because of the known adverse effects often accompanying the administration of cytotoxic agents.
We also established that both personalized-direct and personalized-indirect (see Data Supplement for definition) approaches led to significantly higher RRs and longer PFS and OS times than strategies without patient selection. This observation suggests that impacting aberrant pathways either by directly affecting the cognate target or by mediating an effect downstream of it can be effective (Data Supplement).
Our findings are consistent with prior studies comparing personalized and nonpersonalized therapy specific to tumor types.25–28 For example, Janku et al19 analyzed the outcomes of phase II clinical trials using single agents in advanced or metastatic non–small-cell lung cancer from 2000 to 2009. Treatment arms enriched for patient-specific molecular targets achieved higher RR, longer PFS, and longer OS than those that were not enriched (P = .005). Similarly, an initiative study by The University of Texas MD Anderson Cancer Center published in 201222 and updated in 201428 examining outcome data in 379 patients showed that RR, median PFS, and median OS were significantly greater in individuals treated with matched versus unmatched therapy (median RR, 12% v 5%, respectively; P < .001; median PFS, 3.9 v 2.2 months, respectively; P = .001; median OS, 11.4 v 8.6 months, respectively; P = .04) across diverse histologies.
There are several limitations relevant to this study. First, we only included arms reporting single agents that were published over a limited 3-year period of time (2010 through 2012). Effects of combinations were not evaluated. Second, our analysis included multiple cancer types, and we were unable to confidently assess outcome differences within malignancies because the number of studies pertaining to each histology was small. On the other hand, the observation of correlations in diverse malignancies may support the hypothesis that our results are applicable across histologies.29 Third, trials with RRs of 0% often did not report PFS or OS. Another intrinsic limitation arises from the fact that patient follow-up times may vary between trials, producing heterogeneity in the estimation of median PFS and OS.30 Although this could not be addressed in our meta-analysis, it seems important to acknowledge that the outcomes of certain tumors with actionable alterations (eg, EGFR-mutant lung cancers) could be improved even without a personalized approach because they have a better natural history. Therefore, known prognostic factors, which may differ between patients with or without targetable mutations, can never be fully addressed in a retrospective analysis, even with the use of a multivariable analysis. Only randomized trials stratified by a biomarker or in which treatments are allocated by such a biomarker can fully address this issue, and prospective validation of molecular biomarkers in each specific context is warranted. Other study limitations pertain to the uncertainty of whether Clinical Laboratory Improvement Amendments–certified laboratories were used for biomarker selection, as well as whether or not RR and PFS assessments were performed centrally. Finally, the omission of hormonal agents and nonpublished study results could have lent some bias in favor of any of the variables.
Of note, our definition of a personalized strategy allowed for protein overexpression (tested mostly by immunohistochemistry) if the tested protein was the target of the drug, as well as gene alterations. In the latter, the link with the drug target is less direct given that additional biologic steps can intervene in the process, leading to protein translation. Even so, personalized trials chosen based on protein overexpression produced lower RRs and shorter PFS and OS times than those that were selected based on genomic alterations (Data Supplement), suggesting that the protein expression markers that were chosen to date may be less desirable than genomic markers. The example of epidermal growth factor receptor (EGFR) inhibitors corroborates this observation; EGFR overexpression was not a marker of response to EGFR inhibitors,31 whereas patients with adenocarcinoma of the lung selected based on EGFR mutation status achieved significant responses.32–36
Recently, next-generation sequencing studies have provided a better characterization of the molecular biology of cancer and allowed for analysis of tumor complexity, heterogeneity, progression, and resistance mechanisms.37 Effective anticancer treatment depends on the ability to target the underlying molecular aberrations that contribute to oncogenesis. Our comprehensive analysis of 570 phase II studies including 32,149 patients not only indicates that, across malignancy types, a personalized strategy was independently associated with higher RRs, longer median PFS and OS times, and fewer toxic deaths, but also that nonpersonalized targeted arms led to poorer outcomes than cytotoxic arms. Taken together, the data suggest that personalized therapy resulted in the best outcomes, whereas the worst outcomes were associated with use of targeted agents in a nonpersonalized strategy. However, some important prognostic confounders that might have introduced bias could not be ruled out in our analysis, and the limitations of our analysis should be taken into consideration. For instance, our data set did not include randomized trials within a biomarker-driven population, such as patients with EGFR-mutant lung cancer randomly assigned to EGFR inhibitors versus other drugs. Our results should provide the impetus for the implementation of prospective and controlled trials.
Supplementary Material
Footnotes
Supported in part by National Institutes of Health Grant No. TL1TR00098, the Joan and Irwin Jacobs Fund, and Foundation Medicine.
Presented, in part, at the 51st Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 29-June 2, 2015.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Maria Schwaederle, Alexander M. Eggermont, Richard L. Schilsky, John Mendelsohn, Vladimir Lazar, Razelle Kurzrock
Financial support: Razelle Kurzrock
Administrative support: Maria Schwaederle
Collection and assembly of data: Maria Schwaederle, Melissa Zhao
Data analysis and interpretation: Maria Schwaederle, J. Jack Lee, Razelle Kurzrock
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Maria Schwaederle
No relationship to disclose
Melissa Zhao
No relationship to disclose
J. Jack Lee
No relationship to disclose
Alexander M. Eggermont
Consulting or Advisory Role: GlaxoSmithKline, Bristol-Myers Squibb, MedImune, Merck
Richard L. Schilsky
No relationship to disclose
John Mendelsohn
Leadership: Merrimack
Stock or Other Ownership: Merrimack
Consulting or Advisory Role: Merrimack
Patents, Royalties, Other Intellectual Property: University of California, San Diego patent - royalties to me (former employer)
Vladimir Lazar
No relationship to disclose
Razelle Kurzrock
Stock or Other Ownership: RScueRX
Consulting or Advisory Role: Sequenom
REFERENCES
- 1.Stewart DJ, Kurzrock R. Cancer: The road to Amiens. J Clin Oncol. 2009;27:328–333. doi: 10.1200/JCO.2008.18.9621. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Dancey JE, Bedard PL, Onetto N, et al. The genetic basis for cancer treatment decisions. Cell. 2012;148:409–420. doi: 10.1016/j.cell.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Wong KM, Hudson TJ, McPherson JD. Unraveling the genetics of cancer: Genome sequencing and beyond. Annu Rev Genomics Hum Genet. 2011;12:407–430. doi: 10.1146/annurev-genom-082509-141532. [DOI] [PubMed] [Google Scholar]
- 5.Barrett JC, Frigault MM, Hollingsworth S, et al. Are companion diagnostics useful? Clin Chem. 2013;59:198–201. doi: 10.1373/clinchem.2012.185132. [DOI] [PubMed] [Google Scholar]
- 6.Montemurro F, Valabrega G, Aglietta M. Trastuzumab treatment in breast cancer. N Engl J Med. 2006;354:2186. doi: 10.1056/NEJMc060852. [DOI] [PubMed] [Google Scholar]
- 7.Hudis CA. Trastuzumab: Mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 8.Incorvati JA, Shah S, Mu Y, et al. Targeted therapy for HER2 positive breast cancer. J Hematol OncolJ Hematol Oncol. 2013;6:38. doi: 10.1186/1756-8722-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbany G, Höglund M, Simonsson B. Complete molecular remission in chronic myelogenous leukemia after imatinib therapy. N Engl J Med. 2002;347:539–540. doi: 10.1056/NEJM200208153470719. [DOI] [PubMed] [Google Scholar]
- 10.Boros LG, Lee WN, Cascante M. Imatinib and chronic-phase leukemias. N Engl J Med. 2002;347:67–68. doi: 10.1056/NEJM200207043470116. [DOI] [PubMed] [Google Scholar]
- 11.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 12.Westin JR, Kurzrock R. It's about time: Lessons for solid tumors from chronic myelogenous leukemia therapy. Mol Cancer Ther. 2012;11:2549–2555. doi: 10.1158/1535-7163.MCT-12-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalle S, Poulalhon N, Thomas L. Vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;365:1448–1450. doi: 10.1056/NEJMc1108651. [DOI] [PubMed] [Google Scholar]
- 15.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: A phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez de Castro D, Clarke PA, Al-Lazikani B, et al. Personalized cancer medicine: Molecular diagnostics, predictive biomarkers, and drug resistance. Clin Pharmacol Ther. 2013;93:252–259. doi: 10.1038/clpt.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Said R, Hong DS, Warneke CL, et al. P53 mutations in advanced cancers: Clinical characteristics, outcomes, and correlation between progression-free survival and bevacizumab-containing therapy. Oncotarget. 2013;4:705–714. doi: 10.18632/oncotarget.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janku F, Wheler JJ, Naing A, et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res. 2013;73:276–284. doi: 10.1158/0008-5472.CAN-12-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janku F, Berry DA, Gong J, et al. Outcomes of phase II clinical trials with single-agent therapies in advanced/metastatic non-small cell lung cancer published between 2000 and 2009. Clin Cancer Res. 2012;18:6356–6363. doi: 10.1158/1078-0432.CCR-12-0178. [DOI] [PubMed] [Google Scholar]
- 20.Collins I, Workman P. New approaches to molecular cancer therapeutics. Nat Chem Biol. 2006;2:689–700. doi: 10.1038/nchembio840. [DOI] [PubMed] [Google Scholar]
- 21.Hoelder S, Clarke PA, Workman P. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol Oncol. 2012;6:155–176. doi: 10.1016/j.molonc.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsimberidou AM, Iskander NG, Hong DS, et al. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center initiative. Clin Cancer Res. 2012;18:6373–6383. doi: 10.1158/1078-0432.CCR-12-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Henary H, Hong DS, Falchook GS, et al. Melanoma patients in a phase I clinic: Molecular aberrations, targeted therapy and outcomes. Ann Oncol. 2013;24:2158–2165. doi: 10.1093/annonc/mdt115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Von Hoff DD, Stephenson JJ, Jr, Rosen P, et al. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28:4877–4883. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- 27.Weiss GJ, Liang WS, Demeure MJ, et al. A pilot study using next-generation sequencing in advanced cancers: Feasibility and challenges. PloS One. 2013;8:e76438. doi: 10.1371/journal.pone.0076438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsimberidou AM, Wen S, Hong DS, et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: Validation and landmark analyses. Clin Cancer Res. 2014;20:4827–4836. doi: 10.1158/1078-0432.CCR-14-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz J, Swanton C, Kurzrock R. Molecular profiling and the reclassification of cancer: Divide and conquer. Am Soc Clin Oncol Educ Book. 2013;2013:127–134. doi: 10.14694/EdBook_AM.2013.33.127. [DOI] [PubMed] [Google Scholar]
- 30.Michiels S, Piedbois P, Burdett S, et al. Meta-analysis when only the median survival times are known: A comparison with individual patient data results. Int J Technol Assess Health Care. 2005;21:119–125. doi: 10.1017/s0266462305050154. [DOI] [PubMed] [Google Scholar]
- 31.Lee SM. Is EGFR expression important in non-small cell lung cancer? Thorax. 2006;61:98–99. doi: 10.1136/thx.2005.047936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araki T, Yashima H, Shimizu K, et al. Review of the treatment of non-small cell lung cancer with gefitinib. Clin Med Insights Oncol. 2012;6:407–421. doi: 10.4137/CMO.S7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K-H, Lee K-Y, Jeon Y-J, et al. Gefitinib in selected patients with pre-treated non-small-cell lung cancer: Results from a phase IV, multicenter, non-randomized study (SELINE) Tuberc Respir Dis (Seoul) 2012;73:303–311. doi: 10.4046/trd.2012.73.6.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 35.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 36.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 37.Shyr D, Liu Q. Next generation sequencing in cancer research and clinical application. Biol Proced Online. 2013;15:4. doi: 10.1186/1480-9222-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.