Abstract
Background
Caloric restriction and n-3 polyunsaturated fatty acid (PUFA) supplementation protect from some of the metabolic complications. The aim of this study was to assess the influence of a low calorie diet with or without n-3 PUFA supplementation on glucose dependent insulinotropic polypeptide (GIP) output and insulin sensitivity markers in obese subjects.
Methods
Obese, non-diabetic subjects (BMI 30–40 kg/m2) and aged 25–65 yr. were put on low calorie diet (1200–1500 kcal/day) supplemented with either 1.8 g/day n-3 PUFA (DHA/EPA, 5:1) (n = 24) or placebo capsules (n = 24) for three months in a randomized placebo controlled trial. Insulin resistance markers and GIP levels were analysed from samples obtained at fasting and during an oral glucose tolerance test (OGTT).
Results
Caloric restriction with n-3 PUFA led to a decrease of insulin resistance index (HOMA-IR) and a significant reduction of insulin output as well as decreased GIP secretion during the OGTT. These effects were not seen with caloric restriction alone. Changes in GIP output were inversely associated with changes in red blood cell EPA content whereas fasting GIP level positively correlated with HOMA-IR index. Blood triglyceride level was lowered by caloric restriction with a greater effect when n-3 PUFA were included and correlated positively with fasting GIP level.
Conclusions
Three months of caloric restriction with DHA + EPA supplementation exerts beneficial effects on insulin resistance, GIP and triglycerides.
General significance
Combining caloric restriction and n-3 PUFA improves insulin sensitivity, which may be related to a decrease of GIP levels.
Abbreviations: AUC, area under curve; BMI, body mass index; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; GIP, glucose dependent insulinotropic polypeptide; IGI, insulinogenic index; NEFA, non esterified fatty acids; OGIS, oral glucose insulin sensitivity index; OGTT, oral glucose tolerance test; PC, phosphatidylcholine; PUFA, polyunsaturated fatty acids
Keywords: n-3 polyunsaturated fatty acids, Docosahexaenoic acid, Eicosapentaenoic acid, Caloric restriction, Insulin resistance, Obesity
Highlights
-
•
Caloric restricted diet with n-3 PUFA improves insulin sensitivity in obese subjects
-
•
n-3 PUFA supplementation combined with low calorie diet decrease GIP output
-
•
Blood triglycerides level reduces after caloric restriction diet combined with n-3 PUFA
-
•
GIP level positively correlates with HOMA-IR index and triglycerides
1. Introduction
Obesity and its associated disorders (impaired insulin sensitivity, hyperinsulinemia, dyslipidemia, hypertension) often lead to type 2 diabetes with increased risk for cardiovascular disease [1], [2], [3]. Obesity contributes to type 2 diabetes development by affecting glucose and lipid homeostasis, which depends on the balance between insulin sensitivity and insulin secretion [4]. Incretins account for 50–70% of insulin secretion after meals or an oral glucose load [5]. Glucose-dependent insulinotropic polypeptide (GIP) is released from enteroendocrine K cells in response to oral ingestion of fat or glucose [6] and stimulates insulin secretion from pancreatic β-cells in a glucose-dependent manner [7]. Transduction of its biological effects involves stimulation of G protein-coupled receptors (GPCR) [8], for example GPR 120 [9].
Adequate insulin sensitivity and insulin secretion can be restored by achieving appropriate body mass through adopting a healthy diet, energy restriction, and physical activity [10], [11]. A diet rich in omega-3 (n-3) polyunsaturated fatty acids (PUFA) reduces risk for obesity complications by influencing lipid metabolism, inflammation, coagulation and atherogenesis [12], [13], [14]. Increasing intake of n-3 fatty acids leads to changes in cell membrane phospholipid fatty acid composition, which affects cell and tissue function through alterations in the properties of membranes, altered cell signalling pathways, and modified gene expression profiles [15], [16], [17], [18]. Docosahexaenoic acid (DHA, 22:6n-3) and eicosapentaenoic acid (EPA, 20:5n-3) are considered the most biologically active n-3 PUFA [19], [20].
Beneficial effects of n-3 PUFA on glucose homeostasis have been documented in animal models of obesity and metabolic syndrome [21], [22]. Improvement in glucose tolerance after n-3 PUFA supplementation in obese humans has been less frequently reported and seems to be related to the presence of obesity complications [23], [24], [25], [26]. Whether GIP is involved in the effect of n-3 PUFA on the insulin response to a glucose load has not been reported in human studies. Thus, it is not clear if n-3 PUFA affect the incretin system in humans with obesity and prediabetes.
The aim of this study was to investigate the effects of caloric restriction with or without consumption of n-3 PUFA (DHA + EPA) on GIP, insulin release and metabolic variables at fasting and during an OGTT in obese, non-diabetic subjects.
2. Materials and methods
2.1. Subjects
Obese subjects (BMI 30–40 kg/m2) aged 25–65 years were recruited from the Out-Patient Clinic of Obesity and Lipid Disorders and the Department of Metabolic Disorders, Jagiellonian University Medical College, Krakow, Poland. Exclusion criteria included: diabetes or other endocrine disorders, chronic inflammatory diseases, and kidney or liver dysfunction. Subjects participating in the study had not taken lipid-lowering or anti-inflammatory drugs or supplements containing vitamins A, C, or E, β-carotene or PUFA. Fish consumption was not allowed during the study period.
2.2. Study design and intervention
This clinical trial was randomized, double-blind placebo-controlled parallel and single center. The trial was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and with the Good Clinical Practise guidelines. The trial was approved by Bioethics Committee of the Jagiellonian University in Cracow (written consent, opinion No. KBET/82/B/2009) and all subjects gave written informed consent. The trial was conducted at the Department of Clinical Biochemistry, Jagiellonian University, Medical College, Krakow, Poland. The trial was registered at isrctn.com as ISRCTN11445521.
Recruited subjects were randomly assigned to the n-3 PUFA or the placebo group by using minimalization software balanced for age and sex.
Obese subjects (BMI 30–40 kg/m2) underwent an adaptation period of two weeks. During this time they were advised by a dietician on how to apply an isocaloric diet containing 2300–2400 kcal/d according to individual caloric requirement. The diet contained 57% of energy from carbohydrates, 30% from fat and 13% from protein. After the adaptation period, subjects were advised to use a low calorie diet. The caloric value of the low calorie diet was 1200 kcal/d for women and 1500 kcal/d for men and 60% of energy was provided from carbohydrates (with low glycemic index), 15% from protein and 25% from fat. Subjects were randomly assigned to receive placebo (corn oil) capsules (CR placebo) or n-3 PUFA (EPAX 1050TG; EPAX, Norway) capsules (CR + n-3). Subjects consumed 3 capsules per day for 3 months. Three n-3 PUFA capsules contained 1.8 g DHA + EPA in a ratio of 5:1. Both capsule types were identical in size, shape and appearance and contained 4 mg of vitamin E per capsule. Subjects were advised to swallow their capsules after a meal.
All subjects were given written and verbal instructions by a dietician about the preparation of a caloric restricted diet and consumption of capsules. Body composition changes including fat content, muscle mass and water content were measured by Tanita Body Composition Analyser BC-418 (Tanita, Japan). Hip and waist circumferences, diet and supplementation compliance, and nutritional habits were assessed.
Anthropometric measurements (BMI, hip and waist circumference, systolic and diastolic blood pressure, adipose tissue content) were performed at baseline (after the two week adaptation period) and at the end of three months intervention and after a 12 h overnight fast. An oral glucose tolerance test (OGTT; 75 g glucose load) was performed according to WHO guidelines. At fasting and every 30 min up to 2 h venous blood was collected for measurement of serum glucose, insulin, GIP, non-esterified fatty acids (NEFA), total cholesterol, HDL cholesterol and triglycerides. Additionally, at fasting baseline and at the end of treatment venous blood was collected for determination of plasma phosphatidylcholine (PC) composition and fatty acid content of erythrocytes’ membranes (see below). All samples were centrifuged at 1000 x g for 15 min at 4 °C. Serum, plasma and erythrocytes’ samples were aliquoted and stored at − 80 °C until analysis. Sample size was calculated to 25 per group to detect a 50% change in insulin sensitivity markers at a P value < 0.05 with a power of 80%.
2.3. Compliance
Counting the number of returned capsules during the follow up visits was used to assess compliance to capsules intake. In addition, the fatty acid composition of plasma phosphatidylcholine (PC), and of erythrocytes (RBC) was determined by gas chromatography using methods described previously [27].
Because of concerns about compliance to n-3 PUFA capsules in the n-3 PUFA group and contamination in the placebo group (i.e. intake of n-3 PUFA capsules) it was decided before hand to use a cut-off of a 20% increase in “omega-3 index”, the sum of EPA + DHA in erythrocytes, to identify compliers in the n-3 PUFA group and violators in the placebo group. Using the threshold of a 20% increase in omega-3 index resulted in retention of data for 24 subjects in the n-3 PUFA group and 24 subjects in the placebo group.
2.4. Biochemical measurements
Subjects were instructed to avoid strenuous exercise and alcohol consumption the day before blood collection. Plasma glucose, total cholesterol, HDL-cholesterol and triglycerides were assayed by automated, enzymatic colorimetric methods (ELITech Clinical Systems, France). The intra and inter-assay variability coefficients were as follows: 2.3% and 3.5% (glucose), 1.4% and 3.4% (triglycerides), 1.4% and 3.8% (total cholesterol), 2.1% and 2.8% (HDL-cholesterol), respectively. LDL-cholesterol was calculated from measured values of total cholesterol, triglycerides and HDL-cholesterol according to the Friedewald formula.
Non esterified fatty acids (NEFA) concentration was measured immediately in non-frozen plasma by enzymatic quantitative colorimetric method (Roche Diagnostics GmbH, Germany).
Insulin was determined by an immunoradiometric method (DIAsource ImmunoAssays, Belgium) and read using a gamma counter (LKB Instruments). Within and between-run imprecision CVs were 2.1% and 6.5%, respectively.
GIP was measured using ELISA (EMD Millipore, St. Charles, MO, USA). Within-run CV was 6.1% and between-run CV 8.8%. The limit of detection was 8.2 pg./ml.
2.5. Presentation of results
Area under concentration time curve (AUC) for glucose, insulin, GIP, triglycerides and NEFA during the OGTT was calculated by the trapezoidal method [28]. Β-cell function was assessed by the ratio of the insulin to glucose AUC (AUCI/AUCG) and the insulinogenic index (IGI). IGI is a measure of first-phase insulin secretion [29] and was calculated as the ratio of the difference between the post oral glucose load insulin peak (at 60 min) and basal insulin to the difference in glucose levels (IGI = ∆ I0-60/∆G0-60). Basal insulin resistance was estimated using homeostasis model assessment (HOMA-IR) [30]. Post oral glucose load insulin sensitivity was determined using an oral glucose insulin sensitivity index (OGIS) proposed by Mari et al. [31] which can be computed using a calculator for Excel spread sheet available on the webpage http://webmet.pd.cnr.it/ogis.
2.6. Statistical analysis
Only responders were included in the analysis (n = 24 in the n-3 PUFA group and n = 24 in the placebo group). The Kolmogorov-Smirnov test was used to test the data for a Gaussian distribution. Normally distributed data are presented as mean ± SEM, otherwise as median and interquartile range (IQR). Between group differences were analysed by unpaired t test or non-parametric U-Mann Whitney test. Continuous variables obtained before and after the treatment were log transformed if required and analysed by paired t test (non-normally distributed data were analysed by Wilcoxon's test) to identify treatment-induced differences between individual groups. Spearman rank correlation was used to assess relationship between variables. All analyses were performed with Statistica software (StatSoft). A P value < 0.05 was considered statistically significant.
3. Results
3.1. Study population, intervention and basal characteristics of subjects
This trial aimed to investigate the effect of n-3 PUFA supplementation on insulin sensitivity markers in obese nondiabetic subjects on a calorie restricted diet. The recruitment of obese study participants began in September 2009 and was completed in July 2013.
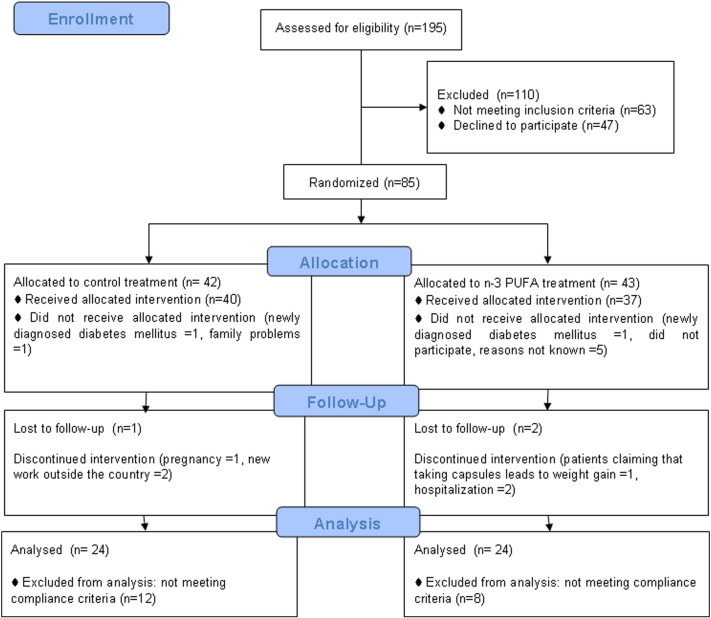
Eighty-five of 195 screened obese subjects were enrolled in the study and randomly assigned to n-3 PUFA or placebo treatment. The progression of subjects through the study is shown in Fig. 1. Three subjects were lost to follow-up (2 in the n-3 PUFA group and 1 in the placebo group). After randomization, 2 subjects were excluded (1 from the n-3 PUFA group and 1 from the placebo group) because they were newly diagnosed with type 2 diabetes during the 2-h OGTT. Six subjects declined to participate after allocation to intervention groups: one from the placebo group due to family problems and five from the n-3 PUFA group (reasons not known). Six subjects were excluded during the follow-up period. Three subjects from the placebo group discontinued intervention: 1 woman due to pregnancy and 2 subjects got new work outside the country. From the n-3 PUFA group 3 subjects were excluded: one subject claimed that taking capsules leads to weight gain, 1 subject developed pneumonia that required hospitalization and 1 subject required hospitalization for decompensation of preexisting heart failure. The n-3 PUFA capsules were safe and well tolerated. No adverse effects were observed after DHA/EPA (5:1) supplementation except one subject who claimed that taking these capsules contributes to weight gain. Finally, 36 patients in placebo group and 32 patients in n-3 PUFA group completed the trial. Twenty subjects were excluded from analysis due to not meeting compliance criteria: 12 subjects from the placebo group (omega-3 index after supplementation increased more than 20%) and 8 subjects from the n-3 PUFA group (increase in omega-3 index was less than 20% after supplementation). Thus, full analyses were performed in 48 subjects: 24 in the placebo group and 24 in the n-3 PUFA group. Baseline characteristics did not differ between CR placebo and n-3 PUFA (CR + n-3) groups (Table 1). Subjects were mainly women (approximately 80%) with a mean age of 48 years, moderately obese (BMI: 34.7 ± 0.5 kg/m2) and without hypertension. The low value of baseline omega-3 fatty acid index (5.63 ± 0.19%) is consistent with the selection of subjects with low fish intake. Subjects had slightly elevated total and LDL cholesterol concentrations although plasma triglyceride concentrations were not elevated (Table 1). Fasting blood glucose concentration was normal but plasma insulin concentration was slightly increased (Table 1).
Fig. 1.
CONSORT flow diagram.
Table 1.
Baseline characteristics of the obese subjects included in the analysis.
| All subjects (n = 48) | Placebo subjects (n = 24) | n-3 PUFA subjects (n = 24) | P1 | |
|---|---|---|---|---|
| Age [y] | 47 ± 22 | 48 ± 2 | 45 ± 2 | 0.47 |
| Sex, female [n] | 38 | 20 | 18 | |
| EPA in plasma PC [weight%] | 2.22 ± 0.22 | 2.27 ± 0.28 | 2.16 ± 0.35 | 0.82 |
| DHA in plasma PC [weight%] | 3.68 ± 0.22 | 3.72 ± 0.33 | 3.62 ± 0.27 | 0.82 |
| EPA in RBC membranes [%] | 1.12 ± 0.06 | 1.19 ± 0.08 | 1.05 ± 0.07 | 0.22 |
| DHA in RBC membranes [%] | 4.51 ± 0.15 | 4.65 ± 0.22 | 4.36 ± 0.19 | 0.32 |
| Omega-3 index [%] | 5.63 ± 0.19 | 5.85 ± 0.28 | 5.41 ± 0.25 | 0.25 |
| Anthropometric measurements: | ||||
| Weight [kg] | 96.18 ± 1.67 | 97.56 ± 2.12 | 94.78 ± 2.59 | 0.41 |
| BMI [kg/m2] | 34.75 ± 0.51 | 35.24 ± 0.72 | 34.25 ± 0.7 | 0.33 |
| Waist to hip ratio | 0.85 (0.82, 0.93)3 | 0.90 (0.84, 0.95) | 0.83 (0.80, 0.91) | 0.10 |
| Adipose tissue mass [%] | 39.68 ± 0.91 | 40.7 ± 1.16 | 38.74 ± 1.33 | 0.28 |
| Systolic BP [mm Hg] | 128.40 ± 2.49 | 132.5 ± 4.08 | 124.09 ± 2.07 | 0.09 |
| Diastolic BP [mm Hg] | 84.30 ± 1.39 | 85.4 ± 2.4 | 83.14 ± 1.06 | 0.42 |
| Metabolic measurements: | ||||
| Total cholesterol [mmol/l] | 5.51 ± 0.16 | 5.56 ± 0.28 | 5.45 ± 0.16 | 0.72 |
| HDL cholesterol [mmol/l] | 1.31 ± 0.04 | 1.38 ± 0.06 | 1.23 ± 0.04 | 0.06 |
| LDL cholesterol [mmol/l] | 3.53 ± 0.13 | 3.52 ± 0.21 | 3.53 ± 0.16 | 0.99 |
| Fasting NEFA [mmol/l] | 0.77 ± 0.04 | 0.8 ± 0.05 | 0.74 ± 0.06 | 0.48 |
| AUC NEFA [mmol/l x min− 1] | 166.59 ± 11.15 | 180.13 ± 16.55 | 153.05 ± 14.75 | 0.23 |
| Fasting Triglycerides [mmol/l] | 1.38 ± 0.09 | 1.34 ± 0.13 | 1.4 ± 0.13 | 0.77 |
| AUC Triglycerides [mmol/l x min− 1] | 641.75 ± 43.63 | 611.62 ± 58.16 | 671.87 ± 65.7 | 0.50 |
| Fasting glucose [mmol/l] | 5.15 ± 0.09 | 5.1 ± 0.13 | 5.19 ± 0.13 | 0.62 |
| AUC Glucose [mmol/l x min− 1] | 3467.88 ± 113.80 | 3613 ± 162.58 | 3322.75 ± 157.03 | 0.21 |
| Fasting insulin [mIU/ml] | 16.65 ± 1.18 | 17.65 ± 1.94 | 15.64 ± 1.35 | 0.40 |
| AUC Insulin [mIU/ml x min− 1] | 46,109.25 ± 3729.84 | 49,504.75 ± 5704.59 | 42,713.75 ± 4828.21 | 0.37 |
| Fasting GIP [pg./ml] | 30.15 ± 2.25 | 30.88 ± 3.54 | 29.41 ± 2.86 | 0.75 |
| AUC GIP [pg./ml x min− 1] | 68,756.03 ± 3585.17 | 67,204.69 ± 5283.18 | 70,307.36 ± 4940.65 | 0.67 |
| Insulin resistance and β cell function: | ||||
| HOMA-IR | 3.88 ± 0.31 | 4.11 ± 0.51 | 3.65 ± 0.34 | 0.46 |
| AUCI/AUCG | 13.37 ± 1.07 | 13.36 ± 1.46 | 13.25 ± 1.58 | 0.96 |
| IGI = ∆ I0-60/∆G0-60 | 186.85 ± 149.60 | 152.79 ± 270.41 | 219.43 ± 140.09 | 0.83 |
| OGIS [ml min− 1 m− 2] | 384.93 ± 9.99 | 379.66 ± 14.25 | 390.18 ± 14.21 | 0.60 |
1Comparison between placebo and n-3 PUFA groups (unpaired t test or Mann–Whitney U test for non-normally distributed variables).
2Mean ± SEM (all such values).
3Median; IQR in parentheses (all such values)
AUC, area under curve during OGTT; BP, blood pressure; BMI, body mass index; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; GIP, glucose dependent insulinotropic polypeptide; IGI, insulinogenic index; NEFA, non esterified fatty acids; OGIS, oral glucose insulin sensitivity index; PC, phosphatidylcholine; RBC red blood cells.
3.2. Anthropometric measurements and lipid parameters after intervention
After the 3 month intervention period, subjects in the CR + n-3 group had higher levels of EPA and DHA in plasma phosphatidylcholine (PC) (average 55% and 80% increase over baseline, respectively) and in erythrocytes (average 80% and 63% increase over baseline, respectively), as well as mean 66% increase in the omega-3 index compared to baseline (9.01 ± 0.36 vs. 5.41 ± 0.25%; P < 0.0001) (Table 2). In the CR placebo group the level of n-3 PUFA in RBC and omega-3 index did not change, although a decrease in plasma PC EPA, but not DHA, was observed (P = 0.02).
Table 2.
Anthropometric and metabolic variables at baseline and at the end of the study in subjects undergoing caloric restriction and supplemented with placebo or n-3 PUFA capsules.
| Placebo (n = 24) |
n-3 PUFA (n = 24) |
|||||
|---|---|---|---|---|---|---|
| Baseline | After supplementation | P1 | Baseline | After supplementation | P1 | |
| Age [y] | 48 ± 22 | 45 ± 2 | ||||
| Sex, female [n] | 20 | 18 | ||||
| EPA in plasmaPC [weight%] | 2.27 ± 0.28 | 1.92 ± 0.21* | 0.02 | 2.16 ± 0.35 | 3.34 ± 0.54**, # | 0.001 |
| DHA in plasma PC [weight%] | 3.72 ± 0.33 | 3.81 ± 0.36 | 0.62 | 3.62 ± 0.27 | 6.47 ± 0.47***, # | < 0.0001 |
| EPA in RBC membranes [%] | 1.19 ± 0.08 | 1.04 ± 0.08 | 0.22 | 1.05 ± 0.07 | 1.89 ± 0.20***, # | 0.0005 |
| DHA in RBC membranes [%] | 4.65 ± 0.22 | 4.49 ± 0.23 | 0.60 | 4.36 ± 0.19 | 7.12 ± 0.19***, # | < 0.0001 |
| Omega 3 index [%] | 5.85 ± 0.28 | 5.54 ± 0.29 | 0.44 | 5.41 ± 0.25 | 9.01 ± 0.36***, # | < 0.0001 |
| Anthropometric measurements: | ||||||
| Weight [kg] | 97.56 ± 2.12 | 89.74 ± 2.38*** | < 0.0001 | 94.78 ± 2.59 | 87.93 ± 2.42*** | < 0.0001 |
| BMI [kg/m2] | 35.24 ± 0.72 | 32.44 ± 0.86*** | < 0.0001 | 34.25 ± 0.7 | 31.66 ± 0.70*** | < 0.0001 |
| Waist to hip ratio | 0.90 (0.84, 0.95)3 | 0.86 (0.83, 0.91)* | 0.03 | 0.83 (0.80, 0.91) | 0.88 (0.80, 0.92) | 0.46 |
| Adipose tissue mass [%] | 40.7 ± 1.16 | 37.45 ± 1.46*** | 0.0001 | 38.74 ± 1.33 | 35.48 ± 1.67*** | 0.0004 |
| Metabolic measurements: | ||||||
| Total cholesterol [mmol/l] | 5.56 ± 0.28 | 5.33 ± 0.25 | 0.28 | 5.45 ± 0.16 | 5.20 ± 0.21 | 0.08 |
| HDL cholesterol [mmol/l] | 1.38 ± 0.06 | 1.42 ± 0.06 | 0.32 | 1.23 ± 0.04 | 1.23 ± 0.04# | 0.98 |
| LDL cholesterol [mmol/l] | 3.52 ± 0.21 | 3.34 ± 0.21 | 0.26 | 3.53 ± 0.16 | 3.40 ± 0.18 | 0.30 |
| Fasting NEFA [mmol/l] | 0.8 ± 0.05 | 0.8 ± 0.07 | 0.99 | 0.74 ± 0.06 | 0.72 ± 0.06 | 0.83 |
| AUC NEFA [mmol/l x min− 1] | 180.13 ± 16.55 | 196.67 ± 19.50 | 0.33 | 153.05 ± 14.75 | 158.35 ± 13.21 | 0.78 |
| Fasting Triglycerides [mmol/l] | 1.34 ± 0.13 | 1.11 ± 0.09* | 0.02 | 1.4 ± 0.13 | 0.96 ± 0.07*** | 0.0004 |
| AUC Triglycerides [mmol/l x min− 1] | 611.62 ± 58.16 | 510.52 ± 40.16* | 0.02 | 671.87 ± 65.7 | 461.85 ± 40.81*** | 0.0003 |
| Fasting glucose [mmol/l] | 5.1 ± 0.13 | 5.16 ± 0.11 | 0.54 | 5.19 ± 0.13 | 5.08 ± 0.07 | 0.39 |
| AUC Glucose [mmol/l x min− 1] | 3613 ± 162.58 | 3468.25 ± 176.61 | 0.30 | 3322.75 ± 157.03 | 3215.5 ± 132.02 | 0.42 |
| Fasting insulin [mIU/ml] | 17.65 ± 1.94 | 15.32 ± 1.72 | 0.10 | 15.64 ± 1.35 | 12.7 ± 1.02* | 0.01 |
| AUC Insulin [mIU/ml x min− 1] | 49,504.75 ± 5704.59 | 42,087.39 ± 5595.32 | 0.12 | 42,713.75 ± 4828.21 | 35,725.25 ± 3733.77* | 0.04 |
| Fasting GIP [pg./ml] | 30.88 ± 3.54 | 30.36 ± 5.38 | 0.91 | 29.41 ± 2.86 | 20.67 ± 2.24* | 0.02 |
| AUC GIP [pg./ml x min− 1] | 67,204.69 ± 5283.18 | 74,080.78 ± 8600.00 | 0.22 | 70,307.36 ± 4940.65 | 56,435.58 ± 3743.82** | 0.003 |
| Insulin resistance and β cell function: | ||||||
| HOMA-IR | 4.11 ± 0.51 | 3.59 ± 0.43 | 0.19 | 3.65 ± 0.34 | 2.89 ± 0.25* | 0.01 |
| AUCI/AUCG | 13.36 ± 1.46 | 11.72 ± 1.25 | 0.08 | 13.25 ± 1.58 | 11.22 ± 1.17* | 0.02 |
| IGI = ∆ I0-60/∆G0-60 | 152.79 ± 270.41 | 269.49 ± 113.57 | 0.70 | 219.43 ± 140.09 | 354.04 ± 153.33 | 0.52 |
| OGIS [ml min− 1 m− 2] | 379.66 ± 14.25 | 383.12 ± 17.65 | 0.78 | 390.18 ± 14.21 | 404.38 ± 13.60 | 0.30 |
*Significantly different between baseline and after supplementation within a group (paired t test or Wilcoxon's test for non-normally distributed variables).
#Significantly different between placebo and n-3 PUFA groups after supplementation (unpaired t test) p < 0.05.
1Comparison within a group (paired t test or Wilcoxon's test for non-normally distributed variables).
2Mean ± SEM (all such values).
3Median; IQR in parentheses (all such values).
AUC, area under curve during OGTT; BP, blood pressure; BMI, body mass index; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; GIP, glucose dependent insulinotropic polypeptide; IGI, insulinogenic index; NEFA, non esterified fatty acids; OGIS, oral glucose insulin sensitivity index; PC, phosphatidylcholine; RBC red blood cells.
Caloric restriction led to an approximately 8% decrease (p < 0.0001) of body weight and adipose tissue mass in both groups, without any statistically significant differences between groups (Table 2). The CR placebo group showed a 17% decrease in fasting TG concentration as well as a 16% decrease in TG AUC during OGTT (Table 2). This effect was greater and more significant in subjects also supplemented with n-3 PUFA (CR + n-3 group) whose decrease in fasting TG concentration and TG AUC during OGTT was approximately 30% below baseline (P = 0.0003) (Table 2). Neither caloric restriction nor n-3 PUFA influenced fasting NEFA or NEFA level during OGTT (Table 2). No changes in plasma total, HDL and LDL cholesterol levels were observed after 3 months of caloric restriction with either placebo (CR placebo) or n-3 PUFA (CR + n-3) (Table 2).
3.3. Changes in the GIP level and insulin sensitivity markers after intervention
Three months of caloric restriction alone (CR placebo), or in combination with n-3 PUFA (CR + n-3) had no significant affect on the fasting glucose concentration or glucose AUC during OGTT (Table 2). Caloric restriction in combination with n-3 PUFA decreased fasting (Table 2) and 60′ OGTT insulin levels (98.81 ± 10.23 vs. 128.53 ± 14.04 ± IU/ml, P < 0.05). The insulin OGTT AUC was also significantly decreased (P < 0.05) (Table 2). These measurements were not affected by caloric restriction alone. Fasting blood GIP level was lowered in the CR + n-3 group (P < 0.05) (Table 2) and a 30% decrease of the GIP AUC from baseline was observed (P < 0.01) (Table 2). In contrast, caloric restriction alone (without n-3 PUFA supplementation) did not affect GIP level at fasting or during the OGTT (Table 2).
In the whole group of subjects (n = 48) changes in GIP AUC were inversely correlated with changes in erythrocyte EPA content (rho = − 0.37; P = 0.009) (Table 3). After 3 months of intervention fasting plasma GIP level correlated positively with fasting plasma TG level and TG AUC during OGTT (rho = 0.4; P = 0.004) in the CR + n-3 group (Table 3).
Table 3.
Spearman rank correlation between changes in variables (∆) during 3 months of low calorie diet enriched placebo or n-3 PUFA capsules.
| rho | P1 | |
|---|---|---|
| ∆ GIP AUC & ∆ EPA RBC | − 0.37 | 0.009 |
| Fasting GIP & Fasting TG | 0.40 | 0.004 |
| Fasting GIP & TG AUC | 0.41 | 0.004 |
| Fasting GIP & HOMA-IR | 0.29 | 0.047 |
1Statistically significant correlations, P < 0.05.
GIP, glucose dependent insulinotropic polypeptide; AUC, area under curve during OGTT; EPA, eicosapentaenoic acid; RBC, red blood cells; TG, triglycerides.
The ratio of insulin to glucose areas under the curve (AUCI/AUCG) (which reflects the secretory ability of β cells [29]) was significantly decreased in the CR + n-3 group (P = 0.02), but not in the group receiving only calorie restriction (Table 2). The insulinogenic index (IGI = ∆ I0-60/∆G0-60), which measures first phase insulin secretion, and the oral glucose insulin sensitivity index (OGIS) were not significantly changed in either group (Table 2).
A significant (P = 0.01) decrease (by 20%) in insulin resistance measured by HOMA-IR index was observed in the CR n-3 group, with no effect in the CR placebo group (Table 2). After intervention the HOMA-IR index was positively correlated with fasting plasma GIP level (rho = 0.29; P = 0.04) in the CR n-3 group (Table 3).
4. Discussion
The study confirms that controlled caloric restriction leads to loss of body weight, fat mass and BMI and shows no additional effect of n-3 PUFA on these measures. The main new findings of the study are that combining caloric restriction with 1.8 g/day of DHA + EPA (in a ratio of 5:1) improves insulin sensitivity, which was not seen with caloric restriction alone, and causes a greater lowering of plasma triglyceride concentration than seen with caloric restriction alone. The subjects studied were obese and without diabetes but with slightly elevated fasting plasma insulin level as well as total and LDL cholesterol concentrations. The finding of improved insulin sensitivity with n-3 PUFA is important since it suggests an improved metabolic function and a possible lowering of risk for future cardiometabolic complications. The beneficial metabolic changes induced by n-3 PUFA in individuals with caloric restriction correlated with a lower output of GIP.
It is well known that a decrease in body weight in obese persons will per se influence glucose and lipid metabolism, decreasing the risk for cardiovascular complications [32]. Similarly, marine n-3 PUFA supplementation is reported as a protective factor against dyslipidemia and atherosclerosis [15], [33], [34]. Several studies suggest that DHA/EPA can contribute to additional weight loss in calorie restricted individuals; this would obviously be a major health benefit. For example, Thorsdottir et al. reported a greater decrease in body weight after 8 weeks of caloric restriction combined with 1.3 g/day EPA + DHA in comparison to placebo in obese adults [11]. However, the current study did not observe any additional effect of 1.8 g/d DHA + EPA supplementation for three months on weight loss and body adipose tissue content compared with what was observed with caloric restriction alone. Subjects participating in the current study did not undertake any specific physical activity regimen and the diet they used was less restricted in comparison to that of Thorsdottir et al. [11]. Similarly to the findings of our study, Munro and Garg observed that 8 week dietary supplementation with 6 g/day of n-3 PUFA improved metabolic profile but did not modify weight of obese patients on a calorie restricted diet [35].
This study demonstrated that in combination with caloric restriction DHA + EPA supplementation decreases GIP output, improves insulin sensitivity, and lowers plasma triglyceride level. It is widely reported that n-3 PUFA at a dose of 3–4 g/day can lower fasting plasma triglycerides by 25 to 50% [36]. The current study demonstrates that calorie restricted obese subjects supplemented with a more moderate dose of DHA + EPA (1.8 g/day) for 3 months show decreases in plasma fasting triglycerides comparable with these findings. Furthermore, we found that n-3 PUFA decrease the triglyceride response seen during an OGTT. This would suggest an improved clearance of triglycerides from the circulation, perhaps due to improved insulin sensitivity. However, we did not observe any effect of n-3 PUFA on total, LDL or HDL cholesterol concentrations. This finding is in agreement with studies by Schuchardt et al. who demonstrated an increase in omega-3 index of more than 4% after 6 months of 1.68 g/day n-3 PUFA which also caused a significant decrease in plasma triglycerides but not in other risk factors of atherosclerosis such as total and LDL cholesterol concentrations [37].
Marine fish intake has been documented to be associated with improved glucose homeostasis. For example, Feskens et al. showed that higher intake of marine fish was associated with lowered relative risk of impaired glucose tolerance [38]. Other studies showed a negative association between fish intake and the risk for type 2 diabetes [39], [40], [41]. These effects of fish may be due to n-3 PUFA. However, less favourable effects on glucose control were sometimes observed in subjects supplemented with more than 3 g/day of n-3 PUFA [26], [42], [43]. Our results demonstrated that n-3 PUFA did not affect fasting glucose but lowered fasting insulin and decreased basal insulin resistance index (HOMA-IR), which suggests a reduction of (mainly) hepatic insulin resistance [44], [45]. It has been reported that three months of a low fat diet enriched with n-3 PUFA in subjects with metabolic syndrome decreased insulin resistance and improved insulin signalling in subcutaneous white adipose tissue [46]. Improved insulin sensitivity could be related to a reduction of post oral glucose load lipaemia or to anti-inflammatory effects of n-3 PUFA [47], [48]. We did not observe statistically significant changes in first phase insulin secretion (IGI index) or in empirical index of insulin sensitivity during OGTT [31].
In our study the combination of 1.8 g/d of n-3 PUFA and calorie restriction, but not calorie restriction itself, resulted in a decrease in GIP concentration in the fasting state and during oral glucose load in obese patients. Decreased GIP could contribute to the observed decrease of β-cell function index measured by post oral glucose load insulin secretion (AUCINS/AUCGLUCOSE). Several studies report that β-cell secretion is sensitive to the degree of unsaturation of fatty acids [49]. We observed that changes in serum GIP levels were inversely correlated with changes in erythrocyte EPA and that fasting GIP levels were positively correlated with insulin resistance index (HOMA-IR). These observations suggest that n-3 PUFA treatment improves insulin sensitivity by lowering GIP level. As a consequence of lower GIP signalling, oxidation of fat is induced, which leads to clearing triglycerides from liver and muscles, suppression of hepatic glucose output and improvement in insulin sensitivity. Studies in animal models are consistent with this. Flachs et al. suggested that in mice fed with a high fat diet, supplementation with DHA + EPA (30 g/kg of diet) could normalize elevated GIP levels and contribute to an increase in whole body insulin sensitivity [50]. It was also reported that reduction of GIP secretion, by deletion of the GIP gene in mice, reduced insulin secretion and lessened the degree of insulin resistance with high fat feeding without changing glucose levels during OGTT [51].
Several studies report that plasma GIP is elevated in obesity and metabolic syndrome [52], [53] and in subjects with impaired glucose tolerance [54], [55], [56]. Interestingly, it was also reported that GIP/GIP receptor signalling was disrupted in type-2 diabetes and this could contribute to elevated GIP secretion. Hyperglycemia per se led to down-regulation of GIP receptor expression. As a consequence, insulin response to GIP was markedly reduced in islets, which resulted in the deterioration of β-cell function found in diabetes. Therefore, normalization of GIP/GIP receptor signalling has been suggested as a potential target in the treatment of obesity-associated type 2 diabetes [57]. Results of this study also argue for the suggestion that an inhibition of GIP signalling is beneficial in insulin resistance. In diabetic patients with β-cell dysfunction as a result of lipo- and glucotoxicity, normalizing of GIP/GIP receptor signalling could improve β-cell function and thus glucose homeostasis.
In vitro studies demonstrated that GIP influences lipid metabolism in adipocytes from rodents [58] and humans [59] modifying lipogenesis [60] and lipolysis [61], [62], [63]. In turn, studies in vivo showed an association between plasma fasting GIP and triglycerides' level (fasting and post oral glucose load) [64]. In this study we have also observed that after intervention with calorie restriction, higher fasting GIP levels were related to higher fasting and post oral glucose load triglycerides' levels.
5. Conclusions
The current study suggests that providing n-3 PUFA to obese subjects undergoing caloric restriction results in beneficial effects on insulin sensitivity, which may be related to a decrease of plasma GIP levels. These findings require confirmation in a larger trial.
Transparency Document
Transparency document.
Acknowledgements
This research was funded by the European Commission through its Seventh Framework Programme “BIOmarkers of Robustness of Metabolic Homeostasis for Nutrigenomics-derived Health CLAIMS Made on Food” project (BIOCLAIMS, Grant agreement no. 244995) and Narodowe Centrum Nauki (PL) (grant number DEC-2011/02/A/NZ2/00022). The authors would like to thank Dr. Morten Bryhn MD PhD for supplying capsules as well as Assoc. Prof. Bogdan Solnica MD PhD for critical revision of the manuscript.
Footnotes
This trial was registered at isrctn.com as ISRCTN11445521.
The Transparency document associated with this article can be found, in online version.
References
- 1.Swinburn B.A., Sacks G., Hall K.D., McPherson K., Finegood D.T., Moodie M.L. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 2.Eckel R.H., York D.A., Rossner S., Hubbard V., Caterson I., St Jeor S.T. American heart association. Prevention conference VII: obesity, a worldwide epidemic related to heart disease and stroke: executive summary. Circulation. 2004;110:2968–2975. doi: 10.1161/01.CIR.0000140086.88453.9A. [DOI] [PubMed] [Google Scholar]
- 3.Poirier P., Giles T.D., Bray G.A., Stern J.S., Pi-Sunyer F.X., Robert H., Eckel R.H. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 4.Cnop M., Vidal J., Hull R.L., Utzschneider K.M., Carr D.B., Schraw T. Progressive loss of beta-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care. 2007;30(3):677–682. doi: 10.2337/dc06-1834. [DOI] [PubMed] [Google Scholar]
- 5.Yabe D., Seino Y. Two incretin hormones GLP-1 and GIP: comparison of their actions in insulin secretion and β cell preservation. Prog. Biophys. Mol. Biol. 2011;107(2):248–256. doi: 10.1016/j.pbiomolbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Karhunen L.J., Juvonen K.R., Huotari A., Purhonen A.K., Herzig K.H. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul. Pept. 2008;149:70–78. doi: 10.1016/j.regpep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Drucker D.J. Glucagon-like peptide-1 and the islet beta-cell: augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144:5145–5148. doi: 10.1210/en.2003-1147. [DOI] [PubMed] [Google Scholar]
- 8.McIntosh C.H., Widenmaier S., Kim S.J. Glucose-dependent insulinotropic polypeptide [gastric inhibitory polypeptide; GIP) Vitam. Horm. 2009;80:409–471. doi: 10.1016/S0083-6729(08)00615-8. [DOI] [PubMed] [Google Scholar]
- 9.Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinberg H.O., Chaker H., Leaming R., Johnson A., Brechtel G., Baron A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Invest. 1996;97(11):2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorsdottir I., Tomasson H., Gunnarsdottir I., Gisladottir E., Kiely M., Parra M.D. Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. Int. J. Obes.(Lond.) 2007;31(10):1560–1566. doi: 10.1038/sj.ijo.0803643. [DOI] [PubMed] [Google Scholar]
- 12.Kidd P.M. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural functional synergies with cell membrane phospholipids. Altern. Med. Rev. 2007;12(3):207–227. [PubMed] [Google Scholar]
- 13.Marik P.E., Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic Review. Clin. Cardiol. 2009;32(7):365–372. doi: 10.1002/clc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui R.A., Harvey K.A., Zaloga G.P. Modulation of enzymatic activities by n − 3 polyunsaturated fatty acids to support cardiovascular health. J. Nutr. Biochem. 2008;19(7):417–437. doi: 10.1016/j.jnutbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Calder P.C. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 2009;91:791–795. doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Calder P.C. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot. Essent. Fat. Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Jump D.B. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 2002;277:8755–8758. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- 18.Adkins Y., Kelley D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010;21:781–792. doi: 10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Flachs P., Rossmeisl M., Bryhn M., Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin. Sci. 2009;116:1–16. doi: 10.1042/CS20070456. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D., Lemaitre R.N., King I.B., Song X., Huang H., Sacks F.M. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann. Intern. Med. 2013;158:515–525. doi: 10.7326/0003-4819-158-7-201304020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lombardo Y.B., Chicco A.G. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J. Nutr. Biochem. 2006;17:1–13. doi: 10.1016/j.jnutbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Peyron-Caso E., Fluteau-Nadler S., Kabir M., Guerre-Millo M., Quignard-Boulange A., Slama G. Regulation of glucose transport and transporter 4 [GLUT − 4) in muscle and adipocytes of sucrose-fed rats: effects of n-3 poly- and monounsaturated fatty acids. Horm. Metab. Res. 2002;34:360–366. doi: 10.1055/s-2002-33467. [DOI] [PubMed] [Google Scholar]
- 23.Bjerregaard P., Pedersen H.S., Mulvad G. The associations of a marine diet with plasma lipids, blood glucose, blood pressure and obesity among the Inuit in Greenland. Eur. J. Clin. Nutr. 2000;54:732–737. doi: 10.1038/sj.ejcn.1601088. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T., Azuma A., Kuribayashi T., Sugihara H., Okuda S., Nakagawa M. Serum fatty acid levels, dietary style and coronary heart disease in three neighbouring areas in Japan: the kumihama study. Br. J. Nutr. 2003;89:267–272. doi: 10.1079/BJN2002747. [DOI] [PubMed] [Google Scholar]
- 25.Krebs J.D., Browning L.M., McLean N.K., Rothwell J.L., Mishra G.D., Moore C.S. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int. J. Obes. 2006;30:1535–1544. doi: 10.1038/sj.ijo.0803309. [DOI] [PubMed] [Google Scholar]
- 26.Glauber H., Wallace P., Griver K., Brechtel G. Adverse metabolic effect of omega-3 fatty acids in noninsulin-dependent diabetes mellitus. Ann. Intern. Med. 1988;108:663–668. doi: 10.7326/0003-4819-108-5-663. [DOI] [PubMed] [Google Scholar]
- 27.Bandarra N.M., Palma P., Batista I., Kiely M., Thorsdottir I. Effect of a supplemented diet with canned sardine on the lipid fraction of human plasma and erythrocytes. J. Aquat. Food Prod. Technol. 2002;11:177–185. [Google Scholar]
- 28.Allison D.B., Paultre F., Maggio C., Mezzitis N., Pi-Sunyer F.X. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 29.Abdul-Ghani M.A., Tripathy D., DeFronzo R.A. Contributions of –cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–1139. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 30.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Mari A., Pacini G., Murphy E., Ludvik B., Nolan J.J. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24:539–548. doi: 10.2337/diacare.24.3.539. [DOI] [PubMed] [Google Scholar]
- 32.Bôas Huguenin G.V., Kimi Uehara S., Nogueira Netto J.F., Gaspar de Moura E., Rosa G., da Fonseca Passos M.C. Short term lowcalorie diet improves insulin sensitivity and metabolic parameters in obese women. Nutr. Hosp. 2014;30(1):53–59. doi: 10.3305/nh.2014.30.1.7464. [DOI] [PubMed] [Google Scholar]
- 33.Thies F., Garry J.M., Yaqoob P., Rerkasem K., Williams J., Shearman C.P. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 34.Vecka M., Dušejovská M., Stankova B., Zeman M., Vavrova L., Kodydkova J. N-3 polyunsaturated fatty acids in the treatment of atherogenic dyslipidemia. Neuro. Endocrinol. Lett. 2012;33:87–92. [PubMed] [Google Scholar]
- 35.Munro I.A., Garg M.L. Dietary supplementation with n-3 PUFA does not promote weight loss when combined with a very-low-energy diet. Br. J. Nutr. 2012;108(8):1466–1474. doi: 10.1017/S0007114511006817. [DOI] [PubMed] [Google Scholar]
- 36.Shearer G.C., Savinova O.V., Harris W.S. Fish oil – how does it reduce plasma Triglycerides? Biochim. Biophys. Acta. 1821;2012:843–851. doi: 10.1016/j.bbalip.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuchardt J.P., Neubronner J., Block R.C., von Schacky C., Hahn A. Associations between omega-3 index increase and triacylglyceride decrease in subjects with hypertriglyceridemia in response to six month of EPA and DHA supplementation. Prostaglandins Leukot. Essent. Fat. Acids. 2014;91(4):129–134. doi: 10.1016/j.plefa.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Feskens E.J., Bowles C.H., Kromhout D. Inverse association between fish intake and risk of glucose intolerance in normoglycemic elderly men and women. Diabetes Care. 1991;14:935–941. doi: 10.2337/diacare.14.11.935. [DOI] [PubMed] [Google Scholar]
- 39.Patel P.S., Forouhi N.G., Kuijsten A., Schulze M.B., Van Woudenbergh G.J., Ardanaz E. The prospective association between total and type of fish intake and type 2 diabetes in 8 European countries: EPIC-InterAct study. Am. J. Clin. Nutr. 2012;95:1445–1453. doi: 10.3945/ajcn.111.029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M., Picard-Deland E., Marette A. Fish and marine omega-3 polyunsatured fatty acid consumption and incidence of type 2 diabetes: a systematic review and meta-analysis. Int. J. Endocrinol. 2013 Sep 8;2013:501015. doi: 10.1155/2013/501015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virtanen J.K., Mursu J., Voutilainen S., Uusitupa M., Tuomainen T.P. Serum omega-3 polyunsaturated fatty acids and risk of incident type 2 diabetes in men: the Kuopio ischaemic heart disease risk factor study. Diabetes Care. 2014;37:189–1896. doi: 10.2337/dc13-1504. [DOI] [PubMed] [Google Scholar]
- 42.Schectman G., Kaul S., Kissebah A.H. Effect of fish oil concentrate on lipoprotein composition in NIDDM. Diabetes. 1988;37:1567–1573. doi: 10.2337/diab.37.11.1567. [DOI] [PubMed] [Google Scholar]
- 43.Zambon S., Friday K.E., Childs M.T., Fujimoto W.Y., Bierman E.L., Ensinck J.W. Effect of glyburide and v3 fatty acid dietary supplements on glucose and lipid metabolism in patients with noninsulin- dependent diabetes mellitus. Am. J. Clin. Nutr. 1992;56:447–454. doi: 10.1093/ajcn/56.2.447. [DOI] [PubMed] [Google Scholar]
- 44.Tripathy D., Almgren P., Tuomi T., Groop L. Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care. 2004;27(9):2204–2210. doi: 10.2337/diacare.27.9.2204. [DOI] [PubMed] [Google Scholar]
- 45.Stubbs R.S., Wickremesekera S.K. Insulin resistance in the severely obese and links with metabolic co-morbidities. Obes. Surg. 2002;12(3):343–348. doi: 10.1381/096089202321088110. [DOI] [PubMed] [Google Scholar]
- 46.Jimenez-Gomez Y., Cruz-Teno C., Rangel-Zuñiga O.A., Peinado J.R., Perez-Martinez P., Delgado-Lista J. Effect of dietary fat modification on subcutaneous white adipose tissue insulin sensitivity in patients with metabolic syndrome. Mol. Nutr. Food Res. 2014;58(11):2177–2188. doi: 10.1002/mnfr.201300901. [DOI] [PubMed] [Google Scholar]
- 47.Calder P.C., Yaqoob P. Omega-3 [n-3) fatty acids, cardiovascular disease and stability of atherosclerotic plaques. Cell. Mol. Biol. [Noisy-le-grand) 2010;56:28–37. [PubMed] [Google Scholar]
- 48.Itariu B.K., Zeyda M., Hochbrugger E.E., Neuhofer A., Prager G., Schindler K. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. Am. J. Clin. Nutr. 2012;96:1137–1149. doi: 10.3945/ajcn.112.037432. [DOI] [PubMed] [Google Scholar]
- 49.Maedler K., Oberholzer J., Bucher P., Spinas G.A., Donath M.Y. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 50.Flachs P., Rossmeisl M., Kopecky J. The effect of n-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiol. Res. 2014;63:93–118. doi: 10.33549/physiolres.932715. [DOI] [PubMed] [Google Scholar]
- 51.Nasteska D., Harada N., Suzuki K., Yamane S., Hamasaki A., Joo E. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes. 2014;63(7):2332–2343. doi: 10.2337/db13-1563. [DOI] [PubMed] [Google Scholar]
- 52.Flatt P.R., Bailey C.J., Kwasowski P., Page T., Marks V. Plasma immunoreactive gastric inhibitory polypeptide in obese hyperglycaemic [ob/ob) mice. J. Endourol. 1984;101:249–256. doi: 10.1677/joe.0.1010249. [DOI] [PubMed] [Google Scholar]
- 53.Calanna S., Urbano F., Piro S., Laferrère B., Gluud L.L., Vilsbøll T. Elevated plasma glucose-dependent insulinotropic polypeptide associates with hyperinsulinemia in metabolic syndrome. Eur. J. Endocrinol. 2012;166(5):917–922. doi: 10.1530/EJE-11-0765. [DOI] [PubMed] [Google Scholar]
- 54.Vilsboll T., Krarup T., Sonne J., Madsbad S., Volund A., Juul A.G. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003;88:2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 55.Vollmer K., Holst J.J., Baller B., Ellrichmann M., Nauck M.A., Schmidt W.E. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 56.Chia C.W., Odetunde J.O., Kim W., Carlson O.D., Ferrucci L., Egan J.M. GIP contributes to islet trihormonal abnormalities in type 2 diabetes. J. Clin. Endocrinol. Metab. 2014;99(7):2477–2485. doi: 10.1210/jc.2013-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ceperuelo-Mallafré V., Duran X., Pachón G., Roche K., Garrido-Sánchez L., Vilarrasa N. Disruption of GIP/GIPR axis in human adipose tissue is linked to obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2014;99(5):908–919. doi: 10.1210/jc.2013-3350. [DOI] [PubMed] [Google Scholar]
- 58.Yip R.G., Boylan M.O., Kieffer T.J., Wolfe M.M. Functional GIP receptors are present on adipocytes. Endocrinology. 1998;139:4004–4007. doi: 10.1210/endo.139.9.6288. [DOI] [PubMed] [Google Scholar]
- 59.Weaver R.E., Donnelly D., Wabitsch M., Grant P.J., Balmforth A.J. Functional expression of glucose-dependent insulinotropic polypeptide receptors is coupled to differentiation in a human adipocyte model. Int. J. Obes. [Lond) 2008;(32):1705–1711. doi: 10.1038/ijo.2008.148. [DOI] [PubMed] [Google Scholar]
- 60.Kim S.J., Nian C., McIntosh C.H. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. J. Biol. Chem. 2007;282:8557–8567. doi: 10.1074/jbc.M609088200. [DOI] [PubMed] [Google Scholar]
- 61.Getty-Kaushik L., Song D.H., Boylan M.O., Corkey B.E., Wolfe M.M. Glucose-Dependent Insulinotropic Polypeptide Modulates Adipocyte Lipolysis and Reesterification. Obesity (Silver Spring) 2006;14:1124–1131. doi: 10.1038/oby.2006.129. [DOI] [PubMed] [Google Scholar]
- 62.Hauner H., Glatting G., Kaminska D., Pfeiffer E.F. Effects of gastric inhibitory polypeptide on glucose and lipid metabolism of isolated rat adipocytes. Ann. Nutr. Metab. 1988;32:282–288. doi: 10.1159/000177467. [DOI] [PubMed] [Google Scholar]
- 63.McIntosh C.H., Bremsak I., Lynn F.C. Glucose-dependent insulinotropic polypeptide stimulation of lipolysis in differentiated 3 T3-L1 cells: wortmannin-sensitive inhibition by insulin. Endocrinology. 1999;140:398–404. doi: 10.1210/endo.140.1.6464. [DOI] [PubMed] [Google Scholar]
- 64.Alssema M., Rijkelijkhuizen J.M., Holst J.J., Teerlink T., Scheffer P.G., Eekhoff E.M. Preserved GLP-1 and exaggerated GIP secretion in type 2 diabetes and relationships with triglycerides and ALT. Eur. J. Endocrinol. 2013;169(4):421–430. doi: 10.1530/EJE-13-0487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.