Abstract
Adiponectin is a key component in multiple metabolic pathways. Studies evaluating associations of adiponectin with clinical outcomes in older adults have reported conflicting results. We investigated the association of adiponectin with mortality and cardiovascular disease (CVD) morbidity in a young, multiethnic adult population. We analyzed data from participants in the Dallas Heart Study without baseline CVD who underwent assessment of total adiponectin between 2000–2002. The primary outcome of all-cause mortality was assessed over median 10.4 years of follow-up using multivariable-adjusted Cox proportional hazards models. Secondary outcomes included CVD mortality, major adverse cardiovascular and cerebrovascular events (MACCE), and heart failure (HF). The study cohort included 3,263 participants mean age 43.4 years; 44% women; 50% black. There were 184 deaths (63 CVD), 207 MACCE, and 46 HF events. In multivariable models adjusted for age, sex, race, hypertension, diabetes, smoking, low HDL-C, hyperlipidemia, hs-CRP level, eGFR, and BMI, increasing adiponectin quartiles were positively associated with all-cause mortality Q4 vs. Q1, HR=2.27 (95% CI 1.47, 3.50); CVD mortality Q4 vs. Q1, HR=2.43 (95% CI 1.15, 5.15); MACCE Q4 vs. Q1, HR=1.71 (95% CI 1.13, 2.60); and HF Q4 vs. Q1, HR=2.95 (95% CI 1.14, 7.67). Findings were similar with adiponectin as a continuous variable and consistent across subgroups defined by age, sex, race, obesity, diabetes, metabolic syndrome, or elevated hs-CRP. In conclusion, higher adiponectin was associated with increased mortality and CVD morbidity in a young, multiethnic population. These findings may have implications for strategies aimed at lowering adiponectin to prevent adverse outcomes.
Keywords: adiponectin, general population, cardiovascular disease, heart failure, obesity
Introduction
Adiponectin is a 247 amino acid peptide secreted by adipose tissue directly involved with multiple metabolic pathways. Plasma levels are inversely related to body weight, insulin resistance, and type 2 diabetes and reflect increased peroxisome proliferator – activated receptor γ activity.1–3 Adiponectin also has anti-inflammatory and anti-atherogenic properties.4 Considering these favorable associations with cardiovascular disease (CVD) risk factors, it is surprising that only a single study in humans found an inverse association with myocardial infarction (MI) risk in individuals without prior CVD.5 In contrast, multiple other studies found a positive association between adiponectin and several adverse outcomes among individuals with established CVD or at high risk for CVD. Higher adiponectin has been associated with increased mortality in patients with heart failure (HF)6,7 and with increased overall and CVD mortality,8–11 coronary artery disease,12 stroke13 and HF14,15 in several large prospective trials among older adults. Importantly, few data are available from younger populations without established CVD. It is plausible that the association of adiponectin with clinical outcomes may differ in younger populations which would allow better delineation of a potential protective association of adiponectin with outcomes. Therefore, we examined the association of adiponectin with fatal and non-fatal CVD outcomes and with imaging biomarkers of subclinical CVD in participants from the Dallas Heart Study (DHS), a large, prospective, multi-ethnic population cohort. We also examined the consistency of associations across a spectrum of participants with different levels of clinical risk factors for cardiometabolic disease.
Methods
The DHS is a multiethnic, probability-based, population cohort study of Dallas County adults with deliberate over-sampling of blacks. Detailed methods of the DHS have been described previously.16 Briefly, between 2000 and 2002, an initial cohort of 6,101 individuals participated in an in-home survey. Of these, 3,398 participants aged 30–65 years participated in a second visit to provide blood samples, and 3,072 individuals came to UT Southwestern Medical Center for a third visit where multimodality imaging, including comprehensive assessments of subclinical CVD and body composition, were performed. For the present study, participants with prevalent CVD (defined as self-reported coronary heart disease, ischemic stroke or transient ischemic attack, or clinical HF) and those with missing adiponectin data were excluded, yielding a final sample size of 3,263. Participants provided written informed consent, and the protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center.
Weight and height were measured by standard scales. BMI was calculated as weight (kilograms)/height2 (meters). Waist circumference was measured 1 cm above the iliac crest and hip circumference at the widest circumference of the buttocks at the area of the greater trochanters. Dual x-ray absorptiometry (DEXA, Delphi W scanner, Hologic Inc., Bedford, MA and Discovery software [version 12.2]) was used to measure total body fat mass, lean mass, and lower body subcutaneous fat mass.17 Visceral (VAT) and abdominal subcutaneous adipose tissue (SAT) mass were measured by a 1.5-T MRI system (Intera, Philips Medical Systems, Best, The Netherlands) using a prospectively designed and validated method of fat mass prediction from a single MRI slice at the L2-L3 inter-vertebral level.18 LV mass, end-systolic and diastolic volumes, and wall thickness were obtained from short-axis, breath-hold, electrocardiographic-gated cine cardiac magnetic resonance images using the same 1.5-T system as previously described.19 Coronary artery calcium was measured as previously described.20 Liver fat was measured using 1.5T 1H magnetic resonance spectroscopy and is reported as a percentage of signal from fat to total signal from fat and water.21
Obesity was defined as a body mass index (BMI) ≥ 30 kg/m2. Race/ethnicity, history of CVD, and smoking status were self-reported. Variable definitions for hypertension, hypercholesterolemia, diabetes, and low / high-density lipoprotein cholesterol have been previously described using conventional clinical definitions.22 The metabolic syndrome was defined by the National Cholesterol Education Program’s (NCEP) Adult Treatment Panel III report. Physical activity was derived using self-reported frequency and type of leisure-time physical activity and a standard conversion for metabolic equivalence units (METs).23 The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated by fasting insulin (μIU/ml) x fasting glucose (mmol/liter)/22.5.24 Estimated glomerular filtration rate (eGFR) was calculated by the MDRD formula.25
Blood samples were obtained from participants following an overnight fast and collected in EDTA-containing tubes. Plasma aliquots were stored at −80°C until assays were performed. Total adiponectin levels were quantified using a commercially available sandwich enzyme linked immunosorbent assay (Millipore, Billerica, MA, USA) according to the manufacturer’s specifications. The measured intra-assay CVs were between 1.0% and 7.4% and the inter-assay CVs between 2.4% and 8.4%.26 Other biomarkers including leptin, high-sensitivity C-reactive protein (hs-CRP), interleukin (IL)-18,27 and NT-proBNP28 were measured as previously described.
The primary end point was all-cause mortality. Secondary endpoints included: CV mortality, incident major adverse cardiovascular and cerebrovascular events (MACCE; a composite of CVD death, nonfatal MI, nonfatal stroke, or coronary revascularization by percutaneous coronary intervention or coronary artery bypass grafting), and incident HF. All non-fatal events were ascertained through December 31, 2011 using 1) a detailed health survey regarding interval cardiovascular events administered by the Data Coordinating Center during annual calls to study subjects and/or 2) for subjects providing informed consent (>90%), quarterly tracking of hospital admissions using the Dallas-Fort Worth Hospital Council Data Initiative Database that includes all hospital admission data for 70 out of 72 hospitals in the Dallas-Fort Worth area. Primary clinical source documents were collected and reviewed for all suspected non-fatal events and all non-fatal events were independently adjudicated by a blinded endpoint committee. Additionally, for all revascularization events, a 3-month blanking period was used to minimize the chance that information obtained during the study visit led to a revascularization procedure. Death events were ascertained through December 31, 2011 from the National Death Index and classified as cardiovascular if the primary cause was related to the cardiovascular system according to the International Statistical Classification of Diseases, 10th Revision codes I00–I99.
Demographic and clinical variables were compared across quartiles of adiponectin levels using the Jonckheere-Terpstra trend test. Associations between adiponectin levels and imaging markers of subclinical CVD were assessed by multivariable adjusted linear regression. Adiponectin was modeled using standardized β-coefficients (per 1-standard deviation of the log-transformed adiponectin level) and as sex-and race-specific quartiles. Cox proportional-hazards models were used to assess the association between adiponectin (both as a continuous measure and by sex-and race-specific quartiles) and the time to a first event, with associations reported as hazard ratio (HR) and 95% confidence interval (95% CI). Multivariable models were adjusted for age, sex, race, hypertension, diabetes, smoking, low high-density lipoprotein cholesterol (HDL-C), hyperlipidemia, hs-CRP level, estimated glomerular filtration rate (eGFR), and BMI. The proportional-hazards assumption was met for all models. Additional analyses further adjusting for relevant covariates associated with both adiponectin and CVD prognosis in the literature (lean mass, natriuretic peptide levels) were also performed. We performed multivariable-adjusted subgroup analyses with stratification by age (<45 years vs. ≥45 years), sex (male vs. female), race (black vs. non-black), BMI (obese vs. non-obese), diabetes status (yes vs. no), components of the metabolic syndrome (0, 1–2, ≥3), and elevated hs-CRP (≤3 vs. >3 mg/L). To evaluate the models for overfitting, a shrinkage coefficient for each outcome was calculated by: [Likelihood model chi-square-p]/Likelihood model chi-square, where p=# of covariates in the model; values close to 1 suggest minimal model overfitting. Linearity of the association between adiponectin and all-cause mortality was tested using adjusted cubic splines. A two-sided P-value <0.05 was considered to be statistically significant. All statistical analyses were performed with the use of SAS software, version 9.3 (SAS Institute).
Results
The study cohort included 3,263 participants with mean age 43.4 (SD±10) years; 44% were women and 50% were black. The median follow up time was 10.4 (IQR, 10.0–10.8) years.
Demographic, clinical, laboratory, and imaging characteristics of the study population stratified by adiponectin quartiles are presented in Table 1. Higher adiponectin quartiles were associated with a lower prevalence of male sex, black race, traditional cardiovascular risk factors, and generally more favorable adiposity and cardiovascular imaging profiles. The associations of log-transformed adiponectin levels with imaging markers of subclinical CVD are shown in Table 2. After multivariable adjustment for age, sex, race, hypertension, diabetes, smoking, low HDL-C, hyperlipidemia, hs-CRP level, eGFR and BMI, higher adiponectin levels remained significantly associated with lower LV wall thickness, higher left ventricular end-diastolic volumes indexed to body surface area, and lower liver fat content but not with other markers of subclinical CVD. Results were similar for adiponectin quartiles in multivariable analyses.
Table 1.
Characteristics of the Study Cohort by Adiponectin Quartiles (N=3,263)
| Variable | Q1 N=815 (0.65 – 4.39 ng/mL) | Q2 N=816 (4.40 – 6.50 ng/mL) | Q3 N=816 (6.51 – 9.53 ng/mL) | Q4 N=816 (9.54 – 34.52 ng/mL) | P-trend |
|---|---|---|---|---|---|
| Age (years) | 42 (35, 51) | 43 (36, 50) | 42 (36, 50) | 45 (37, 53) | 0.003 |
| Men | 477 (58.5%) | 411 (50.4%) | 332 (40.7%) | 217 (26.6%) | <0.0001 |
| Black | 521 (63.9%) | 434 (53.2%) | 364 (44.6%) | 308 (37.7%) | <0.0001 |
| White | 138 (16.9%) | 199 (24.4%) | 280 (34.3%) | 373 (45.7%) | <0.0001 |
| Hispanic | 134 (16.4%) | 162 (19.9%) | 161 (19.7%) | 116 (14.2)% | 0.25 |
| Hypertension | 290 (36.0%) | 261 (32.7%) | 226 (27.9%) | 222 (27.8%) | <0.0001 |
| Diabetes | 136 (16.7%) | 90 (11.0%) | 65 (8.0%) | 46 (5.6%) | <0.0001 |
| Low HDL cholesterol | 436 (53.5%) | 392 (48.0%) | 317 (38.9%) | 194 (23.8%) | <0.0001 |
| Metabolic Syndrome | 361 (44.3%) | 305 (37.4%) | 232 (28.4%) | 145 (17.8%) | <0.0001 |
| Current Smoker | 244 (29.9%) | 226 (27.7%) | 241 (29.6%) | 216 (26.6%) | 0.25 |
| Physical Activity (METS x min/wk) | 120 (0, 540) | 133 (0, 540) | 120 (0, 549.5) | 213 (0, 660) | 0.04 |
| Total cholesterol (mg/dL) | 180 (153, 207) | 178 (158, 204) | 176 (152, 200) | 178 (155, 203) | 0.26 |
| HDL cholesterol (mg/dL) | 43 (36, 50) | 45 (38, 53) | 49 (42, 58) | 56 (47, 66) | <0.0001 |
| LDL cholesterol (mg/dL) | 108 (86, 133) | 109 (88, 130) | 103 (82, 125) | 101 (78, 123) | <0.0001 |
| Triglycerides (mg/dL) | 113 (78, 172) | 102 (71, 155) | 91 (66, 137) | 81 (58, 113) | <0.0001 |
| hs-C-Reactive Protein (mg/L) | 3.9 (1.8, 7.7) | 3 (1.2, 7.3) | 2.7 (1.0, 6.4) | 1.9 (0.7, 4.65) | <0.0001 |
| IL-18 (mg/dL) | 533.1 (372.2, 803.0) | 525.6 (372.0, 753.0) | 506.0 (340.8, 742.6) | 469.5 (331.7, 726.5) | 0.003 |
| NT-pro-BNP (pg/mL) | 16.5 (6.8, 38.9) | 22.6 (10.2, 42.9) | 30 (15.2, 60.2) | 43.55 (23.0, 86.1) | <0.0001 |
| Estimated GFR (mL/min per 1.73 m2) | 101.0 (87.5, 114.2) | 98.8 (87.9, 113.2) | 98.9 (86.0, 112.2) | 95.84 (83.4, 109.7) | <0.0001 |
| Weight (kg) | 90.7 (78.9, 106.8) | 87.5 (74.6, 102.5) | 81.2 (68.9, 95.7) | 73.0 (63.1, 85.3) | <0.0001 |
| BMI (kg/m2) | 30.54(27.1, 35.0) | 29.3 (25.7, 34.0) | 27.8 (24.4, 32.7) | 25.4 (22.4, 29.6) | <0.0001 |
| Waist/Hip ratio | 0.94 (0.89, 0.99) | 0.92 (0.86, 0.97) | 0.9 (0.85, 0.95) | 0.84 (0.79, 0.9) | <0.0001 |
| Total Fat Mass (kg) | 26.8 (19.7, 34.4) | 27.0 (19.3, 35.8) | 25.3 (18.2, 34.7) | 23.9 (16.8, 30.8) | <0.0001 |
| Total Lean Mass (kg) | 61.5 (52.8, 69.2) | 57.8 (48.9, 66.0) | 52.9 (44.8, 62.1) | 46.8 (41.2, 55.2) | <0.0001 |
| Abdominal Subcutaneous Fat (kg) | 4.7 (3.3, 6.8) | 4.5 (3.1, 6.9) | 4.1 (2.8, 6.3) | 3.6 (2.4, 5.1) | <0.0001 |
| Visceral Fat (kg) | 2.4 (1.9, 3.0) | 2.3 (1.7, 3.1) | 1.9 (1.4, 2.6) | 1.5 (1.1, 2.1) | <0.0001 |
| Lower Body Fat (kg) | 8.3 (6.0, 11.8) | 9.0 (6.3, 12.2) | 8.8 (6.2, 12.5) | 9.1 (6.7, 12.0) | 0.03 |
| Liver Fat (%) | 5.2 (2.9, 10.1) | 4.0 (2.5, 7.4) | 3.3 (2.0, 5.6) | 2.6 (1.6, 4.2) | <0.0001 |
| LV Mass/BSA (g/m2) | 85.0 (75.1, 96.4) | 80.9 (70.6, 93.4) | 78.1 (69.0, 91.0) | 74.7 (66.6, 85.7) | <0.0001 |
| LV wall thickness (mm) | 12.2 (11.1, 13.5) | 11.6 (10.6, 12.7) | 11.2 (10.2, 12.3) | 10.6 (9.7, 11.7) | <0.0001 |
| LVEDV/BSA(ml/m2) | 49.3 (43.6, 56.6) | 50.8 (45.8, 57.5) | 51.6 (45.2, 57.9) | 52.0 (45.8, 58.9) | 0.0001 |
| Coronary Artery Calcium (Agatston units) | 1.0 (0.0, 9.5) | 0.7 (0.0, 5.0) | 0.0 (0.0, 4.1) | 0.0 (0.0, 1.6) | <0.0001 |
| CAC >10 | 135 (22.2%) | 119 (18.9%) | 124 (19.3%) | 108 (16.2%) | 0.01 |
| Aortic wall thickness (mm) | 1.7 (1.5, 1.9) | 1.7 (1.5, 1.8) | 1.7 (1.5, 1.8) | 1.6 (1.5, 1.8) | 0.0002 |
Data are presented as median (interquartile range) or proportion (%) as appropriate.
Abbreviations: BMI=body mass index; BSA=body surface area; CAC=coronary artery calcium; EDV=end-diastolic volume; GFR=glomerular filtration rate; HDL=high-density lipoprotein; hs=high-sensitivity; IL=interleukin; LDL=low-density lipoprotein; LV=left ventricular; METS=metabolic equivalents; NT-pro-BNP=N-terminal-pro-B-type natriuretic peptide.
Table 2.
Unadjusted and Multivariable-Adjusted Association of Adiponectin Levels* with Subclinical Markers of Cardiovascular Disease
| Subclinical CVD Marker | Standardized β-coefficient | P-value |
|---|---|---|
| Left ventricular wall thickness | ||
| Unadjusted | −0.32 | <0.0001 |
| Multivariable-adjusted† | −0.03 | 0.04 |
| Aortic wall thickness | ||
| Unadjusted | −0.08 | 0.0002 |
| Multivariable-adjusted† | 0.03 | 0.19 |
| Left ventricular end-diastolic volume indexed to body surface area | ||
| Unadjusted | 0.07 | 0.001 |
| Multivariable-adjusted† | 0.15 | <0.0001 |
| Left ventricular mass indexed to body surface area | ||
| Unadjusted | −0.19 | <0.0001 |
| Multivariable-adjusted† | 0.03 | 0.10 |
| Liver fat | ||
| Unadjusted | −0.28 | <0.0001 |
| Multivariable-adjusted† | −0.29 | <0.0001 |
| Coronary artery calcium (log-transformed) | ||
| Unadjusted | −0.09 | <0.0001 |
| Multivariable-adjusted† | 0.004 | 0.81 |
Adiponectin is modeled per 1-standard deviation of the log-transformed variable
Models are adjusted for age, sex, race, hypertension, diabetes, smoking, high-density lipoprotein cholesterol, hyperlipidemia, C-reactive protein level, estimated glomerular filtration rate, and body mass index.
During the follow up period, 184 participants died (63 from a cardiovascular cause); 207 experienced a MACCE event, and 46 developed HF. In unadjusted analyses, higher adiponectin quartiles were significantly associated with all-cause mortality (Q4 vs. Q1 HR=1.86 [1.27, 2.73], P=0.001), with a non-significant trend toward increased CVD mortality (Q4 vs. Q1 HR=1.80 [0.91, 3.55], P=0.09), MACCE (Q4 vs. Q1 HR=1.21 [0.83, 1.77], P=0.32), and HF (Q4 vs. Q1 HR=1.67 [0.69, 4.02], P=0.26). In unadjusted analysis as a continuous measure, adiponectin levels per 1-standard deviation increase were not significantly associated with any of the clinical outcomes.
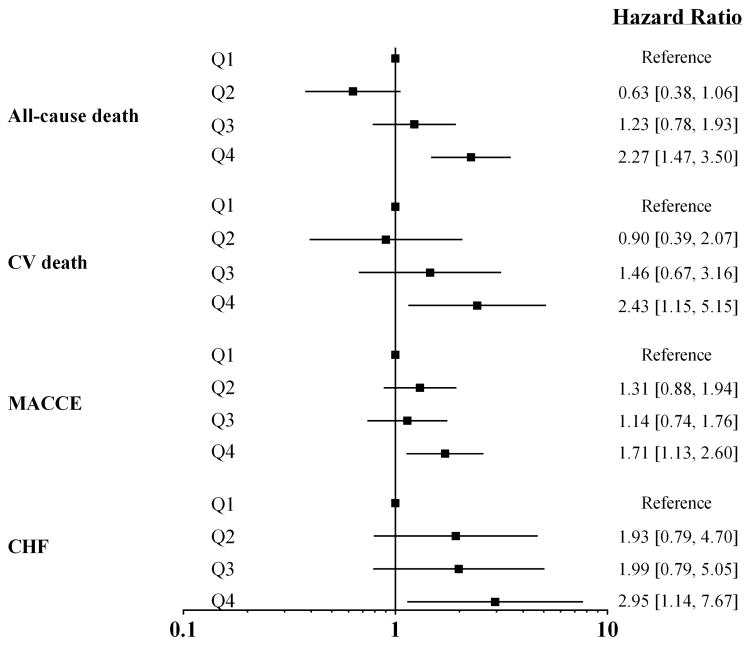
In order to account for the presence of reverse confounding due to lower rates of CVD risk factors with higher adiponectin levels, multivariable modeling of the association between adiponectin and clinical outcomes was performed adjusting for age, sex, race, hypertension, diabetes, smoking, low HDL-C, hyperlipidemia, CRP level, eGFR and BMI. Multivariable-adjusted models showed that increasing adiponectin quartiles were significantly associated with all-cause mortality (Q4 vs. Q1 HR=2.27 [1.47, 3.50], P=0.0002); CVD mortality (Q4 vs. Q1, HR=2.43 [1.15, 5.15], P=0.02); MACCE (Q4 vs. Q1, HR=1.71 [1.13, 2.60], P=0.01); and HF (Q4 vs. Q1, HR=2.95 [1.14, 7.67], P=0.03), Figure 1. Findings were similar when adiponectin was modeled as a continuous measure (Supplemental Table S1). No threshold effect was seen in the relationship between continuous adiponectin levels and all-cause mortality using multivariable-adjusted cubic splines (Supplemental Figure S1). Shrinkage coefficients for the models were 0.95, 0.87, 0.95, and 0.79, respectively, suggesting minimal model overfitting.
Figure 1. Multivariable-adjusted associations between sex-and race-specific quartiles of adiponectin level and clinical outcomes.
Models are adjusted for age, sex, race, hypertension, diabetes, smoking, high-density lipoprotein cholesterol, hyperlipidemia, C-reactive protein level, estimated glomerular filtration rate, and body mass index. CV=cardiovascular, MACCE= major adverse cardiovascular and cerebrovascular events, CHF=congestive heart failure
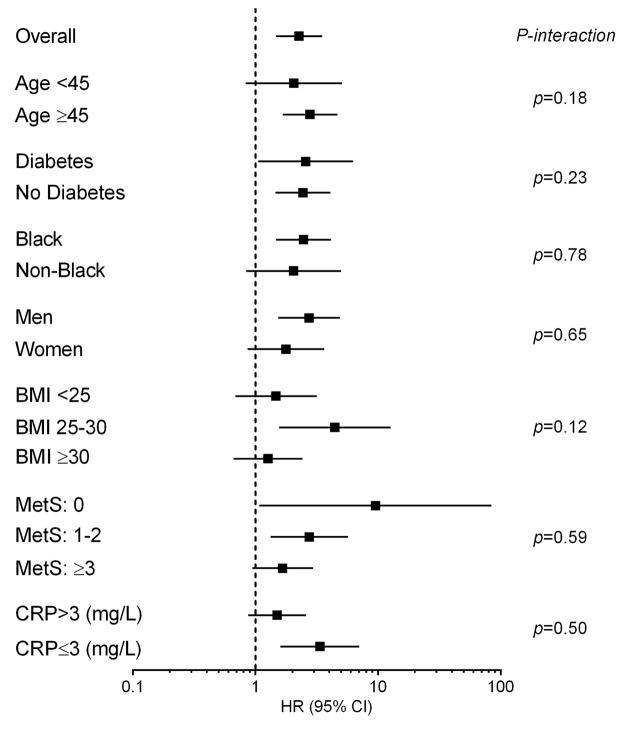
Adjustment for lean mass mildly attenuated the relationship between adiponectin and CVD mortality, but not other outcomes, and lean mass was not associated with morbidity or mortality in these models (Supplemental Figure S2). Stratification of adiponectin and NT-proBNP by median levels demonstrated additive associations of adiponectin with NT-proBNP for all-cause and CVD mortality (Figure 2). Adjustment for NT-proBNP attenuated the association between adiponectin and cardiovascular outcomes (CVD mortality, MACCE, HF) but not with all-cause mortality (Supplemental FigureS3). In subgroup analyses, the multivariable-adjusted association between quartile 4 (vs. quartile 1) adiponectin level and all-cause mortality remained generally consistent across all subgroups with no statistically significant interactions seen (Figure 3).
Figure 2. Incidence of all-cause and cardiovascular mortality stratified by sex-specific median values for adiponectin and NT-proBNP.
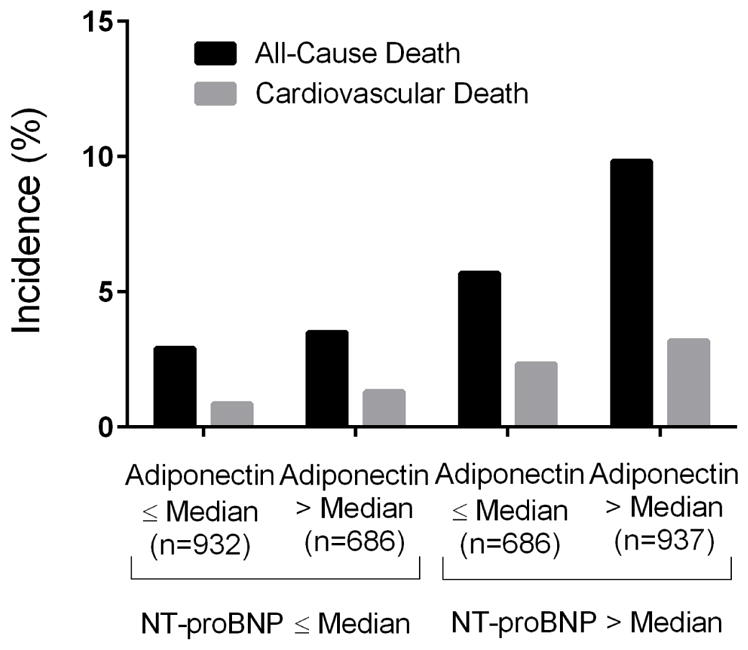
The median cut-points for adiponectin were X for men and X for women. The median cut-points for NT-proBNP were X for men and X for women. P<0.001 for all-cause mortality and P=0.001 for CV mortality.
Figure 3. Associations between adiponectin and all-cause mortality across subgroups of age, sex, race, and by presence or absence of obesity, diabetes, metabolic syndrome, or elevated CRP.
Hazard ratios are for sex-and race-specific quartile 4 of adiponectin with quartile 1 as referent. BMI=body mass index, CRP=C-reactive protein, MetS=metabolic syndrome.
Discussion
To our knowledge, this is the first study to assess the association of adiponectin with overall mortality and CVD morbidity and mortality in a relatively young, multiethnic population cohort. We found independent positive associations between adiponectin and all-cause and CVD mortality, MACCE, and incident HF that became apparent after adjustment for major risk factors. Results for the primary outcome were generally consistent across subgroups of age, sex, race, and presence or absence of obesity, diabetes, metabolic syndrome, or elevated hs-CRP level. The fact that prior to multivariable adjustment, there was minimal association between adiponectin and clinical endpoints suggests reverse confounding; namely that higher adiponectin levels are associated with a favorable cardiometabolic profile and when these factors are accounted for, an adverse prognostic signal is seen between higher adiponectin levels and clinical outcomes. This may reflect an “adiponectin paradox”; namely that a subset of individuals who appear to be metabolically healthy but have high circulating levels of total adiponectin may paradoxically be at high risk for adverse outcomes.
There are several potential explanations as to why increasing adiponectin levels may be associated with adverse clinical outcomes despite its known favorable metabolic effects. First, weight loss and sarcopenia may partially explain the association between higher adiponectin levels and mortality risk mediated through progression of chronic disease;8 however, adjustment for BMI and lean mass had minimal impact on the present findings. Second, at least among certain individuals, elevated adiponectin may reflect a lack of feedback inhibition due to resistance at the level of the cellular receptor, thereby attenuating adiponectin regulatory activity.29 One would expect adiponectin resistance to result in concomitant metabolic derangements; however, adiponectin remained associated with adverse outcomes among those with no metabolic risk factors, suggesting that absence of an effect due to receptor resistance would not fully explain these findings. Third, adiponectin may be increased in pro-inflammatory conditions as a way to counteract systemic inflammation, potentially explaining its association with CVD mortality and morbidity. However, we found generally inverse associations between adiponectin and subclinical CVD markers and the relation of adiponectin to adverse outcomes was not altered by adjustment for markers of inflammation or testing for effect modification by level of inflammation. Fourth, there are emerging data indicating that natriuretic peptides may directly increase adiponectin expression, suggesting that higher adiponectin levels may reflect the increased neurohormonal activation and hemodynamic stress observed in individuals at-risk for or with established HF.30 Our results, in a younger, racially diverse population, showed additive associations between adiponectin and NT-proBNP for all-cause and CVD mortality; however, the associations of adiponectin with CVD-specific outcomes were attenuated after including NT-proBNP as a covariate, suggesting that adiponectin may be a marker of a poor CVD prognosis in the setting of elevated natriuretic peptides, rather than a direct mediator of adverse cardiovascular outcomes. Nevertheless, the present findings suggest that, even among younger adults without prevalent cardiometabolic disease, high levels of adiponectin may be toxic and further research should focus on identifying potential mechanisms underlying the complex relationship between adiponectin, CVD, and mortality.
Our results confirm a growing body of evidence primarily in populations with a mean age over 70 years, linking high adiponectin levels to all-cause and CVD mortality and HF,8–12,14 and extend the association to a relatively younger (mean age 43 years) population with a significant proportion of black participants. The hazard ratio point estimate for both all-cause and CVD mortality was similar to that reported among older patients with no prior CVD8 and consistent with a lack of heterogeneity of effect by age as we observed here. To our knowledge this is only the second study to show an association between adiponectin and incident HF in a population with no CVD at baseline, with our study population being on average much younger and more ethnically diverse compared to other populations previously studied. This is important since excluding a protective association between higher adiponectin levels and mortality and CVD morbidity in younger populations confirms an age-independent, consistently positive relationship with adverse clinical outcomes in this population despite a generally more favorable cardiometabolic profile.
Strengths of the current study include a racially diverse study population with a high percentage of blacks and an extended period of clinical follow up. Several limitations also merit comment. First, our study had a limited number of CVD events and should be confirmed in a larger cohort with higher numbers of events. Second, we did not collect data as to the causes of non-CVD mortality nor did we divide the CVD mortality into HF and non-HF mortality, which limits our ability to draw conclusions as to the mechanisms linking adiponectin to all-cause and CVD mortality. Third, we measured only total adiponectin levels, and not high molecular weight adiponectin, which may be more biologically active; nevertheless there is a strong correlation between total and high molecular weight adiponectin levels.8 Fourth, we did not measure the activity of the adiponectin receptor and thus cannot directly test the adiponectin resistance hypothesis in the current study. Finally, given that our cohort was primarily African-American, Caucasian, and Hispanic, our findings may not be generalizable to other populations such as South or East Asians.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by award number T32HL007360 from the National Heart, Lung, and Blood Institute and by grant 1K23DK106520-01 from the National Institute of Diabetes and Digestive and Kidney Diseases to Dr. Neeland, by grants UL1DE019584 and PL1DK081182 from the National Institutes of Health, and by grant number UL1TR001105 from the National Center for Advancing Translational Sciences. Dr. Neeland is supported as a Dedman Family Scholar in Clinical Care at UT Southwestern Medical Center.
Footnotes
Conflicts of interest: none declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 3.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O’Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 4.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 5.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 6.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 7.Tsutamoto T, Tanaka T, Sakai H, Ishikawa C, Fujii M, Yamamoto T, Horie M. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. Eur Heart J. 2007;28:1723–1730. doi: 10.1093/eurheartj/ehm154. [DOI] [PubMed] [Google Scholar]
- 8.Kizer JR, Benkeser D, Arnold AM, Mukamal KJ, Ix JH, Zieman SJ, Siscovick DS, Tracy RP, Mantzoros CS, Defilippi CR, Newman AB, Djousse L. Associations of total and high-molecular-weight adiponectin with all-cause and cardiovascular mortality in older persons: the Cardiovascular Health Study. Circulation. 2012;126:2951–2961. doi: 10.1161/CIRCULATIONAHA.112.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poehls J, Wassel CL, Harris TB, Havel PJ, Swarbrick MM, Cummings SR, Newman AB, Satterfield S, Kanaya AM, Health ABCS. Association of adiponectin with mortality in older adults: the Health, Aging, and Body Composition Study. Diabetologia. 2009;52:591–595. doi: 10.1007/s00125-009-1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu ZJ, Cheng YJ, Gu WJ, Aung LH. Adiponectin is associated with increased mortality in patients with already established cardiovascular disease: a systematic review and meta-analysis. Metabolism. 2014;63:1157–1166. doi: 10.1016/j.metabol.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Choi SH, Ku EJ, Hong ES, Lim S, Kim KW, Moon JH, Kim KM, Park YJ, Park KS, Jang HC. High serum adiponectin concentration and low body mass index are significantly associated with increased all-cause and cardiovascular mortality in an elderly cohort, “adiponectin paradox”: the Korean Longitudinal Study on Health and Aging (KLoSHA) Int J Cardiol. 2015;183:91–97. doi: 10.1016/j.ijcard.2015.01.057. [DOI] [PubMed] [Google Scholar]
- 12.Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007;165:164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kizer JR, Biggs ML, Ix JH, Mukamal KJ, Zieman SJ, de Boer IH, Mozaffarian D, Barzilay JI, Strotmeyer ES, Luchsinger JA, Elkind MS, Longstreth WT, Jr, Kuller LH, Siscovick DS. Measures of adiposity and future risk of ischemic stroke and coronary heart disease in older men and women. Am J Epidemiol. 2011;173:10–25. doi: 10.1093/aje/kwq311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karas MG, Benkeser D, Arnold AM, Bartz TM, Djousse L, Mukamal KJ, Ix JH, Zieman SJ, Siscovick DS, Tracy RP, Mantzoros CS, Gottdiener JS, deFilippi CR, Kizer JR. Relations of plasma total and high-molecular-weight adiponectin to new-onset heart failure in adults >/=65 years of age (from the Cardiovascular Health study) Am J Cardiol. 2014;113:328–334. doi: 10.1016/j.amjcard.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindberg S, Jensen JS, Bjerre M, Pedersen SH, Frystyk J, Flyvbjerg A, Mogelvang R. Cardio-adipose tissue cross-talk: relationship between adiponectin, plasma pro brain natriuretic peptide and incident heart failure. Eur J Heart Fail. 2014;16:633–638. doi: 10.1002/ejhf.82. [DOI] [PubMed] [Google Scholar]
- 16.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 17.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 18.Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr. 1997;65:403–408. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- 19.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 20.Jain T, Peshock R, McGuire DK, Willett D, Yu Z, Vega GL, Guerra R, Hobbs HH, Grundy SM. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–1017. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 21.Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 22.Deo R, Khera A, McGuire DK, Murphy SA, de Meo Neto JP, Morrow DA, de Lemos JA. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–1818. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 23.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 26.Turer AT, Khera A, Ayers CR, Turer CB, Grundy SM, Vega GL, Scherer PE. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54:2515–2524. doi: 10.1007/s00125-011-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdullah SM, Khera A, Leonard D, Das SR, Canham RM, Kamath SA, Vega GL, Grundy SM, McGuire DK, de Lemos JA. Sex differences in the association between leptin and CRP: results from the Dallas Heart Study. Atherosclerosis. 2007;195:404–410. doi: 10.1016/j.atherosclerosis.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, Canham RM, Chung AK, Leonard D, Wians FH, Jr, de Lemos JA. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–2168. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 29.Rathmann W, Herder C. Adiponectin and cardiovascular mortality: evidence for “reverse epidemiology”. Horm Metab Res. 2007;39:1–2. doi: 10.1055/s-2007-958630. [DOI] [PubMed] [Google Scholar]
- 30.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, Okazaki H, Asai M, Nagamachi Y, Maeda N, Shintani Y, Minamino T, Asakura M, Kishimoto I, Funahashi T, Tomoike H, Kitakaze M. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.