Abstract
Studying host–microbiota interactions are fundamental to understanding the mechanisms involved in intestinal homeostasis and inflammation. In this work, we analyzed these interactions in mice that were mono-associated with six microorganisms that are representative of inflammatory bowel disease (IBD)-associated dysbiosis: the bacteria Bacteroides thetaiotaomicron, adhesive-invasive Escherichia coli (AIEC), Ruminococcus gnavus and Roseburia intestinalis; a yeast used as a probiotic drug, Saccharomyces boulardii CNCM I-745; and another yeast, Candida albicans. Extensive ex vivo analyses including colon transcriptomics, histology, immune response, bile acid metabolism and short-chain fatty acid production were studied. We showed that B. thetaiotaomicron had the highest impact on the immune system because it was almost able to recapitulate the effects of the entire conventional microbiota and notably induced Treg pathways. Furthermore, these analyses uncovered the effects of E. coli AIEC LF82 on indoleamine 2,3-dioxygenase expression and of S. boulardii CNCM I-745 on angiogenesis. These results were confirmed in vitro in human cell lines. Finally, our results suggested that R. gnavus has major effects on metabolism, and notably on tryptophan metabolism. This work therefore reveals that microorganisms with a potential role in intestinal homeostasis and inflammation have specific impacts on the host, and it suggests several tracks to follow to understand intestinal homeostasis and IBD pathogenesis better, providing new insights to identify novel therapeutic targets.
Introduction
The gastrointestinal tract contains a complex mix of epithelial cells, immune cells, food antigens and microorganisms. The intestinal microbiota is estimated to contain approximately 1014 microorganisms, which are primarily bacteria localized in the distal ileum and colon (Ley et al., 2006). Although pathogens are rapidly recognized and destroyed by the immune response, the host tolerates the intestinal microbiota by mechanisms that are still unclear (Maynard et al., 2012). However, this tolerance can be disrupted and evolve into an uncontrolled inflammatory response (Maynard et al., 2012), as observed in pathologies such as inflammatory bowel diseases (IBDs). This deregulation has multiple causes, but dysbiosis (an imbalance in the gut microbiota composition) and genes involved in host–microorganism interactions have been associated with IBD in numerous studies (Sokol et al., 2008a; Khor et al., 2011), supporting the importance of the microbiota and its interactions with the host in IBD pathogenesis. Studying these interactions is therefore of major interest for understanding the mechanisms involved in IBD in addition to normal gut homeostasis.
In this study, we have selected four intestinal bacteria and two yeasts linked to IBD pathogenesis as follows: adhesive-invasive Escherichia coli (AIEC), Ruminococcus gnavus, Bacteroides thetaiotaomicron, Roseburia intestinalis, the probiotic yeast Saccharomyces boulardii CNCM I-745 and the pathogenic yeast Candida albicans. Ileal Crohn's disease (CD) is associated with an increase in E. coli and R. gnavus representation, in addition to a decrease in R. intestinalis representation (Giaffer et al., 1991, 1992; Seksik et al., 2003; Mangin et al., 2004; Willing et al., 2010; Joossens et al., 2011), the latter being a butyrate producer that is among the most abundant bacteria in human intestinal microbiota (Qin et al., 2010). Furthermore, as an original isolate from ileal lesions in a CD patient (Darfeuille-Michaud et al., 1998; Boudeau et al., 1999), AIEC showed an increased prevalence during this pathology (Darfeuille-Michaud et al., 2004) and was reported to worsen the colitis in a mouse model (Carvalho et al., 2008). Although contradictory data are available regarding its link to IBD (Sokol et al., 2008a), we also chose B. thetaiotaomicron because it is a major representative of the Bacteroidetes phylum, one of the three major phyla of intestinal microbiota (Qin et al., 2010). Finally, the two yeasts were reported as having an impact on intestinal inflammation. On the one hand, S. boulardii protects against pathogen-associated diarrhea and colitis in murine models and humans (McFarland et al., 1994; Kirchhelle et al., 1996; Pothoulakis, 2009), and it reportedly had a beneficial effect on IBD in murine models (Jawhara and Poulain, 2007; Lee et al., 2009). On the other hand, C. albicans is the most prevalent fungus in human intestinal microbiota and its concentration is increased in IBD patients (Standaert-Vitse et al., 2009; Richard et al., 2015). Moreover, in the murine colitis model, C. albicans worsens intestinal inflammation, and conversely, its colonization is favored by inflammation (Jawhara et al., 2008).
These six microorganisms are therefore potential actors in the immune deregulation involved in IBD pathogenesis. The aim of this study was to decipher their effects on the host in murine mono-association models.
Materials and methods
Microorganisms and cell lines
Four bacteria and two yeasts were used in this study as follows: E. coli AIEC LF82 (provided by Arlette Darfeuille-Michaud, Clermont Ferrand, France), R. gnavus ATCC 29149, B. thetaiotaomicron VPI-5482 (ATCC 29148), R. intestinalis L1-82 (DSM 14610), S. boulardii CNCM I-745 (syn. HANSEN CBS 5926, Biocodex Laboratories, Gentilly, France) and C. albicans SC5314 (ATCC, Molsheim, France). HT-29 (human colon epithelium; ATCC) and HUVEC (human umbilical vein endothelium; Lonza, Levallois-Perret, France) cell lines were also used for in vitro experiments. The growth and culture conditions are described in Supplementary Information.
Mono-associations
All procedures were carried out according to European guidelines for the care and the use of laboratory animals. Animal experiments were evaluated and approved by the local ethics committee. Germ-free female C3H/HeN mice (ANAXEM platform, INRA, France) were bred in germ-free isolators. Bacterial suspensions (108–109 colony forming unit (CFU) in 400 μl), yeast suspensions (107–108 CFU in 400 μl), or control medium was administered to 6-week-old mice by intragastric gavage (eight mice per group).
For conventionalization, fresh stools from C3H/HeN mice were immediately transferred to an anaerobic chamber, in which the stools were suspended and diluted 1:100 in LYHBHI medium (BD Difco, Le Pont de Claix, France) supplemented with cellobiose (1 mg ml–1; Sigma-Aldrich, Saint Quentin Fallavier, France), maltose (1 mg ml–1; Sigma-Aldrich) and cysteine (0.5 mg ml–1; Sigma-Aldrich). Six-week-old germ-free mice were inoculated by oral gavage with 400 μl of fecal suspension.
After 6 weeks of implantation, the mice were killed and several samples were taken, namely the pieces and content from the jejunum, ileum, cecum and colon, and mesenteric lymph nodes (MLNs).
The microorganism population levels were determined in the feces during implantation (weekly) and in the intestinal contents after killing, all with culture methods (LYHBHI-agar or Sabouraud-agar).
SCFA and bile acid analysis
A measurement of the short-chain fatty acids (SCFAs) was performed on the cecal contents by gas chromatography coupled with mass spectrometry, according to a previously described procedure (Ferchaud-Roucher et al., 2005). D3-acetate, D5-propionate and 13C-butyrate (Sigma-Aldrich) were used as internal standards. The selected ion monitoring mode was used to measure SCFA concentrations with ions at m/z 117 (acetate), 120 (D3-acetate), 131 (propionate), 136 (D5-propionate), 145 (butyrate) and 146 (13C-butyrate).
The bile acid composition and concentration were measured in feces at 6 weeks post-gavage by high-performance liquid chromatography coupled to tandem mass spectrometry, as previously described (Humbert et al., 2012).
Histology
Colon samples for histological studies were fixed in 4% paraformaldehyde acid (Electron Microscopy Sciences, Hatfield, PA, USA), embedded in paraffin, then stained for histological scoring and immunohistochemistry analysis, as described in Supplementary Information.
The quantification of cytokines and immunoglobulins
The MLNs were mashed with a 70-μm cell strainer in cell culture medium, and 2.105 cells per well were cultured in P24 plates for 48 h (37 °C, 10% CO2) with stimulation by anti-CD3/CD28 antibodies (4 μg ml–1 each; eBioscience, Paris, France) or phorbol 12-myristate 13-acetate/ionomycin (50 ng ml–1 and 1 μM, respectively; Sigma-Aldrich). For the IgA quantification, the feces were homogenized in phosphate-buffered saline (1 ml per 100 mg) and centrifuged. All collected supernatants were frozen at −80 °C until processing. The quantification of mouse cytokines or immunoglobulins was performed by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions with interleukin-4 (IL-4), IL-5, interferon-γ (IFNγ), IL-17, IL-10 and IgA (MabTech, Nacka Strand, Sweden); and transforming growth factor-β (TGFβ) and IL-22 (DuoSet ELISA Development System, R&D Systems, Lille, France).
Gene expression
The total RNA was isolated from colon samples and cell lines with an RNeasy Mini Kit (Qiagen, Courtaboeuf, France), according to the manufacturer's instructions. The RNA integrity was checked on a Bioanalyzer 2100 by using RNA 6000 Nano chips (Agilent Technologies, Les Ulis, France), and all the samples had a RNA integrity number score higher than 8.
Transcriptional profiling was performed on the mouse colon samples by using the SurePrint G3 Mouse GE 8 × 60K Microarray (Design ID: 028005, Agilent Technologies), as described in Supplementary Information. The microarray data were submitted to GEO (accession number: GSE63299). In addition, the expression of selected mouse and human genes was assessed in mouse colon and human cells by reverse transcriptase (RT)-quantitative PCR (qPCR), as described in Supplementary Information.
In vitro experiments
HT-29-IDO reporter cells were generated and used to monitor the effect of E. coli LF82 on IDO-1 transcription activity, as well as the HUVEC cell line was used to test the vascular effects of S. boulardii. Detailed protocols are described in Supplementary Information.
Data analysis and statistics
Transcriptomic data obtained from microarrays were analyzed as described in Supplementary Information. For all the data in the graphs, the results were expressed as the mean or mean±s.e.m. (n=3 to 8 per group). Kruskal–Wallis test was used for multiple comparisons, and Mann–Whitney test with Bonferroni correction for 2–2 group comparison.
Results
Microbial implantation and the metabolic and morphological effects of mono-associations
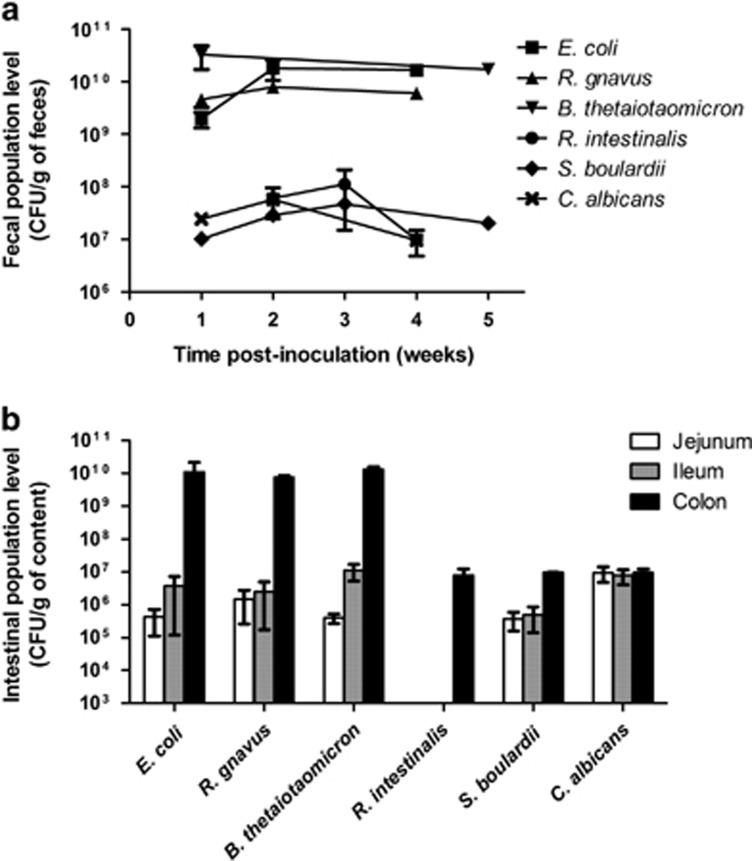
Six mono-association models involving four bacteria in addition to probiotic yeast and pathogenic yeast, all of which were linked to IBD pathogenesis, were studied. All the microorganisms rapidly and stably colonized the guts of germ-free mice as evidenced by the feces quantifications (Figure 1a). However, two distinct patterns were observed (Figure 1a) as follows: E. coli, R. gnavus and B. thetaiotaomicron reached high population levels (1010–1011 CFU per g of feces), and the population levels of R. intestinalis, S. boulardii and C. albicans were lower (107–108 CFU per g of feces). The population levels in the colon contents confirmed these results (Figure 1b). Strikingly, E. coli, R. gnavus and B. thetaiotaomicron but not the two yeasts presented a markedly decreased level in the small intestine (the jejunum and ileum) when compared with the colon. This finding suggests fitness differences between microorganisms with the bacteria being more adapted to the colon environment and fungi being equally adapted to the small and large intestines. R. intestinalis poorly colonized only the colon, suggesting a higher growth requirement (Figure 1b).
Figure 1.
Microbial population levels in mono-associated mice. Population levels were determined in feces during implantation (a) and in different intestinal contents after killing (b) using culture methods on LYHBHI-agar or Sabouraud-agar. Results are expressed as mean±s.e.m. (n=8 per group).
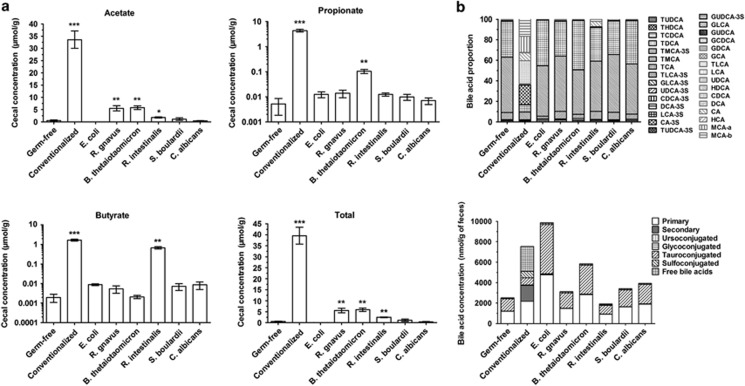
Gut bacteria have high metabolic activity and metabolites that deeply impact the host's physiology. Among these metabolites, SCFA production and bile acid metabolism are among the most important. To gain insight into bacterial metabolism, we thus determined the SCFA and bile acid concentrations in the cecal and fecal contents, respectively. In comparison with germ-free mice, the global SCFA concentration was significantly increased in mice colonized with B. thetaiotaomicron, R. gnavus and R. intestinalis but did not reach the level observed in conventionalized mice (Figure 2a). We observed that the butyrate production by R. intestinalis was similar to that of conventionalized mice, there was moderate acetate production by B. thetaiotaomicron and R. gnavus, and there was moderate propionate production by B. thetaiotaomicron (Figure 2a).
Figure 2.
Cecal concentration of SCFAs and fecal level of bile acids in mono-associated mice. (a) Acetate, propionate and butyrate concentration were determined in cecal content by gas chromatography coupled with mass spectrometry. Results are expressed as mean±s.e.m. (n=5 per group). Statistical comparison with the germ-free group was performed using Mann–Whitney U-test (*P<0.05/**P<0.01/***P<0.001). (b) Bile acid composition was measured in feces at 6 weeks post-gavage by high-performance liquid chromatography coupled to tandem mass spectrometry, and was represented here with the concentration of bile acid families or individual bile acid proportion. Results are expressed as mean of five mice per group.
With regard to the bile acid composition in the stool, major differences were observed between germ-free and conventionalized mice as expected, the most important being the appearance of secondary bile acids (Figure 2b and Supplementary Figure 1). However, mono-associated mice exhibited only minor differences in comparison with germ-free mice (Figure 2b). Although the global bile acid concentration was higher in mice that were mono-colonized with some bacteria (E. coli and B. thetaiotaomicron) in comparison with germ-free mice, no secondary bile acid was detected (Figure 2b). This finding shows that none of the tested microorganisms was able to deconjugate primary bile acids efficiently. Similar results were also observed in a context of simplified microbiota with different cocktails of the tested microorganisms (data not shown), indicating that major actors of primary bile acid deconjugation are lacking.
Finally, the colon histology results were compared between our different models. We observed an increase in the crypt depth and goblet cell density in mono-associated groups in comparison with germ-free mice, as observed in conventionalized mice (Supplementary Figure 2). Strikingly, S. boulardii was the only microorganism that did not induce an increase in the crypt depth (Supplementary Figure 2A). The colon epithelial cell proliferation assessed by the height of Ki67 staining in the crypts showed that B. thetaiotaomicron decreased this proliferation whereas R. intestinalis increased proliferation (Supplementary Figure 2C). As expected, conventionalized mice showed the highest proliferation level.
Host transcriptomics reveal distinct profiles for several mono-associations
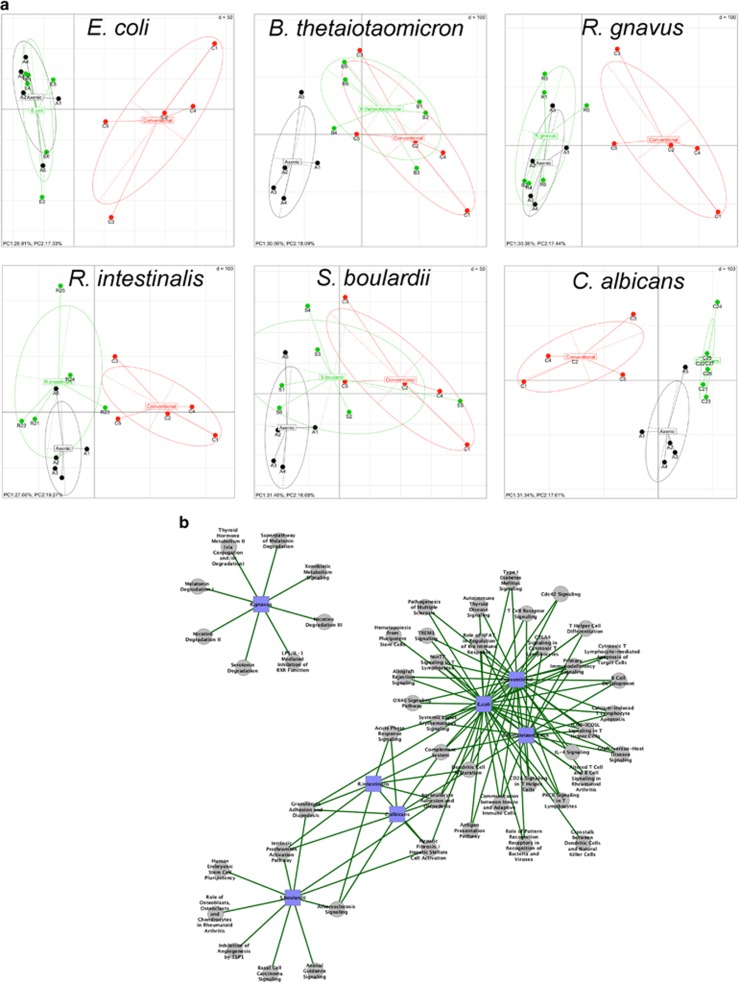
Total RNA was extracted from colon tissues and analyzed by microarrays to determine host transcriptomic profiles. A principal component analysis showed that germ-free and conventionalized mice had markedly different profiles, confirming that the gut microbiota has a major impact on host gene expression. Different types of global expression profiles were observed in mono-colonized mice (Figure 3a). E. coli and R. gnavus induced profiles similar to those of germ-free mice, and R. intestinalis and S. boulardii induced profiles that were intermediate between germ-free and conventionalized mice, whereas C. albicans induced a profile that was totally distinct from those of the control groups. Surprisingly, a mono-association with B. thetaiotaomicron was sufficient to induce an expression profile similar to that of conventionalized mice.
Figure 3.
Colon gene expression in mono-associated mice using microarray technology. (a) Gene expression in mouse colon was analyzed by principal component analysis. The ellipses delimit mono-associated group (monoxenic, in green), germ-free group (axenic, in black) and conventionalized group (conventional, in red). The axes correspond to principal component 1 (x axis) and 2 (y axis). (b) Interaction network established with Cytoscape and showing nodes and connections between significantly enriched ingenuity canonical pathways, in each mono-associated group when compared with germ-free mice. The gray circle nodes represent the ingenuity canonical pathways and the blue square nodes represent the mono-associated group compared with germ-free mice. The green edge links represent significantly upregulated functions/pathways in mono-associated groups compared to germ-free group. Significant level was fixed at α=0.05.
As expected, an analysis of modulated genes and signatures in comparison with germ-free mice showed the high dominance of immune response features (Supplementary Figures 3 and 4, Table 1). This finding was especially clear for the B. thetaiotaomicron model, which shared the highest number of deregulated pathways with conventionalized mice (Figure 3b), and occasionally similar deregulation levels in signature and gene expression levels (Supplementary Figures 3 and 4). Furthermore, the B. thetaiotaomicron model was the only one with a lack of immune response-related pathways in the top downregulated signature when compared with conventionalized mice, rather showing deregulation in DNA and cell division processes (Supplementary Figures 5 and 6,Supplementary Table 2). This finding suggests that B. thetaiotaomicron by itself is able to induce an immune system maturation that is close to the one induced by conventionalization.
Table 1. Selection of significant upregulated genes when compared with germ-free mice.
| Implantation | Genes | Fold change | Description |
|---|---|---|---|
| Conventionalized | Igj | 468.0 | Ig chain linker |
| Trpv6 | 18.8 | Calcium channel | |
| Ly6e | 13.7 | Proliferation of lymphocytes | |
| Reg3b | 12.0 | Antimicrobial peptide | |
| Ifi27l2a/Ifi47/Ifi44 | 11.9/8.3/6.1 | IFN-induced factors | |
| Ccl5 | 8.6 | Chemokine | |
| Cd3d/Cd3g | 7.1/5.8 | CD3 chains | |
| H2-DMb1/H2-Aa/H2-Ab1/H2-DMa | 7.1/7.0/6.4/5.9 | CMH II | |
| B. thetaiotaomicron | Igj | 207.0 | Ig chain linker |
| C1s | 5.1 | Complement subunit | |
| Cd3d/Cd3g | 4.3/3.4 | CD3 chains | |
| Ifitm1 | 4.2 | IFN-induced factor | |
| Mcpt4/Mcpt2/Mcpt1 | 4.2/4.0/3.8 | Mast cell proteases | |
| Trem2 | 3.6 | Inflammatory receptor | |
| Ccl5 | 3.5 | Chemokine | |
| E. coli | Igj | 96.7 | Ig chain linker |
| Reg3b/Reg3g | 63.3/12.9 | Antimicrobial peptides | |
| S100g/Slc10a2 | 11.5/5.4 | Solute transporters | |
| Mmp7 | 7.2 | Matrix metalloproteinase | |
| Ifi47 | 4.7 | IFN-induced factor | |
| Ido1 | 4.5 | Tryptophan metabolism | |
| Tlr13 | 4.4 | Pattern recognition receptor | |
| Cxcl9 | 3.8 | Chemokine | |
| R. gnavus | Igj | 33.0 | Ig chain linker |
| Cyp3a44/3c55/3a16/3a59 | 7.5/5.9/5.2/3.1 | Cytochrome P450 members | |
| Reg1 | 3.8 | Antimicrobial peptide | |
| Tlr13 | 3.7 | Pattern recognition receptor | |
| Ido1 | 3.0 | Tryptophan metabolism | |
| R. intestinalis | Igj | 8.1 | Ig chain linker |
| Dbp/Tef | 7.4/3.1 | Circadian rhythm | |
| C1s | 5.1 | Complement subunit | |
| S100g/Slc2a5/Slc10a2 | 5.0/4.0/3.8 | Solute transporters | |
| Trem2 | 3.6 | Inflammatory receptor | |
| Cxcl12 | 3.5 | Chemokine | |
| Mcpt4 | 3.3 | Mast cell protease | |
| Esm1/Egfl7 | 3.3/3.3 | Produced by endothelial cells | |
| Clec3b | 3.2 | Pattern recognition receptor | |
| S. boulardii | Tex14 | 6.3 | Cell division |
| C1s | 5.2 | Complement subunit | |
| Igj | 4.5 | Ig chain linker | |
| Esm1/Egfl7/Glycam1/Apln | 4.4/3.3/3.1/1.9 | Produced by endothelial cells | |
| Mcpt4 | 3.0 | Mast cell protease | |
| Col1a2/18a1 | 1.8/1.6 | Collagen subunits, linked to angiogenesis | |
| C. albicans | Igj | 9.5 | Ig chain linker |
| Esm1 | 5.3 | Produced by endothelial cells | |
| Trpv6 | 4.7 | Calcium channel | |
| Mcpt4 | 4.7 | Mast cell protease | |
| Col6a3/1a2/5a1/5a3/6a1 | 4.0/3.9/3.9/3.4/3.2 | Collagen subunits | |
| Cd93 | 3.8 | C-type lectin receptor on immune and endothelial cells | |
| Cxcl12 | 3.3 | Chemokine | |
| Clec3b | 3.3 | Pattern recognition receptor |
Strikingly, when compared with germ-free mice, some mono-association models presented top expression signatures that were not related to the immune response. In fact, the top upregulated signature in the R. gnavus model contained only metabolic pathways, and the top upregulated signature of the S. boulardii model contained only pathways related to vascular processes and endothelial cells with genes harboring pro- or anti-angiogenic activity (Figure 3b).
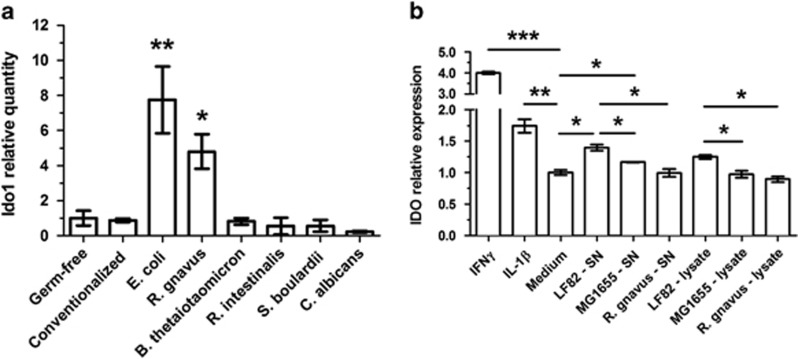
In addition, a comparison of the gene expression with germ-free mice confirmed the metabolic profile induced by R. gnavus with the upregulation of several cytochrome P genes, in addition to the vascular profile induced by S. boulardii with the upregulation of several endothelial genes and genes involved in pro- or anti-angiogenic processes (Table 1). Strikingly, the indoleamine 2,3-dioxygenase gene (Ido1) was particularly upregulated in the colon from E. coli LF82 mono-associated mice (Table 1), but no significant increase was observed in other models or controls except for R. gnavus.
Based on the host transcriptomic profiles, we selected three interesting features for further analysis as follows: (i) B. thetaiotaomicron and the high maturation of the colon immune system; (ii) E. coli LF82 and Ido1 gene induction; and (iii) S. boulardii and the induction of endothelial pathways.
Bacteroides thetaiotaomicron strongly induces the maturation of the colon immune system including Treg pathway activation
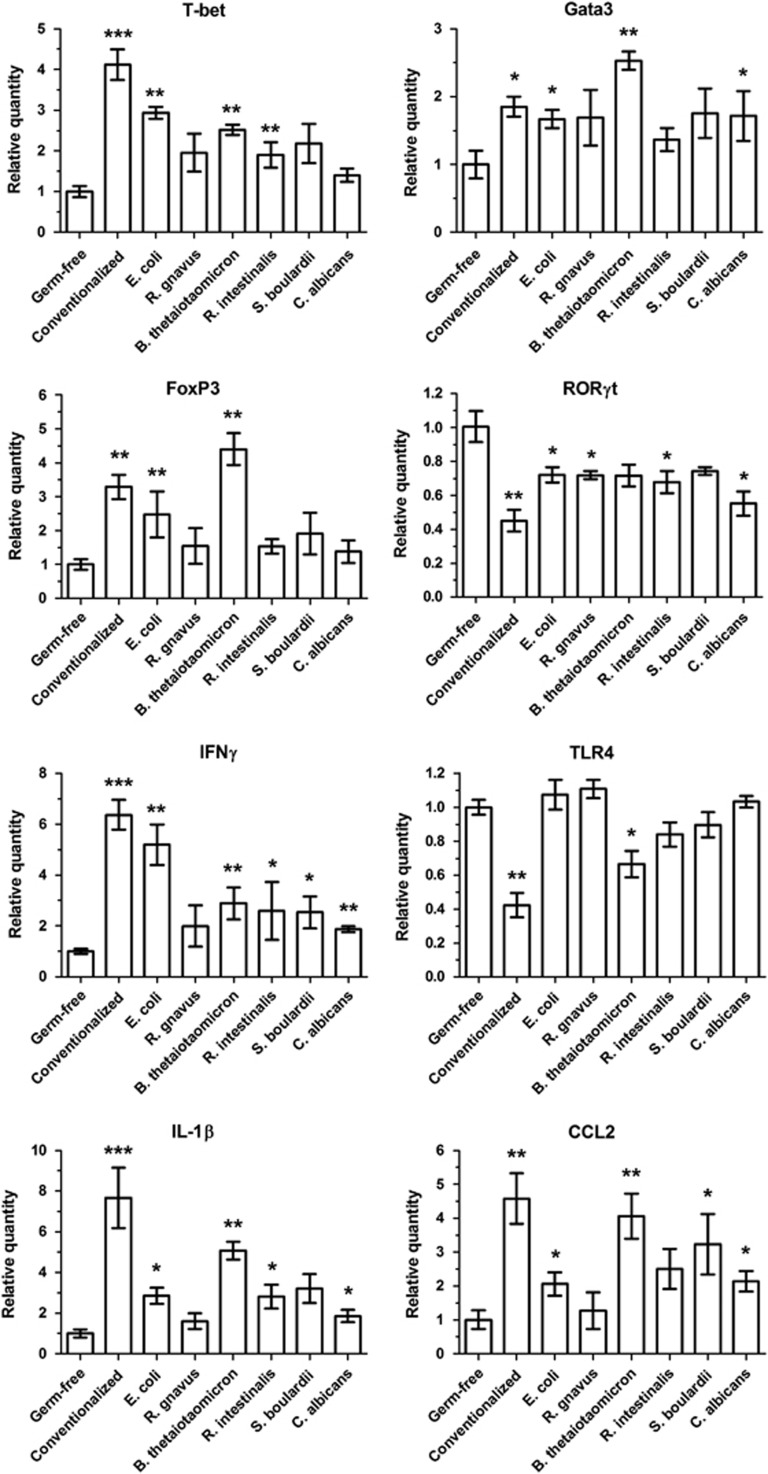
We confirmed the immune response gene deregulation observed in the microarrays in the different models by using RT-qPCR to target major T-cell transcription factors and other selected genes (Figure 4). Consistent with the microarray data, the RT-qPCR results for the B. thetaiotaomicron model were often similar to those found in conventionalized mice (Figure 4). Strikingly, when compared with germ-free mice, this model showed a strong increase in Gata3 and FoxP3 gene expression, even higher than in conventionalized mice (Figure 4).
Figure 4.
Colon immune gene expression in mono-associated mice. To confirm results from microarrays, the genes of eight immune proteins were selected and their RNA expression was determined in colon by RT-qPCR. The ΔΔCt method was used for quantification, with GAPDH as an internal control and germ-free group as a calibrator. Results are expressed as mean±s.e.m. (n=5 per group). Statistical comparison with the germ-free group was performed using Mann–Whitney U-test (*P<0.05/**P<0.01/***P<0.001).
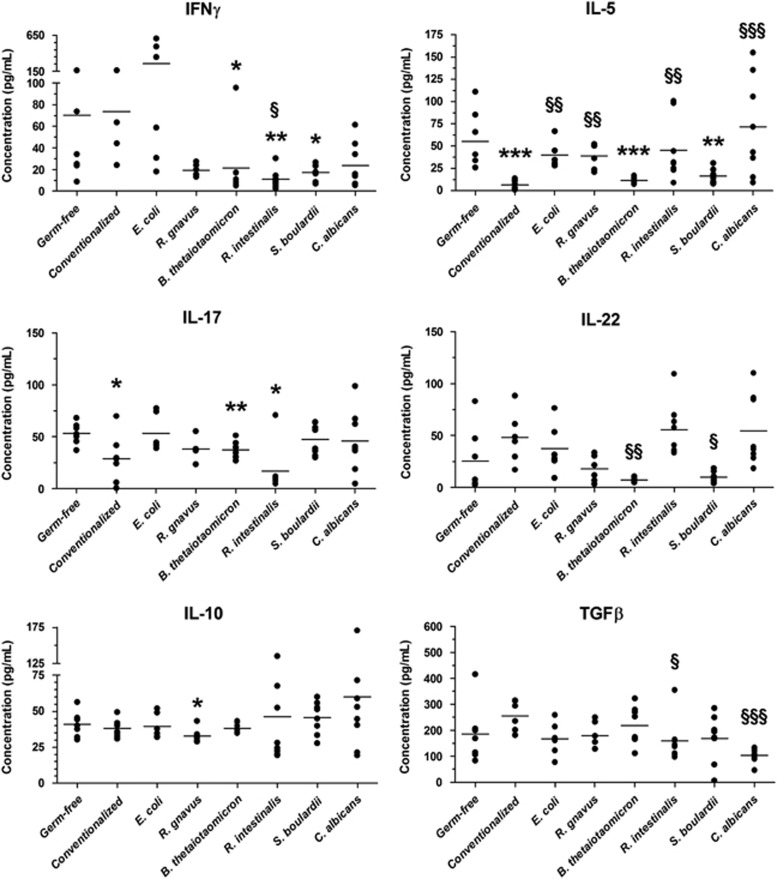
The production of representative cytokines from the major T-helper profiles were quantified in MLNs after stimulation by anti-CD3/CD28 antibodies. For most cytokines, only modest differences were observed between the groups (Figure 5). B. thetaiotaomicron induced a slight but significant reduction in Th1, Th2 and Th17 cytokines (Figure 5). We also observed that R. intestinalis induced decreased IFNγ and IL-17 production with increased IL-22 production, corresponding to an anti-inflammatory pattern. S. boulardii induced decreased IFNγ and IL-5 production (Figure 5). Global stimulation with phorbol 12-myristate 13-acetate/ionomycin showed similar results for B. thetaiotaomicron, R. intestinalis and S. boulardii, but R. gnavus and C. albicans showed a pro-inflammatory profile with increased IFNγ, IL-17 and IL-22 production (Supplementary Figure 7).
Figure 5.
Cytokine production in MLNs from mono-associated mice. Cells were isolated from MLNs and cultured for 48 h with anti-CD3/CD28 antibodies to activate them. ELISA was performed on supernatants to quantify the production of six cytokines representative of major T-helper cytokine profiles. The mean value for each experimental group is indicated by a horizontal bar (n=8 per group). Statistical comparison with the germ-free group (*P<0.05/**P<0.01/***P<0.001) and with conventionalized mice (§P<0.05/§§P<0.01/§§§P<0.001) was performed using Mann–Whitney U-test.
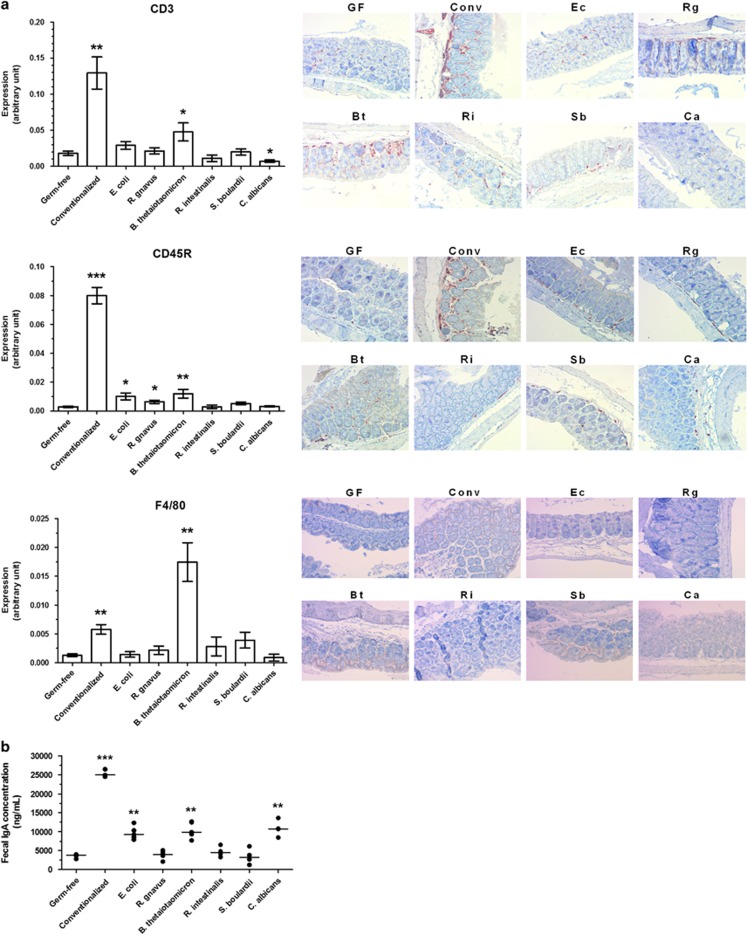
We then explored the local induction of immunity by B. thetaiotaomicron. T cells, B cells and macrophages were quantified in the colon by immunohistochemistry (by using anti-CD3, anti-CD45R and anti-F4/80 antibodies, respectively). In comparison with germ-free mice, only B. thetaiotaomicron increased the presence of these three immune cell types (Figure 6a). B. thetaiotaomicron also induced an IgA increase in the feces (which represents mucosal immunity and, notably, gut plasma cell responses), in addition to E. coli and C. albicans (Figure 6b). Consistent with the MLNs results, R. intestinalis and S. boulardii increased neither the number of immune cells in the colon nor the IgA level in the feces (Figures 6a and b).
Figure 6.
Colon immunity in mono-associated mice. (a) Colon samples were fixed in paraformaldehyde acid, embedded in paraffin and sliced in 4-μm sections, then stained by immunohistochemistry with rabbit polyclonal anti-CD3 antibody (T cells), rat monoclonal anti-CD45R antibody (B cells) and rat monoclonal anti-F4/80 antibody (macrophages). (b) IgA production was determined by ELISA in feces at 6 weeks post-gavage. In graphs, results are expressed as mean±s.e.m. (n=5 per group) and statistical comparison with the germ-free group was performed using Mann–Whitney U-test (*P<0.05/**P<0.01/***P<0.001). Representative photos were taken with x100 magnification. Bt, B. thetaiotaomicron; Ca, C. albicans; Conv, conventionalized; Ec, E. coli; GF, germ-free; Rg, R. gnavus; Ri, R. intestinalis; Sb, S. boulardii.
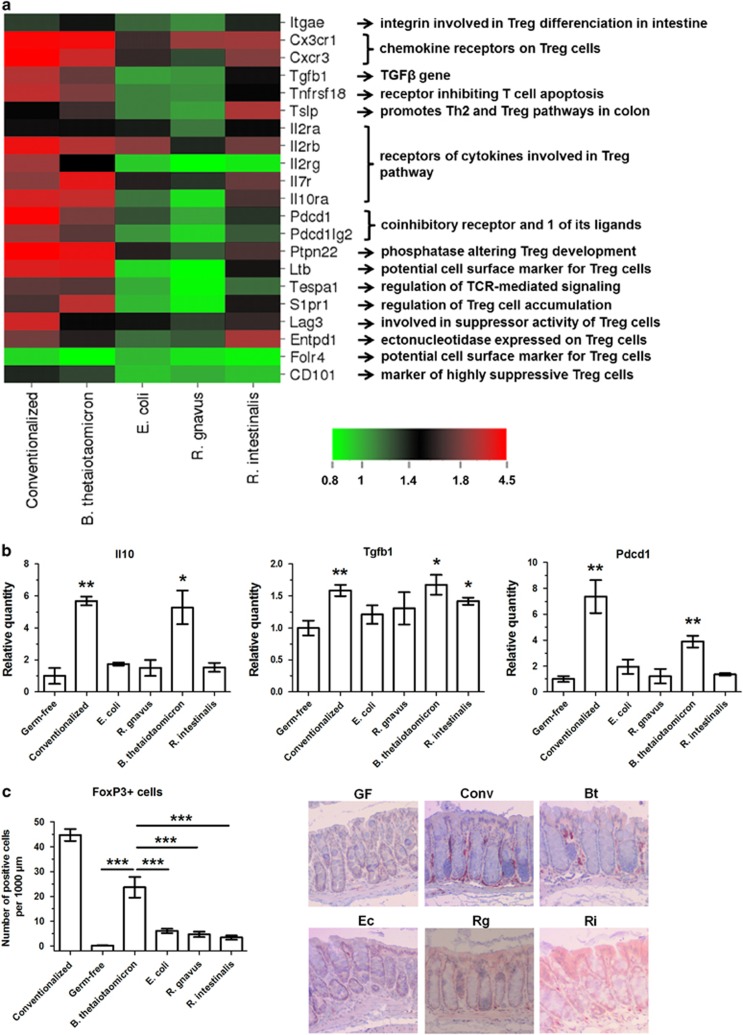
As Foxp3 expression was markedly increased in the colon of B. thetaiotaomicron-colonized mice (RT-qPCR results, Figure 4), we explored the induction of Treg pathways by this bacterium. As observed in Figure 7a, many genes involved in Treg pathways and functions were upregulated by B. thetaiotaomicron in comparison with germ-free mice and other mono-colonized mice, often at the same level as those of conventionalized mice. Three major genes involved in Treg pathways (IL-10, TGFβ and PDCD1) were selected, and we confirmed the upregulation of these three genes by B. thetaiotaomicron (but not by other bacteria) with RT-qPCR (Figure 7b). Finally, Treg cells in colon samples were stained by immunohistochemistry with anti-FoxP3 antibodies, and the induction of Treg cells by B. thetaiotaomicron was confirmed, whereas other bacteria presented low induction (Figure 7c).
Figure 7.
B. thetaiotaomicron mono-association upregulates Treg pathways. (a) Heatmap representing the microarray colon expression of selected genes involved in Treg pathways, when compared with the germ-free group. (b) The expression of three major genes involved in Treg pathways was determined in colon by RT-qPCR, using ΔΔCt quantification method with GAPDH as an internal control and germ-free group as a calibrator. (c) Colon samples were fixed in paraformaldehyde acid, embedded in paraffin and sliced in 4-μm sections, then stained by immunohistochemistry with rabbit polyclonal anti-Foxp3 antibody (Treg cells). In graphs, results are expressed as mean±s.e.m. (n=5 per group) and statistical comparison with the germ-free group (panel b) or between groups (panel c) was performed using Mann–Whitney U-test (*P<0.05/**P<0.01/***P<0.001). Representative photos were taken with x100 magnification. Bt, B. thetaiotaomicron; Conv, conventionalized; Ec, E. coli; GF, germ-free; Rg, R. gnavus; Ri, R. intestinalis.
Escherichia coli LF82 induces IDO gene expression in the colon
To confirm the microarray results with E. coli LF82, Ido1 expression was tested by RT-qPCR in colon samples. This gene was strongly induced in E. coli LF82 mono-associated mice when compared with germ-free mice and its expression was not modified in conventionalized mice or in other mono-associations except for R. gnavus (Figure 8a). We then tested IDO gene expression in humans using an HT-29 colon epithelial cell line with a luciferase reporter system. The IDO gene was induced by an E. coli LF82 supernatant or pellet, but it was not induced by the non-CD-associated E. coli MG1655 strain or by the R. gnavus strain used in our mono-association study (Figure 8b). However, this induction was lower than it was with the common IDO inducers IFNγ and IL-1β (Figure 8b). As R. gnavus mono-associated mice presented Ido1 upregulation in the colon and this increase was not observed in the luciferase reporter epithelial cell system, it suggests a possible effect on other cell types such as dendritic cells or an indirect effect via IDO-activating cytokines (such as type I or type II IFN).
Figure 8.
E. coli LF82 induces IDO expression in colon epithelial cells. To confirm microarrays, the expression of IDO was determined by RT-qPCR in the colon of mono-associated mice (a) and in human colon HT-29 cell line with luciferase reporter submitted to E. coli strains, our R. gnavus strain, or controls (b). For RT-qPCR, ΔΔCt quantification method was used, with GAPDH as an internal control and germ-free group as a calibrator. Ido1 relative quantity and IDO relative expression are expressed as mean±s.e.m. (n=3 or 5 per group). Statistical comparison with the germ-free group or between strains was performed using Mann–Whitney U-test (*P<0.05/**P<0.01/***P<0.001). Panel b is representative of two independent assays.
S. boulardii stimulates endothelial gene expression and matures blood vessels in the colon
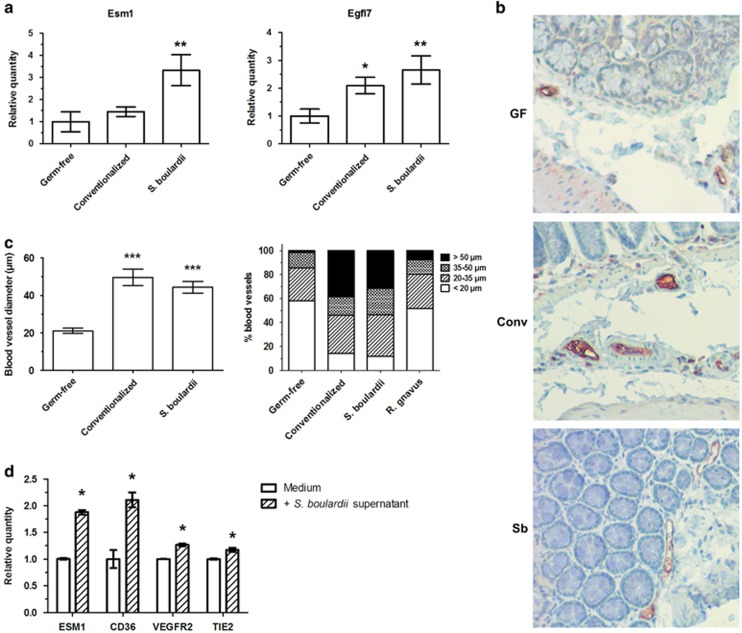
Two of the endothelial genes upregulated by S. boulardii in colon microarrays, namely Esm1 and Egfl7, were selected and their expression was tested by RT-qPCR in colon samples. These genes are involved in angiogenesis stimulation and are upregulated during angiogenesis (Nichol and Stuhlmann, 2012; Rocha et al., 2014). The upregulation of these two genes was confirmed, with expression higher than that in conventionalized mice (Figure 9a). We then wanted to confirm this endothelial effect at the morphological level in mice. To confirm these observations, endothelial cells were stained by immunohistochemistry (anti-CD31 antibody) and the vessels were analyzed in the colon. In comparison with germ-free mice, S. boulardii promoted a twofold increase in the blood vessel diameter, at a level similar to that of conventionalized mice (Figures 9b and c). A trend was observed with regards to an increase in the blood vessel number (Supplementary Figure 8). The S. boulardii mono-associated model was the only one to exhibit a profile of vessel diameters similar to that of conventionalized mice, whereas other mono-colonized mice models were more similar to germ-free mice or intermediate between conventionalized and germ-free mice (Figure 9c and Supplementary Figure 8).
Figure 9.
S. boulardii modulate angiogenesis and increases blood vessel diameter in colon. (a) To confirm microarrays, the expression of two endothelial genes was determined in colon by RT-qPCR, using ΔΔCt quantification method with GAPDH as an internal control and germ-free group as a calibrator. (b and c) Colon samples were fixed in paraformaldehyde acid, embedded in paraffin and sliced in 4-μm sections, then stained by immunohistochemistry with rabbit polyclonal anti-CD31 antibody (endothelial cells) to determine blood vessel diameter. (d) The expression of four endothelial genes was determined in HUVEC cell line with or without S. boulardii supernatant by RT-qPCR, using ΔΔCt quantification method with GAPDH as an internal control and medium as a calibrator. In graphs, results are expressed as mean or mean±s.e.m. (n=5 per group) and statistical comparison with the germ-free group or calibrator was performed using Mann–Whitney U-test (*P<0.05/**P<0.01/***P<0.001). Representative photos were taken with x100 magnification. Conv, conventionalized; GF, germ-free; Sb, S. boulardii.
To assess the effect of S. boulardii on vessels directly, a human endothelial cell line (HUVEC) was stimulated with S. boulardii culture supernatant and the expression of Esm1, CD36, Vegfr2 and Tie2 was assessed. These genes are involved in pro-angiogenic (Esm1, Vegfr2 and Tie2) or anti-angiogenic (CD36) processes (Febbraio et al., 2001; Roskoski, 2008; Cascone and Heymach, 2012; Rocha et al., 2014), and they were upregulated and/or present in the top upregulated expression signature as shown by the microarray assays performed on S. boulardii mono-colonized mice. We observed that S. boulardii culture supernatant induced the expression of the four endothelial genes, when compared with the control medium (Figure 9d), confirming the modulation of angiogenesis and the maturation of blood vessels by the yeast.
Discussion
Gut microbiota–host crosstalk is crucial for intestinal homeostasis in addition to the pathogenesis of diseases such as IBD. In this study, we selected four bacteria in addition to a probiotic yeast and a pathogenic yeast, all of which are representative of intestinal microbiota and/or are linked to IBD (and are therefore considered potential actors in the immune deregulation associated with these diseases), and we studied their impacts on the host in the context of mono-association. Although a decrease in Faecalibacterium prausnitzii was reported as a key factor in CD pathogenesis (Sokol et al., 2008b), this bacterium was not selected for study because we were not able to establish mono-associated mice with it. Some of these microorganisms were previously used for mono-association in rodents, such as B. thetaiotaomicron (Hooper et al., 2001; Lecuit et al., 2007; Wrzosek et al., 2013; Perez-Munoz et al., 2014), S. boulardii (Rodrigues et al., 1996; Martins et al., 2009), C. albicans (Phillips and Balish, 1966; Sundstrom et al., 2002; Mason et al., 2012) and E. coli LF82 (Chassaing et al., 2014), but this study was the first to use R. intestinalis and our strain of R. gnavus. In addition, this is the first time, to our knowledge that all of these mono-associations were studied together and to such an extent.
First, we observed that the global colonic transcriptional response of mono-colonized mice was much closer to that of the germ-free mice than the conventionalized mice. Nevertheless, some mono-associated models showed very specific and original transcriptomic patterns. The B. thetaiotaomicron model was surprisingly and particularly close to conventionalized mice in terms of global transcriptomics and to some extent in immune maturation. The colonization of germ-free mice with this bacterium alone seemed to promote the maturation of the colonic immune system. In comparison with conventionalized mice, it also decreased colon epithelial cell proliferation, as shown in the Ki67 immunochemistry staining and as illustrated by the downregulation of pathways involved in DNA and cell division processes. Notably, the Treg pathways were highly induced by B. thetaiotaomicron, with the FoxP3 gene being even more expressed than it was in conventionalized mice. The number of Treg in colon was also increased by the bacterium. Although the exact mechanism must be investigated, this Treg cell induction could be linked to SCFA production by B. thetaiotaomicron. In fact, propionate and acetate, which were shown to promote Treg cell expansion directly in the colon (Arpaia et al., 2013; Furusawa et al., 2013), were found to be significantly increased in the cecum of B. thetaiotaomicron mono-colonized mice. Interestingly, other species from the Bacteroides genus have been shown to induce Treg cells in the colon. Polysaccharide A from Bacteroides fragilis reportedly led the cellular and physical maturation of the developing immune system (Mazmanian et al., 2005), especially with the induction of colon Treg cells to promote immune tolerance (Round et al., 2011). As B. thetaiotaomicron can also produce polysaccharidic capsules (Burt et al., 1978), identifying a polysaccharide A-like molecule and assessing its effects could lead to interesting insights to explain the Treg-associated gene induction by B. thetaiotaomicron. Furthermore, the induction or expansion of Treg cells can be induced in mice by altered Schaedler flora that consist of eight species of microbiota dominated by a bacterium related to Bacteroides distasonis (Geuking et al., 2011). It would therefore be of great interest to confirm the expansion of Treg cells into the colon of B. thetaiotaomicron mono-associated mice and to identify the mechanisms involved. Bacteroides species may also have pro-inflammatory effects as some of them have been shown to induce colitis in genetically susceptible mice deficient for TGFβ and IL-10 receptors (Bloom et al., 2011). Interestingly, these pathways are among the key one involved in Treg cells regulatory effects.
R. gnavus mono-associated mice exhibited a remarkable transcriptomic profile with a top upregulated signature that contained metabolic pathways and was not linked to the immune response when compared with germ-free mice. Notably, R. gnavus induced the activation of several genes and pathways involved in tryptophan metabolism. Indeed, tryptophan hydroxylase 1 and IDO expression were induced by R. gnavus, and these two enzymes are responsible for tryptophan degradation into 5-hydroxytryptophane (the first step in serotonin and melatonin synthesis) and kynurenine, respectively. Moreover, the melatonin, nicotin and serotonin degradation pathways were activated downstream of these reactions (Figure 3b). As these tryptophan metabolites are involved in neurological and intestinal functions (Chen et al., 2011; Yao et al., 2011; Anderson et al., 2012; Mawe and Hoffman, 2013), the R. gnavus effect should be further investigated with respect to these issues.
S. boulardii mono-associated mice also presented a ‘non-immune' top upregulated signature when compared with germ-free mice. Vascular genes and functions were particularly regulated by S. boulardii and these results were confirmed by colon histology, showing an increased vessel size in comparison with germ-free mice. S. boulardii was previously associated with vascular events and angiogenesis. In fact, it reportedly inhibited vascular endothelial growth factor receptor signaling and angiogenesis in intestinal inflammation to limit this inflammation and promote mucosal tissue repair (Chen et al., 2013). However, our results significantly differed. Instead of inhibition, S. boulardii led to a modulation of angiogenesis and a maturation of blood vessels in our hands, given that we observed the upregulation of genes involved in pro-angiogenic (Esm1, Egfl7, Vegfr2, Tie2) and anti-angiogenic (Col18a1, CD36) pathways, in addition to larger blood vessels without significantly modified numbers. This apparent discrepancy is certainly related to the differences between the selected models. Conventional mice with DSS-induced colitis were used in Chen et al., and healthy mono-associated mice were used in our study. In any case, because angiogenesis has a major role in IBD pathogenesis (Hatoum and Binion, 2005; Chidlow et al., 2006; Danese et al., 2007; Scaldaferri et al., 2009), precisely unraveling the mechanisms by which S. boulardii modulates endothelial cells and angiogenesis to mature blood vessels may be of great interest. Submitting a S. boulardii mono-associated model to colitis and comparing the vascular results with that of colitis in conventional or conventionalized mice could yield important information. Polyamines were reported to modulate angiogenesis (Gao et al., 2013; LaRocca et al., 2013) and were produced by S. boulardii (Buts and De Keyser, 2006, 2010), and they could present an interesting direction to follow. Interestingly, although it is also a fungus, C. albicans induced very different effects in the host compared with S. boulardii. This difference was clear in the transcriptomics results in addition to the colon histology and cytokine production. Notably, the S. boulardii-associated transcriptomic profile was intermediate between germ-free and conventionalized mice profiles, whereas the C. albicans-associated transcriptomic profile was totally different from that of the control groups. Moreover, the cytokine production pattern in MLNs was dominantly pro-inflammatory with C. albicans (with high levels of IFNγ, IL-17 and IL-22), whereas it was mostly anti-inflammatory with S. boulardii (with low levels of IFNγ and IL-17). These results are in accordance with the reported effects of C. albicans and S. boulardii in colitis models, exhibiting pro- and anti-inflammatory properties, respectively (Jawhara and Poulain, 2007; Jawhara et al., 2008; Lee et al., 2009).
Finally, we showed that E. coli LF82 strongly induced IDO gene expression in mice and in human intestinal epithelial cells, in contrast with commensal E. coli MG1655. This induction has already been reported for E. coli in several cellular models (Loughman and Hunstad, 2011; Kassianos et al., 2012; Loughman and Hunstad, 2012), but this is the first time that it was found in colon cells and for the LF82 strain. IDO gene induction may be caused by E. coli-induced type I and type II IFN responses because these cytokines are potent inducers of IDO (Dai and Gupta, 1990a) or may be a direct effect of the bacterium. With regards to the indirect effect of E. coli through IFNγ, we showed that E. coli LF82 highly induced IFNγ gene expression in mice (Figure 4), and intestinal epithelial cells do not express IDO at steady state (Choudhry et al., 2009a, 2009b; Ogawa et al., 2012). However, we showed that E. coli LF82 induced IDO in the colon epithelial cell line independently from IFNγ, which was also reported for uropathogenic E. coli in bladder epithelial cells (Loughman and Hunstad, 2012). In addition, the same study also reported that commensal non-pathogenic E. coli did not induce IDO upregulation. Therefore, a direct induction of IDO by E. coli LF82 seems to be most likely. As IDO presents immunomodulatory effects (Mellor and Munn, 2004), we can hypothesize that the bacterium set up this mechanism to protect itself from the immune response. In fact, this strategy is used by other pathogens (Boasso, 2011; de Souza Sales et al., 2011; Makala et al., 2011). Another possibility would be that E. coli LF82 uses the anti-infectious effects of IDO activation via tryptophan starvation (Pfefferkorn, 1984; Byrne et al., 1986; Zelante et al., 2009) to keep other competitors away from its niche. In any case, further tests are needed to understand this IDO induction by E. coli LF82.
Thus, the microorganisms we studied have very different effects on host. Based on the genome-wide transcriptomics data in mono-colonized mice, we notably pointed out and confirmed some specific effects such as Treg induction or angiogenesis. Although the monoxenic models we used are artificial and the obtained results could be different in a complex microbiota context, our results suggest that, beside global and possibly nonspecific effects of the microbiota on host, some microorganisms may have more specific effects with limited redundancy within the microbiota. These effects may be strain or species specific or even conserved at the genus level. However, a common perturbation in the number or activity of a microbial population involved in one of these specific effects could have major effects on host physiology. This may be the case in the numerous human disease associated with dysbiosis.
In conclusion, although our study have been performed in monoxenic models that are physiologically very different from a complex microbiota context, it revealed that microorganisms with a potential role in intestinal homeostasis and inflammation may have specific impacts on the host, highlighting the complexity of microbiota–host interactions. Gut microbiota and most of the studied microorganisms had major effects on immune functions. Among them, B. thetaiotaomicron had the most impressive effect, being almost able to recapitulate the effects of the whole microbiota. In addition, we noted the effects of E. coli AIEC LF82 on IDO activation and of S. boulardii CNCM I-745 on angiogenesis modulation and the maturation of blood vessels. Our results also suggest that R. gnavus has major effects on metabolism. Interestingly, microorganisms known to be increased in IBD patients' microbiota (E. coli AIEC LF82, R. gnavus and C. albicans) exhibited a mostly pro-inflammatory pattern, whereas those known to be decreased or potentially protective in IBD (R. intestinalis, S. boulardii) exhibited a mostly anti-inflammatory pattern. Beyond the information on the role of each microorganism, this work proposes several new directions to better understand IBD pathogenesis and identify therapeutic targets.
Acknowledgments
This study was funded by Biocodex Laboratories, and TWH received a grant from Biocodex Laboratories to complete this work. BL and HS received financial support from the ATIP-AVENIR program. We thank the members of the ANAXEM germ-free platform for their valued assistance in mouse care, in addition to J Lecardonnel from the CRB GADIE core facility (INRA, Jouy en Josas, France) for his technical assistance and expertize in microarrays. We also thank the Centre de Recherche en Nutrition Humaine (Nantes), especially V Ferchaud-Roucher, for the SCFA quantification.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Anderson G, Maes M, Berk M. (2012). Inflammation-related disorders in the tryptophan catabolite pathway in depression and somatization. Adv Protein Chem Struct Biol 88: 27–48. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL et al. (2011). Commensal bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe 9: 390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boasso A. (2011). Wounding the immune system with its own blade: HIV-induced tryptophan catabolism and pathogenesis. Curr Med Chem 18: 2247–2256. [DOI] [PubMed] [Google Scholar]
- Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. (1999). Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun 67: 4499–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S, Meldrum S, Woods DR, Jones DT. (1978). Colonial variation, capsule formation, and bacteriophage resistance in Bacteroides thetaiotaomicron. Appl Environ Microbiol 35: 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buts JP, De Keyser N. (2006). Effects of Saccharomyces boulardii on intestinal mucosa. Dig Dis Sci 51: 1485–1492. [DOI] [PubMed] [Google Scholar]
- Buts JP, De Keyser N. (2010). Transduction pathways regulating the trophic effects of Saccharomyces boulardii in rat intestinal mucosa. Scand J Gastroenterol 45: 175–185. [DOI] [PubMed] [Google Scholar]
- Byrne GI, Lehmann LK, Landry GJ. (1986). Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun 53: 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FA, Barnich N, Sauvanet P, Darcha C, Gelot A, Darfeuille-Michaud A. (2008). Crohn's disease-associated Escherichia coli LF82 aggravates colitis in injured mouse colon via signaling by flagellin. Inflamm Bowel Dis 14: 1051–1060. [DOI] [PubMed] [Google Scholar]
- Cascone T, Heymach JV. (2012). Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double-edged sword? J Clin Oncol 30: 441–444. [DOI] [PubMed] [Google Scholar]
- Chassaing B, Koren O, Carvalho FA, Ley RE, Gewirtz AT. (2014). AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut 63: 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. (2011). Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol 17: 3888–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yang G, Song JH, Xu H, Li D, Goldsmith J et al. (2013). Probiotic yeast inhibits VEGFR signaling and angiogenesis in intestinal inflammation. PLoS One 8: e64227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow JH Jr, Langston W, Greer JJ, Ostanin D, Abdelbaqi M, Houghton J et al. (2006). Differential angiogenic regulation of experimental colitis. Am J Pathol 169: 2014–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry N, Korbel DS, Edwards LA, Bajaj-Elliott M, McDonald V. (2009. a). Dysregulation of interferon-gamma-mediated signalling pathway in intestinal epithelial cells by Cryptosporidium parvum infection. Cell Microbiol 11: 1354–1364. [DOI] [PubMed] [Google Scholar]
- Choudhry N, Korbel DS, Zaalouk TK, Blanshard C, Bajaj-Elliott M, McDonald V. (2009. b). Interferon-gamma-mediated activation of enterocytes in immunological control of Encephalitozoon intestinalis infection. Parasite Immunol 31: 2–9. [DOI] [PubMed] [Google Scholar]
- Dai W, Gupta SL. (1990. a). Molecular cloning, sequencing and expression of human interferon-gamma-inducible indoleamine 2,3-dioxygenase cDNA. Biochem Biophys Res Commun 168: 1–8. [DOI] [PubMed] [Google Scholar]
- Dai W, Gupta SL. (1990. b). Regulation of indoleamine 2,3-dioxygenase gene expression in human fibroblasts by interferon-gamma. Upstream control region discriminates between interferon-gamma and interferon-alpha. J Biol Chem 265: 19871–19877. [PubMed] [Google Scholar]
- Danese S, Sans M, Spencer DM, Beck I, Donate F, Plunkett ML et al. (2007). Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut 56: 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P et al. (1998). Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 115: 1405–1413. [DOI] [PubMed] [Google Scholar]
- Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N et al. (2004). High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 127: 412–421. [DOI] [PubMed] [Google Scholar]
- de Souza Sales J, Lara FA, Amadeu TP, de Oliveira Fulco T, da Costa Nery JA, Sampaio EP et al. (2011). The role of indoleamine 2, 3-dioxygenase in lepromatous leprosy immunosuppression. Clin Exp Immunol 165: 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Hajjar DP, Silverstein RL. (2001). CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest 108: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferchaud-Roucher V, Pouteau E, Piloquet H, Zair Y, Krempf M. (2005). Colonic fermentation from lactulose inhibits lipolysis in overweight subjects. Am J Physiol Endocrinol Metab 289: E716–E720. [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D et al. (2013). Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450. [DOI] [PubMed] [Google Scholar]
- Gao JH, Guo LJ, Huang ZY, Rao JN, Tang CW. (2013). Roles of cellular polyamines in mucosal healing in the gastrointestinal tract. J Physiol Pharmacol 64: 681–693. [PubMed] [Google Scholar]
- Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S et al. (2011). Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34: 794–806. [DOI] [PubMed] [Google Scholar]
- Giaffer MH, Holdsworth CD, Duerden BI. (1991). The assessment of faecal flora in patients with inflammatory bowel disease by a simplified bacteriological technique. J Med Microbiol 35: 238–243. [DOI] [PubMed] [Google Scholar]
- Giaffer MH, Holdsworth CD, Duerden BI. (1992). Virulence properties of Escherichia coli strains isolated from patients with inflammatory bowel disease. Gut 33: 646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum OA, Binion DG. (2005). The vasculature and inflammatory bowel disease: contribution to pathogenesis and clinical pathology. Inflamm Bowel Dis 11: 304–313. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. (2001). Molecular analysis of commensal host-microbial relationships in the intestine. Science 291: 881–884. [DOI] [PubMed] [Google Scholar]
- Humbert L, Maubert MA, Wolf C, Duboc H, Mahe M, Farabos D et al. (2012). Bile acid profiling in human biological samples: comparison of extraction procedures and application to normal and cholestatic patients. J Chromatogr B Analyt Technol Biomed Life Sci 899: 135–145. [DOI] [PubMed] [Google Scholar]
- Jawhara S, Poulain D. (2007). Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med Mycol 45: 691–700. [DOI] [PubMed] [Google Scholar]
- Jawhara S, Thuru X, Standaert-Vitse A, Jouault T, Mordon S, Sendid B et al. (2008). Colonization of mice by Candidaalbicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J Infect Dis 197: 972–980. [DOI] [PubMed] [Google Scholar]
- Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P et al. (2011). Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut 60: 631–637. [DOI] [PubMed] [Google Scholar]
- Kassianos AJ, Hardy MY, Ju X, Vijayan D, Ding Y, Vulink AJ et al. (2012). Human CD1c (BDCA-1)+ myeloid dendritic cells secrete IL-10 and display an immuno-regulatory phenotype and function in response to Escherichia coli. Eur J Immunol 42: 1512–1522. [DOI] [PubMed] [Google Scholar]
- Khor B, Gardet A, Xavier RJ. (2011). Genetics and pathogenesis of inflammatory bowel disease. Nature 474: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhelle A, Fruhwein N, Toburen D. (1996). [Treatment of persistent diarrhea with S. boulardii in returning travelers. Results of a prospective study]. Fortschr Med 114: 136–140. [PubMed] [Google Scholar]
- LaRocca TJ, Gioscia-Ryan RA, Hearon CM Jr, Seals DR. (2013). The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev 134: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M, Sonnenburg JL, Cossart P, Gordon JI. (2007). Functional genomic studies of the intestinal response to a foodborne enteropathogen in a humanized gnotobiotic mouse model. J Biol Chem 282: 15065–15072. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim YW, Chi SG, Joo YS, Kim HJ. (2009). The effect of Saccharomyces boulardii on human colon cells and inflammation in rats with trinitrobenzene sulfonic acid-induced colitis. Dig Dis Sci 54: 255–263. [DOI] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, Gordon JI. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124: 837–848. [DOI] [PubMed] [Google Scholar]
- Loughman JA, Hunstad DA. (2011). Attenuation of human neutrophil migration and function by uropathogenic bacteria. Microbes Infect 13: 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughman JA, Hunstad DA. (2012). Induction of indoleamine 2,3-dioxygenase by uropathogenic bacteria attenuates innate responses to epithelial infection. J Infect Dis 205: 1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makala LH, Baban B, Lemos H, El-Awady AR, Chandler PR, Hou DY et al. (2011). Leishmania major attenuates host immunity by stimulating local indoleamine 2,3-dioxygenase expression. J Infect Dis 203: 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin I, Bonnet R, Seksik P, Rigottier-Gois L, Sutren M, Bouhnik Y et al. (2004). Molecular inventory of faecal microflora in patients with Crohn's disease. FEMS Microbiol Ecol 50: 25–36. [DOI] [PubMed] [Google Scholar]
- Martins FS, Silva AA, Vieira AT, Barbosa FH, Arantes RM, Teixeira MM et al. (2009). Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch Microbiol 191: 623–630. [DOI] [PubMed] [Google Scholar]
- Mason KL, Erb Downward JR, Mason KD, Falkowski NR, Eaton KA, Kao JY et al. (2012). Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect Immun 80: 3371–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe GM, Hoffman JM. (2013). Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10: 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard CL, Elson CO, Hatton RD, Weaver CT. (2012). Reciprocal interactions of the intestinal microbiota and immune system. Nature 489: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. (2005). An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122: 107–118. [DOI] [PubMed] [Google Scholar]
- McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA et al. (1994) A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease JAMA 271: 1913–1918. [PubMed] [Google Scholar]
- Mellor AL, Munn DH. (2004). IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4: 762–774. [DOI] [PubMed] [Google Scholar]
- Nichol D, Stuhlmann H. (2012). EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood 119: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Hara T, Shimizu M, Nagano J, Ohno T, Hoshi M et al. (2012). (-)-Epigallocatechin gallate inhibits the expression of indoleamine 2,3-dioxygenase in human colorectal cancer cells. Oncol Lett 4: 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Munoz ME, Bergstrom K, Peng V, Schmaltz R, Jimenez-Cardona R, Marsteller N et al. (2014). Discordance between changes in the gut microbiota and pathogenicity in a mouse model of spontaneous colitis. Gut Microbes 5: 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn ER. (1984). Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA 81: 908–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AW, Balish E. (1966). Growth and invasiveness of Candida albicans in the germ-free and conventional mouse after oral challenge. Appl Microbiol 14: 737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoulakis C. (2009). Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii Aliment Pharmacol Ther 30: 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard ML, Lamas B, Liguori G, Hoffmann TW, Sokol H. (2015). The gut fungal microbiota: the Yin and Yang of inflammatory bowel disease. Inflamm Bowel 21: 656–665. [DOI] [PubMed] [Google Scholar]
- Rocha SF, Schiller M, Jing D, Li H, Butz S, Vestweber D et al. (2014). Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ Res 115: 581–590. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Nardi RM, Bambirra EA, Vieira EC, Nicoli JR. (1996). Effect of Saccharomyces boulardii against experimental oral infection with Salmonella typhimurium and Shigella flexneri in conventional and gnotobiotic mice. J Appl Bacteriol 81: 251–256. [DOI] [PubMed] [Google Scholar]
- Roskoski Jr R. (2008). VEGF receptor protein-tyrosine kinases: structure and regulation. Biochem Biophys Res Commun 375: 287–291. [DOI] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA et al. (2011). The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332: 974–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, Stigliano E et al. (2009). VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology 136: 585–595 e585. [DOI] [PubMed] [Google Scholar]
- Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P et al. (2003). Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut 52: 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Lay C, Seksik P, Tannock GW. (2008. a). Analysis of bacterial bowel communities of IBD patients: what has it revealed? Inflamm Bowel Dis 14: 858–867. [DOI] [PubMed] [Google Scholar]
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ et al. (2008. b). Faecalibacteriumprausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 105: 16731–16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert-Vitse A, Sendid B, Joossens M, Francois N, Vandewalle-El Khoury P, Branche J et al. (2009). Candidaalbicans colonization and ASCA in familial Crohn's disease. Am J Gastroenterol 104: 1745–1753. [DOI] [PubMed] [Google Scholar]
- Sundstrom P, Balish E, Allen CM. (2002). Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis 185: 521–530. [DOI] [PubMed] [Google Scholar]
- Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z et al. (2010). A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139: 1844–1854 e1841. [DOI] [PubMed] [Google Scholar]
- Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V et al. (2013). Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Fang J, Yin YL, Feng ZM, Tang ZR, Wu G. (2011). Tryptophan metabolism in animals: important roles in nutrition and health. Front Biosci (Schol Ed) 3: 286–297. [DOI] [PubMed] [Google Scholar]
- Zelante T, Fallarino F, Bistoni F, Puccetti P, Romani L. (2009). Indoleamine 2,3-dioxygenase in infection: the paradox of an evasive strategy that benefits the host. Microbes Infect 11: 133–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.