Abstract
The emergence of malarial resistance to most antimalarial drugs is the main factor driving the continued effort to identify/discover new agents for combating the disease. Moreover, the unacceptably high mortality rate in severe malaria has led to the consideration of adjuvant therapies. Senna singueana leaves are traditionally used against malaria and fever. Extracts from the leaves of this plant demonstrated in vitro and in vivo antioxidant activities, which in turn could reduce the severity of malaria. Extracts from the root bark of this plant exhibited antiplasmodial activity; however, the leaves are the more sustainable resource. Thus, S. singueana leaf was selected for in vivo evaluation as a potential alternative or adjuvant therapy for malaria. Using malaria [Plasmodium berghei ANKA, chloroquine (CQ) sensitive]-infected Swiss albino mice of both sexes, 70% ethanol extract of S. singueana leaves (alone and in combination with CQ) was tested for antimalarial activity and adjuvancy potential. The 4-day suppressive test was used to evaluate antimalarial activity. The dose of S. singueana extract administered was safe to mice and exhibited some parasite suppression effect: extract doses of 200 mg/kg/d, 400 mg/kg/d, and 800 mg/kg/d caused 34.54%, 44.52%, and 47.32% parasite suppression, respectively. Concurrent administration of the extract with CQ phosphate at varied dose levels indicated that the percentage of parasite suppression of this combination was higher than administering CQ alone, but less than the sum of the effects of the extract and CQ acting separately. In conclusion, the study indicated that 70% ethanol extract of S. singueana leaf was safe to mice and possessed some parasite suppression effect. Coadministration of the extract with CQ appeared to boost the overall antimalarial effect, indicating that the combination may have a net health benefit if used as an adjuvant therapy.
Keywords: adjuvant, antimalarial, in vivo, Plasmodium berghei, Senna singueana
Graphical abstract
1. Introduction
Human malaria is mainly caused by four plasmodium species (Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax). Recently, a fifth human parasite (Plasmodium knowlesi) has been reported. Of these, P. falciparum is the most widespread, causes the most severe infections, and is responsible for nearly all malaria-related deaths.1, 2, 3, 4 Malaria remains a major cause of morbidity and mortality worldwide, with the vast majority of cases reported in African countries.5 The global spread of multidrug-resistant malarial parasites has led to an urgent need to develop new chemotherapeutic agents.6 Furthermore, a significant number of malarial patients progress to severe malaria with organ dysfunction, which is more common in immunologically naïve individuals, especially young children.7 The current primary treatment for severe malaria is parenteral quinine or administration of artemisinin derivatives. Despite improved survival with artemisinin derivatives, mortality rate in severe malarial cases treated with either artemisinin derivatives or quinine remains unacceptably high, and therefore, adjuvant therapies (additional therapies that modify the pathophysiologic processes caused by malaria) are urgently needed.5, 7 Accordingly, numerous adjuvant therapies have been tested and some of those under clinical trials for severe malaria are immune response modulators, antioxidants, anticoagulants, and agents with antiseizure activity.5
Medicinal plants are the most important sources of new antimalarial drugs as well as adjuvant therapies for severe malaria. Historically, plants have provided two major drugs for the treatment of malaria, namely, quinine from Cinchona species and artemisinin from Artemisia annua.8, 9 Major drugs that are available for infectious diseases, particularly for malaria, have been obtained from plants. In malaria-endemic regions, people use traditional medicinal plants to treat malaria and the fever or symptoms associated with this pathology.9 One such medicinal plant is Senna singueana (Del.) Lock (Syn: Cassia singueana; Fabaceae), which has many medicinal uses throughout Africa,10 including traditional antimalarial uses. Its leaf sap is drank to combat malaria in Tanzania11 and a hot decoction of powdered leaves, taken orally, is indicated for malaria and fever in Kenya12 and Burkina Faso.11, 13 Previous studies have indicated that methanolic extract of the root bark of the plant exhibited significant antiplasmodial activity against Plasmodium berghei.14 The root bark is also reported to contain lupeol,10 a triterpene that exhibits a wide spectrum of biological activity such as antimalarial effects against chloroquine (CQ)-resistant P. falciparum.15 Although S. singueana has numerous medicinal uses, research into its pharmacology has been scarce and is restricted to the root bark. Because of its many medicinal uses, research into the properties of its leaves is warranted, as they are a more sustainable source of medicine than the root or stem bark.10 Furthermore, scientific claims indicate that various extracts of the leaf possess in vitro and in vivo antioxidant activities16, 17, 18. Antioxidant therapy using the leaf extract of S. singueana is claimed to reduce malarial severity.7 Taking these considerations into account, the aim of this work was to evaluate the effectiveness of S. singueana as an antimalarial agent or as an adjuvant therapy for severe malaria.
2. Materials and methods
2.1. Collection of plant material and preparation of extract
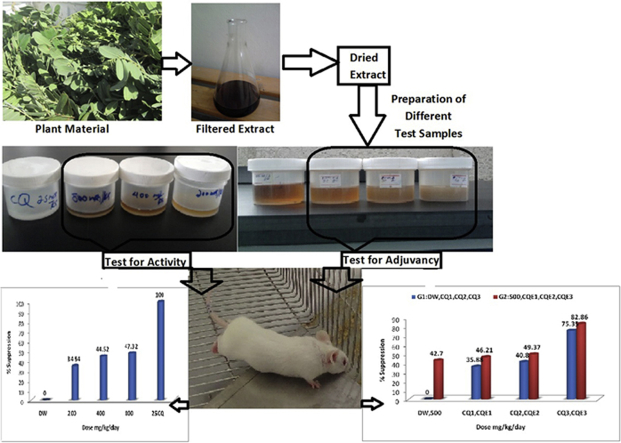
Sufficient amounts of S. singueana leaves were collected in March 2013 from the northwest and central zones of Tigray, Northern Ethiopia. The plant material was authenticated at the National Herbarium, Department of Biology, Addis Ababa University (Addis Ababa, Ethiopia). Photographs of S. singueana taken during collection at the collection site and collected leaves are shown in Fig. 1. The collected leaves were sorted, dried, powdered, and then defatted using petroleum ether (HiMedia Laboratories, Mumbai, India) for 48 hours. The marc was then allowed to dry and extracted three times by maceration using 70% ethanol with intermittent agitation. Each maceration was carried out for 72 hours. The extracts were filtered, collected, concentrated under reduced pressure using Rotavapor (BIBBY Sterlin Ltd, stuart®, UK), and dried in a vacuum oven at 35°C. The dried extracts were then transferred into vials and stored for further use.
Fig. 1.
Photographs of Senna singueana (Del.) Lock (Fabaceae) and the collected leaves.
2.2. Experimental animals and parasite
Swiss albino mice of both sexes (8–12 weeks of age) were obtained from the Pharmacology Animal House of the Department of Pharmacy, College of Health Sciences (Mekelle University, Mekelle, Ethiopia). The animals were housed in an air-conditioned room and were allowed to acclimatize for 1 week before the study. Before and during the experiment, the animals were provided with standard animal feed or pellets and clean water ad libitum. P. berghei ANKA strain (CQ sensitive)-infected donor mice were obtained from the Faculty of Science, Addis Ababa University (Addis Ababa, Ethiopia). The parasite was subsequently maintained in the Pharmacology Laboratory of the Department of Pharmacy, College of Health Sciences, Mekelle University by serial blood passage from one mouse to another.
2.3. Ethical considerations
The study was performed after obtaining ethical approval from the Institutional Review Committee of the College of Health Sciences, Mekelle University. All experiments were carried out in accordance with scientific procedures. Ethical issues, especially the handling of experimental animals, were respected.
2.4. Acute oral toxicity test
An acute oral toxicity study was performed according to the Organization for Economic Cooperation and Development guidelines 423,19 but with a slight modification in the number of mice used. The study animals (n = 20 mice) were divided into four groups of five mice per cage. The animals were physically active and their consumption of food and water was normal. Before the administration of a single dose of the extract, the mice fasted for 2 hours. First, an acute oral toxicity study was performed on five female mice (weight, 30–32 g). The mice were given 2000 mg/kg of the extract dissolved in distilled water. The test was then performed on the remaining 15 mice (10 female and 5 male; weight, 23–25 g), which were divided into three groups of five each: The first group (5 female mice) was given distilled water, whereas the second (5 female mice) and third (5 male mice) groups were given the hydroalcoholic leaf extract of S. singueana (5000 mg/kg dissolved in distilled water). After the oral administration of 0.5 mL of the test extract (2000 mg/kg and 5000 mg/kg), the animals were continuously observed during the first 30 minutes, periodically observed during the first 24 hours, with particular attention during the first 4 hours, and observed daily thereafter. The mice in the control group received 0.5 mL of distilled water. The animals were observed for 14 days for gross behavioral changes such as loss of appetite, hair erection, lacrimation, tremors, convulsions, salivation, diarrhea, mortality, and other signs of toxicity manifestation.19
2.5. Antiplasmodial activity test of extract alone and in combination with CQ
The commonly used method of a 4-day suppressive test (Peters' test) was used to evaluate antimalarial activity. Swiss albino mice were randomly divided into five groups of five mice per cage: three test groups and two control groups (CQ was used as a standard drug and distilled water as a negative control). Blood was taken from donor mice with approximately 24% parasitemia and diluted in physiological saline (2 mL blood was diluted with normal saline to 8 mL). Mice weighing 25–30 g were infected with 0.2 mL (≈1 × 107 P. berghei-parasitized erythrocytes) diluted blood intraperitoneally. Test extracts were prepared in three different doses (200 mg/kg, 400 mg/kg, and 800 mg/kg of body weight). CQ phosphate was prepared at a dose of 25 mg/kg. The total volume of each dose was 0.5 mL. The extract (0.5 mL) or standard drug was administered as a single dose per day using oral gavages. Treatment was started 2 hours after the infection on Day 0 and was then continued daily for 4 days (i.e., from Day 0 to Day 3). On the 5th day (Day 4), thin blood smears, on microscopic slides, were obtained from the tail of each mouse. The blood smears were fixed with methanol and stained with 10% Giemsa for 15 minutes. The parasitemia level was determined by counting the number of parasitized erythrocytes from six random fields of the microscope. The average percentage of parasitemia and suppression were calculated as follows:
The 4-day suppression test was used to assess the adjuvant potential of the extract. The antiplasmodial effect of the hydroalcoholic leaf extract in combination with CQ phosphate against P. berghei infection in mice was determined. Forty Swiss albino mice were randomly divided into eight groups of five mice per cage: three groups received different doses of CQ alone (0.1 mg/kg, 1 mg/kg, and 10 mg/kg), another three groups received different doses of CQ (0.1 mg/kg, 1 mg/kg, and 10 mg/kg) in combination with 500 mg/kg of extract. The seventh group received only 500 mg/kg of the extract and the eighth group received distilled water as a negative control. All mice were infected with 0.2 mL of diluted blood. The remaining steps of the procedure were carried out as stated earlier.
2.6. Data analysis
Data were analyzed using Windows SPSS Version 16 (SPSS Inc., Chicago, USA). The results were compared among and within groups by one-way analysis of variance followed by Tukey's honestly significant difference post hoc test. The results were considered significant at a value of p < 0.05.
3. Results and discussion
3.1. Preparation of plant extract
Traditional medicines are often used in the form of aqueous or hydroalcohol preparations. S. singueana juice or decoction is used as the traditional antimalarial remedy.11, 12, 13 Ethanol is the solvent of choice for obtaining classical extracts and ethanol is usually mixed with water to optimize extraction. Thus, 70% ethanol (ethanol/water ratio 7:3) was used in this study to prepare the plant extract. The percentage yield was 19.6%.
3.2. Acute toxicity
In general, historical use of a material in traditional medicine is an indicator that the material is nontoxic.8 However, the activity of the material still has to be supported with scientific evidence. In acute toxicity studies, no mortality or sign of toxicity was observed in study animals after the oral administration of S. singueana hydroalcoholic leaf extract even at doses as high as 5000 mg/kg, indicating that S. singueana leaf can be used as a safe herbal product. However, while doing the first acute toxicity test at a dose of 2000 mg/kg, a slight reduction in the average weight of the mice was observed during the initial 14-day observation period, which could be an indication of possible toxicity. Thus, to further verify whether the extract actually caused the weight reduction, the test was repeated while making some modifications as follows: (1) the dose was increased to 5000 mg/kg; (2) mice of both sexes were used; and (3) mice below the age of 8 weeks were used. The results of this test confirmed that the S. singueana leaf extract was not the cause of the weight reduction. Thus, the extract does not have any obvious toxicity even at a single dose as high as 5000 mg/kg. Supporting reports include the following: 80% methanolic extract of S. singueana leaves was safe for rats and it did not cause mortality at an oral dose of up 4000 mg/kg. In addition, it was indicated that the use of the extract for therapeutic medication may not be a health hazard to humans.20, 21, 22 By contrast, Ruffo et al23 suggested that S. singueana is known to be toxic and care should be taken while using it medicinally. Thus, although the existing evidence indicates that S. singueana leaf can be used as a safe herbal product, care is still warranted, especially if long-term use is intended.
3.3. Screening for antimalarial activity and adjuvancy potential
Experimental results are presented as a tabular summary and include mean percentage of parasitemia for evaluating antimalarial activity (Table 1), adjuvancy potential testing (Table 2), and the mean survival time of animals during testing of both antimalarial activity and adjuvancy potential (Table 3).
Table 1.
Mean percentage of parasitemia in the different treatment groups.
| Treatment groups | Mean % parasitemia |
|---|---|
| Distilled water | 24.26 ± 0.41 |
| 200 mg/kg extract | 15.88 ± 3.75 |
| 400 mg/kg extract | 13.46 ± 2.57 |
| 800 mg/kg extract | 12.77 ± 1.58 |
| 25 mg/kg chloroquine | 0.00 ± 0.00 |
Distilled water served as the negative control, whereas chloroquine was the positive control.
Table 2.
Mean percentage of parasitemia in the different treatment groups.
| Treatment groups | Mean % parasitemia |
|---|---|
| Distilled water | 14.24 ± 0.72 |
| 0.1 mg/kg CQ | 9.13 ± 0.74 |
| 1 mg/kg CQ | 8.43 ± 1.37 |
| 10 mg/kg CQ | 3.51 ± 0.12 |
| 500 mg/kg extract | 8.16 ± 0.19 |
| 0.1 mg/kg CQ + 500 mg/kg extract | 7.66 ± 0.47 |
| 1 mg/kg CQ + 500 mg/kg extract | 7.21 ± 0.60 |
| 10 mg/kg CQ + 500 mg/kg extract | 2.44 ± 0.06 |
Distilled water was used as the negative control.
CQ = chloroquine.
Table 3.
Mean survival time of mice during antimalarial testing and adjuvancy potential in the different treatment groups.
| Treatment groups | Mean survival time (d) |
|---|---|
| Mean survival time of mice during antimalarial testing | |
| GI (distilled water) | 7.7 ± 0.43589 |
| GII (200 mg/kg extract) | 7.8 ± 0.25495 |
| GIII (400 mg/kg extract) | 8.4 ± 0.18708 |
| GIV (800 mg/kg extract) | 8.7 ± 0.12247 |
| GV (25 mg/kg CQ) | All survived |
| Mean survival time of mice during testing for adjuvancy potential | |
| Distilled water | 9.9 ± 0.24495 |
| 0.1 mg/kg CQ | 10.3 ± 0.20000 |
| 1 mg/kg CQ | 11.4 ± 0.50990 |
| 10 mg/kg CQ | Four survived but one died on Day 12 |
| 500 mg/kg extract | 11 ± 0.52440 |
| 0.1 mg/kg CQ + 500 mg/kg extract | 11.4 ± 0.18708 |
| 1 mg/kg CQ + 500 mg/kg extract | 13.00 ± 0.35355 |
| 10 mg/kg CQ + 500 mg/kg extract | All survived |
In antimalarial testing, distilled water was used as the negative control, whereas chloroquine was used as the positive control.
CQ = chloroquine.
3.4. Antimalarial activity of S. singueana leaf extract
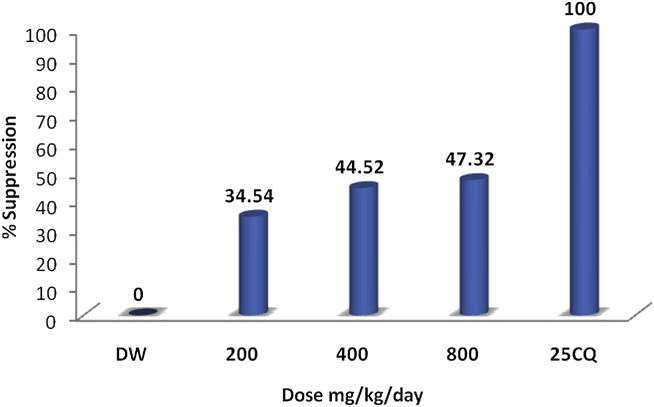
The results of this study indicated that S. singueana hydroalcoholic leaf extract possesses antiplasmodial activity against P. berghei in vivo. Compared with the negative control, the extract reduced the percentage of parasitemia (Table 1); however, none of the animals survived in any of the doses administered. Extract doses of 200 mg/kg/d, 400 mg/kg/d, and 800 mg/kg/d caused 34.54%, 44.52%, and 47.32% chemosuppression, respectively (Fig. 2). These results indicate that the extract exhibited some dose-dependent activity but dose dependency was not statistically significant (p > 0.05). When compared with the negative control, the chemosuppressive effect of S. singueana hydroalcoholic leaf extract was significant (p < 0.05) for doses ≥400 mg/kg/d. CQ had 100% chemosuppression at a dose of 25 mg/kg/d. Although the difference was not statistically significant at 95% confidence interval, better mean survival time was observed in the extract-treated mice than in mice that received distilled water (Table 3). This may be because the relatively lower antimalarial activity and low potency of the extract may not be able to inhibit the growth of the parasite especially during the late stages (Days 3 and 4) of infection, which is normally associated with a critically high percentage of parasitemia.24
Fig. 2.
Percentage of parasitemia suppression at different doses (200 mg/kg/d, 400 mg/kg/d, and 800 mg/kg/d) of Senna singueana leaf extract compared with the negative control [distilled water (DW)] and the positive control [25 mg/kg/d of chloroquine phosphate (25 CQ)].
The study of antiprotozoal compounds from plants has benefited from the development of bioassay techniques. The 4-day suppressive test (Peters' test) is the commonly applied method that uses P. berghei in mice for antimalarial screening,8, 9 and determination of percentage of parasitemia suppression is the most reliable parameter for evaluating antimalarial efficiency. Based on the measurement results, the in vivo antiplasmodial activity of an extract can be classified as moderate, good, and very good if an extract displays a percentage of parasite suppression ≥50% at a dose of 500 mg/kg body weight/d, 250 mg/kg body weight/d, and 100 mg/kg body weight/d, respectively.25, 26 According to this classification, the results of this study show that the hydroalcoholic extract of S. singueana leaf exhibited more or less moderate antiplasmodial activity as the percentage of parasite suppression was less than 50% even at a dose of 800 mg/kg, although it was incomparable to the standard drug, CQ. This perhaps might be due to the crude nature of the extract.27 Previous studies reported a percentage of parasite suppression of 66.51% for the methanolic extract of the root bark at a dose of 100 mg/kg, which indicates that the methanolic extract exhibited very good antiplasmodial activity against P. berghei.14 In comparison, the leaf part, although a relatively sustainable resource, displayed lower antiplasmodial activity than the root part. However, the study provides evidence to support the traditional use of S. singueana leaves as an antimalarial remedy. Furthermore, diterpene phytol is reported to be a major constituent of S. singueana leaves18; phytol in the leaves exhibits antiplasmodial activity against CQ-sensitive and CQ-resistant strains of P. falciparum.28, 29 Phytol also showed antitrypanosomal activity.30 Thus, among others, the antimalarial activity of S. singueana leaves might be attributed to the presence of the terpene class of constituents, which have been implicated in antiplasmodial activities of many plants.31, 32, 33
3.5. Antimalarial activity of the combination of S. singueana extract and CQ
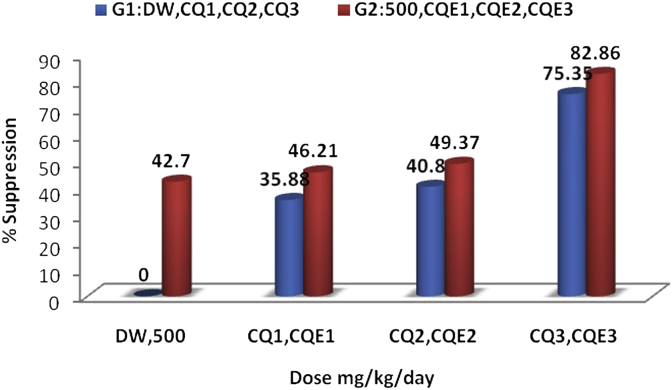
The effect of the combination of S. singueana extract and CQ phosphate in all the tested dose levels (Table 2) showed that the percentage of parasite suppression of the combination was higher than CQ alone, but less than the sum of the effects of the extract and CQ acting separately (Fig. 3). These results show that although the overall net effect of the combination was better than CQ alone, there existed an interaction between CQ and the extract. Moreover, while combining the different doses of CQ phosphate solutions with the extract solution, each combination was changed to suspension, which could indicate the occurrence of at least some physical interaction. Such an interaction in turn may influence the pharmacokinetics of both chloroqhine (CQ) and constituents of the extract. It is possible to consider a pharmacodynamic influence but it may be quite complex to explain. Literature supports the fact that various plant extracts influence the pharmacokinetic parameters of CQ both negatively and positively. For example, it has been reported that most of the pharmacokinetic parameters of CQ were decreased and the bioavailability and effectiveness impaired following concurrent administration of CQ with an aqueous extract of Azadirachta indica leaf,34 ethanol extracts of leaves of Gnetum africana, Heinsia crinita, Telfairia occidentalis, and Vernonia amygdalina.35, 36, 37, 38 By contrast, grapefruit juice39 and spinach40 were shown to increase the blood level of CQ. Some extracts even demonstrated synergistic interaction41, and various studies support the use of plant preparations as potential adjuvants to CQ therapy.6, 42 Despite the possible extract–drug interaction, coadministration of S. singueana hydroalcoholic leaf extract with CQ appeared to increase the overall antimalarial effect. Moreover, a better mean survival time was observed in the mice that received a combination of extract and CQ than CQ alone at all tested concentrations (Table 3). A statistically significant mean survival time was seen between a (500 mg/kg extract + 1 mg/kg CQ)-combination and 1 mg/kg CQ alone, whereas this was not true between a (500 mg/kg extract + 0.1 mg/kg CQ)-combination and 0.1 mg/kg CQ alone. All five mice that received the (500 mg/kg extract + 10 mg/kg CQ) combination survived, of which only four survived after receiving 10 mg/kg CQ alone. The fifth mouse died on the 12th day. Therefore, it can be suggested that the extract may boast a net health benefit if used as an adjuvant therapy to support CQ for the treatment of malaria.
Fig. 3.
Percentage of parasitemia suppression using chloroquine alone and using a combination of Senna singueana extract and chloroquine. G1 = four groups [distilled water (DW) and three doses of chloroquine (CQ1–3; 0.1 mg/kg/d, 1 mg/kg/d, and 10 mg/kg/d)]; G2 = four groups [extract (500 mg/kg/d), extract combined with three doses of chloroquine (CQE1–3; 0.1 + 500 mg/kg/d), 1 + 500 mg/kg/d), and 10 + 500 mg/kg/d)].
4. Conclusion
In this study, results of acute toxicity and antiplasmodial activity tests on Swiss albino mice indicated that 70% ethanol extract of S. singueana leaves was relatively safe to mice and demonstrated antimalarial activity, which may contribute to its traditional use for the management of malaria and related symptoms such as fever. The results also indicated that despite the possible extract–drug interaction, concurrent use of CQ and S. singueana leaf extract appeared to increase the overall antimalarial effect, and therefore, the extract may have a net health benefit if used as an adjuvant therapy to support CQ for the treatment of malaria.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
The project has been funded from the recurrent budget of College of Health Science, Mekelle University (Mekelle, Ethiopia). The malarial parasite was obtained from the Faculty of Science, Addis Ababa University (Addis Ababa, Ethiopia). The authors are very grateful to both institutes.
M.G.H. designed the study, collected the plant material, performed laboratory work, data analysis, data interpretation, and wrote the paper. G.G.S. participated in the selection and handling of Plasmodium berghei-infected donor mice. Both G.G.S. and B.S.F. participated in the study design, laboratory work, data analysis, and interpretation. H.D.G. and S.B.T. organized and helped with the parasite count component of the laboratory work. All authors read, commented, and approved the final manuscript.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.World Health Organization . 2nd ed. World Health Organization; Geneva, Switzerland: 1995. WHO Model Prescribing Information: Drugs Used in Parasitic Diseases. [Google Scholar]
- 2.Cox-Singh J., Hiu J., Lucas S.B., Divis P.C., Zulkarnaen M., Chandran P. Severe malaria—a case of fatal Plasmodium knowlesi infection with post-mortem findings: a case report. Malar J. 2010;9:10. doi: 10.1186/1475-2875-9-10. http://dx.doi.org/10.1186/1475-2875-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figtree M., Lee R., Bain L., Kennedy T., Mackertich S., Urban M. Plasmodium knowlesi in Human, Indonesian Borneo. Emerg Infect Dis. 2010;16:672–674. doi: 10.3201/eid1604.091624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sermwittayawong N., Singh B., Nishibuchi M., Sawangjaroen N., Vuddhakul V. Human Plasmodium knowlesi infection in Ranong province, southwestern border of Thailand. Malar J. 2012;11:36. doi: 10.1186/1475-2875-11-36. http://dx.doi.org/10.1186/1475-2875-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John C.C., Kutamba E., Mugarura K., Opoka R.O. Adjunctive therapy for cerebral malaria and other severe forms of Plasmodium falciparum malaria. Expert Rev Anti Infect Ther. 2010;8:997–1008. doi: 10.1586/eri.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carraz M., Jossang A., Franetich J.F. A plant-derived morphinan as a novel lead compound active against malaria liver stages. PLoS Med. 2006;3:e513. doi: 10.1371/journal.pmed.0030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackerman H.C., Beaudry S.D., Fairhurst R.M. Antioxidant therapy: reducing malaria severity? Crit Care Med. 2009;37:758–760. doi: 10.1097/CCM.0b013e318194d5de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trease G.E., Evans W.C. 15th ed. Bailliere Tindall; London, UK: 2002. Pharmacognosy. [Google Scholar]
- 9.Mambu L., Grellier P. Antimalarial compounds from traditionally used medicinal plants. In: Colegate S.M., Molyneux R.J., editors. Bioactive Natural Products: Detection, Isolation, and Structural Determination. 2nd ed. CRC; Boca Raton, FL: 2008. pp. 491–529. [Google Scholar]
- 10.Kawanga V., Bosch C.H. 2007. Senna singueana (Delile) Lock.http://database.prota.org/search.htm [accessed 16.02.14] [Google Scholar]
- 11.Fowler D.G. 2006. Traditional Fever Remedies: A List of Zambian Plants.http://www.giftsofhealth.org/ritam/news/Traditional_Fever_remedie1.pdf [last accessed 08.12.13] [Google Scholar]
- 12.Nanyingi M.O., Mbaria J.M., Lanyasunya A.L. Ethnopharmacological survey of Samburu district, Kenya. J Ethnobiol Ethnomed. 2008;4:14. doi: 10.1186/1746-4269-4-14. http://dx.doi.org/10.1186/1746-4269-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadembega P., Boussim J.I., Nikiema J.B., Poli F., Antognoni F. Medicinal plants in Baskoure, Kourittenga Province, Burkina Faso: an ethnobotanical study. J Ethnopharmacol. 2011;133:378–395. doi: 10.1016/j.jep.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Adzu B., Abbah J., Vongtau H., Gamaniel K. Studies on the use of Cassia singueana in malaria ethnopharmacy. J Ethnopharmacol. 2003;88:261–267. doi: 10.1016/s0378-8741(03)00257-5. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan T., Srivastava G.K., Pathak A. Solid-phase synthesis and bioevaluation of Lupeol-based libraries as antimalarial agents. Bioorg Med Chem Lett. 2002;12:2803–2806. doi: 10.1016/s0960-894x(02)00623-6. [DOI] [PubMed] [Google Scholar]
- 16.Gebrelibanos M., Asres K., Veeresham C. In vitro radical scavenging activity of the leaf and bark extracts of Senna singueana (Del.) Lock. Ethiop Pharm J. 2007;25:77–84. [Google Scholar]
- 17.Madubunyi I.I., Ode O.J. In vitro and in vivo antioxidant potential of the methanolic extract of Cassia singueana Delile (Fabaceae) Lock leaves. Comp Clin Path. 2012;21:1565–1569. [Google Scholar]
- 18.Ibrahim M.A., Koorbanally N.A., Islam M.S. In vitro anti-oxidative activities and GC-MS analysis of various solvent extracts of Cassia singueana parts. Acta Pol Pharm. 2013;70:709–719. [PubMed] [Google Scholar]
- 19.OECD . OECD; Paris, France: 2001. OECD Test Guideline 423. Acute Oral Toxicity—Acute Toxic Class Method. [Google Scholar]
- 20.Ode O.J., Nwaehujor C.O. The haematological effects of chronic toxicity with Cassia singueana leaf extract in rats. J Pharm Biomed Sci. 2010;8:1–5. [Google Scholar]
- 21.Ode O.J. The antiulcer activities of the methanol extract of Cassia singueana leaves using indomethacin-induced gastric ulcer model in rats. Adv Sci Res. 2011;2:66–69. [Google Scholar]
- 22.Ode O.J., Asuzu O.V., Oladele G.M. The biochemical changes in rats following chronic toxicity with Cassia singueana leaf extract. J Pharm Biomed Sci. 2011;8:1–4. [Google Scholar]
- 23.Ruffo C.K., Birnie A., Tengnäs B. Regional Land Management Unit (RELMA) and Swedish International Development Cooperation Agency (SIDA); Nairobi, Kenya: 2002. Edible wild plants of Tanzania. RELMA Technical Handbook Series 27. [Google Scholar]
- 24.Basir R., Chan K.L., Yam M.F. Antimalarial activity of selected Malaysian medicinal plants. Phytopharmacology. 2012;3:82–92. [Google Scholar]
- 25.Muñoz V., Sauvain M., Bourdy G. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part I. Evaluation of the antimalarial activity of plants used by the Chacobo Indians. J Ethnopharmacol. 2000;69:127–137. doi: 10.1016/s0378-8741(99)00148-8. [DOI] [PubMed] [Google Scholar]
- 26.Deharo E., Bourdy G., Quenevo C., Muñoz V., Ruiz G., Sauvain M. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part V. Evaluation of the antimalarial activity of plants used by the Tacana Indians. J Ethnopharmacol. 2001;77:91–98. doi: 10.1016/s0378-8741(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 27.Okokon J.E., Antia B.S., Igboasoiyi A.C., Essien E.E., Mbagwu H.O. Evaluation of antiplasmodial activity of ethanolic seed extract of Picralima nitida. J Ethnopharmacol. 2007;111:464–467. doi: 10.1016/j.jep.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Köhler I., Jenett-Siems K., Kraft C. Herbal remedies traditionally used against malaria in Ghana: bioassay-guided fractionation of Microglossa pyrifolia (Asteraceae) Z Naturforsch C. 2002;57:1022–1027. doi: 10.1515/znc-2002-11-1212. [DOI] [PubMed] [Google Scholar]
- 29.Grace M.H., Lategan C., Graziose R., Smith P.J., Raskin I., Lila M.A. Antiplasmodial activity of the ethnobotanical plant Cassia fistula. Nat Prod Commun. 2012;7:1263–1266. [PubMed] [Google Scholar]
- 30.Bero J., Beaufay C., Hannaert V., Hérent M.F., Michels P.A., Quetin-Leclercq J. Antitrypanosomal compounds from the essential oil and extracts of Keetia leucantha leaves with inhibitor activity on Trypanosoma brucei glyceraldehyde-3-phosphate dehydrogenase. Phytomedicine. 2013;20:270–274. doi: 10.1016/j.phymed.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Batista R., Silva A.J., Jr., de Oliveira A.B. Plant-derived antimalarial agents: new leads and efficient phytomedicines. Part II. Non-alkaloidal natural products. Molecules. 2009;14:3037–3072. doi: 10.3390/molecules14083037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ene A.C., Atawodi S.E., Ameh D.A., Ndukwe G.I., Kwanashie H.O. Bioassay-guided fractionation and in vivo antiplasmodial effect of fractions of chloroform extract of Artemisia maciverae Linn. Acta Trop. 2009;112:288–294. doi: 10.1016/j.actatropica.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Kaur K., Jain M., Kaur T., Jain R. Antimalarials from nature. Bioorg Med Chem. 2009;17:3229–3256. doi: 10.1016/j.bmc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 34.Nwafor S.V., Akah P.A., Okoli C.O., Onyirioha A.C., Nworu C.S. Interaction between chloroquine sulphate and aqueous extract of Azadirachta indica A. Juss (Meliaceae) in rabbits. Acta Pharm. 2003;53:305–311. [PubMed] [Google Scholar]
- 35.Eseyin O.A., Edoho E.J., Godwin N.E., Igboasoiyi A.C., Ekpo A. Effects of the leaf extract of Telfairia occidentalis on the pharmacokinetics of chloroquine in rats. Int J Biol Chem. 2007;1:256–260. [Google Scholar]
- 36.Igboasoiyia C., Eseyin O.A., Udoma N.F. The effect of ethanolic extract of Vernonia amygdalina leaves on some pharmacokinetics parameters of chloroquine in rats. Res J Pharmacol. 2008;2:24–27. [Google Scholar]
- 37.Olorunfemi E., Aniekan E., Emmanuel A., Udoh I. Effects of the leaf of Heinsia crinita on the pharmacokinetics of chloroquine in rats. Int J Biol Pharm Res. 2010;1:88–93. [Google Scholar]
- 38.Eseyin O., Ebong A., Ubobre A., Ekarika J., Udo I. Changes in some pharmacokinetics parameters of chloroquine by Gnetum africana. Macedon J Med Sci. 2012;5:275–279. [Google Scholar]
- 39.Ali B.H., Al-Qarawi A., Mousa H.M. Effect of grapefruit juice on plasma chloroquine kinetics in mice. Clin Exp Pharmacol Physiol. 2002;29:704–706. doi: 10.1046/j.1440-1681.2002.03722.x. [DOI] [PubMed] [Google Scholar]
- 40.Mason P. Drug–food interactions (1). Food and medicines. Continuing professional development. Pharmaceutical J. 2002;269:571–573. [Google Scholar]
- 41.Muregi F.W., Ishih A., Miyase T. Antimalarial activity of methanolic extracts from plants used in Kenyan ethnomedicine and their interactions with chloroquine (CQ) against a CQ-tolerant rodent parasite, in mice. J Ethnopharmacol. 2007;111:190–195. doi: 10.1016/j.jep.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Singh R.K. Tinospora cordifolia as an adjuvant drug in the treatment of hyper-reactive malarious splenomegaly—case reports. J Vector Borne Dis. 2005;42:36–38. [PubMed] [Google Scholar]