Abstract
Purpose
To compare the ability of several visual functional tests in terms of the strength of their associations with the earliest phases of age-related macular degeneration (AMD), which bears on their potential to serve as functional endpoints in evaluating treatments for early AMD and prevention strategies.
Materials and Methods
Eyes from adults ≥ 60 years old were identified as being in normal macular health or in the earliest stages of AMD (steps 2, 3 or 4) through grading of color stereo-fundus photos by an experienced grader masked to all other study variables who used the 9-step Age-Related Eye Disease Study (AREDS) classification system for AMD severity. Visual function was assessed using the following tests: best-corrected visual acuity, low luminance visual acuity, spatial contrast sensitivity, macular cone-mediated light sensitivity, and rod-mediated dark adaptation.
Results
A total of 1,260 eyes were tested from 640 participants; 1,007 eyes were in normal macular health (defined as step 1 in AREDS system) and 253 eyes had early AMD (defined as step 2, 3, or 4). Adjusting for age and gender, early AMD eyes had 2 times the odds of having delayed rod-mediated dark adaptation than eyes in normal macular health (p = 0.0019). Visual acuity, low luminance acuity, spatial contrast sensitivity, and macular light sensitivity did not differ between normal eyes and early AMD eyes.
Conclusions
Eyes in the earliest phases of AMD were two times more likely to have delayed rod-mediated dark adaptation, as assessed by the rod-intercept, as compared to older eyes in normal macular health, whereas there was no difference in early AMD versus normal eyes in tests of visual acuity, low luminance acuity, macular light sensitivity, and spatial contrast sensitivity.
Keywords: age-related macular degeneration, aging, dark adaptation, functional endpoints, visual function
Visual acuity is the established and accepted functional endpoint for evaluating interventions in the treatment of exudative age-related macular degeneration (AMD).1–3 However visual acuity is inadequate as an endpoint for evaluating treatments for early and intermediate AMD since visual acuity is largely undisturbed during these disease stages. Furthermore, for the same reason, acuity is not suitable as an endpoint in assessing AMD disease prevention strategies in those with normal macular health. Thus a challenge for the field is to identify visual functional measures that are impacted very early in the AMD disease course so that they can be evaluated in terms of their suitability as functional endpoints in trials targeted at early AMD or its prevention.
Previous research has suggested several aspects of macular visual function can be impaired in early AMD, both cone-mediated and rod-mediated functions. These include spatial contrast sensitivity, visual acuity under low luminance and/or low contrast, photopic and scotopic light sensitivity, flicker sensitivity, and dark adaptation.4–20 The purpose of this study was to assess several visual functional measures in eyes in normal macular health and eyes in the very earliest stages of AMD. Our goal is to understand which aspects of visual function are significantly impacted in the initial stages of the disease. The major advantages of the current approach is that these measures are evaluated in the same eyes and are based on a very large sample of older adults whose macular health has been evaluated by a standard and accepted AMD classification system.
METHOD
This study was approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB). The research adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants. The sample was part of the baseline cohort assembled for the Alabama Study on Early Age-Related Macular Degeneration (ALSTAR),21 a prospective study of older adults in normal macular health at baseline whose purpose is to understand what medical, behavioral and functional characteristics are associated with the incident development of AMD three years later. Participants were recruited from two primary care ophthalmology practices in the Callahan Eye Hospital at UAB. Eligibility criteria for eyes in normal macular health were as follows: (1) Age ≥ 60 years old; (2) Normal macular health in both eyes as determined by 3-field digital stereo-fundus photos (Carl Zeiss Meditec 450 Plus camera, Dublin CA) evaluated by an experienced grader masked to other study variables. The eye’s grade had to be 1 in the AREDS 9-step classification system,22 indicating normal macular health. (3) No previous diagnoses of glaucoma, other retinal conditions, optic nerve conditions, corneal disease, diabetes, Alzheimer’s disease, Parkinson’s disease, brain injury, other neurological or psychiatric conditions as revealed by the medical record or by self-report. (4) Did not reside in a nursing home or was not bed-bound. (5) Was willing to participate in a study that included a baseline visit to the Clinical Research Unit in the UAB Department of Ophthalmology and a follow-up visit three years later. Eligibility criteria for those with early AMD were identical to the criteria listed above except that eyes were required to have an AREDS grade in the 9-step system22 of 2, 3, or 4. These grades correspond to the earliest phases of AMD, as outlined in Table 1.
Table 1.
Steps 1 through 4 in the AREDS 9-Step severity scale22 which pertain to normal macular health and early AMD
| Step | Description |
|---|---|
| 1 | Drusen area is < 125 µm; no increased pigment and no depigmentation/geographic atrophy. Step 1 defines normal macular health. |
| Steps 2 – 4 define early AMD. | |
| 2 | Drusen area is ≥ 125 µm but < 250 µm; no increased pigment and no depigmentation/geographic atrophy; Or Drusen area is < 125 µm and ≥ Questionable increased pigment and/or ≥ Questionable but < 350 µm depigmentation/geographic atrophy. |
| 3 | Drusen area ≥ 250 µm but < 350 µm and no increased pigment and no depigmentation/geographic atrophy. |
| 4 | Drusen area ≥ 350 µm but < 650 µm and no increased pigment and no depigmentation/geographic atrophy.\; Or Drusen area ≥ 125 µm but < 350 µm and ≥ Questionable increased pigment and/or ≥ Questionable but < 350 µm depigmentation/geographic atrophy; Or Drusen area < 250 µm and ≥ 0 increased pigment and ≥ 350 µm but < 750 µm depigmentation/geographic atrophy |
Demographic characteristics (age, sex, race/ethnicity) were confirmed with participants through interview. Best-corrected visual acuity for each eye was assessed via the Electronic Visual Acuity tester23 (EVA; JAEB Center, Tampa FL) under photopic conditions (100 cd/m2) and expressed as the logarithm of the minimum angle resolvable (logMAR). Low luminance visual acuity was also assessed using the EVA for each eye with participants viewing letters through a 1.5 log unit neutral density filter, a method described by Sunness et al.,24 which reduced background luminance to 3.16 cd/m2. To determine how much logMAR decreased under conditions of the lower light level as compared to the photopic (100 cd/m2) assessment, we defined a decrease in visual acuity under low luminance by the increase in logMAR; an increase in logMAR by 0.1 is equivalent to a decrease in visual acuity by one line (5 letters) on the ETDRS chart.25 Contrast sensitivity for each eye was estimated by the Pelli-Robson chart26 (Precision Vision, La Salle IL) with mean luminance of 100 cd/m2, the letter-by-letter scoring method,27 and expressed as logarithm of sensitivity.
Macular light sensitivity for each eye was assessed using the Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA). The 24-2 SITA standard protocol was used following the instrument’s recommended white stimulus on white background testing procedure. To obtain an estimate of light sensitivity in the macula, sensitivity for those test targets in the macular region were averaged; these test targets consisted of 16 targets falling within the region −9° to 9° on the horizontal and vertical meridia. Average sensitivity was expressed as decibels (dB).
Rod-mediated dark adaptation was measured in only one eye in each participant because of time constraints in the protocol. The eye with better visual acuity was selected for testing. Dark adaptation was measured psychophysically using the AdaptDx (MacuLogix, Hummelstown, PA), a computer-automated dark adaptometer described previously.13, 28, 29 Before testing, the eye was dilated with 1% tropicamide and 2.5% phenylephrine hydrochloride so that a pupil diameter of ≥ 6 mm was achieved. Trial lenses were added for the 30 cm viewing distance if needed to correct for optical blur. The fellow eye was occluded with an opaque patch. The participant placed his/her head in the forehead-chinrest of the adaptometer. An infrared camera positioned behind the fixation light displayed the eye on a monitor viewed by the examiner, who facilitated the positioning of the participant’s test eye to the red fixation light using a reticule displayed on the eye’s image. The procedure began with a photo-bleach exposure to a flash (0.25 ms duration, 58,000 scotopic cd/m2 s intensity; equivalent ~ 83% bleach) while the participant was focused on the fixation light. This bleach has been shown to be sufficiently intense to generate impaired dark adaptation parameters in early AMD patients using a 20-minute duration test protocol.28 The photo-bleach flash, subtending 4°, was centered at 5° on the inferior vertical meridian (i.e., superior to the fovea on the retina) which was also the test target’s position for measuring light sensitivity. Threshold measurement for a 2° diameter, 500 nm circular target began 15 seconds after bleach offset. During threshold measurement, the participant was instructed to always maintain fixation on the red fixation light and to press a response button when a flashing target first became visible within the bleached area. Threshold was estimated using a three-down/one-up modified staircase estimate procedure described previously,28 and continued at 30 seconds intervals for 20 minutes. Log thresholds were expressed as sensitivity in decibel (dB) units as a function of time from bleach offset. The speed of dark adaptation was characterized by the rod intercept value. The rod intercept is defined as the duration required for sensitivity to recover to a criterion sensitivity value of 5.0 × 10−3 scotopic cd/m2 (3.0 log units of attenuation of the stimulus).28 The criterion sensitivity level is located in the latter half of the second component of rod recovery.30 An increase in the rod intercept is caused by a slowing of the second component of rod-mediated dark adaptation and thus a rightward shift of the dark adaptation function.
Statistical Analysis
In defining normal versus impairment for each visual function, we selected cutpoints already established in the literature.21, 24, 28, 31 Demographic and functional data were shown as means and standard deviations or percentages. Logistic regression using generalized estimating equations (GEE) were used to evaluate the relationship between impairment status and each functional measure; the GEE allows the model to account for the clustering within study participants that occurs when two eyes from the same individual are used. Age and gender were identified in the univariate analysis as potential confounders and were included in the adjusted model as covariates. Crude and adjusted odds ratios (OR) and 95% confidence intervals (CI) were presented. A p-value <0.05 (two-tailed) was considered statistically significant.
To examine the relative strength of the association between each of the visual function measures and early AMD, standardized beta coefficients were computed. The standardized beta coefficients control for scale differences among various tests of visual function and were calculated from the adjusted model as the parameter estimate multiplied by the sample standard deviation. Visual functional measures having larger standardized beta coefficients (in absolute value) indicate stronger relationships with the outcome (early AMD).
RESULTS
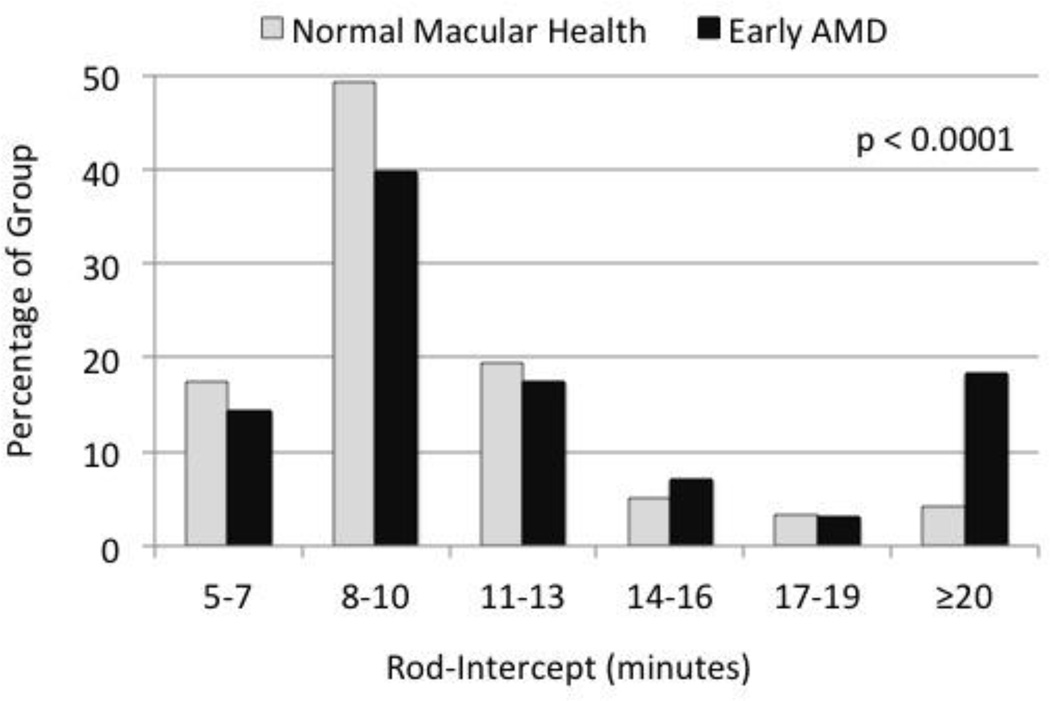
There were 1,260 eyes from 640 participants in the analysis sample. In terms of eyes, 1,007 eyes were in normal macular health and 253 eyes had early AMD (Table 2). Early AMD eyes were more likely to be from older individuals (p < 0.0001) and from men (p = 0.062), as compared to eyes in normal macular health. Means and standard deviations for the visual functional tests are also presented in Table 2. Visual acuity under photopic conditions on the ETDRS chart25 was on average only 2 letters worse in eyes with early AMD, and on average 1 letter worse under low luminance conditions, although both findings were statistically significant. There were no differences between the two groups in spatial contrast sensitivity, light sensitivity in the macula, or the magnitude of decrease in acuity under low luminance. Rod-mediated dark adaptation as measured by the rod-intercept was significantly slowed in eyes with AMD as compared to those in normal macular health. Figure 1 shows the distribution of rod-intercept for both groups.
Table 2.
Demographic and visual function characteristics of eyes in analysis sample stratified by normal macular health and early AMD
| Characteristic | Normal eyes | Early AMD Eyes | p-value |
|---|---|---|---|
| n = 1,007 | n = 253 | ||
| Age, years, n (%) | |||
| 60 – 69 | 636 (63.2%) | 116 (46.0%) | <0.0001 |
| 70 –79 | 333 (33.1%) | 105 (41.7%) | |
| 80 – 89 | 38 (3.8%) | 31 (12.3%) | |
| Mean (SD) | 68.8 (5.7) | 71.1 (7.0) | <0.0001 |
| Sex, n (%) | |||
| Female | 670 (66.5%) | 150 (59.3%) | 0.062 |
| Male | 337 (33.5%) | 103 (40.7%) | |
| Race/Ethnicity, n (%) | |||
| White | 950 (94.3%) | 243 (96.1%) | 0.41 |
| Black | 46 (4.6%) | 7 (2.8%) | |
| Other | 11 (1.1%) | 3 (1.2%) | |
| Visual acuity, logMAR, mean (SD) | 0.043 (0.13) | 0.069 (0.13) | 0.0090 |
| Low luminance visual acuity, logMAR, mean (SD) | 0.35 (0.13) | 0.37 (0.14) | 0.034 |
| Number of acuity lines lost under low luminance, mean (SD) | 0.31 (0.10) | 0.30 (0.11) | 0.45 |
| Contrast sensitivity, log sensitivity, mean (SD) | 1.61 (0.10) | 1.60 (0.11) | 0.21 |
| Light sensitivity, dB, mean (SD) | 30.40 (2.03) | 30.40 (1.65) | 0.96 |
| Rod-mediated dark adaptation, rod-intercept, minutes, mean (SD) 1 | 11.2 (5.9) | 15.3 (10.9) | <0.0001 |
Dark adaptation based on 430 normal eyes and 126 early AMD eyes since dark adaptation was tested on one eye only.
Figure 1.
Frequency histogram of the rod-intercept as measured by dark adaptation testing for the participants in normal macular health and those with early AMD. Frequency in each group is expressed as a percentage of the group with rod-intercept values in each of the indicated ranges. The p-value comparing the distributions of the two groups is p < 0.0001.
Table 3 shows the crude and the age- and gender-adjusted odds ratio (OR) for the association between impaired vision on that particular visual function measure and early AMD. Eyes with early AMD were 35% more likely to have worse than 20/20 visual acuity (0.0 logMAR) when adjusted for age and gender, although this was of borderline significance. After adjustment a similar proportion of early AMD eyes and normal eyes were worse than 0.30 logMAR (20/40). When the decrease in visual acuity under low luminance conditions was expressed in terms of the number of lines “lost” compared to photopic conditions, the proportion of eyes dropping more than 3 lines (15 letters) on the ETDRS chart was similar in the two groups. Similarly there was no significant difference with respect to impaired contrast sensitivity or reduced macular light sensitivity. Early AMD eyes had two times the odds of having delayed rod-mediated dark adaptation compared to eyes in normal macular health (p = 0.0019).
Table 3.
Visual function for eyes in normal macular health versus early AMD eyes
| Normal Eyes (n=1007) |
Early AMD Eyes (n=253) |
Crude | Adjusted1 | ||||
|---|---|---|---|---|---|---|---|
| OR | p-value | OR | 95% CI | p-value | |||
| Visual acuity, logMAR, n (%) | |||||||
| ≤0.0 | 460 (45.8) | 91 (36.0) | Ref | --- | Ref | --- | |
| >0.0 | 545 (54.2) | 162 (64.0) | 1.50 | 0.0097 | 1.35 | 1.00–1.84 | 0.053 |
| Low luminance visual acuity, logMAR, n (%) | |||||||
| ≤0.30 | 399 (39.7) | 83 (32.8) | Ref | --- | Ref | --- | |
| >0.30 | 606 (60.3) | 170 (67.2) | 1.35 | 0.058 | 1.19 | 0.87–1.62 | 0.28 |
| Number of acuity lines lost under low luminance, n (%) | |||||||
| ≤ 3 | 500 (49.8) | 127 (50.2) | Ref | --- | Ref | --- | |
| > 3 | 505 (50.3) | 126 (49.8) | 0.98 | 0.90 | 0.94 | 0.71–1.25 | 0.69 |
| Contrast sensitivity, log sensitivity, n (%) | |||||||
| ≥1.5 | 918 (91.3) | 223 (88.1) | Ref | --- | Ref | --- | |
| <1.5 | 87 (8.7) | 30 (11.9) | 1.42 | 0.11 | 1.17 | 0.76–1.79 | 0.48 |
| Light sensitivity, dB, n (%) | |||||||
| ≥ 30 | 690 (68.8) | 163 (65.5) | Ref | --- | Ref | --- | |
| < 30 | 313 (31.2) | 86 (34.5) | 1.16 | 0.36 | 0.94 | 0.68–1.32 | 0.74 |
| Rod-mediated dark adaptation, rod-intercept, minutes, n (%)2 | |||||||
| < 12.3 | 334 (77.7) | 75 (59.5) | Ref | --- | Ref | --- | |
| ≥ 12.3 | 96 (22.3) | 51 (40.5) | 2.37 | <0.0001 | 2.06 | 1.30–3.24 | 0.0019 |
Adjusted for age and sex.
Dark adaptation based on 430 normal eyes and 126 early AMD eyes since dark adaptation was tested on one eye only.
Standardized beta coefficients for each visual function test were computed in order to assess the strength of association for each measure independent of the units of measurement (Table 4). The coefficient for rod-mediated dark adaptation demonstrated the strongest association, nearly three times that of the next strongest measure (i.e., visual acuity).
Table 4.
Standardized beta coefficients
| Standardized Beta Coefficient |
|
|---|---|
| Visual acuity | 0.139 |
| Low luminance visual acuity | 0.079 |
| Number of acuity lines lost under low luminance | −0.074 |
| Contrast sensitivity | 0.016 |
| Light sensitivity | 0.093 |
| Rod-mediated dark adaptation | 0.386 |
Adjusted for age and sex.
DISCUSSION
Several visual functional tests have been suggested in the literature as potentially useful endpoint measures for evaluating treatments for the earliest phases of AMD or evaluating AMD prevention strategies. In this study we evaluated the association between the earliest phases of AMD and visual acuity, low luminance visual acuity, the magnitude of drop in acuity under low luminance conditons, spatial contrast sensitivity, light sensitivity in the macula, and rod-mediated dark adaptation. Our results indicate that among the tests evaluated, the only functional measure that was significantly associated with the earliest phases of AMD when adjusted for age is rod-mediated dark adaptation. The standardized beta coefficients, which remove differences in units of measurement across the various tests of visual function, also verified these findings. While the current findings suggest that dark adaptation is a promising candidate as a functional endpoint in evaluating treatments for the earliest phases of AMD or its prevention, future research will need to examine the natural history of dark adaptation in eyes transitioning from normal macular health to early AMD. Previous research has already demonstrated significant worsening of dark adaptation in eyes with early or intermediate AMD over a 12-month period, despite stable visual acuity and fundus appearance.29
There is growing evidence supporting the biological plausibility of rod-mediated dark adaptation as a sensitive marker for early AMD,32–34 and our psychophysical findings here are consistent with this framework. Metabolic exchange between the choroid and photoreceptors in early AMD eyes may be hampered by depositions rich in hydrophobic esterified cholesterol in Bruch’s membrane and in the sub-retina pigment epithelium (RPE) space,35, 36 which can create a diffusion barrier that impairs transport of essential nutrients such as vitamin A. These depositions impede translocation of multimolecular complexes, such as plasma lipoproteins, that deliver lipophilic essentials for rapid outer retinal uptake and distribution.37, 38 These events can have negative ramifications for rod-mediated vision. Vitamin A deficiency preferentially causes rod dysfunction and eventual photoreceptor death.39–43 In addition, slowing in the regeneration rate of the visual pigment rhodopsin and the recovery of light sensitivity after photopigment bleaching can result from diminished amounts of 11-cis-retinal (a derivative of vitamin A) available to combine with the protein opsin to form rhodopsin.30 Cones and cone-mediated sensitivity may be less impacted by this nutritional barrier since; unlike rods that derive vitamin A preferentially from the RPE, cones have alternative sources of vitamin A through the retinal vasculature (i.e., Müller cells) and cone-selective retinoid targeting mechanisms.44, 45 In addition, persons with early AMD tend to exhibit deficits in rod-mediated vision that are more severe than cone-mediated definitions measured in the same retinal areas.6, 16, 46, 47 Our previous work has provided evidence in support of the nutritional barrier/retinoid deficiency hypothesis in that the rate of rod-mediated dark adaptation in older adults with normal retinal health or AMD became more rapid after a 30-day course of high-dose retinol, where as cone-mediated dark adaptation parameters were unaffected.48
This study has strengths and limitations. Strengths include the assessment of several different candidate psychophysical tests in the same eyes in order to evaluate their ability to differentiate normal and early AMD. In addition, the sample of eyes studied was very large compared to samples from previous psychophysical studies. Normal macular health and early AMD disease presence were determined by an accepted and a commonly used disease classification system22 based on the grading of stereo fundus photos by graders with established reliability who were masked to all other participant characteristics. This study focused exclusively on AMD eyes in the earliest phases, as opposed to combining data from both early and intermediate disease. Limitations also must be acknowledged. Some visual function tests previously shown as having potential as functional endpoints in evaluating treatments for early AMD were not included in our study (e.g., flicker sensitivity, short wavelength light sensitivity, multi-focal electroretinogram, scotopic perimetry).5, 14, 46, 49 Further investigation of this issue is merited, especially in light of a recent study suggesting that 14 Hz flicker and blue color thresholds have good diagnostic sensitivity and ease of test administration.20 Rod-mediated dark adaptation was assessed in one eye only, unlike the other visual function tests measured in both eyes; however, even with the reduced sample size, there was adequate statistical power to demonstrate significant differences in rod-intercept between the two groups. In addition, the pattern of results was unchanged when analyses were restricted to eyes for which dark adaptation was measured.
In summary, rod-mediated dark adaptation, as assessed by the rod-intercept, was associated with the earliest phases of AMD, whereas tests of visual acuity, low luminance visual acuity, macular light sensitivity, and spatial contrast sensitivity were not. The analyses presented here are cross-sectional in nature, however the prospective nature of the ALSTAR study will ultimately be able to address to what extent these visual functions change over three years as eyes undergo aging with some transitioning from normal macular health to early AMD.
Acknowledgments
FUNDING
This research was supported by the National Institute on Aging of the National Institutes of Health (R01AG04212), Research to Prevent Blindness, the EyeSight Foundation of Alabama, and the Alfreda J. Schueler Trust.
Footnotes
DECLARATION OF INTEREST
Cynthia Owsley is a patent holder on the technology used to measure dark adaptation in this study. Gregory Jackson is an employee of and investor in MacuLogix, the manufacturer of the AdaptDx used in this study. The other authors report no conflicts of interest. The authors alone are responsible for the content of this paper.
REFERENCES
- 1.Csaky KG, Richman EA, Ferris FL., III Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49:479–489. doi: 10.1167/iovs.07-1132. [DOI] [PubMed] [Google Scholar]
- 2.The Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. NEJM. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Midena E, Angeli CD, Blarzino MC, Valenti M, Segato T. Macular function impairment in eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997;38:469–477. [PubMed] [Google Scholar]
- 5.Eisner A, Fleming SA, Klein ML, Mauldin WM. Sensitivities in older eyes with good acuity: eyes whose fellow eye has exudative AMD. Invest Ophthalmol Vis Sci. 1987;28:1832–1837. [PubMed] [Google Scholar]
- 6.Owsley C, Jackson GR, Cideciyan AV, Huang Y, Fine SL, Ho AC, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:267–273. [PubMed] [Google Scholar]
- 7.Lovie-Kitchin JE, Feigl B. Assessment of age-related maculopathy using subjective vision tests. Clin Exp Optom. 2005;88:292–303. doi: 10.1111/j.1444-0938.2005.tb06713.x. [DOI] [PubMed] [Google Scholar]
- 8.Haymes SA, Roberts KF, Cruess AF, Nicolela MT, LeBlanc RP, Ramsey MS, et al. The letter contrast sensitivity test: Clinical evaluation of a new design. Invest Ophthalmol Vis Sci. 2006;47:2739–2745. doi: 10.1167/iovs.05-1419. [DOI] [PubMed] [Google Scholar]
- 9.Patel PJ, Chen FK, Rubin GS, Tufail A. Intersession repeatability of contrast sensitivity scores in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:2621–2625. doi: 10.1167/iovs.08-2407. [DOI] [PubMed] [Google Scholar]
- 10.Midena E, Vujosevic S, Convento E, Manfre A, Cavarzeran F, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91:1499–1503. doi: 10.1136/bjo.2007.119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feigl B, Brown B, Lovie-Kitchen J, Swann P. Cone-mediated multifocal eletroretinogram in early age-related maculopathy and its relationships with subjective macular function tests. Curr Eye Res. 2004;29:327–336. doi: 10.1080/02713680490516198. [DOI] [PubMed] [Google Scholar]
- 12.Owsley C, Jackson GR, White MF, Feist R, Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001;108:1196–1202. doi: 10.1016/s0161-6420(01)00580-2. [DOI] [PubMed] [Google Scholar]
- 13.Jackson GR, Scott IU, Kim IK, Quillen DA, Iannaccone A, Edwards JG. Diagnostic test sensitivity and specificity of the AdaptDx for the detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:1427–1431. doi: 10.1167/iovs.13-13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phipps JA, Dang TM, Vingrys AJ, Guymer RH. Flicker perimetry losses in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:3355–3360. doi: 10.1167/iovs.04-0253. [DOI] [PubMed] [Google Scholar]
- 15.Dimitrov PN, Guymer RH, Zele AJ, Anderson AJ, Vingrys AJ. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49 doi: 10.1167/iovs.06-1048. [DOI] [PubMed] [Google Scholar]
- 16.Owsley C, McGwin G, Jackson G, Kallies K, Clark M. Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007;114:1728–1735. doi: 10.1016/j.ophtha.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Vujosevic S, Smolek M, Lebow K, Notaroberto N, Pallikaris A, Casciano M. Detection of macular function changes in early (AREDS 2) and intermediate (AREDS 3) age-related macular degeneration. Ophthalmologica. 2011;225:155–160. doi: 10.1159/000320340. [DOI] [PubMed] [Google Scholar]
- 18.Elsner AE, Burns SA, Weiter JJ. Cone photopigment in older adults: decreased optical density in early age-related macular degeneration. J Opt Soc Am A Opt Image Sci Vis. 2002;19:214–222. doi: 10.1364/josaa.19.000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisner A, Stoumbos VD, Klein ML, Fleming SA. Relations between fundus appearance and function: Eyes whose fellow eye has exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 1991;32:8–20. [PubMed] [Google Scholar]
- 20.Dimitrov PN, Robman LD, Marsamidis M, Aung KZ, Makeyeva G, Busija L, et al. Relationship between clinical macular changes and retinal function in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:5213–5220. doi: 10.1167/iovs.11-8958. [DOI] [PubMed] [Google Scholar]
- 21.Owsley C, Huisingh C, Jackson GR, Curcio CA, Szalai AJ, Dashti N, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Invest Ophthalmol Vis Sci. 2014;55:4776–4789. doi: 10.1167/iovs.14-14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration. AREDS Report No. 17. Arch Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck RW, Moke PS, Turpin AH, Ferris FLI, SanGiovanni JP, Johnson CA, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 24.Sunness JS, Rubin GS, Applegate CA, Bressler NM, Marsh MJ, Hawkins BS, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104:1677–1691. doi: 10.1016/s0161-6420(97)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 26.Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vision Sci. 1988;2:187–199. [Google Scholar]
- 27.Elliott DB, Bullimore MA, Bailey IL. Improving the reliability of the Pelli-Robson contrast sensitivity test. Clin Vision Sci. 1991;6:471–475. [Google Scholar]
- 28.Jackson GR, Edwards JG. A short-duration dark adaptation protocol for assessment of age-related maculopathy. J Ocul Biol Dis Infor. 2008;1:7–11. doi: 10.1007/s12177-008-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson GR, Clark ME, Scott IU, Walter LE, Quillen DA, Brigell MG. Twelve-month natural history of dark adaptation in patients with AMD. Optom Vis Sci. 2014;91:925–931. doi: 10.1097/OPX.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 30.Lamb TD, Pugh ENJ. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Brenton RS, Phelps CD. The normal visual field on the Humphrey field analyzer. Ophthalmologica. 1986;193:56–74. doi: 10.1159/000309679. [DOI] [PubMed] [Google Scholar]
- 32.Curcio CA, Jackson GR, Owsley C. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000;41:2015–2018. [PubMed] [Google Scholar]
- 33.Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Aging Res Rev. 2002;1:381–386. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 34.Jackson GR, Curcio CA, Sloan KR, Owsley C. Photoreceptor degeneration in aging and age-related maculopathy. In: Penfold PL, Provis JM, editors. Macular Degeneration: Science and Medicine in Practice. New York: Springer-Verlag Inc; 2004. pp. 45–62. [Google Scholar]
- 35.Curcio C, Johnson M, Huang J, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res. 2009;28:393–422. doi: 10.1016/j.preteyeres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curcio CA, Johnson M, Rudolf M, Huang J-D. The oil spill in ageing Bruch's membrane. Br J Ophthalmol. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cankova Z, Huang J-D, Kruth H, Johnson M. Passage of low-density lipoproteins through Bruch's membrane and choroid. Exp Eye Res. 2011;93:947–955. doi: 10.1016/j.exer.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tserentsoodol N, Sztein J, Campos M, Gordiyenko NV, Fariss RN, Lee JW, et al. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol Vis. 2006;12:1306–1318. [PubMed] [Google Scholar]
- 39.Dowling JE, Wald G. Vitamin A deficiency and night blindness. Proc Natl Acad Sci U S A. 1958;44:648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kemp CM, Jacobson SG, Faulkner DJ. The effects of vitamin A deficiency on human visual function. Exp Eye Res. 1988;46:185–197. doi: 10.1016/s0014-4835(88)80076-9. [DOI] [PubMed] [Google Scholar]
- 41.Kemp CM, Jacobson SG, Borruat F-X, Chaitlin MH. Rhodopsin levels and retinal function in cats during recovery from vitamin A deficiency. Exp Eye Res. 1989;49:49–65. doi: 10.1016/0014-4835(89)90075-4. [DOI] [PubMed] [Google Scholar]
- 42.Katz ML, Chen D-E, Stientjes HJ, Stark WS. Photoreceptor recovery in retinoid-deprived rats after vitamin A replenishment. Exp Eye Res. 1993;56:671–682. doi: 10.1006/exer.1993.1084. [DOI] [PubMed] [Google Scholar]
- 43.Kemp CM, Jacobson SG, Faulkner DJ, Walt RW. Visual function and rhodopsin levels in humans with vitamin A deficiency. Exp Eye Res. 1988;46:185–196. doi: 10.1016/s0014-4835(88)80076-9. [DOI] [PubMed] [Google Scholar]
- 44.Mata NL, Radu RA, Clemmons RS, Travis GH. Isomerization and oxidation of vitamin A in cone-dominant retinas: A novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garlipp MA, Gonzalez-Fernandez F. Cone outer segment and Muller microvilli pericellular matrices provide binding domains for interphotoreceptor retinoid-binding protein (IRBP) Exp Eye Res. 2013;113:192–202. doi: 10.1016/j.exer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Scholl HPN, Bellmann C, Dandekar SS, Bird AC, Fitzke FW. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci. 2004;45:574–583. doi: 10.1167/iovs.03-0495. [DOI] [PubMed] [Google Scholar]
- 47.Chen JC, Fitzke FW, Pauleikhoff D, Bird AC. Functional loss in age-related Bruch's membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992;33:334–340. [PubMed] [Google Scholar]
- 48.Owsley C, McGwin G, Jackson GR, Heimburger DC, Piyathilake CJ, Klein R, et al. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47:1310–1318. doi: 10.1167/iovs.05-1292. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Tso M, Lam T. Reduced amplitude and delayed latency in foveal response of multifocal electroretinogram in early age related macular degeneration. Br J Ophthalmol. 2001;85:287–290. doi: 10.1136/bjo.85.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]