Abstract
We investigated the role of N2-fixation by the colony-forming cyanobacterium, Aphanizomenon spp., for the plankton community and N-budget of the N-limited Baltic Sea during summer by using stable isotope tracers combined with novel secondary ion mass spectrometry, conventional mass spectrometry and nutrient analysis. When incubated with 15N2, Aphanizomenon spp. showed a strong 15N-enrichment implying substantial 15N2-fixation. Intriguingly, Aphanizomenon did not assimilate tracers of 15NH4+ from the surrounding water. These findings are in line with model calculations that confirmed a negligible N-source by diffusion-limited NH4+ fluxes to Aphanizomenon colonies at low bulk concentrations (<250 nm) as compared with N2-fixation within colonies. No N2-fixation was detected in autotrophic microorganisms <5 μm, which relied on NH4+ uptake from the surrounding water. Aphanizomenon released about 50% of its newly fixed N2 as NH4+. However, NH4+ did not accumulate in the water but was transferred to heterotrophic and autotrophic microorganisms as well as to diatoms (Chaetoceros sp.) and copepods with a turnover time of ~5 h. We provide direct quantitative evidence that colony-forming Aphanizomenon releases about half of its recently fixed N2 as NH4+, which is transferred to the prokaryotic and eukaryotic plankton forming the basis of the food web in the plankton community. Transfer of newly fixed nitrogen to diatoms and copepods furthermore implies a fast export to shallow sediments via fast-sinking fecal pellets and aggregates. Hence, N2-fixing colony-forming cyanobacteria can have profound impact on ecosystem productivity and biogeochemical processes at shorter time scales (hours to days) than previously thought.
Introduction
Vast regions in the tropical ocean as well as brackish waters (for example, the Baltic Sea) and lakes are (periodically) characterized by low concentrations of dissolved inorganic nitrogen. N2-fixation by cyanobacteria is a major source of new nitrogen and can have an important role sequestrating carbon in these regions and habitats. Extensive blooms of Trichodesmium occur in the tropical ocean, whereas the genera Nodularia and Aphanizomenon bloom in brackish waters, and Aphanizomenon in lakes (Capone, et al., 1997; Ibelings and Maberly, 1998; Larsson et al., 2001). These N2-fixing cyanobacteria form colonies and leak a substantial fraction of newly fixed N2 to the surrounding water (Mulholland and Capone 2001; Ploug et al., 2010, 2011). However, quantitatively little is known about the fate of this newly fixed nitrogen, its role for the microbial and classical food webs and for large-scale biogeochemical fluxes (Mulholland, 2007).
In the Baltic Sea Proper, N2-fixation contributes a yearly N input close to that of the entire riverine load (Wasmund et al., 2001). Here the large N2-fixing cyanobacteria are dominated by Aphanizomenon sp. (sometimes referred to as A. flos-aquae), Nodularia spumigena and, to a lesser extent, Dolichospermum spp. (formerly Anabaena spp.), (Wasmund 1997; Larsson et al., 2001; Wasmund et al., 2001). These filamentous, N2-fixing cyanobacteria make up approximately 20–40% of the C biomass of phytoplankton during summer, while the remaining phytoplankton biomass is represented by a variety of prokaryotic and eukaryotic plankton organisms, for example, diatoms, dinoflagellates and picoplankton (Stal et al., 2003; Hajdu et al., 2007). Experimental studies as well as nitrogen budgets have suggested that N2-fixing cyanobacteria leak a substantial fraction of their fixed nitrogen that is subsequently assimilated by picoplankton in the Baltic Sea (Ohlendieck et al., 2000; Larsson et al., 2001; Stal et al., 2003). Various routes by which diazotrophic nitrogen may enter the pelagic food web have been investigated indirectly through experimental studies and by using the natural abundance (δ15N) signature of N2-fixation in field studies (Wannicke et al., 2013; Woodland et al., 2013; Lesutiene et al., 2014; Karlson et al., 2015). Direct measurements of the routes and overall significance of N release by N2-fixing cyanobacteria and subsequent N uptake within the phytoplankton community are missing, largely owing to technical and methodological limitations. Hence, the role of large, colony-forming N2-fixng cyanobacteria in nutrient cycling, food webs and biogeochemical fluxes has remained unresolved.
Nanoscale secondary ion mass spectrometry (nanoSIMS) is a novel high spatial resolution (50 nm) technique that combines the qualities of a microscope with those of a mass spectrometer. Thus its use in isotope tracer experiments allows simultaneous determination of cell identity and activity in field samples of mixed microbial populations (Kuypers and Jørgensen, 2007; Wagner, 2009; Musat et al., 2012). Using this technique, we recently demonstrated that the volume-specific N2-fixation and NH4+ release were similar in Aphanizomenon sp. and Nodularia spumigena in the Baltic Sea. Furthermore, N2-fixation by both cyanobacteria covered their cellular N-demands, and both species released ~35% of their newly fixed nitrogen to the surrounding water (Ploug et al., 2010, 2011). Aphanizomenon does not form the conspicuous surface accumulations typical of Nodularia, but it often comprises >50% of the biomass of the N2-fixing cyanobacteria during the summer season (Wasmund 1997, Larsson et al., 2001, Walve and Larsson, 2007). In the present study, we combined nutrient measurements, stable isotope tracers analyzed with conventional mass-spectrometry and single-cell uptake-rate measurements by SIMS and nanoSIMS to directly identify and quantify the transfer of newly fixed N2 by Aphanizomenon within the N-limited plankton community during early summer in the Baltic Sea.
Materials and methods
Sampling
Aphanizomenon colonies were sampled in the upper 10 m of the water column using a plankton net (Hydrobios, 0.5 mø, 90-μm mesh) at station B1 (N 58° 48' 28, E 17° 37' 60) in the southern Stockholm archipelago of the NW Baltic Proper during the early phase of the Aphanizomenon bloom in June 2010, 2011, 2012 and 2013. Additional water from 5-m depth was collected using a 2-l water sampler (NM Tech AB, Stockholm, Sweden). The salinity was ~6 and the temperature ~10 °C. The samples were brought to the laboratory within 30 min and poured into a light trap in a thermostated room at in situ temperature to separate zooplankton from the cyanobacterial colonies. The light trap consisted of a funnel, which was covered by black foil except at its bottom where it was illuminated to attract zooplankton, while cyanobacterial colonies floated to the surface.
Microscopy
Aphanizomenon colonies disaggregate in 1% Lugol's solution. Lugol-fixed samples (each 1 ml) for the various incubations were transferred to a gridded Sedgewick Rafter counting chamber (Wildlife supply Company, New York, NY, USA). Numbers and dimensions of dispersed trichomes and vegetative cells were measured under an inverted microscope (Leica DMIRB, Wetzlar, Germany) at × 100 or × 400 magnification. Heterocyst frequency was measured as the number of heterocysts (trichome length)−1 and in percentage of vegetative cells. Total cumulative trichome length per field was measured until its mean value was stable and the s.e. <2% of the mean. For each incubation which included Aphanizomenon, we counted and measured six replicates each with 32.5 mm trichomes and enriched the community with Aphanizomenon cells to the same final concentration of 5.0 × 107 (±0.3 × 107 cells l−1; s.e.) by adding net-harvested colonies to bulk water samples.
Incubations with 13C-labeled bicarbonate and 15N2
During 2010, bulk samples enriched with Aphanizomenon were incubated in 280-ml serum bottles, whereas other bottles (1-L Duran, Main, Germany) only contained the fraction of cells <5 μm after gentle filtration of the bulk water community through a 5-μm polycarbonate filter. 13C-labeled bicarbonate and 15N2 gas (ISOTECH, #CX0937; Sigma-Aldrich, Stockholm, Germany) was injected to a final concentration of 11% and 25% or 33% labeling, respectively, except for three control bottles. We started our experiments during the night at 0200 hours to prolong the equilibration time prior to N2-fixation during day time, and incubations were stopped after 12 and 24 h when the underestimation of N2-fixation rate relative to true N2-fixation rates is <20% owing to disequilibrium between the injected 15N2 gas bubble and the surrounding water (Mohr et al., 2010). We injected a 15N2 gas bubble instead of using a large aliquot of 15N2-enriched water because a relatively high labeling—percentage of 15N2 (>25%)—is required to track its fixation by cyanobacteria, 15NH4+ release and transfer to other phytoplankton, as well as grazing within 24 h. In 2012 and 2013, we repeated the experiments using a small aliquot of 15N2-enriched, 0.2-μm-filtered Baltic Sea water to a final labeling of 1% to ensure that measured N2-fixation by Aphanizomenon was independent of labeling% and that lack of measured N2-fixation by cells <5 μm when adding a 15N2-bubble was not due to a long equilibration time relative to the incubation time and a long diffusion distance to small cells in the bottles (Mohr et al., 2010).
During 2010, 2011 and 2012, all samples were incubated at 0.5-m depth in an outdoor mesocosm at ambient light with running sea water at in situ temperature (10 °C). Incident light intensity was measured using a LiCor irradiance sensor (Biospherical Instruments, San Diego, CA, USA) and varied from 0.2 μmol photons m−2 s−1 (at night) to 1800 μmol photons m−2 s−1 (no clouds at noon). In 2013, we repeated the experiment with cells <5 μm in a thermostated room at in situ temperature at 200 μmol photons m−2 s−1 during 18 h followed by 6 h darkness, with samples taken at T0, T6, T12 and T24. In parallel to the incubation of cells <5 μm, field samples of Aphanizomenon sp., large diatoms and zooplankton were concentrated using a plankton net (90 μm) and incubated in 2-L Duran bottles. They contained 5.3 × 107±0.3 × 107 Aphanizomenon cells l−1 (±denotes s.e.). 13C-labeled bicarbonate and 15N2 gas were injected to 11% and 25% final labeling, respectively, except for three control bottles, and incubated in the thermostated room at in situ temperature and 200 μmol photons m−2 s−1 for 18 h followed by 6 h darkness. Afterwards, copepods were separated from the rest of the sample in the light trap and collected on precombusted GF/F filters (~120 copepods each) for elemental analysis isotope ratio mass spectrometry (EA-IRMS) analysis.
After all incubations during all years, sub-samples of 50 ml for SIMS and nanoSIMS analysis were preserved with 2% paraformaldehyde for 24 h at 4 °C and then filtered onto gold–palladium-coated GTTP filters (pore size 0.22 μm; diameter 25 mm; Millipore, Eschborn, Germany), washed, dried and stored at −20 °C. The remaining water of each incubation was immediately filtered onto precombusted GF/F filters for determining bulk carbon and nitrogen assimilation rates of the total plankton community using EA-IRMS (please see below). The filtrate from each incubation was filled into 7.7 ml Exetainers (Labco, Lampeter, Wales, UK) and biological activity immediately stopped by adding 100 μl saturated HgCl2 solution for 13C-labeled bicarbonate and 15NH4+ analysis. Our 15N2 gas did not belong to the batches that have recently been documented to contain contaminations by 15NH4+ and 15NO3− (Dabundo et al., 2014). However, we examined leftover fixed filtrates from the start of our experiments and detected low contamination of 15NH4+ (<1 nm final concentration) and 15NO3− (<16 nm final concentration) (Warembourg, 1993; Füssel et al., 2012). These concentrations were insignificant relative to the measured 15NH4+ released from 15N2-fixation during experiments (please see Discussion).
Elemental analysis-isotope ratio mass spectrometry
The GF/F filters were freeze-dried and decalcified overnight with the fumes of 37% HCl in a desiccator, packed into tin cups and analyzed by a Thermo Flash EA 1112 elemental analyzer coupled to an isotopic ratio mass spectrometer (Thermo Delta Plus XP, Thermo Fisher Scientific, Waltham, MA, USA) at MPI, Bremen, Germany. Caffeine was used as a standard for isotope correction and C/N quantification of bulk carbon and nitrogen assimilation.
NanoSIMS analysis of microbial single cells
NanoSIMS analysis of single cells was performed on gold–palladium-coated polycarbonate filters (0.22-μm pore size, GTTP type, Millipore) containing the chemically fixed cells. To assess the distribution of autotrophic bacteria, autofluorescent cells on the filters were marked using a laser microdissection microscope (Leica, Wetzlar, Germany) and then re-identified after loading filters into the nanoSIMS 50L instrument at the MPI Bremen (Cameca, Gennevilliers Cedex, France) using the internal charge coupled device, as described by Polerecky et al. (2012). The areas were presputtered with a primary Cs+ ion beam of around 300 pA. During nanoSIMS analysis, the Cs+ ion beam was focused to a nominal spot size <100 nm and a beam current between 0.8 and 1.2 pA was used. Secondary ion images for 12C−, 13C−, 12C14N−, 12C15N−, 31P− and 32S− were simultaneously recorded from the analysis area. To minimize interferences for 13C−, the instrument was tuned with an average mass resolution of 7000. The image and data processing were performed as previously described (Polerecky et al., 2012). An overlay analysis with the Look@NanoSIMS software confirmed the single-cellular correlation of previously detected autofluorescence and autotrophic uptake ratios of 13C (data not shown).
SIMS analysis of Aphanizomenon and diatom cells
As diatoms are difficult to analyze by nanoSIMS owing to their silicified cells walls, we used a large geometry SIMS instrument (NORDSIM facility, Swedish Museum of Natural History, Stockholm, Sweden) capable of a primary beam with higher energy output for diatom cells as compared with that of the nanoSIMS. Furthermore, we analyzed additional Aphanizomenon sp. cells on the SIMS. The filters containing fixed cells were cut into ca. 4 × 4 mm2 pieces and mounted on a sample holder. The analysis was performed using an IMS 1280 instrument manufactured by Cameca (Gennevilliers, France) using a Cs+ primary beam with a spatial resolution of ca. 1 μm. Each diatom cell was presputtered with a beam of 10 nA for <5 min to remove the silicified cell wall and then imaged using a 40–80 pA primary beam for 200 cycles. For each cell chain, we recorded secondary ion images of 13C14N− and 12C14N− and 12C15N− using a peak-switching routine at a mass resolution of ca. 6000 (M/ΔM). Image and data processing using the Cameca WinImage (Gennevilliers, France) software defined regions of interest corresponding to individual cells from which isotope ratios were calculated.
Ammonium analysis
Total NH4+ concentration in the surrounding water was measured on a fluorometer (Turner Designs, Trilogy, Sunnyvale, CA, USA) using the method by Holmes et al. (1999). 15NH4+ was added to the bulk water enriched with Aphanizomenon to a final labeling of 28% in 1-L Duran bottles, which were carefully shaken after addition of the stable isotope tracer. Triplicate bottles and one control were incubated for 0, 2 and 5 h in light. Triplicate sub-samples of 40 ml were transferred into acid-washed falcon tubes and 10 ml OPA solution was added immediately afterwards for NH4+ concentration analysis. Triplicate subsamples for isotopic analysis were transferred into 7.7 ml Exetainer, and biological activity was immediately stopped by adding 100 μl saturated HgCl2 solution. The isotopic ratio of 15NH4+: 14NH4+ was analyzed after evacuation to remove 15N2 and 14N2 and subsequent chemical conversion of NH4+ into N2 with alkaline hypobromite (NaOBr) (Warembourg, 1993, Preisler et al., 2007). The isotopic ratios of 28N2, 29N2 and 30N2 were analyzed by a gas chromatographic IRMS (VG Optima, Micromass, Manchester, UK) calibrated with known concentrations of 15NH4+ in the nm range (1–100 nm). The net NH4+ production rate was calculated from the change in 15NH4+ concentration over time (Holtappels et al., 2011).
C- and N-growth rates by cells
SIMS and nanoSIMS measure the ratio of stable isotopes of the same element, whereas EA-IRMS measures atom percentage (AT%) of stable isotopes of the same element. The C-specific assimilation rates and N-specific assimilation rates, k(t), in the various organisms were calculated from the isotope ratios (IR) of solid matter after the incubation time (T1−T0) with stable isotope tracers as:
 |
where Fbulk is the fraction of labeled isotope in the bulk. The generation time was calculated assuming exponential growth, where k is expressed by Equation 1.
Model calculations of diffusion-limited NH4+ uptake and N-generation time by different phytoplankton types
The size-specific cellular N-content of dinoflagellates and diatoms was calculated using empirical relations between cell size and C- and N-content reported by Menden-Deuer and Lessard (2000). Extrapolated to a cell size of 1 μm, the cellular N-content was similar to those reported in different Synechococcus strains (Bertilsson et al., 2003). Diffusive boundary layers and the cellular N-content of Aphanizomenon colonies of different sizes were previously quantified (Ploug et al., 2010). The equivalent spherical diameters of cells, cell chains, trichomes and colonies were calculated from their respective volume. The diffusion-limited NH4+ supply to these cells was calculated according to (Sherwood et al., 1975):
where Q is the uptake rate (nmol N s−1), D is the molecular diffusion coefficient of NH4+ (1.2 × 10−5 cm2 s−1 at 10 °C and a salinity of 6; Li and Gregory, 1974), r0 is the average radius of the cell or colony and (C∞−C0) is the concentration difference between that of the bulk (250 nm) and that at the cell or colony surface. The maximum diffusion-limited supply of NH4+ occurs when C0 is zero at the cell surface. The minimum N-doubling time was calculated as the ratio between the cellular N-content of cells or colonies and the diffusion-limited NH4+ uptake.
Results
Single-cell analysis of N2-fixation and transfer of newly fixed N to other organisms in the plankton community
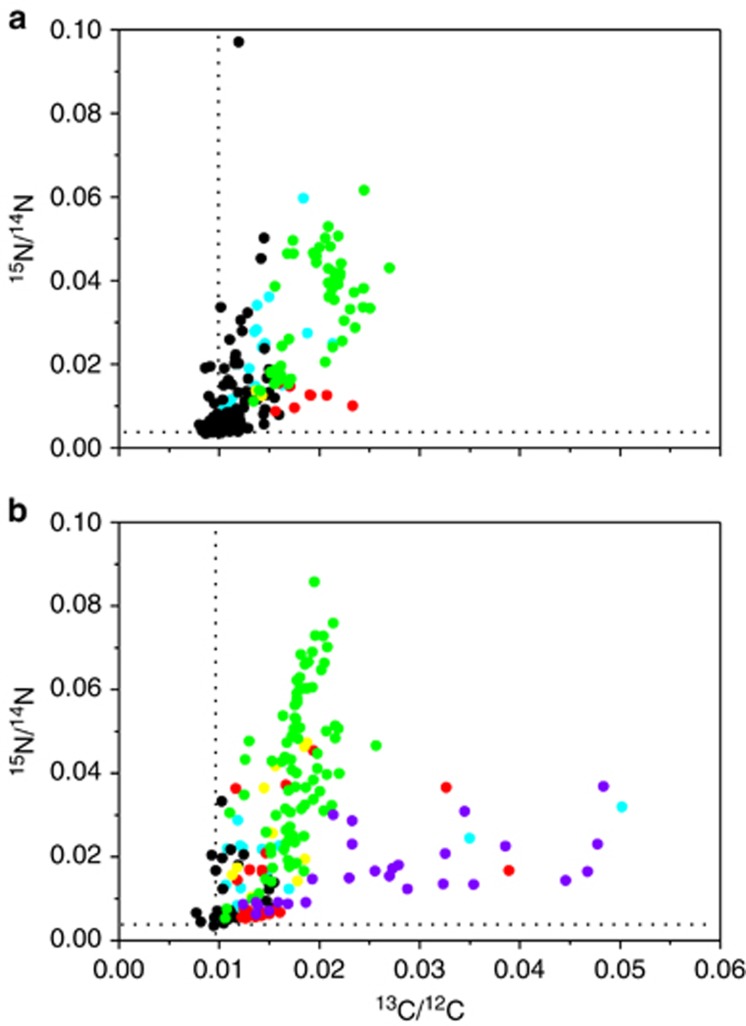
Aphanizomenon dominated the large cyanobacteria population (>99% of large cyanobacteria) as is usual during June in the Baltic Sea plankton community. After 12 h incubation with 13C-labeled bicarbonate and 15N2 gas, the 15N/14N isotope ratio was enriched above that of the natural abundance within Aphanizomenon, autotrophic and heterotrophic bacteria attached to Aphanizomenon and free-living autotrophic and heterotrophic bacteria (Figure 1a). Diatom cells (Chaetoceros sp.), in addition to the other cell types from the same incubation, were analyzed after 24 h and showed a high enrichment of 15N above that of the natural abundance (Figure 1b). The highest 15N-enrichment within the plankton community was measured in Aphanizomenon indicating a transfer of 15N derived from recently fixed 15N by Aphanizomenon to the other cells in the plankton community. Copepods were also enriched with 15N, after incubation with Aphanizomenon and diatoms during 24 h, demonstrating assimilation and transfer of newly fixed 15N also to higher tropic levels (Table 1).
Figure 1.
Bulk sample enriched with Aphanizomenon sp. and incubated with 13C-labeled bicarbonate and 15N2. Isotope ratios of 15N/14N versus 13C/12C in various organisms (a) incubated from 0300 to 1500 hours and (b) incubated from 0300 to 0300 hours: Aphanizomenon sp. vegetative cells (green symbols); autotrophic bacteria attached to Aphanizomenon sp. (yellow symbols); free-living autotrophic bacteria (red symbols); heterotrophic bacteria attached to Aphanizomenon sp. (cyan symbols); free-living heterotrophic bacteria (black symbols); and diatoms (violet symbols). The dotted lines represent the isotope ratios of the natural abundance of 15N/14N and 13C/12C.
Table 1. AT% of 13C/12C and 15N/14N measured in the <5-μm size fraction and copepods (EA-IRMS), and in Aphanizomenon sp. and Chaetoceros sp., autotrophic bacteria analyzed by SIMS or nanoSIMS after incubation with 13C-labeled bicarbonate and 15N2 tracers.
| Organism or cell size fraction | AT% 13C/12C | AT% 15N/14N |
|---|---|---|
| <5-μm size fraction, T0 (n=3) | 1.075±0.001 | 0.3711±0.0011 |
| <5-μm size fraction, T6 (n=3) | 1.205±0.005 | 0.3694±0.0002 |
| <5-μm size fraction, T12 (n=3) | 1.292±0.085 | 0.3709±0.0002 |
| <5-μm size fraction, T24 (n=3) | 1.600±0.008 | 0.3711±0.0003 |
| Copepods, T24 (n=3) | 1.195±0.021 | 0.542±0.030 |
| Chaetoceros sp., T24 (n=27) | 2.7±1.1 | 1.6±1.1 |
| Aphanizomenon sp., T24 (n=85) | 1.8±0.2 | 4.2±1.8 |
| Autotrophic bacteria att. to Aphan, T24 (n=10) | 1.6±0.3 | 2.7±1.3 |
| Autotrophic bacteria, free-living, T24 (n=24) | 1.6±0.6 | 1.5±1.2 |
| Autotrophic bacteria, T24 (n=40)a | 2.4±0.7 | 0.37±0.11 |
| Bacteria att. to Aphan, T24 (n=15) | 1.6±1.1 | 1.7±0.8 |
| Free-living bacteria, T24 (n=28) | 1.2±0.2 | 1.1±0.7 |
Abbreviations: AT%, atom percentage; EA-IRMS, elemental analysis isotope ratio mass spectrometry; nanoSIMS, nanoscale secondary ion mass spectrometry; SIMS, secondary ion mass spectrometry.
Incubated in the <5-μm size fraction.
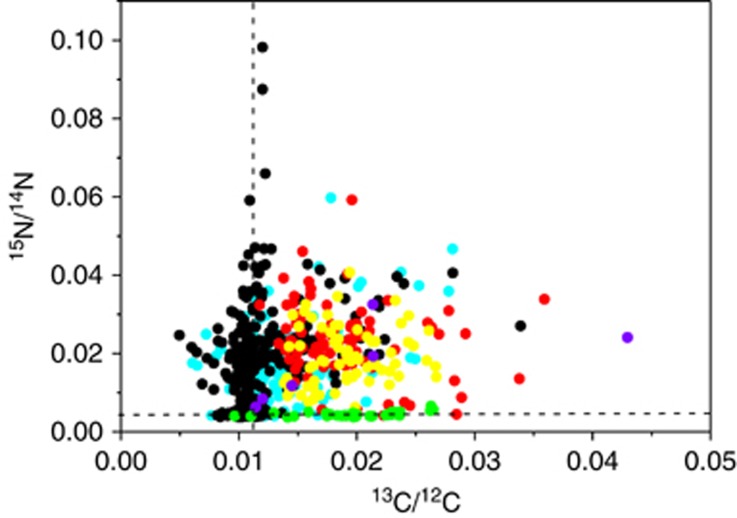
Cells >5 μm were removed by filtration, leaving small eukaryotic cells and the phototrophic and heterotrophic bacteria in the community. This smaller size fraction was incubated with 13C-labeled bicarbonate and 15N2-tracer to examine whether they were able to fix dissolved inorganic C and N2. Cells <5 μm were 13C-fixing (Figure 2), and the isotope enrichments corresponded to an average C-generation time of 1.4 days. However, no 15N assimilation was detectable in this size fraction as the 15N/14N ratio remained stable at the natural abundance level (Figure 2). These observations were also confirmed by EA-IRMS analysis of particulate matter <5 μm, which did also not reveal any 15N assimilation during incubations ⩽24 h (Table 1). The high cellular rates of 13CO2 uptake in combination with the non-detectable 15N uptake show that there were no active N2-fixers in the <5 μm fraction. In contrast, when cells <5 μm were incubated together with Aphanizomenon, their 15N/14N ratios increased above that of the natural abundance owing to the uptake of released diazotrophic 15N.
Figure 2.
Bulk (<5-μm fraction) sample incubated with 13C-labeled bicarbonate and 15N2. Isotope ratios of 15N/14N versus 13C/12C in cells incubated from 2100 to 0900 hours (open symbols) and incubated from 0900 to 2100 hours (closed symbols). The dotted lines represent the natural abundance levels of 13C/12C and 15N/14N isotope ratios.
Ammonium release and uptake rates by different plankton organisms
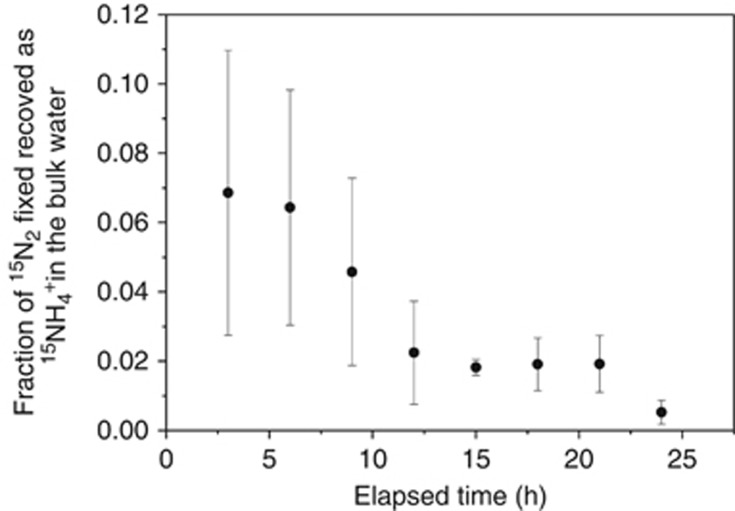
The net 15NH4+ release in the bulk water (tracked from 15N2-fixation) is shown as a fraction of the 15N2-fixation during the 24-h experiment (Figure 3). Its average value varied from 0.069±0.041 to 0.005±0.003 and it decreased throughout the 24-h (from 0200 to 0200 hours) experiment. Tracers of 13C-labeled bicarbonate and 15NH4+ were added to the phytoplankton community to study C-fixation and NH4+ uptake rates by the different (micro)organisms and the NH4+ turnover time in the bulk water. All cell types, except Aphanizomenon, showed enrichment in their 15N/14N isotopic ratio above that of the natural abundance after 5 h (Figure 4). Thus, Aphanizomenon apparently did not take up NH4+ from the bulk water. However, bacteria attached to Aphanizomenon cells showed high isotope ratios of both 13C/12C and 15N/14N. The high 15N/14N ratios observed in attached microbiota relative to that of Aphanizomenon were due to 15NH4+ uptake from the bulk water. Hence, attached and free-living microbiota showed much higher affinity to 15NH4+ in the nm concentration range than Aphanizomenon did. A large fraction of free-living bacteria showed high but variable 15N/14N ratios while the 13C/12C isotope ratio was close to the natural abundance of these isotopes, that is, these cells were most likely heterotrophic bacteria. Other free-living bacteria showed autofluorescence and their 13C/12C and 15N/14N ratios were high. These cells were photoautotrophic relying on NH4+ from the bulk water.
Figure 3.
Ratio of 15NH4+ tracked in the bulk water to 15N2-fixation during the 24-h experiment. The symbols show the average value with the s.d. (n=3) of the average value shown as bars.
Figure 4.
Bulk sample enriched with Aphanizomenon sp. incubated with 13C-labeled bicarbonate and 15NH4+ during 5 h in light: isotope ratios of 15N/14N versus 13C/12C in various organisms: Aphanizomenon sp. vegetative cells (green symbols); autotrophic bacteria attached to Aphanizomenon sp. (yellow symbols); free-living autotrophic bacteria (red symbols); heterotrophic bacteria attached to Aphanizomenon sp. (cyan symbols); free-living heterotrophic bacteria (black symbols); and eukaryotes (violet symbols). The dotted lines represent the isotope ratios of the natural abundance of 15N/14N and 13C/12C.
The isotopic composition of the various cell types was used to calculate average C-specific growth rates, N-specific growth rates based on NH4+ and N-doubling times (Table 2). Aphanizomenon showed C-specific growth rates similar to those of small autotrophic picoplankton cells, but its low N-specific growth rate based on NH4+ uptake resulted in a long N-doubling time of approximately 1 month with NH4+ from the surrounding water as the main N-source. All other cells showed similar N-doubling times of 1–2 days. Hence, the NH4+ uptake rate was insufficient to sustain the growth of Aphanizomenon and N2-fixation must have been its major N-source.
Table 2. C-specific C-fixation rate with respect to 13C-labeled bicarbonate uptake and N-specific NH4 +-growth rate and N-doubling time of N-biomass calculated from the isotope ratios in the various cell types (Figure 4).
| Cell type | C-specific C-growth rate (h−1) | N-specific NH4+-growth rate (h−1) | N-doubling time (days) |
|---|---|---|---|
| Aphanizomenon sp. (n=39) | 0.025±0.008 | 0.0011±0.0008 | 38 |
| Heterotrophic bacteria attached to Aphan (n=110) | 0.009±0.011 | 0.025±0.012 | 1.7 |
| Free-living heterotrophic bacteria (n=264) | 0.003±0.007 | 0.026±0.016 | 1.6 |
| Autotrophic bacteria attached to Aphan (n=68) | 0.024±0.007 | 0.028±0.009 | 1.2 |
| Free-living autotrophic bacteria (n=84) | 0.022±0.009 | 0.035±0.012 | 1.2 |
| Eukaryotes (n=6) | 0.041±0.025 | 0.032±0.011 | 1.3 |
Autotrophic bacteria were identified by autofluorescence.
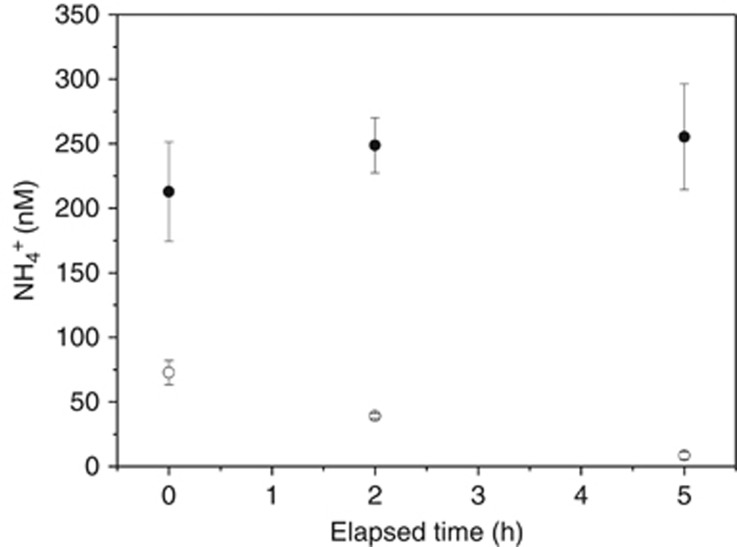
The total NH4+ concentration and the 15NH4+ concentration in the surrounding water during the experiment are shown as a function of time after addition of 15NH4+ tracers (Figure 5). The total bulk NH4+ concentration was relatively stable during the entire 5 h of the incubation. However, the 15NH4+ concentration decreased linearly over time. Thus the NH4+ concentration of the bulk was at steady state, with NH4+ uptake being balanced by NH4+ production, that is, it was taken up by the organisms in the community at the same rate as it was produced. The turnover time of NH4+ in the bulk water was ~5.5 h.
Figure 5.
Total NH4+ (15NH4+ and 14NH4+) (closed symbols) in the bulk and the added tracer 15NH4+ (open symbols) measured as a function of time in light.
Discussion
Using 13C- and 15N-tracers in combination with SIMS and nanoSIMS, we could directly demonstrate that recently fixed N2 by Aphanizomenon is released as NH4+ to the surrounding water and consequently supports the primary production by other phytoplankton and the microbial as well as the eukaryotic food web in the Baltic Sea.
Very recently, it was reported that 15N2 gas used for N2-fixation studies can be contaminated with other 15N-labeled compounds, for example, 15NH4+ and 15NO3− potentially leading to biased estimates of N2-fixation rates as well as of diazotrophic 15NH4+ transferred to other organisms (Dabundo et al., 2014). We detected low contaminations of 15NH4+ and 15NO3− in our samples, which corresponded to <1% and <15% of total 15NH4+ release and transfer from 15N2-fixation, respectively. The in situ NO3− concentration was low (<100 nm; Swedish monitoring program, SMHI), and phytoplankton is known to preferentially use excreted and remineralized NH4+ as N-sources above NO3− at low concentrations (McCarthy and Goldman, 1979).The detection of 13C-fixation but no enrichment of 15N from 15N2-fixation (or uptake of contaminated 15NH4+ and 15NO3−) by cells <5 μm after 12–24 h incubation confirmed that our results were not biased by contaminated 15N2 gas (Figure 2, Table 1). When 70 nm 15NH4+ (final concentration) was added as tracer to the natural bulk water (<250 nm) the community showed a very fast uptake rates by cells <5 μm as directly demonstrated by nanoSIMS (Figure 4, Table 2). These cells had on average 2% enrichment of 15N/14N above that of the natural abundance after 5 h incubation time. Hence, the cells <5 μm showed a high affinity for 15NH4+, and these cells would quickly have assimilated 15NH4+ and shown an enrichment of 15N/14N above that of the natural background if exposed to 15N2 gas contaminated by this nutrient. The same 15N2 gas bottles used for N2-fixation measurements in the <5-μm cell fraction were also used for all transfer experiments. We conducted and partly repeated our experiments during 4 years, with highly reproducible results between different years. Hence, we consider our data to be robust and not compromised by contaminations in the added 15N2 gas.
Simultaneous measurements of C- and N2-fixation rates in the Baltic Proper have suggested that N2-fixation supports 5–37% of the N-demand for the measured C-fixation by the whole phytoplankton community assuming a C:N molar ratio of 6.6 in phytoplankton (Karlson et al., 2015). In the present study, NH4+ release by Aphanizomenon was substantial although we did not measure any high accumulation of 15NH4+ in the bulk water during our 15N2-fixation experiments. NH4+ was produced and consumed within the phytoplankton community at a faster rate than previously measured by tracking 15NH4+ from 15N2-fixation rates in the bulk water (Ploug et al., 2010, 2011). The average net N2-fixation rate was 31 nm N h−1 in samples enriched with Aphanizomenon. At steady state of the NH4+ concentration in the bulk water, the net NH4+ production and consumption rate of 45 nm h−1 implies an average release rate equal to 59% of the gross N2-fixation rate. The N2-fixation has been estimated to contribute about as much to the new production in the Baltic Proper as riverine sources (Wasmund et al., 2001). Hence, NH4+ release by N2-fixing cyanobacteria and its uptake by other phytoplankton may therefore correspond to about half or more of the riverine input to the Baltic Sea Proper.
Interestingly, nanoSIMS analysis demonstrated NH4+ uptake by bacteria attached to Aphanizomenon cells while no NH4+ assimilation was detected in the Aphanizomenon cells (Figure 4, Table 2). The NH4+ and NO3− concentrations are generally <250 nm during summer in the Baltic Sea (Swedish Monitoring Program, Stockholm University and SMHI). A 3-year, large-scale field study in the Baltic Sea demonstrated that Aphanizomenon colonies show persistently low δ15N-values owing to high N2-fixation independent of variations in concentrations of combined inorganic nitrogen in the surrounding water (Zakrisson et al., 2014). Theoretical N-doubling time in biomass of various organisms at diffusion limitation by NH4+ from the bulk water depends on cell or colony size (Figure 6). Larger colonies, for example, chain-forming diatoms or bundles of trichomes of Aphanizomenon, will have much longer theoretical N-doubling times than small cells such as picocyanobacteria at diffusion limitation owing to larger diffusion distances and lower surface area-to-volume ratio of colonies relative to those of single cells. The calculated N-doubling times with respect to biomass must be considered as the theoretical lower limit at low bulk NH4+ concentrations (<250 nm) observed in the Baltic Sea during summer. Small, autotrophic bacteria can theoretically cover their N-demand for one cell division within hours owing to their small size and large surface area:volume ratio. This is faster than the measured rates of about 1 day (Table 2). The NH4+ uptake rates by these small cells may likely be reaction-limited (partly due to temperature limitation at 10 °C) by enzymatic activities rather than by diffusion-limited transport of nutrients toward the cell surface. Using the frequency of dividing cells as a proxy for growth rates, the minimum generation time was estimated to 0.7 days for the <3-μm cell fraction at 15 °C during summer in the same area of the Baltic Sea (Larsson and Hagström, 1982). Larger phytoplankton (flagellates, diatom chains and single trichomes of large cyanobacteria) with diameters of 10–100 μm need several days to cover their N-demand for one cell division owing to their larger size and lower surface area:volume ratio. This was also confirmed by our observation of the 13C/12C and 15N/14N ratios in diatoms, which on average corresponded to C-generation times of 3–4 days (Figure 1). The Aphanizomenon colonies are represented by sizes between 100 and 1000 μm, and cells within such colonies would need about 1 month to cover their N-demand for one cell doubling from NH4+ uptake from the surrounding water as also indicated by our tracer experiments (Table 2). Colonies normally contain thousands of densely packed cells, which must share the low NH4+ flux to the whole colony at low bulk concentrations (Ploug et al., 2010). Thus, given the low NH4+ flux at bulk concentrations <250 nm, colony-formation and generation times on the order of days would be impossible in Aphanizomenon colonies without N2-fixation. Low NH4+ uptake capacity, however, may also lead to its release from colonies as NH4+ is the first product during N2-fixation.
Figure 6.
The theoretical shortest time required to cover N-demand for one cell division at diffusion limitation at a NH4+ bulk concentration of 250 nm (for details, please see text).
Release of newly fixed N2 may appear as a poor growth strategy because N2-fixation is a highly energy-demanding process. However, commensalism and mutualism are well-documented life strategies within plankton communities. In cyanobacterial colonies, attached bacteria may benefit from NH4+ release from the large cyanobacteria while the heterotrophic bacteria through respiration may change the O2 and pH microenvironment for carbon acquisition and trace metal (Fe) speciation to improve the growth conditions for the cyanobacteria (Ploug, 2008). Interestingly, we detected a high NH4+ uptake by heterotrophic bacteria (Table 2, Figure 4), which suggest that NH4+ released from cyanobacteria was quantitatively more important for heterotrophic bacterial growth than other N-sources, that is, the concentration of dissolved organic matter was low and/or its C:N ratio was high. Hence, the present study showed that NH4+ released by N2-fixing cyanobacteria can be a key nutrient source for pelagic production in N-limited environments during a cyanobacteria bloom in addition to remineralization. We could demonstrate N-transfer to very different functional plankton types encompassing heterotrophic and autotrophic bacteria and picoeukaryotes as well as diatoms and zooplankton. Transfer of diazotrophic nitrogen to diatoms and zooplankton has profound consequences for both the classical food web, including fish, as well as for large-scale biogeochemical carbon and nitrogen fluxes. The average C-specific growth rate by Chaetoceros sp. was higher than that of Aphanizomenon. However, its abundance was low, possibly due to preferential grazing on diatoms by copepods because their feeding appendices tend to be clogged by cyanobacterial colonies (Engström et al., 2001). Diatom frustules act as ballast in sinking zooplankton fecal pellets and marine snow aggregates, which comprise a large fraction of the fast-sinking flux of organic matter in the ocean (Ploug et al., 2008). Thus our study demonstrates that diazotrohpic nitrogen is not only re-distributed to other organisms during the decay and remineralization of cyanobacterial blooms. Instead, a substantial release and transfer of recently fixed diazotrophic nitrogen during the early bloom can stimulate pelagic production, microbial and classical food webs as well as large-scale CO2 sequestration and export production more directly and at much shorter timescales (hours to days) than previously thought.
Acknowledgments
We thank the staff at Stockholm University's Baltic Sea Centre for their hospitality and support during this study at the Askö field station. We also thank Marcel Günter for his assistance in the field and during experiments, Gabriele Klockgether and Hannah Marchant for assistance with the MS analysis and Ragnar Elmgren for constructive comments to the manuscript. The Nordsim ion microprobe facility is operated under an agreement between the research funding agencies of Denmark, Iceland, Norway and Sweden, the Geological Survey of Finland and the Swedish Museum of Natural History. This work was supported by The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning, FORMAS (Grant no. 215-2009-813 to Ragnar Elmgren, JW and HP, and Grant no. 215-2010-779 to HP), the Max Planck Society (MPG), by the Baltic Ecosystem Adaptive Management programme (http://www.su.se/beam) to HP, the University of Stockholm, the University of Gothenburg and the Swedish Research Council (VR, Dnr: 621-2011-4406 to HP).
The authors declare no conflict of interest.
References
- Bertilsson S, Berglund O, Karl DM, Chrisholm SW. (2003). Elemental composition of marine Prochlorococcus and Synechococcus: Implications for the ecological stoichiometry of the sea. Limnol Oceanogr 48: 1721–1731. [Google Scholar]
- Capone DG, Zehr JP, Pearl HW, Bergmann B, Carpenter EJ. (1997). Trichodesmium, a globally significant marine Cyanobacterium. Science 276: 1221–1229. [Google Scholar]
- Dabundo R, Lehmann MF, Treibergs L, Tobias CR, Altabet MA, Moisander PH et al. (2014). The contamination of commercial N-15(2) gas stocks with N-15-labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS One 9: e110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström J, Viherluoto M, Viitasalo M. (2001). Effects of toxic and non-toxic cyanobacteria on grazing, zooplanktivory, and survival of the mysid shrimp Mysis mixta. J Exp Mar Biol Ecol 257: 269–280. [DOI] [PubMed] [Google Scholar]
- Füssel J, Lam P, Lavik G, Jensen MM, Holtappel M, Günter M et al. (2012). Nitrit oxidation in the Namibian Oxygen Minimum Zone. ISME J 6: 1200–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu S, Höglander H, Larsson U. (2007). Phytoplankton vertical distributions and composition in Baltic Sea cyanobacterial blooms. Harmful Algae 6: 189–205. [Google Scholar]
- Holmes RM, Aminot A, Keroul R, Hooker BA, Peterson BJ. (1999). A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci 56: 1801–1808. [Google Scholar]
- Holtappels M, Lavik G, Jensen MM, Kuypers MMM (2011). 15N-labeling experiments to dissect the contributions of heterotrophic denitrification and anammox to nitrogen removal in the OMZ waters of the ocean. In: Martin GK (ed). Methods in Enzymology, vol. 486, Academic Press: Burlington, VT, USA, pp 223–251. [DOI] [PubMed]
- Ibelings BW, Maberly SC. (1998). Photoinhibition and the availability of inorganic carbon restrict photosynthesis by surface blooms of cyanobacteria. Limnol Oceanogr 43: 408–419. [Google Scholar]
- Karlson AM, Duberg J, Motwani NH, Hogfors H, Klawonn I, Ploug H et al. (2015). Nitrogen fixation by cyanobacteria stimulates production in Baltic food-webs. Ambio 44: 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers MMM, Jørgensen BB. (2007). The future of single-cell environmental microbiology. Environ Microbiol 9: 6–7. [DOI] [PubMed] [Google Scholar]
- Larsson U, Hagström Å. (1982). Fractionated phytoplankton primary production, exudate release, and bacterial production in a Baltic eutrophication gradient. Mar Biol 67: 57–70. [Google Scholar]
- Larsson U, Hajdu S, Walve J, Elmgren R. (2001). Baltic Sea nitrogen fixation estimated from the summer increase in upper mixed layer total nitrogen. Limnol Oceanogr 46: 811–820. [Google Scholar]
- Lesutiene J, Bukaveckas PA, Gasiunaite ZR, Pilkaityte R, Razinkovas-Baziukas A. (2014). Tracing the isotopic signal of a cyanobacteria bloom through the food web of a Baltic Sea coastal lagoon. Estuar Coast Shelf Sci 138: 47–56. [Google Scholar]
- Li Y-H, Gregory S. (1974). Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta 38: 703–714. [Google Scholar]
- McCarthy JJ, Goldman JC. (1979). Nitrogenous nutrition of marine phytoplankton in nutrient depleted waters. Science 203: 670–672. [DOI] [PubMed] [Google Scholar]
- Mohr W, Grosskopf T, Wallace DW, LaRoche J. (2010). Methodological underestimation of oceanic nitrogen fixation rates. PLoS One 5: e12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menden-Deuer S, Lessard EJ. (2000). Carbon to volume relationships for dinoflagellates, diatoms, and other protest plankton. Limnol Oceanogr 45: 569–579. [Google Scholar]
- Mulholland MR. (2007). The fate of nitrogen fixed by diazotrophs in the ocean. Biogeosciences 1: 37–51. [Google Scholar]
- Mulholland MR, Capone DG. (2001). Stoichiometry of nitrogen and carbon utilization in cultured populations of Trichodesmium IMS101: implications for growth. Limnol Oceanogr 46: 436–443. [Google Scholar]
- Musat N, Foster RA, Vagner T, Adam B, Kuypers MMM. (2012). Detecting metabolic activities in single cells with emphasis on nanoSIMS. FEMS Microbiol Rev 36: 486–511. [DOI] [PubMed] [Google Scholar]
- Ohlendieck U, Stuhr A, Siegmund H. (2000). Nitrogen fixation by diazotrophic cyanobacteria in the Baltic Sea and transfer of the newly fixed nitrogen to picoplankton organisms. J Mar Syst 25: 213–219. [Google Scholar]
- Ploug H. (2008). Cyanobacterial aggregates formed by Aphanizomenon sp. and Nodularia spumigena in the Baltic Sea: Small-scale fluxes, pH and oxygen microenvironments. Limnol Oceanogr 53: 914–921. [Google Scholar]
- Ploug H, Iversen MH, Fischer G. (2008). Ballast, sinking velocity and apparent diffusivity in marine snow and zooplankton fecal pellets: Implications for substrate turnover by attached bacteria. Limnol Oceanogr 53: 1878–1886. [Google Scholar]
- Ploug H, Musat N, Adam B, Moraru CM, Lavik G, Vagner T et al. (2010). Carbon and nitrogen fluxes associated with the cyanobacterium Aphanizomenon sp. in the Baltic Sea. ISME J 4: 1215–1223. [DOI] [PubMed] [Google Scholar]
- Ploug H, Adam B, Musat N, Kalvelage T, Lavik G, Wolf-Gladrow D et al. (2011). Carbon, nitrogen, and O2 fluxes associated with the cyanobacterium Nodularia spumigena in the Baltic Sea. ISME J 5: 1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polerecky L, Adam B, Milucka J, Musat N, Vagner T, Kuypers MMM. (2012). Look@nanosims – a tool for the analysis of nanoSIMS data in environmental microbiologyemi. Environ Microbiol 14: 1009–1023. [DOI] [PubMed] [Google Scholar]
- Preisler A, de Beer D, Lichtslag A, Lavik G, Boetius A, Jørgensen BB. (2007). Biological and chemical sulfide oxidation in a Beggiatoa inhabited marine sediment. ISME J 1: 314–353. [DOI] [PubMed] [Google Scholar]
- Sherwood TK, Pigford RL, Wilke CR. (1975) Mass Transfer. McGraw-Hill Book Company: New York, USA. [Google Scholar]
- Stal LJ, Albertano P, Bergman B, von Bröckel K, Gallon JR, Hayes PK et al. (2003). BASIC: Baltic Sea cyanobacteria. An investigation of the structure and dynamics of water blooms of cyanobacteria in the Baltic Sea-responses to a changing environment. Cont Shelf Res 23: 1695–1714. [Google Scholar]
- Wagner M. (2009). Single-cell ecophysiology of microbes as revealed by Raman microspectroscopy or secondary ion mass spectrometry imaging. Annu Rev Microbiol 63: 411–429. [DOI] [PubMed] [Google Scholar]
- Walve J, Larsson U. (2007). Blooms of Baltic Sea Aphanizomenon sp.(Cyanobacteria) collapse after internal phosphorus depletion. Aquat Microb Ecol 49: 57–69. [Google Scholar]
- Wannicke N, Korth F, Liskow I, Voss M. (2013). Incorporation of diazotrophic fixed N2 by mesozooplankton — case studies in the southern Baltic Sea. J Mar Syst 117–118: 1–13. [Google Scholar]
- Warembourg FR (1993). Nitrogen Isotopes Techniques, Nitrogen Fixation in Soil and Plant Systems. In: Knowles K, Blackburn TH (eds). Academic: New York, USA, pp 157–180.
- Wasmund N. (1997). Occurrence of cyanobacterial blooms in the Baltic Sea in relation to environmental factors. Int Rev Hydrobiol 82: 169–184. [Google Scholar]
- Wasmund N, Voss M, Lochte K. (2001). Evidence of nitrogen fixation by non-heterocystous cyanobacteria in the Baltic Sea and re-calculation of a budget of nitrogen fixation. Mar Ecol Prog Ser 214: 1–14. [Google Scholar]
- Woodland RJ, Holland DP, Beardall J, Smith J, Scicluna T, Cook PLM. (2013). Assimilation of diazotrophic nitrogen into pelagic food webs. PLoS One 8: e67588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrisson A, Larsson U, Höglander H. (2014). Do Baltic Sea diazotrophic cyanobacteria take up combined nitrogen in situ? J Plankton Res 36: 1368–1380. [Google Scholar]