Abstract
The blood-red sap of Dragon's blood has been used in folk medicine for fractures, wounds, inflammation, gastrointestinal disorders, rheumatism, blood circulation dysfunctions, and cancer. Existing in vitro and in vivo bioactivity of this herb on different mechanisms of healing shows strong potential of this sap in wound healing. This clinical trial study was designated to evaluate the wound healing effect of Dragon's blood on human wounds. Sixty patients, between the ages of 14–65 years, who were referred to remove their skin tag, were assigned to this double-blind, placebo-controlled, randomized clinical trial and received either Dragon's blood or a placebo cream. They were visited on the 3rd, 5th, 7th, 10th, 14th, and 20th day of the trial to check the process of healing and to measure the wound's surface. At the end of trial, there was a significant difference in the mean duration of wound healing between the two groups (p = 0.0001). The phenolic compounds and the alkaloid taspine, which exist in Dragon's-blood resin, are probably the main reasons for the wound healing property of this plant. Being natural accessible, safe, and affordable makes Dragon's blood cream, a good choice for addition to the wound healing armamentarium. Further studies on wounds with different causes and among larger populations are suggested to ensure the effectiveness and safety of Dragon's blood.
Keywords: clinical trial, Dragon's blood, wound healing
Graphical abstract
1. Introduction
Dragon's blood, a deep red resin, is a well-known traditional medicine, obtained from four different sources: Croton spp. (syn. Sangre de draco, Euphorbiaceae), Dracaena spp. (syn. Zanzibar drop, Dracaenaceae), Daemonorops spp. (syn. Jerang or Djerang, Palmaceae), and Pterocarpus spp. (syn. East India Kino or Malabar Kino, Fabaceae). Dragon's blood has been used by different civilizations such as the Greeks, the Romans, and the Arabs.1 Croton lechleri has several medicinal properties, such as wound healing,1, 2 cicatrizant,3 immunomodulator,1, 4 analgesic, antiulcer, antidiarrheal,1 antibacterial,5 antiviral,6 antihemorrhagic,1 anti-inflammatory, antioxidant,1, 3 mutagenic and antimutagenic,1, 3, 7 antitumor,1, 8 anticancer,9 and cytotoxic effects.1, 3 Proanthocyanidins are the main chemical constituent of the resin, >90% of the dry weight.10 It also contains taspin, an alkaloid, and catechin, epigalocatechin, epicatechin, and a small percentage of terpene compounds.11, 12
Wounds, the physical damage to the skin and its underlying structure, can result from trauma, burns, or chemicals.13 Wound healing is a complicated process. According to cellular and molecular mechanisms, there are three overlapping phases of wound healing as follows: inflammation due to the migration of fibroblasts and inflammatory cells, such as neutrophils and monocytes, into the wound site; then, reconstruction of the epithelial barrier and production matrix at the site of injury leading to new tissue formation; and finally, maturation.14, 15, 16
Dragon's blood has the immunomodulatory property by influence on complement system.4 The antioxidant agents from Dragon's blood leaves and fruit, such as phenolic profile and organic acids, are able to protect against free radicals.17, 18 Noteworthy, the anti-inflammatory effect of the alkaloid taspine has been reported.19 These studies have shown this plant can promote healing by affecting the inflammatory phase. Studies confirm that after only 1 day of treatment with Dragon's blood, the wound contracts and a dark crust forms on the wound surface which prevents secondary infection.2, 20 It also stimulates the proliferation and migration of fibroblasts and the production of collagen, resulting in epithelial regeneration and wound healing which can affect second and third phases of the healing process.2, 12
Although there are some studies addressing the healing effect of Dragon's blood on cell lines in animal models,2, 20, 27 to best of our knowledge, the effect on human skin has not been studied yet. In this study, we designated to evaluate the healing effect of Dragon's blood on human skin.
2. Materials and methods
2.1. Trial design
This double-blind, placebo-controlled, randomized clinical trial was conducted on 60 patients referred to the dermatology clinic of Imam Khomeini Hospital (Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.) for the removal of their skin tags between October 2010 to November 2011. Written consent was obtained from all participants. The trial was registered by the IRCT Iranian Registry of Clinical Trials (IRCT201008224610N1), in accordance with the Helsinki Declaration of 1975, and was confirmed by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (Eth NO. 715).
The patients, aged between 14 years and 65 years, were referred for skin tag removal using cautery. Inclusion criteria were lesions between 3 mm and 10 mm in diameter and exclusion criteria were uncontrolled or chronic diseases, pregnancy, or breastfeeding. Demographic characteristics such as sex, age, and skin type, and number and anatomical area of lesions were recorded in the questionnaire. After measuring the wound's surface using checkered transparent paper (the surface of each square was 1 mm2), a therapeutic or placebo cream was given to the patients randomly (according to block method randomization). All patients were asked to use the cream twice a day and store it at room temperature. They were also asked not to use any other medicament for wound healing. The wound's surface and recovery was considered 100% and 0%, respectively, on the 1st day. The patients were visited on the 3rd, 5th, 7th, 10th, 14th, and 20th day of the trial to check the healing process and to measure the wound's surface. If recovery was not 100% on the 20th day, they were followed up until the wound's surface and recovery were 0% and 100%, respectively.
2.2. Plant material
Croton lechleri powder was purchased from the Maya Ethnobotanicals, Harleem, Netherland Company. Extraction was performed using a Soxhlet apparatus with ethanol (80%) for 4 hours,12 and was then filtrated and concentrated using a rotary evaporator (Heidolph, Germany). Finally, the extract was dried in a freeze drier (Operon, Korea).
2.3. Phytochemical study
Total phenolic content was determined using the Folin-Ciocaltue method.21 Briefly, to 0.5 mL of each sample (tannic acid as positive standard or extract) 2.5 mL 1/10 diluted Folin–Ciocalteu reagent was added. After 5 minutes, 2 mL of Na2CO3 (7.5% w/v) was added and incubated at room temperature in a dark place for 2 hours. The absorbance of all samples was measured at 765 nm. The results were expressed as g of tannic acid equivalent/100 g of dry extract powder.
2.4. Preparation of Dragon's blood cream
The formulation was as follows: 10% cetyl alcohol, 7% isopropylmeristat, and 21% Vaseline(cream base); 1.5% span20 and 1.5% tween80 (emulsifying agent); 0.02% propylparaben and 0.18% methylparaben (preservative); 5% propylene glycol (humectant); 15% ethanolic extract of C. lechleri, and distilled water.
The same ingredients were used to prepare the placebo cream with the exception of the herbal extract. Permitted food colors were used to achieve the color of the therapeutic cream (reddish brown). Similar tubes were filled with the therapeutic and placebo cream and labeled. Both creams were differentiated using codes that were unknown to the participants and researcher.
2.5. Statistical analysis
The data were expressed as mean ± standard deviation and analyzed using the two-tailed Student t test to compare the mean duration of wound healing between the two groups and the percentage of wound healing per day, and the analysis of variance (ANOVA) method was used to compare the mean duration of healing between different anatomical areas. A value of p < 0.05 was considered significant.
3. Results
The presence of alkaloid was confirmed by black spot in Dragandrof, and cream and brown precipitation in Mayer and Wagner reagents, respectively.
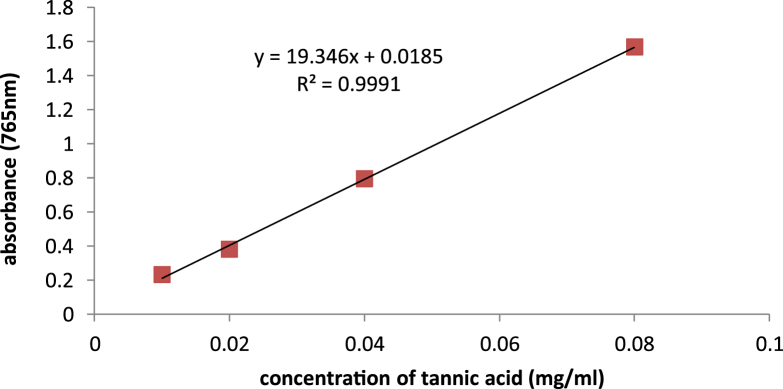
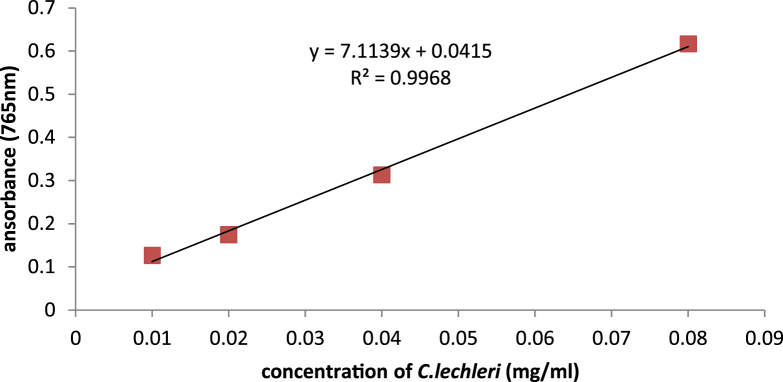
Total phenolic content was measured using the Folin–Ciocalteu method and tannic acid was used as a positive standard (Fig. 1, Fig. 2).
Fig. 1.
Tannic acid Standard curve in measurement of total phenolic content using Folin- Ciocaltue method.
Fig. 2.
Measurement of total phenolic content of C. lechleri using Folin- Ciocaltue method.
Tannic acid equivalent in 100 g Dragonʼs blood powder was 6.632 ± 0.65 g, and in 100 g dry extract was 43.35 ± 4.27 g. The prepared cream contained 15% extract, therefore, it included 6.5025 g total phenolic compounds.
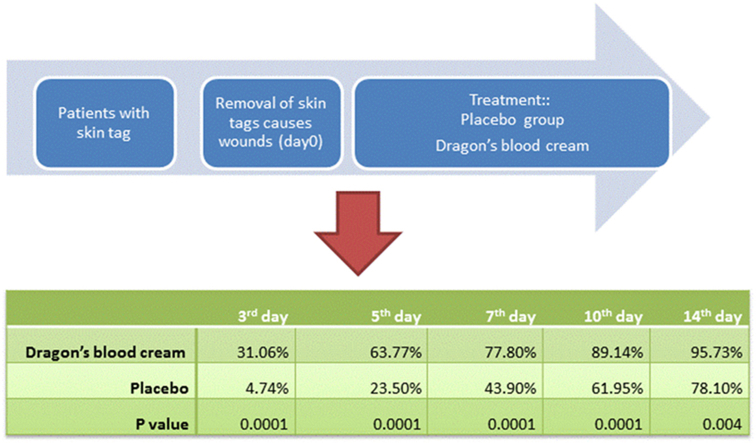
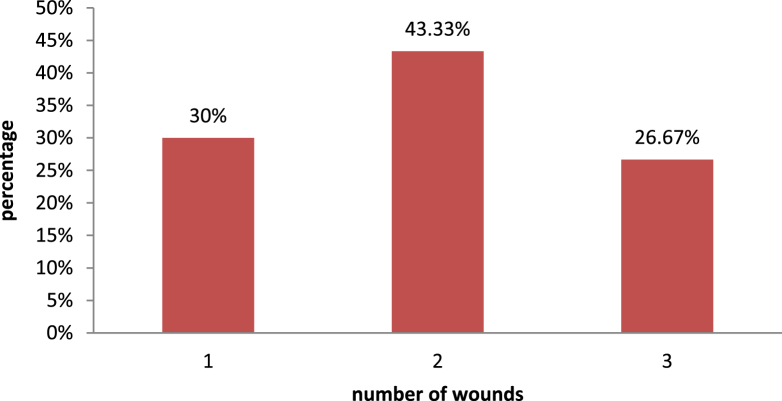
Sixty participants (31 women and 29 men) entered into the trial. Eighteen patients had one wound, 26 patients had two wounds, and 16 patients had three wounds (Fig. 3). We investigated 100 wounds in total. Forty-five wounds were assigned to the therapeutic group and 55 wounds were assigned in the placebo group randomly. Thirty-eight wounds (63.34%) were on the neck, 20 wounds (43.33%) were on the trunk, and 16 wounds (26.67%) were on the lower limbs. There were no significant differences between the two groups for age (p = 0.799) and wound surface (p = 0.946) before starting the study (Table 1, Table 2). Twelve patients (17 wounds) had poor compliance and withdrew from the study. There were no irritations or wound infections among the therapeutic participants, whereas one patient in the placebo group showed wound infection, and was treated with mupirocin topical ointment and dropped out of the study. Eventually, the trial was completed with 37 wounds in the therapeutic group and 46 wounds in the placebo group.
Fig. 3.
Number of wounds in studied population.
Table 1.
Comparison of mean age between two groups before starting the study.
| Group | Mean of age | SD | P value |
|---|---|---|---|
| Therapeutic | 38.4 | 10.98 | 0.799 |
| Placebo | 48.96 | 10.99 | 0.799 |
Table 2.
Comparison of wound's surface between two groups before starting the study.
| Group | Number of wounds | Mean of wound's surface | SD | P value |
|---|---|---|---|---|
| Therapeutic | 45 | 8.54 | 4.1 | 0.946 |
| placebo | 55 | 8.6 | 4.048 | 0.946 |
Two-tailed Student t test was used to compare the mean duration of healing and the percentage of wound healing between the two groups. The differences in the percentage of wound healing between the two groups were significant on all days of the trial (Table 3).
Table 3.
The mean percentage of wound healing in different days for two groups.
| 3rd day | 5th day | 7th day | 10th day | 14th day | |
|---|---|---|---|---|---|
| Therapeutic | 31.06% | 63.77% | 77.80% | 89.14% | 95.73% |
| Placebo | 4.74% | 23.50% | 43.90% | 61.95% | 78.10% |
| P value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.004 |
To compare the mean duration of healing between different anatomical areas, the ANOVA test was used. There were no significant differences between anatomical areas in both groups (p > 0.05).
4. Discussion
The results of this clinical trial showed that Dragon's blood cream can significantly improve the wound healing duration (p = 0.0001).
Previous studies reported the antioxidant and anti-inflammatory effects of Dragon's blood sap.1, 3, 22 It is also reported to inhibit the lipid peroxidation in the liver of mice.23 In this study, we observed a significant improvement of wound healing from the 3rd day, which may be due to a shortening of the inflammation process because of the presence of phenolic compounds such as proanthocyanidins and catechin.1, 20
Our results are in agreement with two studies which investigated the healing effect of Dragon's blood on rats. One of these studies showed the wound healing effect of the alkaloid taspine in rats. It suggested taspine stimulated chemotaxis of fibroblasts.24 Another study showed the wound healing activity of Dragon's blood was because of the high percentage of polyphenolic compounds in this plant.1, 2
Inflammation is usually a suitable media for infections, and healing is delayed in these situations.25 The polyphenolic compounds of the sap create a protective layer on the wound surface, and this physical barrier prevents microbial contamination. Some compounds of the resin, 2,4,6-trimethoxyphenol, 1,3,5-trimethoxybenzene, crolechinic acid, and korberin, which showed antibacterial effects, can indirectly improve the healing process.1, 20
Wound contraction is one of the factors that facilitates re-epithelialization.25 The polyphenolic compounds condense and clog the wounds by binding to proteins, and enzymes lead to ailment.20 Froldi et al26 have suggested that the vasoconstriction effect of Dragon's blood affects wound healing probably by fastening the wound.
Angiogenesis and matrix reformation phases occur after re-epithelialization. The migration and proliferation of fibroblasts causes reformation of the matrix.25 Dragon's blood sap probably affects wound healing through increasing the migration of human fibroblasts in the cell culture and proliferation of the endothelial cells.12, 24
5. Conclusion
This clinical trial suggests Dragon's blood is a potent, available, affordable, and safe healing agent. The exact role of Dragon's blood in the pathogenesis of wound healing regarding its effect on stimulation or hindering mediator's synthesis is still absent and further studies are required. Our study encourages evaluating the healing process on other wounds such as diabetic ulcers, bedsores, or burns.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Gupta D., Bleakley B., Gupta R.K. Dragon's blood: botany, chemistry and therapeutic uses. J Ethnopharmacol. 2008;115:361–380. doi: 10.1016/j.jep.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Pieters L., De Bruyne T., Van Poel B. In vivo wound healing activity of Dragon's blood (Croton spp.), a traditional South American drug, and its constituents. Phytomedicine. 1995;2:17–22. doi: 10.1016/S0944-7113(11)80043-7. [DOI] [PubMed] [Google Scholar]
- 3.Lopes M.I., Saffi J., Echeverrigaray S., Henriques J.A.P., Salvador M. Mutagenic and antioxidant activities of Croton lechleri sap in biological systems. J Ethnopharmacol. 2004;95:437–445. doi: 10.1016/j.jep.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Risco E., Ghia F., Vila R., Iglesias J., Álvarez E., Cañigueral S. Immunomodulatory activity and chemical characterisation of sangre de drago (dragon's blood) from Croton lechleri. Planta Med. 2003;69:785–794. doi: 10.1055/s-2003-43208. [DOI] [PubMed] [Google Scholar]
- 5.Edward H.G.M., de Oliveira L.F.C., Quye A. Raman spectroscopy of coloured resins used in antiquity: dragon's blood and related substances. Spectrochim Acta A Mol Biomol Spectrosc. 2001;57:2831–2842. doi: 10.1016/s1386-1425(01)00602-3. [DOI] [PubMed] [Google Scholar]
- 6.Ubillas R., Jolad S.D., Bruening R.C. SP-303, an antiviral oligomeric proanthocyanidin from the latex of Croton lechleri (sangre ded) Phytomedicine. 1994;1:77–106. doi: 10.1016/S0944-7113(11)80026-7. [DOI] [PubMed] [Google Scholar]
- 7.Rossi D., Guerrini A., Paganetto G. Croton lechleri Müll Arg. (Euphorbiaceae) stem bark essential oil as possible mutagen-protective food ingredient against heterocyclic amines from cooked food. Food Chem. 2013;139:439–447. doi: 10.1016/j.foodchem.2013.01.076. [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Castro A.J., Ortiz-Sánchez E., Domínguez F. Antitumor effect of Croton lechleri Mull. Arg. (Euphorbiaceae) J Ethnopharmacol. 2012;140:438–442. doi: 10.1016/j.jep.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Montopoli M., Bertin R., Chen Z., Bolcato J., Caparrotta L., Froldi G. Croton lechleri sap and isolated alkaloid taspine exhibit inhibition against human melanoma SK23 and colon cancer HT29 cell lines. J Ethnopharmacol. 2012;144:747–753. doi: 10.1016/j.jep.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Cai Y., Evans F.J., Roberts M.F., Phillipson J.D., Zenk M.H., Gleba Y.Y. Polyphenolic compounds from Croton lechleri. Phytochemistry. 1991;30:2033–2040. [Google Scholar]
- 11.Phillipson J.D. A matter of some sensitivity. Phytochemistry. 1995;38:1319–1343. doi: 10.1016/0031-9422(94)00780-w. [DOI] [PubMed] [Google Scholar]
- 12.Vaisberg A.J., Milla M., Planas M.C., Cordova J.L., de Agusti E.R., Ferreyra R., Mustiga M.C., Carlin L., Hammond G.B. Taspine is the Cicatrizant Principle in Sangre de Grado Extracted from Croton lechleri. Planta Med. 1989;55:140–143. doi: 10.1055/s-2006-961907. [DOI] [PubMed] [Google Scholar]
- 13.Martindale. The complete drug reference. 36th ed. Pharmaceutical Press; United Kingdom: 2009. [Google Scholar]
- 14.Jia Y., Zhao G., Jia J. Preliminary evaluation: the effects of i Miller and Aloe arborescens Miller on wound healing. J Ethnopharmacol. 2008;120:181–189. doi: 10.1016/j.jep.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Ashcroft G.S., Roberts A.B. Loss of Smad3 modulates wound healing. Cytokine Growth Factor Rev. 2000;11:125–131. doi: 10.1016/s1359-6101(99)00036-2. [DOI] [PubMed] [Google Scholar]
- 16.Wang A.S., Armstrong E.J., Armstrong A.W. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am JSurg. 2013;206:410–417. doi: 10.1016/j.amjsurg.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Santos R.P., Mendes L.S., Silva B.M. Phytochemical profiles and inhibitory effect on free radical-induced human erythrocyte damage of Dracaena draco leaf: a potential novel antioxidant agent. Food Chem. 2011;124:927–934. [Google Scholar]
- 18.Silva B.M., Santos R.P., Mendes L.S. Dracaena draco L. fruit: phytochemical and antioxidant activity assessment. Food Res Int. 2011;44:2182–2189. [Google Scholar]
- 19.Perdue G.P., Blomster R.N., Blake D.A., Farnsworth N.R. South American plants II:taspine isolation an anti-inflammatory activity. J Pharm Sci. 1979;68:124–125. doi: 10.1002/jps.2600680145. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z.P.C.Y., Phillipson J.D. Studies on the anti-tumor,anti-bactrial,and wound-healing properties of Dragons blood. Planta Medica. 1994;60:541–545. doi: 10.1055/s-2006-959567. [DOI] [PubMed] [Google Scholar]
- 21.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In: Lester P., editor. vol. 299. Academic Press; 1999. pp. 152–178. (Methods in Enzymology). [Google Scholar]
- 22.Desmarchelier C., Schaus F.W., Coussio J., Cicca G. Effects of Sangre de Drago from Croton lechleri Muell.-Arg. on the production of active oxygen radicals. J Ethnopharmacol. 1997;58:103–108. doi: 10.1016/s0378-8741(97)00087-1. [DOI] [PubMed] [Google Scholar]
- 23.Desmarchelier C.J., de Moraes Barros S.B. Pharmacological activity of South American plants: effects on spontaneous in vivo lipid peroxidation. Phytother Res. 2003;17:80–82. doi: 10.1002/ptr.1080. [DOI] [PubMed] [Google Scholar]
- 24.Porras-Reyes B.H., Lewis W.H., Roman J., Simchowitz L., Mustoe T.A., editors. Enhancement of wound healing by the alkaloid taspine defining mechanism of action. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. Royal Society of Medicine; New York, NY: 1993. [DOI] [PubMed] [Google Scholar]
- 25.McGibbon D. Rook's Textbook of Dermatology. Clin Exp Dermatol. 2006;31:178–179. [Google Scholar]
- 26.Froldi G., Zagotto G., Filippini R., Montopoli M., Dorigo P., Caparrotta L. Activity of sap from Croton lechleri on rat vascular and gastric smooth muscles. Phytomedicine. 2009;16:768–775. doi: 10.1016/j.phymed.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 27.He Xuan-ling, Wang Shen-zhi, Huang Zheng-de. Effects of dracaena on expression of Smads protein in skin ulcers in diabetic rats. J Trad Chin Med Univ Hunan. 2010–11 [Google Scholar]