Abstract
Background/Objectives
Inconsistent data from randomized trials suggest cholinesterase inhibitors may cause weight loss. We sought to determine if the initiation of cholinesterase inhibitors is associated with significant weight loss in a real-word clinical setting.
Design
Retrospective cohort study from 2007-2010, comparing weight loss in patients with dementia newly prescribed cholinesterase inhibitors and patients newly prescribed other chronic medications
Setting
National Veterans Affairs (VA) data
Participants
Patients 65 years or older with a diagnosis of dementia who received a new prescription for a cholinesterase inhibitor or other new other chronic medication.
Measurements
The primary outcome was time to 10 pound weight loss over 12 months. We used propensity score matching patients to control for the likelihood of receiving a cholinesterase inhibitor based on baseline characteristics. Data were analyzed in a priori defined subgroups by age, comorbid burden, and initial weight.
Results
Of 6,504 patients that met study criteria, 1188 patients started on cholinesterase inhibitors were matched to 2189 patients started on other medications. The propensity-matched cohorts were well balanced on baseline covariates. Patients initiated on cholinesterase inhibitors had a higher risk of weight loss compared to matched controls at 12 months, HR 1.23 (95% CI 1.07 - 1.41). At twelve months, 29.3% of patients on cholinesterase inhibitors had experienced weight loss compared to 22.8% of non-users, corresponding to a number needed to harm of 21.2 (95% CI 12.5 – 71.4) over one year. There were no significant differences across subgroups.
Conclusion
Patients with dementia started on cholinesterase inhibitors had a higher risk of clinically significant weight loss over a 12-month period compared to matched controls. These results are consistent with the available data from randomized controlled trials. Clinicians should consider the risk of weight loss when prescribing cholinesterase inhibitors.
Keywords: cholinesterase inhibitors, dementia, weight loss
Introduction
Cholinesterase inhibitors are thought to provide a modest improvement in cognition in patients with dementia1,2 and are the most frequently recommended pharmacologic treatment for this condition.3,4 However, patients frequently experience adverse effects such as gastrointestinal (GI) symptoms, as well as bradycardia.
Weight loss and anorexia are reported adverse effects of cholinesterase inhibitors that may be under-recognized. This is an important issue because involuntary weight loss has been associated with increased mortality in older adults,5,6 and patients with dementia may represent an additionally vulnerable population. However, much is unknown about cholinesterase inhibitors and their effect on weight. There is inconsistent evidence from clinical trials that weight loss can occur in some patients started on cholinesterase inhibitors.7,8 However, the available data provide highly variable information on the degree of weight loss experienced, and many studies were underpowered to provide meaningful data about this outcome. In addition, the limited weight loss data from clinical trials may not generalize well to clinical practice, since the spectrum of patients typically treated with cholinesterase inhibitors is older and has more comorbidities than those in clinical trials.9 These vulnerabilities may place patients in clinical practice at higher risk of weight loss than the selected populations studied in clinical trials.
Despite the importance of this potential adverse effect, we are unaware of studies that have attempted to evaluate the impact of cholinesterase inhibitors on weight in real-world populations. As a result, we used national data from the Veterans Affairs healthcare system to determine if starting cholinesterase inhibitors is associated with clinically significant weight loss in older adults with dementia in a real-world setting, and to identify subgroups that may be at higher risk for weight loss.
Methods
Subjects
Using data from Fiscal Year (FY) 2007-2010, we studied a cohort of patients in the Veterans Affairs healthcare system age 65 years or older with a diagnosis of dementia who were not receiving a cholinesterase inhibitor during a one-year baseline period. Employing a new-user design, we identified patients who subsequently received a new prescription for a cholinesterase inhibitor over the following year, and a control group of patients who received a prescription for different new chronic medication over the same time period.
To maximize completeness of medication prescribing data, we excluded patients who received less than 80% of primary care or specialty visits at VA medical centers or were enrolled in Medicare managed care programs during the study period, as those patients may have received cholinesterase inhibitors from other sources that we would not capture.
We applied several weight-based exclusion criteria to focus on patients who had relatively stable weight at baseline and did not have comorbid conditions associated with large fluctuations in weight. We excluded patients with congestive heart failure or cancer (other than prostate or basal-cell skin cancer), patients with more than a 10-pound weight change in the year prior, and patients with insufficient weight data during the study period.
Among eligible patients, we identified patients who were newly prescribed a cholinesterase inhibitor (donepezil, galantamine, rivastigmine, or tacrine) through a VA outpatient pharmacy during FY 2008 – 2009. We also identified a separate control group of patients not prescribed cholinesterase inhibitors, but who were started on other new chronic medications (which we defined as medications prescribed with a >=25 days supply) during that time period. If a patient was started on more than one new chronic medication, we randomly selected one as the control medication for this study. An index date for each patient was defined as the day the new medication (cholinesterase inhibitor or other chronic medication) was first dispensed.
Measures
We used ICD-9 codes to define patients diagnosed with dementia or mild cognitive impairment using established methods10,11 that included patients who had at least one inpatient or two outpatient encounter ICD-9 codes for dementia (290-290.9, 294-294.9, 331-331.9, 291.1, 291.2, 292.82, 292.83, 438.0, or 780.93). These include ICD-9 codes for Alzheimer's, vascular, and Lewy body dementia, and exclude codes for dementia due to alcohol and drug-induced dementia, and general senility or frailty. In validation studies, the sensitivity of using similar ICD-9 codes to identify dementia ranges from 30-87%, and specificity from 84–100%.10
We obtained baseline demographics including age and race from national VA and Medicare databases. We defined the presence of each of 22 comorbid conditions by the presence of 2 or more outpatient encounter codes or 1 or more inpatient discharge diagnosis, following previously described methods.12 We measured health services utilization by examining the number of primary and specialty clinic visits by each patient to the VA in the year before the index date for new drug. We used both VA and Medicare date to determine whether patients were hospitalized in the baseline year.
Weight data in VA are collected during clinic visits and entered manually into the electronic medical record. To reduce impact of erroneous readings or data entry errors, we eliminated biologically implausible values of <70 pounds or >500 pounds.13 To further exclude clearly erroneous weights from the data, in the one-year baseline period we excluded weight measures for each patient that were more than 20% different from their median weight in the baseline period. Similarly, in the follow-up period weight measures more then 20% different from the starting weight were excluded, to avoid classifying patients to as having weight loss based on a single aberrant weight measure.
We then defined weights during three periods. First, we established the weight one year prior to drug initiation by determining the mean of weights recorded between 245-485 days before the index date. Then, we established the weight at the index date (the “index weight”) by calculating the mean of recorded weights for each patient between 30 days before to 5 days after the index date. We used this date range because patients did not always have a weight on their exact index date. We considered the difference between the one-year-prior weight and the index weight to be the weight trajectory for each patient during the baseline period. Finally, we evaluated weights over the year following initiation of the cholinesterase inhibitor or control drug. We defined 10-pound weight loss as the first recorded weight in the follow-up period that was >=10 pounds lower than the weight at the time the drug was started.
Our primary outcome was the time to 10-pound weight loss during the first year after initiating a cholinesterase inhibitor, or in the control group, after starting a different chronic medication. We chose 10 pound weight loss as our primary outcome, as this represents a degree of weight loss that would be noticed by a clinician and may prompt further action in in considering causes and work-up of weight loss.
Analysis
Propensity score matching was used to match new users of cholinesterase inhibitors with new users of other chronic medications who had similar baseline characteristics, using a propensity score model with 3:1 nearest neighbors matching. The propensity score model included demographic factors (age, sex, race); baseline degree of comorbid burden and medication use (presence or absence of 22 common conditions in older adults,12 number of chronic medications, use of memantine); weight status (baseline weight, weight trajectory over prior year); and intensity of medical care (frequency of weight measurements, number of primary care and specialty visits, and hospitalizations over the baseline year).
We used Cox-proportional hazards models to compare the risk of weight loss between cholinesterase inhibitor and matched controls. To determine if there were clinically important subgroups in which cholinesterase inhibitors were associated with greater risk of weight loss, we evaluated treatment-by-subgroup interaction terms for a priori-defined subgroups by age, comorbid burden, and initial weight. Number needed to harm was calculated using survival probabilities, which does not assume a constant hazard ratio over time.
A secondary outcome was the mean change in weight over one year. We used mixed-effects linear regression with random slopes and intercepts to estimate a one-year weight trajectory for each patient. We compared the predicted weight trajectory in the two groups using Student's t-test.
We did several sensitivity analyses. First, we examined the outcome of time to 5% weight loss instead of 10-pound weight loss. Second, we used regression analysis incorporating the propensity score to evaluate weight loss instead of matching using the propensity score. Third, we evaluated weight loss in an on-treatment analysis, censoring patients from further weight loss assessments 30 days after they stopped taking the medication. In the on-treatment analysis, in addition to matching on propensity score we also matched new users of cholinesterase inhibitors to control patients by the length of time they continued filling prescriptions for the new medication. Fourth, we used Cox regression to compare the risk of weight loss among donepezil and galantamine, the two most commonly used cholinesterase inhibitors in our sample, controlling for the same types of variables used in the propensity score.
STATA version 12 was used for analysis. The Committee on Human Research at the University of California, San Francisco, and the Research Committee at the San Francisco VA Medical Center approved this research.
Results
There were 6,504 patients who met all of the inclusion and exclusion criteria. Initially, 50,330 patients were identified as 65 years or older with a diagnosis of dementia and a new user of a cholinesterase inhibitor or other chronic medication. Of those patients, 13,651 had a diagnosis code for heart failure or cancer within the last year and were excluded. An additional 21,606 patients were excluded for having had <80% primary care or specialty visits at VA medical centers during the study period. There were 8,569 patients excluded based on specific weight-measure exclusion criteria; most of these patients (6,389) had incomplete weight data, meaning they did not have at least one weight measure in each of three time periods of the study: at time of starting the new medication, during the year prior, and during follow-up period.
Of the 6,504 patients who met the inclusion and exclusion criteria, 1,190 were new users of cholinesterase inhibitors and 5,314 were new users of other chronic medications. Patients prescribed cholinesterase inhibitors were generally older, took fewer medications at baseline, and had a lower baseline weight (Table 1).
Table 1. Characteristics Of Participants (Unmatched Cohort), N = 6,504.
| Characteristic | Cholinesterase Inhibitor New-User N = 1,190 | Non-Cholinesterase New-User N = 5314 | p-value |
|---|---|---|---|
| Age, mean (± SD) | 77.9 (6.3) | 76.6 (6.8) | <0.001 |
| Male, n (%) | 1161 (97.6) | 5148 (96.9) | 0.21 |
| Race, n (%) | |||
| White, non Hispanic | 883 (74.2) | 4108 (77.3) | |
| Black, non Hispanic | 197 (16.6) | 890 (16.7) | |
| Hispanic | 66 (5.6) | 147 (2.8) | <0.001 |
| Asian | 14 (1.2) | 33 (0.6) | |
| Other | 30 (2.5) | 136 (2.6) | |
| Number of Co-Morbid Conditions, mean (± SD) | 3.1 (2.1) | 3.2 (2.1) | 0.20 |
| Cerebrovascular Accident, n (%) | 135 (11.3) | 812 (15.3) | <0.001 |
| COPD/Asthma, n (%) | 159 (13.4) | 780 (14.7) | 0.24 |
| Hypertension, n (%) | 826 (69.4) | 3428 (64.5) | 0.001 |
| Hyperlipidemia, n (%) | 507 (42.6) | 2247 (42.3) | 0.84 |
| Diabetes Mellitus, n (%) | 341 (28.7) | 1734 (32.6) | 0.008 |
| Gastroesophageal Reflux Disease, n (%) | 144 (12.1) | 776 (14.6) | 0.03 |
| Depression, n (%) | 209 (17.6) | 991 (18.6) | 0.38 |
| Atrial Fibrillation, n (%) | 119 (10.0) | 465 (8.8) | 0.17 |
| Number of chronic medications, mean (± SD) | 9.7 (5.7) | 10.1 (5.7) | 0.02 |
| Prescribed Memantine, n (%) | 46 (3.9) | 120 (2.3) | 0.001 |
| Hospitalized in past year, n (%) | 336 (28.2) | 1556 (29.3) | 0.47 |
| Number of outpatient visits (primary care/specialty) in baseline year, median [IQR] | 6 (4, 9) | 6 (3, 9) | 0.03 |
| Baseline weight in pounds, mean (± SD) | 174.8 (32.8) | 181.5 (36.6) | <0.001 |
| Frequency of Weight Measures in year prior, n (%) | |||
| 2-5 | 400 (33.6) | 1766 (33.2) | |
| 6-10 | 499 (41.9) | 2111 (39.7) | 0.16 |
| ≥ 11 | 291 (24.5) | 1437 (27.0) | |
| Weight Trajectory in Year Prior in pounds, mean (± SD) | 0.9 (4.8) | 0.4 (5.0) | 0.002 |
Propensity score matching of new users vs. non-users yielded 1,188 new users of cholinesterase inhibitors who were matched with 2,189 new users of other chronic medications. After propensity score matching, the two groups were well balanced on all baseline covariates (Table 2). The mean age was 78 years, 98% were male, and mean weight at baseline was 175-177 pounds. The number of weight measures in the follow-up period was slightly higher in new users of cholinesterase inhibitors, with a median [interquartile range] of 6 [IQR 4-9] weight measures in the cholinesterase inhibitor group and 5 [IQR 3-8] weight measures in the control group.
Table 2. Characteristics Of Propensity Score Matched Cohort, All Matched Subjects, N = 3,377.
| Characteristic | Cholinesterase Inhibitor New User N = 1,188 | Non-Cholinesterase New User N = 2,189 | p-value |
|---|---|---|---|
| Age, mean (± SD) | 77.9 (6.2) | 77.7 (6.8) | 0.38 |
| Male, n (%) | 1159 (97.6) | 2136 (97.6) | 0.97 |
| Race, n (%) | |||
| White, non Hispanic | 883 (74.3) | 1663 (76.0) | |
| Black, non Hispanic | 197 (16.6) | 374 (17.1) | |
| Hispanic | 64 (5.4) | 76 (3.5) | 0.09 |
| Asian | 14 (1.2) | 19 (0.9) | |
| Other | 30 (2.5) | 57 (2.6) | |
| Number of Comorbid Conditions, mean (± SD) | 3.1 (2.1) | 3.1 (2.1) | 0.86 |
| Cerebrovascular Accident, n (%) | 135 (11.4) | 264 (12.1) | 0.55 |
| COPD/Asthma, n (%) | 158 (13.3) | 317 (14.5) | 0.35 |
| Hypertension, n (%) | 824 (69.4) | 1476 (67.4) | 0.25 |
| Hyperlipidemia, n (%) | 505 (42.5) | 913 (41.7) | 0.65 |
| Diabetes Mellitus, n (%) | 341 (28.7) | 645 (29.5) | 0.64 |
| Gastroesophageal Reflux Disease, n (%) | 144 (12.1) | 288 (13.2) | 0.39 |
| Depression, n (%) | 209 (17.6) | 388 (17.7) | 0.92 |
| Atrial Fibrillation, n (%) | 119 (10.0) | 200 (9.1) | 0.40 |
| Number of chronic medications, mean (± SD) | 9.7 (5.7) | 9.9 (5.4) | 0.40 |
| Prescribed Memantine, n (%) | 46 (3.9) | 68 (3.1) | 0.24 |
| Hospitalized in past year, n (%) | 336 (28.3) | 635 (29.0) | 0.66 |
| Number of outpatient visits in baseline year, median [IQR] | 6 (4, 9) | 6 (3, 9) | 0.06 |
| Baseline weight in pounds, mean (± SD) | 174.9 (32.8) | 176.7 (34.5) | 0.12 |
| Frequency of weight measures in year prior, n (%) | |||
| 2-5 | 398 (33.5) | 793 (36.2) | |
| 6-10 | 499 (42.0) | 841 (38.4) | 0.11 |
| ≥ 11 | 291 (24.5) | 555 (25.4) | |
| Weight trajectory in year prior in pounds, mean (± SD) | 0.9 (4.8) | 0.8 (5.0) | 0.59 |
Of patients who started cholinesterase inhibitors, 58% (n = 694) were prescribed donepezil, 41% (n= 482) galantamine, and 1% (n= 12) rivastigmine. No patients were prescribed tacrine. Of the patients who started other chronic medications, the most common prescriptions were for amlodipine, simvastatin, omeprazole, hydrochlorothiazide, and docusate. At 12 months, 78% of patients started on cholinesterase inhibitors were still receiving the drug. Of patients started on other medications, 66% still had a prescription for that medication at 12 months.
Patients initiated on cholinesterase inhibitors had a higher risk of weight loss over 12 months compared to patients started on other medications, with a hazard ratio of 1.23 (95% CI 1.07 -1.41). At twelve months, 29.3% of patients on cholinesterase inhibitors had experienced >=10lbs weight loss compared to 22.8% of non-users (Figure 1) corresponding to a number needed to harm of 21.2 over one year (95% CI 12.5 – 71.4).
Figure 1. Time to >=10 pound weight loss in new users of cholinesterase inhibitors vs. matched controls.
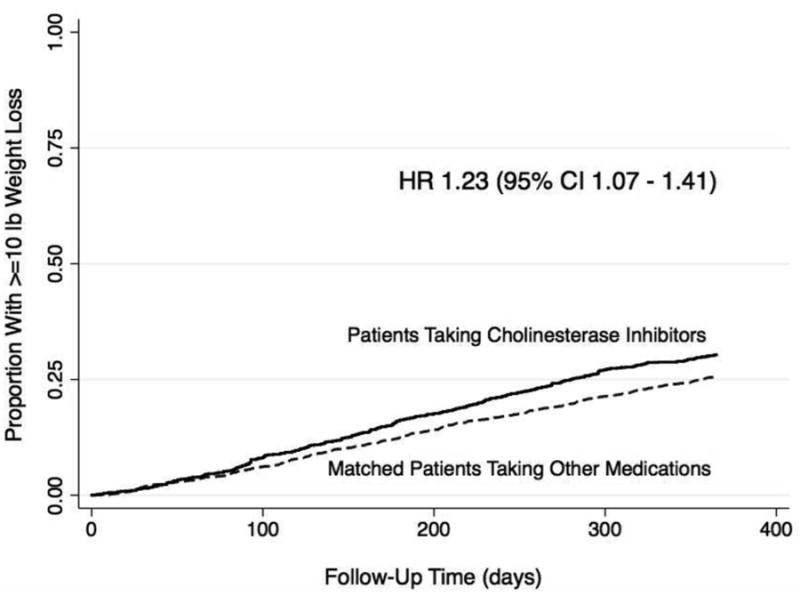
There were no significant differences in the impact of cholinesterase inhibitor use on weight loss across age groups, levels of comorbid burden, or levels of baseline weight (Table 3). There was no statistically significant difference in the risk of weight loss between patients treated with galantamine and those treated with donepezil (HR 1.15 for galantamine vs. donepezil, 95% CI 0.93-1.42).
Table 3. Risk of Weight Loss With Cholinesterase Inhibitors, Stratified By Age, Comorbid Burden, And Initial Weight.
| Subgroup | Number in Group | Hazard Ratio for Weight Loss (95% CI) | P-value for Difference in Hazard Ratio (Interaction Term) |
|---|---|---|---|
| Age | |||
| Age <=77 years | 1,541 | 1.16 (0.93 – 1.43) | 0.46 |
| Age >77 years | 1,836 | 1.28 (1.07 – 1.53) | |
| Comorbid Burden | |||
| 0-3 conditions | 2,035 | 1.33 (1.11 – 1.59) | 0.24 |
| >3 conditions | 1,342 | 1.13 (0.91 – 1.39) | |
| Initial Weight | |||
| Weight <=170 pounds | 1,592 | 1.26 (1.01 – 1.56) | 0.94 |
| Weight >170 pounds | 1,785 | 1.25 (1.04 – 1.49) |
As a different approach to evaluate weight loss, in secondary analyses we assessed the weight trajectory over one year in each group. There was a mean weight loss of 3.1 lbs over one year in patients on cholinesterase inhibitors, compared to a mean weight loss 2.5 lbs over one year in non-users (p=0.02).
In sensitivity analyses, we examined the outcome of time to 5% weight loss. We found a similar increased risk of weight loss in new users of cholinesterase inhibitors compared to matched controls (HR 1.22, 95% CI 1.08 - 1.38). We also used regression analysis incorporating the propensity score to evaluate weight loss instead of matching using the propensity score. This analysis of all 6,504 patients yielded similar results for risk of weight loss in new users of cholinesterase inhibitors compared to unmatched controls (HR 1.32, 95% CI 1.16 – 1.50).
To control for possible ascertainment bias, we did additional sensitivity analysis controlling for the number of weight measures in follow-up by adding this value to the Cox proportional hazards model. Adjusting for the number weight measures, there remained an increased risk of weight loss in new users of cholinesterase inhibitors compared to matched controls HR 1.20 (95% CI 1.04 – 1.38).
In an on-treatment analysis, outcomes were similar with a persistently higher risk of weight loss in patients started on cholinesterase inhibitors compared to patients started on other medications (HR 1.28, 95% CI 1.11 - 1.48). Mean weight change in the on-treatment analysis was similar to the main analysis, with a mean of 3.2 lbs weight loss in the cholinesterase inhibitor group compared to mean of 2.8 lbs in the control group.
Discussion
In this study of patients with dementia in the VA healthcare system, starting a cholinesterase inhibitor was associated with a 24% increased risk of developing weight loss (HR 1.23, 95% CI 1.07-1.41), corresponding to a number needed to harm of 21 over one year. We did not find significant differences in the impact of cholinesterase inhibitor use on weight loss across subgroups of age, comorbid burden, or baseline weight.
Unintentional weight loss in older adults is associated with many adverse outcomes, including increased rates of institutionalization and mortality, a decline in functional status, and poorer quality of life.6,14-16 Our study provides evidence in a large, real-world population that cholinesterase inhibitors may contribute to clinically significant weight loss in a substantial proportion of older adults with dementia. This is consistent with prior studies, almost all randomized controlled trials, that have suggested weight loss can be an adverse event in patients treated with cholinesterase inhibitors. Although concerning, results from these trials have been difficult to interpret for two reasons. First, patients in these trials may not be similar to real-world patients. Patients with dementia included in clinical trials are highly selected, and often younger and with fewer comorbidities than the general population of patients with dementia.9 Second, there is a wide range of methods and results across the trials. For example, among 22 studies of cholinesterase inhibitors covered in a 2004 meta-analysis and 14 additional studies published more recently,1,8,17-30 weight loss was reported in 14/37 trials. Of those that reported weight loss, there was a substantial range in the degree of weight loss between trials, ranging from 0% absolute risk increase to 15% absolute risk increase, with a trend of patients on higher doses experiencing a higher frequency of weight loss. In addition, the definition of weight loss varied; some studies defined weight loss as >=7% change in body weight, >5% change from baseline, and in others the definition of weight loss was unspecified.
Of note, only two studies of cholinesterase inhibitors, both observational, evaluated weight loss as the primary outcome of interest. One study from France of 486 patients treated with cholinesterase inhibitors found that 21% of patients experienced a weight loss of >=4%, but there was no meaningful control group for comparison. Another observational study in the Netherlands found no difference in weight loss between galantamine and donepezil,31 although there was no placebo or control group for comparison. This is consistent with data from meta-analysis revealing a similar side effect profile between donepezil, galantamine, and rivastigmine.8
Cholinesterase inhibitors may cause weight loss through adverse GI tract effects that frequently occur with drug initiation. The common symptoms of diarrhea, nausea, vomiting, and anorexia are likely due the drugs effect of increased cholinergic activity in the GI tract. Increased acetylcholine reaching the muscarinic receptors leads to an increase in smooth muscle contraction, increased peristalsis, and sphincter relaxation.32 However anorexia is also very common with initiation of cholinesterase inhibitors, and it possible that weight loss is mediated through poor oral intake due to anorexia and nausea. While diarrhea and vomiting usually resolve quickly,1 anorexia and nausea may be harder to detect and go unnoticed.
These findings are important for clinical practice. This degree of weight loss, 10 pounds or greater, is clinically significant in this vulnerable patient population and may lead to increased institutionalization and mortality.6,14,16 Morbidity and morality risks associated with weight loss may differ depending on the cause of weight loss, however, because cholinesterase inhibitors are widely used, this adverse effect may have large implications for both individual patients and society. The decision to prescribe a cholinesterase inhibitor is a complex one; physicians must weigh the modest possible benefits with the increased pill burden and possibility of side effects. This study provides more information about the possible harm of weight loss, which clinicians should take into account when prescribing a cholinesterase inhibitor. A trial of a cholinesterase inhibitor may be appropriate for some patients with dementia. However, clinicians need to be vigilant about assessing for weight loss over time. Though a hazard ratio of 1.23 represents a small absolute risk of weight loss, this study provides evidence that a newly started cholinesterase inhibitors should be strongly considered a possible source of weight loss when evaluating unintentional weight loss in older adults.
Our study has some limitations. A measure of cognitive function was not available in national VA data, which may be a concern as patients with severe dementia may experience weight loss due to their underlying disease. While were not able to directly measure dementia severity, we were able to measure and include weight trajectory of the year prior. This may largely address the potential confounding, as dementia induced anorexia from anosmia, decreased access to food, and other causes are likely to be reflected in weight changes prior to starting the drug.
Furthermore, we matched patients on their baseline weight and weight trajectory in the past year, in addition to the intensity of medical care they were receiving and degree of comorbid burden. However, the possibility of unmeasured confounding still exists, and further studies would be helpful to further validate our findings and address limitations we were not able to attend to in this study. Due to restrictions in the VA formulary, only 1% of the new users of cholinesterase inhibitors in our study were prescribed rivastigmine. Because some randomized controlled trials suggest rivastigmine has greater GI side effects,8 the results may have been different had more patients been prescribed rivastigmine in our study. We did not have information about if patients received medications through Medicare Part D from stand-alone prescription drug plans. However, if some patients classified as non-users had received cholinesterase inhibitors through Medicare Part D, this would serve to bias our results in the direction of the null. We were not able to explore dose response and dose duration response relationships with cholinesterase inhibitors in this study. We did not control for other drugs associated with weight loss, such as metformin, topiramate, or methylphenidate, though we would not expect it to influence our propensity-matched results. As the study sample included mainly older male veterans, the generalizability of our findings to women is uncertain. Finally, although we did not find a specific subgroup in which starting cholinesterase inhibitors had a higher risk of weight loss, we may have been underpowered to find these interactions.
Patients with dementia started on cholinesterase inhibitors had a substantially higher risk of clinically significant weight loss over a 12-month period compared to matched controls. Clinicians should take into account the risk of weight loss when weighing the risks and benefits of prescribing cholinesterase inhibitors in patients with dementia. In addition, clinicians should monitor for weight loss if these medications are prescribed, and consider discontinuing cholinesterase inhibitors if significant weight loss occurs.
Acknowledgments
Dr. Sheffrin and Dr. Steinman report grant funding from the National Institute on Aging and the American Federation on Aging Research (T32 AG000212, K23-AG030999, and RC1-AG036377).
We wish to acknowledge Kiya Komaiko, BA of (Northern California Institute for Research and Education) and Kathy Fung, MS (San Francisco VA Medical Center) for their assistance with data collection and cleaning. They were not compensated for their contributions.
Sponsor's Role: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
This work was supported by National Institute on Aging and the American Federation on Aging Research (T32 AG000212-21, K23-AG030999, and RC1-AG036377). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Footnotes
Conflict of Interest: None of the authors report any conflicts of interest or financial relationships to disclose. Please see accompanying COI checklist.
| Elements of Financial/Personal Conflicts | Author 1 – Meera Sheffrin | Author 2 – Yinghui Miao | Author 3- W. John Boscardin | Author 4 – Michael A. Steinman | ||||
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
Author Contributions: Dr. Sheffrin and Dr. Steinman had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of the study: Meera Sheffrin, Michael Steinman
Collection, management, analysis, and interpretation of the data: Meera Sheffrin, Michael Steinman, Yinghui Miao, W. John Boscardin
Review and final approval of the manuscript: Meera Sheffrin, Michael Steinman, Yinghui Miao, W. John Boscardin
References
- 1.Wilkinson D, Doody R, Helme R, Taubman K, Mintzer J, Kertesz A, et al. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology. 2003 Aug 26;61(4):479–486. doi: 10.1212/01.wnl.0000078943.50032.fc. [DOI] [PubMed] [Google Scholar]
- 2.Birks J. Cholinesterase inhibitors for Alzheimer's disease. The Cochrane database of systematic reviews. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doody RS, Stevens JC, Beck C, Dubinsky RM, Kaye JA, Gwyther L, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001 May 8;56(9):1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 4.Qaseem A, Snow V, Cross JT, Jr, Forciea MA, Hopkins R, Jr, Shekelle P, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Annals of internal medicine. 2008 Mar 4;148(5):370–378. doi: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- 5.Wallace JI, Schwartz RS, LaCroix AZ, Uhlmann RF, Pearlman RA. Involuntary weight loss in older outpatients: incidence and clinical significance. Journal of the American Geriatrics Society. 1995 Apr;43(4):329–337. doi: 10.1111/j.1532-5415.1995.tb05803.x. [DOI] [PubMed] [Google Scholar]
- 6.Knudtson MD, Klein BE, Klein R, Shankar A. Associations with weight loss and subsequent mortality risk. Annals of epidemiology. 2005 Aug;15(7):483–491. doi: 10.1016/j.annepidem.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology. 1998 Jan;50(1):136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 8.Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. BMJ. 2005 Aug 6;331(7512):321–327. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoenmaker N, Van Gool WA. The age gap between patients in clinical studies and in the general population: a pitfall for dementia research. Lancet neurology. 2004 Oct;3(10):627–630. doi: 10.1016/S1474-4422(04)00884-1. [DOI] [PubMed] [Google Scholar]
- 10.St Germaine-Smith C, Metcalfe A, Pringsheim T, Roberts JI, Beck CA, Hemmelgarn BR, et al. Recommendations for optimal ICD codes to study neurologic conditions: a systematic review. Neurology. 2012 Sep 4;79(10):1049–1055. doi: 10.1212/WNL.0b013e3182684707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martens PJ, Fransoo R, Burland E, Burchill C, Prior HJ, Ekuma O. Prevalence of mental illness and its impact on the use of home care and nursing homes: a population-based study of older adults in Manitoba. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 2007 Sep;52(9):581–590. doi: 10.1177/070674370705200906. [DOI] [PubMed] [Google Scholar]
- 12.Steinman MA, Lee SJ, John Boscardin W, Miao Y, Fung KZ, Moore KL, et al. Patterns of multimorbidity in elderly veterans. Journal of the American Geriatrics Society. 2012 Oct;60(10):1872–1880. doi: 10.1111/j.1532-5415.2012.04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das SR, Kinsinger LS, Yancy WS, Jr, Wang A, Ciesco E, Burdick M, et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. American journal of preventive medicine. 2005 Apr;28(3):291–294. doi: 10.1016/j.amepre.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Alibhai SM, Greenwood C, Payette H. An approach to the management of unintentional weight loss in elderly people. CMAJ. 2005 Mar 15;172(6):773–780. doi: 10.1503/cmaj.1031527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wedick NM, Barrett-Connor E, Knoke JD, Wingard DL. The relationship between weight loss and all-cause mortality in older men and women with and without diabetes mellitus: the Rancho Bernardo study. Journal of the American Geriatrics Society. 2002 Nov;50(11):1810–1815. doi: 10.1046/j.1532-5415.2002.50509.x. [DOI] [PubMed] [Google Scholar]
- 16.Payette H, Coulombe C, Boutier V, Gray-Donald K. Nutrition risk factors for institutionalization in a free-living functionally dependent elderly population. Journal of clinical epidemiology. 2000 Jun;53(6):579–587. doi: 10.1016/s0895-4356(99)00186-9. [DOI] [PubMed] [Google Scholar]
- 17.Mori E, Ikeda M, Kosaka K. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Annals of neurology. 2012 Jul;72(1):41–52. doi: 10.1002/ana.23557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda M, Mori E, Kosaka K, Iseki E, Hashimoto M, Matsukawa N, et al. Long-term safety and efficacy of donepezil in patients with dementia with Lewy bodies: results from a 52-week, open-label, multicenter extension study. Dementia and geriatric cognitive disorders. 2013;36(3-4):229–241. doi: 10.1159/000351672. [DOI] [PubMed] [Google Scholar]
- 19.Farlow M, Veloso F, Moline M, Yardley J, Brand-Schieber E, Bibbiani F, et al. Safety and tolerability of donepezil 23 mg in moderate to severe Alzheimer's disease. BMC neurology. 2011 May 25;11:57. doi: 10.1186/1471-2377-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois B, Tolosa E, Katzenschlager R, Emre M, Lees AJ, Schumann G, et al. Donepezil in Parkinson's disease dementia: a randomized, double-blind efficacy and safety study. Movement disorders. 2012 Sep 1;27(10):1230–1238. doi: 10.1002/mds.25098. [DOI] [PubMed] [Google Scholar]
- 21.Roman GC, Salloway S, Black SE, Royall DR, Decarli C, Weiner MW, et al. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke; a journal of cerebral circulation. 2010 Jun;41(6):1213–1221. doi: 10.1161/STROKEAHA.109.570077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkinson D, Roman G, Salloway S, Hecker J, Boundy K, Kumar D, et al. The long-term efficacy and tolerability of donepezil in patients with vascular dementia. International journal of geriatric psychiatry. 2010 Mar;25(3):305–313. doi: 10.1002/gps.2340. [DOI] [PubMed] [Google Scholar]
- 23.Black S, Roman GC, Geldmacher DS, Salloway S, Hecker J, Burns A, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke; a journal of cerebral circulation. 2003 Oct;34(10):2323–2330. doi: 10.1161/01.STR.0000091396.95360.E1. [DOI] [PubMed] [Google Scholar]
- 24.Dichgans M, Markus HS, Salloway S, Verkkoniemi A, Moline M, Wang Q, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet neurology. 2008 Apr;7(4):310–318. doi: 10.1016/S1474-4422(08)70046-2. [DOI] [PubMed] [Google Scholar]
- 25.Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet. 2002 Apr 13;359(9314):1283–1290. doi: 10.1016/S0140-6736(02)08267-3. [DOI] [PubMed] [Google Scholar]
- 26.Hager K, Baseman AS, Nye JS, Brashear HR, Han J, Sano M, et al. Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer's disease. Neuropsychiatric disease and treatment. 2014 Feb 21;10:391–401. doi: 10.2147/NDT.S57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auchus AP, Brashear HR, Salloway S, Korczyn AD, De Deyn PP, Gassmann-Mayer C. Galantamine treatment of vascular dementia: a randomized trial. Neurology. 2007 Jul 31;69(5):448–458. doi: 10.1212/01.wnl.0000266625.31615.f6. [DOI] [PubMed] [Google Scholar]
- 28.McKeith I, Del Ser T, Spano P, Emre M, Wesnes K, Anand R, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000 Dec 16;356(9247):2031–2036. doi: 10.1016/S0140-6736(00)03399-7. [DOI] [PubMed] [Google Scholar]
- 29.Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, et al. Rivastigmine for dementia associated with Parkinson's disease. The New England journal of medicine. 2004 Dec 9;351(24):2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- 30.Ballard C, Sauter M, Scheltens P, He Y, Barkhof F, van Straaten EC, et al. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the VantagE study. Current medical research and opinion. 2008 Sep;24(9):2561–2574. doi: 10.1185/03007990802328142. [DOI] [PubMed] [Google Scholar]
- 31.Droogsma E, van Asselt DZ, van Steijn JH, Schuur T, Huinink EJ. Effect of long-term treatment with galantamine on weight of patients with Alzheimer's dementia. The journal of nutrition, health & aging. 2013;17(5):461–465. doi: 10.1007/s12603-012-0420-6. [DOI] [PubMed] [Google Scholar]
- 32.Uchiyama T, Chess-Williams R. Muscarinic receptor subtypes of the bladder and gastrointestinal tract. Journal of smooth muscle research. 2004 Dec;40(6):237–247. doi: 10.1540/jsmr.40.237. [DOI] [PubMed] [Google Scholar]


