Abstract
Liver cancer is the second leading cause of cancer deaths in Taiwan as per the 2011 statistics and ranks fourth in cancer-related mortality in the world. Recent researches have shown that Antrodia cinnamomea, a Taiwan-specific medicinal mushroom, has biological activities, including hepatoprotection, anti-inflammation, antihepatitis B virus activity, and anticancer activity. In the present study, the antiproliferative activity and molecular mechanisms of antcin K, the most abundant ergostane triterpenoid from the fruiting bodies of basswood cultivated A. cinnamomea, were investigated using human hepatoma Hep 3B cells. The results showed that antcin K effectively reduced Hep 3B cells viability within 48 hours. Antcin K induced phosphatidylserine exposure, chromatin condensation, and DNA damage, but did not significantly increase autophagosome content or cause cell expansion and cell lysis. Thus, the principal mode of Hep 3B cells death induced by antcin K was apoptosis, rather than autophagy or necrosis. In-depth investigation of the molecular mechanisms revealed that antcin K first promoted reactive oxygen species generation and adenosine triphosphate depletion, leading to endoplasmic reticulum stress and resulting in mitochondrial membrane permeability changes. After losing the mitochondrial membrane potential, caspase-independent and caspase-dependent apoptosis-related proteins were released, including HtrA2, apoptotic-induced factor, endonuclease G, and cytochrome c. Cytochrome c activated caspase-9 and caspase-3, and cut downstream protein PARP, ultimately leading to cell apoptosis. These results suggested that antcin K induced mitochondrial and endoplasmic reticulum stress-mediated apoptosis in human hepatoma cells. Coupled with these findings, antcin K has a potential to be a complementary agent in liver cancer therapy.
Keywords: antcin K, Antrodia cinnamomea, apoptosis, autophagy, Hep 3B cells
1. Introduction
Antrodia cinnamomea, a Taiwan-specific mushroom, has been reported to have numerous biological activities including hepatoprotection, anti-inflammation,1 antihepatitis C virus activity,2 and anticancer activity.3 Liver cancer ranks fourth in cancer-related mortality around the world (WHO 2008) and is the second leading cause of cancer deaths in Taiwan. Twenty years ago, the major cause of liver cancer in Taiwan was hepatitis virus infection, such as hepatitis B virus (HBV) infection.4 The incidence of HBV infection is very low now, mostly due to the HBV vaccine policy of Taiwan government. However, the mortality of liver cancer is still high, which may be due to social culture and unhealthy living habits such as drugs abuse, drinking, and overworking. There are three common types of cell death: apoptosis, autophagy, and necrosis. Apoptosis, also called type I programmed cell death, involves DNA fragmentation, caspase induction, and phosphatidylserine translocation from the inner side of the cell membrane to its outer side5; autophagy, also recognized as type II programmed cell death, forms autophagosome as the major phenomenon6; and necrosis would cause inflammation and disrupt organelles.7 There is no study on the determination of major mechanism of antcin K-induced cell death in human liver cancer cells.
Proteins must go through a series of post-translational modifications and be fully folded in order to be transported from the endoplasmic reticulum (ER). Proteins with incomplete or incorrect folding will remain in the ER or be degraded by the proteasome in the cytoplasm. Many physiological and pathological circumstances such as hypoxia, oxidative damage, deficiency in ER calcium ion, and viral infection may cause disorders of protein folding in the ER, in which proteins with incomplete folding will accumulate on the ER surface and cause ER stress. In order to resist ER stress, cells will start a series of reactions to adapt to the circumstance and regulate the ER stress, which include promotion of protein degradation, inhibition of protein translation, and activation of relevant genes. However, if the ER stress persists, apoptosis or autophagy would be induced, which will eventually lead to cell death.
ER stress can induce unfolded protein response and calcium signals. The unfolded protein response is primarily a survival response, acting to resolve dysregulation of protein-folding pathways. If the normal luminal environment cannot be restored, the response to ER stress would be switched from survival to apoptosis. PERK, IRE1, and ATF-6 are all involved in the induction of proapoptotic as well as prosurvival pathways.8 Both PERK and ATF-6 induce expression of the transcription factor C/EBP homologous protein (CHOP), which in turn leads to reduced expression of B-cell lymphoma 2 (Bcl-xL) and increased expression of a number of proapoptotic proteins, including BH3-only protein BIM and its downstream protein B-cell lymphoma—extra-large (Bax)/Bak.9, 10, 11 Furthermore, Bcl-2 family proteins at the ER also regulate apoptosis through both direct modulation of signaling pathways leading to caspase activation and modulation of Ca2+ signaling of ER.12, 13 Calcium is a common metal ion, which plays a dominant role in cell death signaling transduction. It has been reported that the concentration of calcium ion can regulate the stability of organelles such as mitochondria and ER.14 Here, we will demonstrate how antcin K modulates cell death through the change of calcium distribution in human liver cancer cells.
Mitochondria is the organelle with its own DNA in matriclinous. It plays a very important role in cell death. In some cancer therapy, it would induce reactive oxygen species (ROS) production, and the ROS may attack some organelles such as mitochondria. Next, the permeability transition pore of mitochondria would be opened by ROS or other stimulation, and then by some proapoptotic protein Bax/Bak overexpression, which could release cytochrome c into the cytoplasm. Finally, cytochrome c would increase the cleavage caspase-9 protein expression, which triggers caspase-3 activation. There are other caspase-independent death pathways in mitochondria, such as HtrA2/Omi, endonuclease G (Endo G), and apoptotic-induced factor (AIF) released from mitochondria.15, 16
2. Materials and methods
2.1. Chemicals and reagents
Basswood cultivated A. cinnamomea (BCRC930103) mycelial powder was supplied by PO-ZO Co., Ltd (Taipei, Taiwan).
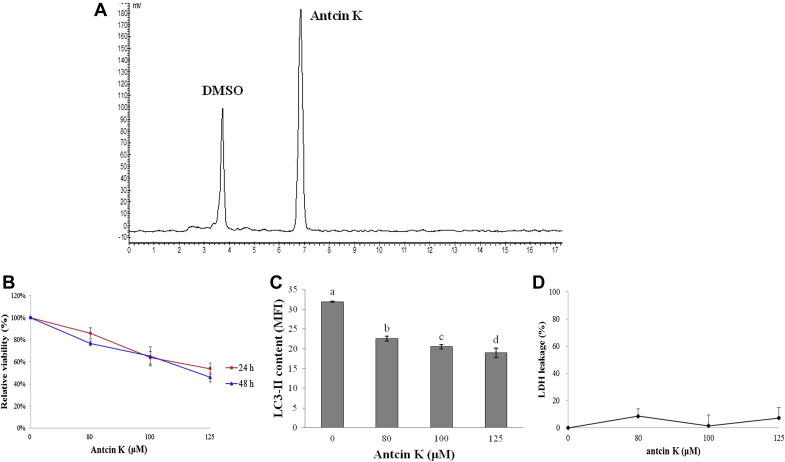
Ethyl acetate (EA) was added to 500 g dry power of A. cinnamomea to a total volume of 4 L and stirred for 3 days. The extract was decanted, and the solvent was removed using a rotary evaporator at 50°C three times for each sample. A silica gel column was used to fractionate the extracted sample. The column was consecutively eluted with 10%, 15%, 20%, 30%, 50%, 70%, and 100% EA/hexane. The fraction with 100% EA/hexane contained the highest amount of antcin K. It was further purified by high-performance liquid chromatography to obtain antcin K with >90% purity (Fig. 1A).
Fig. 1.
(A) High-performance chromatogram of the purified compound antcin K (retention time 6.5 minutes). Conditions: column, COSMOSIL 5C18-AR-II RP-C18; flow rate, 1 mL/min; detector, 254 nm; mobile phase, methanol (70%)/water (30%). (B) Effect of antcin K on cell viability in Hep 3B cells. After incubation of the cells with 0μM, 80μM, 100μM, and 125μM antcin K for 24 hours and 48 hours, cell viability was determined by MTT assay. Data are expressed as percentage of negative control (0.2% DMSO) and mean ± SD from one of three independent experiments. (C) Effect of antcin K on the degree of LC3-II fluorescence intensity in Hep3B cells. After incubation of the cells with 0μM, 80μM, 100μM, and 125μM antcin K for 48 hours, the degree of LC3-II fluorescence intensity was analyzed by flow cytometry. (D) Effect of antcin K on degree of cell disruption in Hep 3B cells. After incubation of the cells with 0μM, 80μM, 100μM, and 125μM antcin K and lysis solution for 48 hours, the degree of cell disruption was determined by LDH leakage assay. Data are expressed as percentage between positive control (lysis solution) and negative control (0.2% DMSO) and mean ± SD from one of three independent experiments, and analyzed statistically using one-way ANOVA and Duncan's test. Different letters (a–d) represent statistically significant differences among treatments (p < 0.05). ANOVA = analysis of variance; DMSO = dimethyl sulfoxide; LDH = lactate dehydrogenase; SD = standard deviation.
Alexa Fluor 488 antirabbit IgG antibody, antibiotic–antimycotic, 2,7-dihydrodichlorofluorescein diacetate, Dulbecco's modified Eagle's medium, fetal bovine serum, fluo-3-acetoxymethyl ester, nonessential amino acids, Rhod-2-acetoxymethyl ester, and 3,3′-dihexyloxacarbocyanine iodide were purchased from Invitrogen (Carlsbad, CA, USA). Anti-β-actin antibody, anti-AIF antibody, anti-Bcl-xL antibody, anti-Bax antibody, anti-Bak (D4E4) rabbit mAb antibody, anti-caspase-9 antibody, anticleaved caspase-3 rabbit mAb (Asp175)(5A1E) antibody, anti-CHOP (L63F7) mouse mAb antibody, anti-cytochrome c antibody, anti-Endo G antibody, anti-HtrA2/omi antibody, anti-PARP antibody, antirabbit IgG HRP-linked antibody, and antimouse IgG HRP-linked antibody were obtained from Cell Signaling Technology (Beverly, MA, USA). Caspase-3 assay kit and annexin V-FITC apoptosis detection kit were purchased from BD Biosciences (San Jose, CA, USA). Anti-LC3B antibody was purchased from GeneTex (Irvine, CA, USA). Adenosine diphosphate/adenosine triphosphate (ADP/ATP) ratio assay kit and lactate dehydrogenase (LDH) cytotoxicity assay kit were purchased from BioChain Institute (Hayward, CA, USA). All other chemicals were of analytical or reagent grade and obtained from Sigma-Aldrich (St Louis, MO, USA).
2.2. Cell culture and treatment
Human hepatoma Hep 3B cell line was a kind gift from Professor Ming-Shi Shiao (Medical Research and Education Department, Taipei Veterans General Hospital, Taipei, Taiwan). Hep 3B cells were cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum, 1.5 g/L sodium bicarbonate, 1% nonessential amino acids, and 1% antibiotic–antimycotic at 37°C, 5% CO2, and 90% relative humidity. Antcin K was diluted in dimethyl sulfoxide (DMSO) prior to being added to cultures. Negative control cultures were treated with 0.2% DMSO.
2.3. MTT assay
Liver cancer cells at a concentration of 5 × 103 cells/well were seeded in 96-well plates and incubated for 24 hours, followed by treatment with 0μM (0.3% DMSO), 80μM, 100μM, and 125μM antcin K and incubated further for 24 hours and 48 hours. At the end of the stipulated period, 100 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) solution (0.5 mg/mL) was added, and the cells were incubated at 37°C for 4 hours. The resulting MTT formazan was dissolved in 100 μL DMSO and the absorbance recorded at 570 nm using a PowerWave HT microplate spectrophotometer (Bio-Tek, Winooski, VT, USA).17
2.4. LDH leakage assay
Hep3B cells (1 × 104 cells/well) were seeded in 96-well plates for 24 hours, followed by treatment with 200 μL medium containing 0μM, 80μM, 100μM, and 125μM antcin K and lysis solution (as a positive control), and incubated for another 48 hours. The plates were then centrifuged at 250g for 10 minutes, and 100 μL of supernatant was transferred to corresponding cells of a new 96-well plate. Following this, 45 μL of assay mixture containing lactate, nicotinamide adenine dinucleotide, iodonitrotetrazolium, and diaphorase was added to each well, protected from light, and incubated for 60 minutes. The absorbance was recorded at 490 nm using a Bio-Tek PowerWave HT microplate spectrophotometer.18
2.5. Immunofluorescence
The cells (1.75 × 104 cells/well) were seeded in a four-well plate for 24 hours. For the 4,6-diamidini-2-phenylindole (DAPI) staining, after 24 hours of incubation, the cells were treated with 350 μL medium containing 0μM, 80μM, 100μM, and 125μM antcin K for 24 hours. After 24 hours of treating, the cells were washed and fixed by 4% paraformaldehyde for 30 minutes, and then washed twice. After washing, the cells were blocked for 1 hour by 5% bovine serum albumin and 0.1% Triton X-100. Then the cells were stained by DAPI for 15 minutes and protected from light. After washing, the slides were mounted and examined under fluorescence microscopy (Olympus IX51; Olympus, Tokyo, Japan). For the mitochondria calcium staining, after 24 hours of incubation, the cells were treated with 350 μL medium containing 0μM, 80μM, 100μM, and 125μM antcin K for 30 minutes. After 30 minutes of treatment, the cells were incubated with 350 μL medium containing 5μM Rhod-2-acetoxymethyl ester for 1 hour and protected from light. After washing, the cells were incubated with 5% bovine serum albumin at 37°C for 30 minutes. The slides were mounted and examined under a Leica TCS SP5 II confocal fluorescence microscope (Leica, Solms, Germany).19
2.6. Bioluminescence
The cells (5 × 103 cells/well) were seeded in 96-well plates for 24 hours. After 24 hours of incubation, the cells were treated with 100 μL medium containing 0μM, 80μM, 100μM, and 125μM antcin K for 24 hours. After 24 hours of treatment, 90 μL lysis solution, which contains luciferin and luciferase, was added to each well and incubated for 10 minutes. The luminescence was integrated for 5 seconds using a Beckman Coulter DTX-880 microplate reader (Beckman Coulter, Brea, CA, USA).20
2.7. Flow cytometry
The cells (3 × 105 cells/dish) were seeded in 6 cm2 dishes for 24 h hours. After 24 hours of incubation, the cells were treated with 6 mL medium containing 0μM, 80μM, 100μM, and 125μM antcin K for 30 minutes to detect Ca2+, 24 hours to detect ROS, 48 hours to detect autophagosome, 48 hours to determine mitochondrial membrane potential, and 24 hours to determine the percentage of cells undergoing apoptosis, using an annexin V-FITC/propidium iodide (PI) assay kit (BD Biosciences). At the end of the stipulated period, the cells were harvested and washed. For the detection of Ca2+, ROS, and mitochondrial membrane potential, the cells were separately stained by 4μM fluo-3-acetoxymethyl ester, 5μM dihydrodichlorofluorescein diacetate, and 4μM 3,3′-dihexyloxacarbocyanine iodide at 37°C for 30 minutes. For the detection of autophagosome, the cells were permeabilized with 0.25 mg/mL digitonin for 5 minutes. Following this, the cells were washed twice, pelleted by centrifugation at 1000g, and incubated with anti-LC3-II antibody (1:2000) for 30 minutes. After two washes, the cells were stained by Alexa Fluor 488 anti-rabbit IgG antibody (1:500) for 1 hours and protected from light. All these cells were washed and filtered prior to being analyzed by a Becton-Dickinson FACScan flow cytometer (Becton-Dickinson, Hercules, CA, USA). A total of 10,000 cells per sample were collected, and the mean fluorescence intensity and percentage of mitochondrial membrane potential detected by FL1-H (530 ± 15 nm) were analyzed using WinMDI 2.8 software. For the detection of the percentage of apoptosis cells, the cells were resuspended in binding buffer [10 mmol/L HEPES/NaOH (pH 7.4), 140 mmol/L NaCl, 2.5 mmol/L CaCl2] and stained with annexin V-FITC and PI at room temperature for 15 minutes in the dark. Cells were washed and filtered prior to being analyzed by a Beckman Coulter FC500 flow cytometer (Beckman Coulter, Pembroke Pines, FL, USA). A total of 10,000 cells per sample were collected, and the apoptotic cells were defined as annexin V-FITC-positive and PI-negative cells.19, 21, 22
2.8. Electrophoresis
For western blotting, the cells (5 × 105 cells/10 mL/dish) were seeded in 10 cm2 dishes for 24 hours. After 24 hours of incubation, the cells were treated with 10 mL medium containing 0μM, 80μM, 100μM, and 125μM antcin K for 48 hours. After 48 hours of treating, total cell extracts were prepared in protein extraction solution, which contained 1mM phenylmethanesulfonylfluoride, 1mM EDTA, 1μM pepstatin A, 1μM leupeptin, and 0.1μM aprotinin. The cell lysates were sonicated and cleared by centrifugation, and the protein concentration in the lysates was measured by Lowry's method.23 Protein (50 μg) was loaded over 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, transferred to polyvinylidene fluoride membranes, blotted with specific primary antibodies, and then labeled by horseradish peroxidase-conjugated secondary antibody according to the manufacturer's instructions. The membranes were performed using the enhanced chemiluminescence and the EC3 300 Corning UVP biospectrum AC system (Corning, NY, USA), and the relative density of each band after normalization for β-actin was analyzed using Image J 1.45 software.24 For the comet assay, the cells (5 × 105 cells/10 mL/dish) were seeded in 6 cm2 dishes for 24 hours. After 24 hours of incubation, the cells were treated with 6 mL medium containing 0μM, 80μM, 100μM, and 125μM antcin K for 24 hours. After 24 hours of treatment, cells were examined for DNA damage using the comet assay described previously.25
2.9. Statistical analysis
All results are reported as mean ± standard deviation (SD), and the differences between the antcin K-treated group and the control group were analyzed by one-way analysis of variance (ANOVA) and Duncan's multiple comparison tests (SAS Institute Inc., Cary, NC, USA) to determine significant differences among treatments (p < 0.05).
3. Results
3.1. Cell proliferation inhibitory effect of antcin K in Hep 3B cells
After treating Hep 3B cells with 0μM, 80μM, 100μM, and 125μM antcin K for 24 hours or 48 hours, cell viability was analyzed by MTT assay. Fig. 1B shows that after being treated with 80μM, 100μM, and 125μM antcin K for 24 hours, compared to the negative control (0.2% DMSO, cell survival rate set at 100%), the cell survival rates were 85.7 ± 5.0%, 63.8 ± 5.8%, and 53.8 ± 5.3%, respectively; after 48 hours of treatment, cell survival rates decreased to 76.7 ± 2.1%, 65.0 ± 8.6%, and 46.0 ± 4.2%, respectively. In addition, the maximal half inhibitory concentration for 48-hour treatment was 119.3μM.
3.2. Antcin K could not induce autophagy or necrosis in Hep 3B cells
After treating Hep 3B cells with 0μM, 80μM, 100μM, and 125μM antcin K, for 48 hours, the autophagosome formation was analyzed by flow cytometry. Fig. 1C shows that after being treated with 80μM, 100μM, and 125μM antcin K, the LC3-II contents were significantly decreased, and not increased. After treating Hep 3B cells with 0μM, 80μM, 100μM, and 125μM antcin K and lysis solution for 48 hours, the degree of cell membrane damage was analyzed by LDH leakage assay. Fig. 1D shows that after being treated with 80μM, 100μM, and 125μM antcin K for 48 hours, compared with the positive control (lysis solution, lactate dehydrogenase leakage rate set at 100%) and negative control (0.2% DMSO, lactate dehydrogenase leakage rate set at 0%), the lactate dehydrogenase leakage rates were 8.56 ± 5.24%, 1.46 ± 7.85%, and 7.21 ± 7.48%, respectively, which were all low.
3.3. Effects of antcin K on induction of apoptosis in Hep 3B cells were determined by DAPI staining
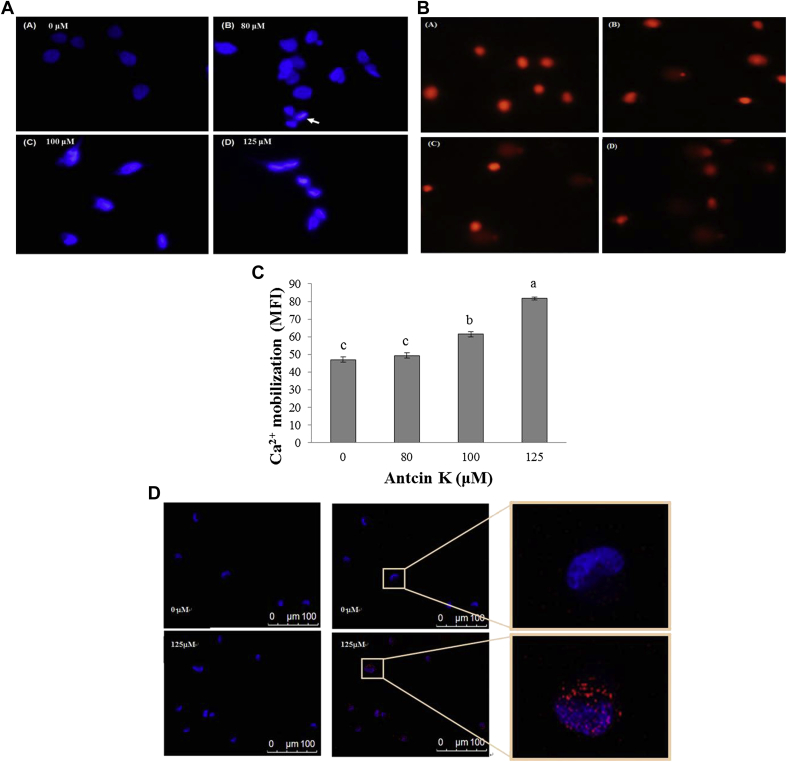
We assessed the effect of antcin K on the induction of DNA aggregation in Hep 3B cells by DAPI staining. The immunofluorescence at 24 hours showed that 80μM, 100μM, and 125μM antcin K treatment resulted in the formation of chromatin condensation in Hep 3B cells. The nuclei of control cells were round and dim, whereas those became condensed and bright after antcin K treatment (Fig. 2A).
Fig. 2.
(A) Detection of chromatin condensation in Hep 3B cells by DAPI staining. Typical apoptotic changes comprise condensation of chromatin, its presence along the periphery of the nucleus, and segmentation of the nucleus. Cells were treated with 0μM, 80μM, 100μM, and 125μM antcin K for 24 hours. DAPI fluorescence of nuclei was visualized by excitation at 330–385 nm with a 420 nm barrier filter. The experiments were repeated two times with similar results. (B) Detection of DNA damage in Hep 3B cells by comet assay. Cells were treated with 0μM, 80μM, 100μM, and 125μM antcin K for 24 hours. EtBr fluorescence of DNA was visualized by excitation at 510–550 nm with a 590 nm barrier filter. The experiments were repeated two times with similar results. (C) Effect of antcin K on calcium ion mobilization in Hep 3B cells. After incubation of the cells with 0μM, 80μM, 100μM, and 125μM antcin K for 30 minutes, Fluo 3-Ca complex was analyzed by flow cytometry. (D) Effect of antcin K on mitochondrial calcium distribution in Hep 3B cells. After incubation of the cells with 0μM and 125μM antcin K for 30 minutes, mitochondrial calcium distribution was observed by immunofluorescence. The cells were fixed and stained with Rhod-2-AM (red fluorescence). Nuclear DNA in the cells was then counterstained with DAPI (blue fluorescence). The fluorescent images were recorded and superimposed (merged). Data are expressed as mean ± SD from one of three independent experiments and analyzed statistically using one-way ANOVA and Duncan's test. Different letters (a–c) represent statistically significant differences among treatments (p < 0.05). ANOVA = analysis of variance; DAPI = 4,6-diamidini-2-phenylindole; MFI = mean fluorescence intensity; Rhod-2-AM = Rhod-2-acetoxymethyl ester; SD = standard deviation.
3.4. Antcin K-induced DNA damage in Hep 3B cells
The comet assay showed that antcin K induced DNA damage in Hep 3B cells. Higher concentrations of antcin K led to a longer DNA migration smear (comet tail) (Fig. 2B), indicating that damage to DNA in the cells was more.
3.5. Effects of antcin K on induction of apoptosis in Hep 3B cells were determined by annexin V-FITC/PI assay
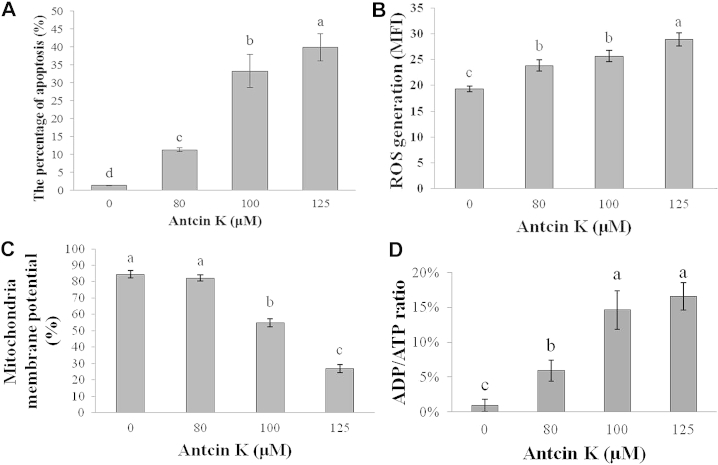
A quantitative evaluation was sought using annexin V-FITC dye to detect the translocation of phosphatidylserine from the inner (cytoplasmic) leaflet of the plasma membrane to the outer side (cell surface). Compared with negative control cells, 80μM, 100μM, and 125μM antcin K induced, respectively, 11.25 ± 0.51%, 33.28 ± 4.67%, and 39.87 ± 3.83% of apoptotic cells in Hep 3B cells in 24 hours (Fig. 3A).
Fig. 3.
(A) Percentage of apoptotic cell induction by antcin K-treated Hep 3B cells. Cells were exposed to 0μM, 80μM, 100μM, and 125μM antcin K for 24 hours, and the distribution of apoptosis (lower right panel) was assessed by annexin V-FITC/PI assay. Effect of antcin K on ROS generation and ADP/ATP ratio in Hep 3B cells. (B) After incubation of the cells with 0μM, 80μM, 100μM, and 125μM antcin K for 24 hours, the fluorescence intensity of DCFH-DA was analyzed by flow cytometry. (C) Effect of antcin K on the degree of fluorescence intensity of DiOC6 in Hep 3B cells. After incubation of the cells with 0μM, 80μM, 100μM, and 125μM antcin K for 48 hours, the mitochondrial membrane potential was analyzed by flow cytometry. (D) After incubation of the cells with 0μM, 80μM, 100μM, and 125μM antcin K for 48 hours, the ADP/ATP ratio was determined by bioluminescence. Data are expressed as mean ± SD from one of three independent experiments and analyzed statistically using one-way ANOVA and Duncan's test. Different letters (a–d) represent statistically significant differences among treatments (p < 0.05). ADP/ATP = adenosine diphosphate/adenosine triphosphate; ANOVA = analysis of variance; DCFH-DA = dihydrodichlorofluorescein diacetate; DiOC6 = 3,3′-dihexyloxacarbocyanine iodide; MFI = mean fluorescence intensity; PI = propidium iodide; ROS = reactive oxygen species; SD = standard deviation.
3.6. Antcin K promoted ROS generation and ATP depletion in Hep 3B cells
After treating Hep 3B cells with 0μM, 80μM, 100μM, and 125μM antcin K for 24 hours, ROS generation was analyzed by flow cytometry; in addition, after treating Hep 3B cells with the same concentrations of antcin K for 48 hours, the ADP/ATP ratio was analyzed with bioluminescence. Fig. 3B shows that as the antcin K dose increased, ROS generation in cells increased significantly (the maximum value being 1.49 times greater than the minimum) and had a dose-dependent effect. Fig. 3D shows that as the antcin K dose increased, the ADP/ATP ratio in cells increased significantly. The ADP/ATP ratios were 0.9 ± 0.9%, 5.9 ± 1.5%, 14.7 ± 2.8%, and 16.1 ± 2.0%, respectively, for 0μM, 80μM, 100μM, and 125μM antcin K treatment.
3.7. Antcin K induced Ca2+ flux from cytoplasm to mitochondria
Using fluo-3-acetoxymethyl ester, a cell-permeable Ca2+ indicator, we assessed the effect of antcin K treatment on cytosolic Ca2+. The level of cytosolic Ca2+ induced significantly by 100μM and 125μM antcin K at 30 minutes. In addition, treatment with 125μM antcin K similarly induced a 1.74-fold increase in Ca2+ level (Fig. 2C). Mitochondrial free calcium levels were quantitated in cells that were still viable with the cell-permeability acetoxymethyl ester of the fluorescent marker Rhod-2. Using Rhod-2-acetoxymethyl ester, a selective indicator for mitochondrial Ca2+, the distribution of calcium in 0μM and 125μM antcin K-treated Hep 3B cells was revealed by confocal immunofluorescence microscopy (Fig. 2D).
3.8. Antcin K promoted mitochondrial depolarization in Hep 3B cells
Hep 3B cells were treated with 0μM, 80μM, 100μM, and 125μM antcin K for 48 hours, after which the mitochondrial membrane potential was analyzed and quantified by flow cytometry. Fig. 3C shows that as the antcin K dose increased, the mitochondrial membrane potential in cells decreased significantly (the maximum potential being 0.34 times greater than the minimum) and had a dose-dependent effect.
3.9. Effect of antcin K on the expression of mitochondria and ER stress-mediated apoptosis-related protein
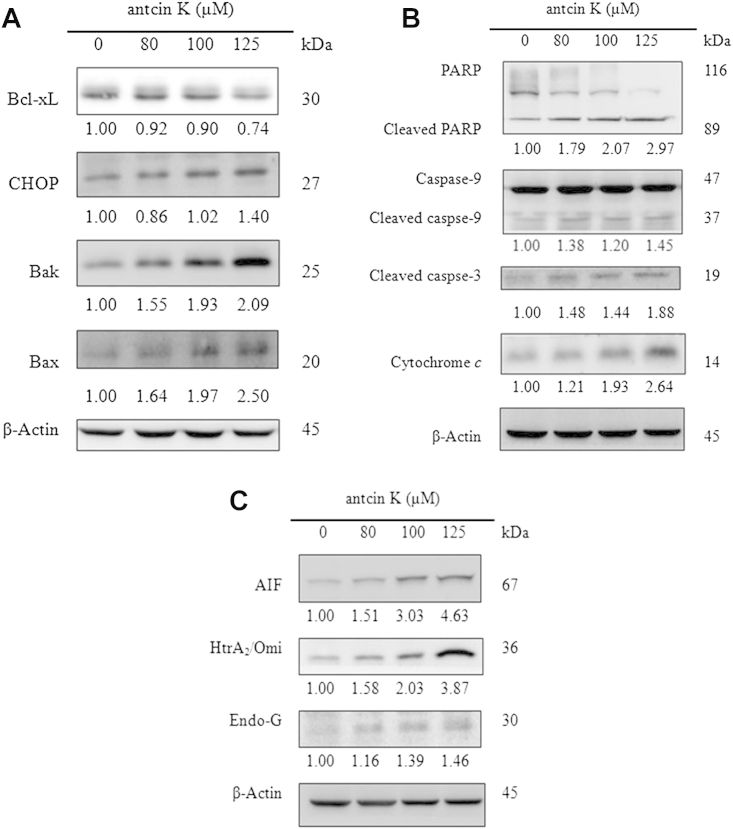
After treating Hep 3B cells with 0μM, 80μM, 100μM, and 125μM antcin K for 48 hours, unfolded protein response-related protein expression was analyzed by western blotting. Fig. 4A shows that as the concentration of antcin K increased, the CHOP expressions increased in dose-dependent manners, compared with the negative control (0.2% DMSO). As Bcl-2 family proteins play an important regulatory role in calcium flux and mitochondrial apoptosis, we next studied the effect of antcin K on the protein expressions of Bcl-xL, Bax, and Bak in Hep 3B cells. The results showed that the Bcl-xL expressions were decreased in dose-dependent manners and the protein expressions of Bax and Bak were increased in dose-dependent manners compared with the negative control, after treatment with various concentrations of antcin K in 48 hours.
Fig. 4.
(A) Effects of antcin K on the expressions of mitochondria and ER stress-mediated apoptosis-related protein in Hep 3B cells. After incubation of the cells with 0μM, 80μM, 100μM, and 125μM antcin K for 48 hours, expressions of mitochondria and ER stress-mediated apoptosis-related protein were assessed by western blotting. The experiments were repeated at least two times with similar results, and β-actin was used as a loading control. Protein levels are expressed as multiples of negative control (0.2% DMSO) by β-actin-normalized densitometry and shown at the bottom of each band. Effects of antcin K on expressions of (B) caspase-dependent and (C) caspase-independent apoptosis-related proteins in Hep 3B cells. After incubation of the cells with 0μM, 80μM, 100μM, and 125μM antcin K for 48 hours, expressions of proteins were assessed by western blotting. The experiments were repeated at least two times with similar results, and β-actin was used as a loading control. Protein levels are expressed as multiples of negative control (0.2% DMSO) by β-actin-normalized densitometry and shown at the bottom of each band. CHOP = C/EBP homologous protein; Bcl-xL = B-cell lymphoma 2; Bax = B-cell lymphoma—extra-large; DMSO = dimethyl sulfoxide; ER = endoplasmic reticulum.
3.10. Effect of antcin K on the expression of caspase-dependent and caspase-independent apoptosis-related proteins
After treating Hep 3B cells with 0μM, 80μM, 100μM, and 125μM antcin K for 48 hours, caspase-independent and caspase-dependent apoptosis-related protein expression was analyzed by western blotting. Fig. 4B shows that antcin K significantly increased the protein expression of cytochrome c in a dose-dependent manner, and the protein expressions of cleaved caspase-9, PARP, and caspase-3 were also increased. In addition, Fig. 4C shows that antcin K truly increased the caspase-independent apoptosis-related protein expressions of AIF, HtrA2, and Endo G at 48 hours in Hep 3B cells.
4. Discussion
Currently known active ingredients of A. cinnamomea include polysaccharides, benzenoids, triterpenoids, and steroids.26 Among them, triterpenoids have received more attention due to their potent anticancer effects.27 Out of three artificial cultivation methods for A. cinnamomea, basswood cultivation is most valuable and yields the highest content of triterpenoids, followed by solid culture, and liquid fermentation material, which is almost free of triterpenoids. Antcin K is a triterpenoid from fruiting bodies of basswood cultivated A. cinnamomea and accounts for 0.5% of A. cinnamomea.28 Furthermore, it has previously been reported that antcin K can inhibit ROS generation in human neutrophils to attain anti-inflammatory effects29 and inhibit Na+/K+-ATPase.30 Our current study demonstrated that antcin K inhibited the proliferation of human liver cancer cells. Treatment of Hep 3B cells with antcin K caused the cells to undergo apoptotic cell death through the mitochondrial and ER stress signaling pathway.
ER and mitochondria interact both physiologically and functionally, and one of the most critical aspects of this interaction is calcium signaling between the two organelles.31 As evident from ROS production and the results of ATP level analysis (Fig. 3B and D), antcin K can increase ROS generation in human hepatoma Hep 3B cells and also reduce the ATP level of the cells, suggesting that ER may be damaged by oxidation and in a hypoxic state.32 Shown by the results of the western blotting (Fig. 4A), when ER stress was induced, the protein expression of CHOP increased, downregulated Bcl-xL, and activated Bax/Bak channel to promote intracellular calcium ion release from the ER then transfer into the mitochondria. We studied the interaction of ER and the mitochondrial pathway in our ongoing efforts to determine the apoptotic mechanism of antcin K against Hep 3B cells. Apoptosis occurs upon the perturbation of cellular Ca2+ homeostasis, such as cytosolic Ca2+ overload, ER Ca2+ depletion, and mitochondrial Ca2+ increase.33, 34 Ca2+ overload can induce mitochondrial depolarization and, subsequently, the release of apoptosis-inducing factors (such as cytochrome c), which leads to the sequential activation of caspase-9 and caspase-3.35, 36 As evident from the results of the calcium ion signal analysis (Fig. 2C and D), mitochondrial calcium ion signals were increased obviously after antcin K treatment; antcin K might cause release of calcium ions from the ER into the cytoplasm, leading to the mitochondrial uptake of these ions. In addition, as shown by the results of the mitochondrial membrane potential (Fig. 3C), antcin K can affect the cytosolic Ca2+ homeostasis and induce mitochondrial depolarization. As shown by the results of the western blotting (Fig. 4B and C), caspase-independent and caspase-dependent apoptosis-related proteins were released, including HtrA2, AIF, Endo G, and cytochrome c; cytochrome c activated caspase-9 and caspase-3, and cut downstream protein PARP, ultimately leading to cell apoptosis.
Su et al37 have shown that eburicoic acid, a triterpenoid from A. cinnamomea, induced autophagy in human hepatoma Hep 3B cells of the ER stress pathway and increased the calcium ion concentration as well as the protein expressions of Bcl-2 and Beclin-1. This study reported that antcin K could cause ER stress in Hep 3B cells and also increase calcium ion concentration in the cytoplasm, which were consistent with the findings of the previous studies conducted in our laboratory. However, the most interesting issue is that antcin K could not induce autophagy in Hep 3B cells; instead, it caused apoptosis, decreased the protein expression of Bcl-xL, and changed mitochondrial membrane potential. Why did eburicoic acid induce autophagy whereas antcin K cause apoptosis in Hep 3B cells, although both these active components (eburicoic acid and antcin K) were from A. cinnamomea? The reason for different cell death modes may be the different protein expressions of Bcl-2 and Bcl-xL.38 Persisting ER pressure will cause apoptosis or autophagy, and eventually lead to cell death; the cell death mode, on the other hand, depends on factors such as cell types, growth conditions, and drug structures. After comparing the protein expressions of apoptosis and autophagy, we observed that apoptosis would inhibit Bcl-2 and Bcl-xL, while autophagy could activate Bcl-2 and Bcl-xL, which happened to be opposite effects.39 Previous studies revealed that Bcl-2 and Bcl-xL exert two separate functions, depending on their subcellular localization. On the one hand, they induce apoptosis by (directly or indirectly) changing mitochondrial membrane potential. On the other hand, they can activate autophagy by liberating Beclin-1 from its inhibition by Bcl-2 or Bcl-xL, allowing it to activate the lipid kinase activity of VPS34 at the level of the ER.40 However, the cell death mechanism of triterpenoids from A. cinnamomea was not the same. It may be caused by the different chemical structures, cell lines, environments, bioactivities, and bioavailabilities; even some pure components had the effect of coordination. Although we have limited references to clarify the cell death mechanism of the triterpenoids from A. cinnamomea, future studies will certainly reveal the anticancer mechanism of A. cinnamomea.
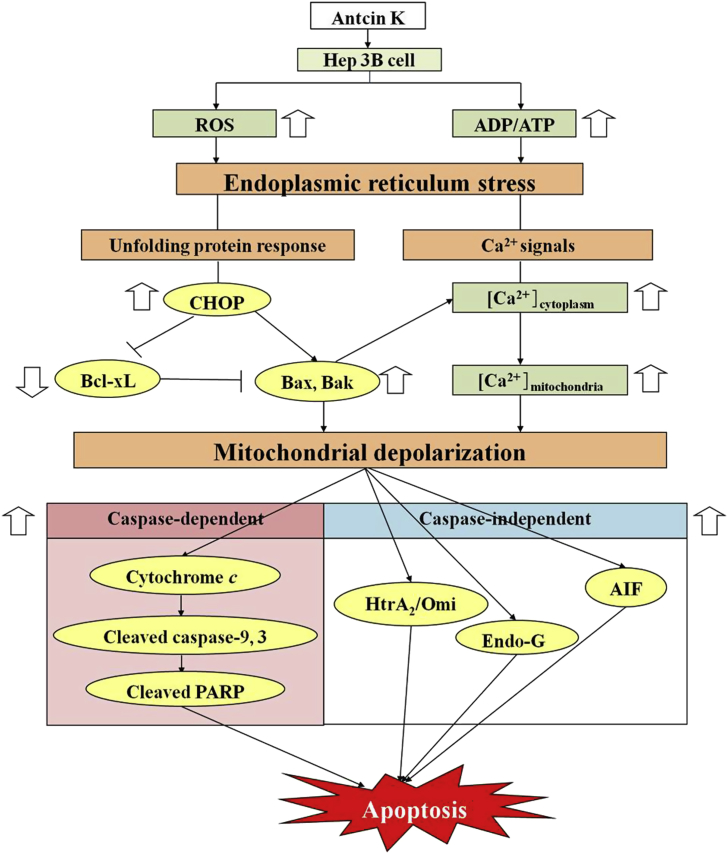
Several studies have demonstrated that A. cinnamomea has a good inhibitory effect on cancer.3, 41, 42 This study is the first of its kind to show that antcin K has an antiproliferative effect in liver cancer cells. A summary of our results is given in Fig. 5. Antcin K treatment induced Hep 3B cell death via promotion of intracellular ROS generation and ATP depletion, leading to ER stress. Moreover, the detail mechanism are followed by antcin K treatment induced ER stress and mitochondria membrane permeability change through the induction of Ca2+ release, CHOP and Bax/Bak protein expression but downregulation of Bcl-xL. In addition, it also induced caspase-dependent apoptosis by activating caspase-9 and caspase-3, and caspase-independent apoptosis by inducing protein expressions of AIF, Endo G, and HtrA2. In conclusion, antcin K has good inhibitory effects in human hepatoma cells.
Fig. 5.
Possible cell death mechanism pathway of antcin K in human liver cancer Hep 3B cells. ADP/ATP = adenosine diphosphate/adenosine triphosphate; AIF = apoptotic-induced factor; CHOP = C/EBP homologous protein; Bcl-xL = B-cell lymphoma 2; Bax = B-cell lymphoma—extra-large; Endo-G = endonuclease G; ROS = reactive oxygen species.
5. Conclusion
Summary, antcin K has good inhibitory effects in human hepatoma cells.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Joint Center for Instruments and Researches, College of Bioresources and Agriculture, National Taiwan University. In addition, this research work was partially funded by the National Science Council (NSC-101-2313-B-002-061-MY2) and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan and Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Yueh-Hsiung Kuo, Email: kuoyh@mail.cmu.edu.tw.
Lee-Yan Sheen, Email: lysheen@ntu.edu.tw.
References
- 1.Wu M.D., Cheng M.J., Yech Y.J., Yuan G.F., Chen J.J. Inhibitory effects of maleimide derivatives from the mycelia of the fungus Antrodia cinnamomea BCRC 36799 on nitric oxide production in lipopolysaccharide (LPS)-activated RAW264.7 macrophages. Chem Biodivers. 2013;10:434–441. doi: 10.1002/cbdv.201200258. [DOI] [PubMed] [Google Scholar]
- 2.Phuong do T., Ma C.M., Hattori M., Jin J.S. Inhibitory effects of antrodins A–E from Antrodia cinnamomea and their metabolites on hepatitis C virus protease. Phytother Res. 2009;23:582–584. doi: 10.1002/ptr.2657. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y.M., Liu Y.K., Lan K.L., Lee Y.W., Tsai T.H., Chen Y.J. Medicinal fungus Antrodia cinnamomea inhibits growth and cancer stem cell characteristics of hepatocellular carcinoma. Evid Based Complement Alternat Med. 2013:569737. doi: 10.1155/2013/569737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freitas N., Salisse J., Cunha C., Toshkov I., Menne S., Gudima S.O. Hepatitis delta virus infects the cells of hepadnavirus-induced hepatocellular carcinoma in woodchucks. Hepatology. 2012;56:76–85. doi: 10.1002/hep.25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demchenko A.P. Beyond annexin V: fluorescence response of cellular membranes to apoptosis. Cytotechnology. 2013;65:157–172. doi: 10.1007/s10616-012-9481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskelinen E.L., Doctor Jekyll, Hyde Mister. autophagy can promote both cell survival and cell death. Cell Death Differ. 2005;12(Suppl. 2):1468–1472. doi: 10.1038/sj.cdd.4401721. [DOI] [PubMed] [Google Scholar]
- 7.Dutta S., Going J.J., Crumley A.B. The relationship between tumour necrosis, tumour proliferation, local and systemic inflammation, microvessel density and survival in patients undergoing potentially curative resection of oesophageal adenocarcinoma. Br J Cancer. 2012;106:702–710. doi: 10.1038/bjc.2011.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malhotra J.D., Kaufman R.J. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCullough K.D., Martindale J.L., Klotz L.O., Aw T.Y., Holbrook N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bc12 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y.J., Brewer J.W., Diehl J.A., Hendershot L.M. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 11.Marciniak S.J., Yun C.Y., Oyadomari S. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakes S.A., Lin S.S., Bassik M.C. The control of endoplasmic reticulum-initiated apoptosis by the BCL-2 family of proteins. Curr Mol Med. 2006;6:99–109. doi: 10.2174/156652406775574587. [DOI] [PubMed] [Google Scholar]
- 13.Rong Y., Distelhorst C.W. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 14.Verkhratsky A. Calcium and cell death. Sub-Cell Biochem. 2007;45:465–480. doi: 10.1007/978-1-4020-6191-2_17. [DOI] [PubMed] [Google Scholar]
- 15.Ip S.W., Chu Y.L., Yu C.S. Bee venom induces apoptosis through intracellular Ca2+-modulated intrinsic death pathway in human bladder cancer cells. Int J Urol. 2012;19:61–70. doi: 10.1111/j.1442-2042.2011.02876.x. [DOI] [PubMed] [Google Scholar]
- 16.Orsolic N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012;31:173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 18.Decker T., Lohmann-Matthes M.L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;15:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 19.Kaminskyy V., Abdi A., Zhivotovsky B. A quantitative assay for the monitoring of autophagosome accumulation in different phases of the cell cycle. Autophagy. 2011;7:83–90. doi: 10.4161/auto.7.1.13893. [DOI] [PubMed] [Google Scholar]
- 20.Crouch S.P., Kozlowski R., Slater K.J., Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 21.Wallen C.A., Higashikubo R., Dethlefsen L.A. Comparison of two flow cytometric assays for cellular RNA—acridine orange and propidium iodide. Cytometry. 1982;3:155–160. doi: 10.1002/cyto.990030303. [DOI] [PubMed] [Google Scholar]
- 22.Zachwieja J., Zaniew M., Bobkowski W. Beneficial in vitro effect of N-acetyl-cysteine on oxidative stress and apoptosis. Pediatr Nephrol. 2005;20:725–731. doi: 10.1007/s00467-004-1806-4. [DOI] [PubMed] [Google Scholar]
- 23.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 24.Wu C.C., Chung J.G., Tsai S.J., Yang J.H., Sheen L.Y. Differential effects of allyl sulfides from garlic essential oil on cell cycle regulation in human liver tumor cells. Food Chem Toxicol. 2004;42:1937–1947. doi: 10.1016/j.fct.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Yang J.H., Hsia T.C., Kuo H.M. Inhibition of lung cancer cell growth by quercetin glucuronides via G(2)/M arrest and induction of apoptosis. Drug Metab Dispos. 2006;34:296–304. doi: 10.1124/dmd.105.005280. [DOI] [PubMed] [Google Scholar]
- 26.Ao Z.H., Xu Z.H., Lu Z.M., Xu H.Y., Zhang X.M., Dou W.F. Niuchangchih (Antrodia camphorata) and its potential in treating liver diseases. J Ethnopharmacol. 2009;121:194–212. doi: 10.1016/j.jep.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 27.Laszczyk M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75:1549–1560. doi: 10.1055/s-0029-1186102. [DOI] [PubMed] [Google Scholar]
- 28.Kuo Y.H., Lin B.F. Official Gazette of the United States Patent and Trademark Office Patents; 2011. Compounds from Antrodia camphorata. [Google Scholar]
- 29.Shen Y.C., Wang Y.H., Chou Y.C. Evaluation of the anti-inflammatory activity of zhankuic acids isolated from the fruiting bodies of Antrodia camphorata. Planta Med. 2004;70:310–314. doi: 10.1055/s-2004-818941. [DOI] [PubMed] [Google Scholar]
- 30.Chung T.Y., Li F.Y., Chang C.I., Jinn T.R., Tzen J.T. Inhibition of Na(+)/K(+)-ATPase by antcins, unique steroid-like compounds in Antrodia camphorate. Am J Chin Med. 2012;40:953–965. doi: 10.1142/S0192415X1250070X. [DOI] [PubMed] [Google Scholar]
- 31.Kornmann B., Currie E., Collins S.R. An ER–mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verfaillie T., Salazar M., Velasco G., Agostinis P. Linking ER Stress to autophagy: potential implications for cancer therapy. Int J Cell Biol. 2010;2010:930509. doi: 10.1155/2010/930509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Y.J., Hendershot L.M. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 34.Orrenius S., Zhivotovsky B., Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 35.Chandra D., Choy G., Deng X.D., Bhatia B., Daniel P., Tang D.G. Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion–endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol. 2004;24:6592–6607. doi: 10.1128/MCB.24.15.6592-6607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breckenridge D.G., Stojanovic M., Marcellus R.C., Shore G.C. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su Y.C., Liu C.T., Chu Y.L., Raghu R., Kuo Y.H., Sheen L.Y. Eburicoic acid, an active triterpenoid from the fruiting bodies of basswood cultivated Antrodia cinnamomea, induces ER stress-mediated autophagy in human hepatoma cells. J Tradit Complement Med. 2012;2:312–322. doi: 10.1016/s2225-4110(16)30117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litaudon M., Jolly C., Le Callonec C. Cytotoxic pentacyclic triterpenoids from Combretum sundaicum and Lantana camara as inhibitors of Bcl-xL/BakBH3 domain peptide interaction. J Nat Products. 2009;72:1314–1320. doi: 10.1021/np900192r. [DOI] [PubMed] [Google Scholar]
- 39.Zhou F., Yang Y., Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278:403–413. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- 40.Maiuri M.C., Zalckvar E., Kimchi A., Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 41.Chang C.W., Chen C.C., Wu M.J. Active component of Antrodia cinnamomea mycelia targeting head and neck cancer initiating cells through exaggerated autophagic cell death. Evid Based Complement Alternat Med. 2013;2013:946451. doi: 10.1155/2013/946451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y.Y., Chou P.Y., Chien Y.C., Wu C.H., Wu T.S., Sheu M.J. Ethanol extracts of fruiting bodies of Antrodia cinnamomea exhibit anti-migration action in human adenocarcinoma CL1-0 cells through the MAPK and PI3K/AKT signaling pathways. Phytomed Int J Phytother Phytopharmacol. 2012;19:768–778. doi: 10.1016/j.phymed.2012.02.016. [DOI] [PubMed] [Google Scholar]