Abstract
Elucidating mechanisms of chemoresistance is critical to improve cancer therapy, especially for the treatment of pancreatic ductal adenocarcinoma (PDAC). Genome-wide association studies have suggested the little studied gene HEATR1 as a possible determinant of cellular sensitivity to different chemotherapeutic drugs. In this study, we assessed this hypothesized link in PDAC, where HEATR1 expression is downregulated significantly. HEATR1 silencing in PDAC cells increased resistance to gemcitabine and other chemotherapeutics, where this effect was associated with increased AKT kinase phosphorylation at the Thr308 regulatory site. Mechanistically, HEATR1 enhanced cell responsiveness to gemcitabine by acting as a scaffold to facilitate interactions between AKT and the protein phosphatase PP2A, thereby promoting Thr308 de-phosphorylation. Consistent with these findings, treatment with the AKT inhibitor TCN sensitized HEATR1-depleted PDAC cells to gemcitabine, suggesting this therapeutic combination may overcome gemcitabine resistance in patients with low HEATR1 expression. Clinically, we found that HEATR1 downregulation in PDAC patients was associated with increased AKT phosphorylation, poor response to tumor resection plus gemcitabine standard-of-care treatment and shorter overall survival. Collectively, our findings establish HEATR1 as a novel regulator of AKT and a candidate predictive and prognostic indicator of drug responsiveness and outcome in PDAC patients.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains a lethal malignancy. The prognosis of patients with PDAC is dismal with a five-year survival of less than 5%. Anti-tumor drugs and radiation therapy are current treatment options for PDAC, however drug resistance frequently occurs. Thus, understanding molecular mechanisms contributing to the resistance of PDAC to chemotherapy will provide clues for new targeted therapies.
Akt is a central module to regulate cell proliferation and survival, angiogenesis and glucose metabolism (1, 2). Aberrant Akt activation is associated with diverse pathophysiological states including cancers and chemoresistance (3, 4). Akt controls these cellular functions through phosphorylating substrates. Akt directly phosphorylates BAD, preventing it from inhibiting prosurvival Bcl-2 family members (5, 6). Akt regulates glucose metabolism through phosphorylating and inactivating GSK3 (7). In addition, Akt negatively regulates FOXO and p53 and blocks the transcription of BIM, Puma and Noxa (8, 9). Furthermore, Akt promotes protein synthesis and cell growth through activation of mammalian target of rapamycin(10).
Akt activity is tightly controlled at multiple levels. Phosphoinositide 3-kinase (PI-3K), a critical upstream kinase of Akt signaling, is activated by growth factors, cytokine and other (2) and converts phosphatidylinositol–4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3). PIP3 recruits Akt to plasma membrane, where Akt is phosphorylated at Thr308 (11). Ubiquitination of Akt by TRAF6 and Skp2-SCF E3 ligase is required for the recruitment of Akt to plasma membrane (12, 13). Full Akt activity requires phosphorylation of both Thr308 and Ser473 mediated by phosphoinositide-dependent kinase 1 (PDK1) (14) and mammalian target of rapamycin (mTOR) complex 2 (mTORC2) (15), respectively. On the other hand, protein phosphatase 2A (PP2A) (16–18) and PH domain leucine-rich repeat protein phosphatase (PHLPP) (19, 20) dephosphorylate AktThr308 and Ser473, respectively. FKBP51 promotes dephosphorylation of Akt Ser473 through acting as a scaffolding protein for Akt and PHLPP (21). However, how Akt is targeted to PP2A is not clear.
HEAT repeat-containing protein 1 (HEATR1) contains HEAT repeats, which was initially found in a diverse family of proteins including huntingtin, elongation factor-3 and the PR65/A subunit of protein phosphatase 2A (22). Except for a couple of reports suggesting HEATR1 may regulate rRNA synthesis and cytotoxic T lymphocytes in patients with glioma (23, 24), the cellular function of HEATR1 remains largely unknown. Here, we report that HEATR1 regulates cancer cell response to multiple classes of chemotherapeutic drugs. Mechanistically, HEATR1 affects survival of pancreatic cancer cells to chemotherapy through affecting Akt activity. We demonstrate that HEATR1 functions as a scaffold protein to regulate Akt phosphorylation by PP2A. Furthermore, our study identifies HEATR1 as a potential prognostic marker of pancreatic cancers.
Materials and Methods
Cell Culture and Plasmids
Human pancreatic cancer cell lines SU86.86, ASPC-1, and PANC-1 were purchased from ATCC in 2014 and the identities of all cell lines were confirmed by the medical genome facility at Mayo Clinic Center using short tandem repeat profiling upon receipt. The cell lines were maintained in RPMI 1640 with 10% FBS. HEATR1 cDNA was purchased from Thermo Scientific and full length and mutants were subcloned into pIRES-EGFP. PP2A-Aα, B55α, B56β, Cα were purchased from addgene and subcloned into HA-pcmv and pGex4T-1. HEATR1 siRNA and shRNA were from Dharmacon and sigma, respectively.
MTS Assay
Gemcitabine was from Eli Lilly (Indianapolis, IN). Paclitaxel, SN-38, mitomycin c, oxaliplatin and etopside were from Sigma-Aldrich (St. Louis, MO). Triciribine was from EMD Biosciences (San Diego, CA). Ctrl or HEATR1 siRNA or shRNA were transfected, and the growth-inhibitory effect was performed as described previously (21).
Tumor xenograft study
Female athymic nu/nu mice were obtained from the National Cancer Institute. Experiments were performed under the approval of the Institutional Animal Care and Use Committee at Mayo Clinic. PANC-1 cells stably expressing Ctrl and HEATR1 shRNA were injected subcutaneously in mouse flanks (106cells/mouse flank). Tumor volumes were measured every three days. When tumors reached 100mm3, mice were randomized to four groups (n=8): vehicle; TCN (0.5mg/kg/day), administered i.p. once daily; gemcitabine (50mg/kg), administered i.p. every 3 days; a combination of two treatments. After sacrificing the mice, tumors were surgically removed and weighted.
Patients’ specimens and Follow-Up
From 2007 to 2012, the same surgical team in our institute performed curative resection for pancreatic cancer on 100 consecutive patients, defined as macroscopically complete removal of the tumor. All patients were observed until Feburary 2014. Overall survival (OS) was defined as the interval between the date of surgery and death. None of the patients received any preoperative anticancer treatment, and all of them received standardized postoperative chemotherapeutic regimen of Gemcitabine. Of the clinicopathological features, tumor stage was determined according to the 2002 International Union Against Cancer TNM classification system. Tumor differentiation was graded by the Edmondson grading system. The study was approved by the Zhongshan Hospital Research Ethics Committee.
Immunohistochemical Staining and image analysis
Tissue microarray was constructed following standard tissue array producing protocols as described previously (25). Primary antibodies were both rabbit polyclonal antibody for HEATR1 and pAkt308, followed by incubation with the secondary antibody. High-sensitivity diaminobenzidine chromogenic substrate system was used for colorimetric visualization. The density of positive staining was measured by a computerized image system including Leica-CCD camera connected to a Leica-DM-IRE2 microscope. Under high-power magnification, photographs of representative fields were captured by the Leica QWin Plus v3 software. HEATR1 density was counted by Image-Pro Plus v6.2 software.
Statistical Analysis
Data were presented as mean ± SD and analyzed using t-test or ANOVA. Significance was set at p< 0.05. SPSS 16.0 for Windows (SPSS Inc) were used for survival analysis in patients; Pearson χ2 test or Fisher’s exact test was used to compare qualitative variables; and quantitative variables were analyzed by t test or Pearson’s correlation test. Kaplan-Meier analysis was used to determine the survival. Log-rank test was used to compare patients’ survival between subgroups; the Cox regression model was used to perform multivariate analysis.
Other methods and information are available in Supplement Materials and Methods.
Results
HEATR Regulates pancreatic cancer cells response to chemotherapy
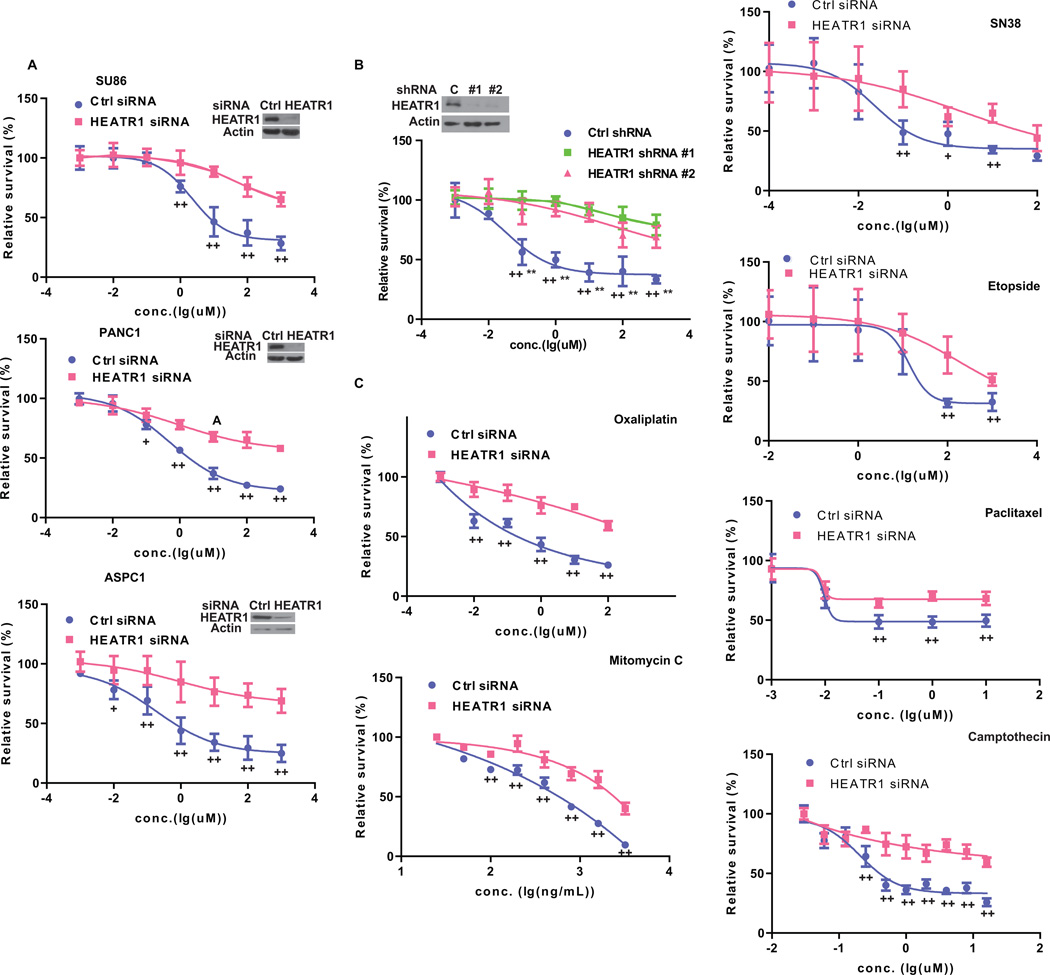
We have performed several genome wide association studies to identify genes whose expression associates with sensitivity to chemotherapy. Interestingly, we found that HEATR1 gene, although not among the top hits in these studies, associates with response to several drugs, including gemcitabine and 1-beta-d-arabinofuranosylcytosine(26). We depleted HEATR1 with siRNA in cells and treated cells with gemcitabine. Downregulation of HEATR1 resulted in increased resistance to gemcitabine (Figure 1A). Similar results were obtained with two HEATR1 specific shRNAs (Figure 1B and Supplementary Fig.S1A). Next, multiple classes of chemotherapeutic drugs including oxaliplatin, SN-38, mitomycin c (MMC), paclitaxel, Camptothecin and etopside were used to treat cells. Similarly, cells with HEATR1 knockdown were significantly resistant to these drugs (Figure 1C and Supplementary Fig.S1B). These results establish an important role of HEATR1 in regulating chemo-response to different anti-tumor agents.
Figure 1. HEATR1 regulates cancer cell response to chemotherapy.
(A) Cells were transfected with indicated siRNA and treated with gemcitabine. Cell survival was determined. The data presented are mean ± SD (n=6). +P<0.05, ++ P<0.01 (Ctrl vs HEATR1 siRNA) (B) SU86.86 cells stably expressing indicated shRNA were treated with gemcitabine. Cell survival was determined. +*P<0.05, ++** P<0.01 (Ctrl vs HEATR1 shRNA #1 or #2 respectively). (C) SU86.86 cells were transfected with indicated siRNA and treated. Cell survival was determined.
HEATR1 regulates Akt phosphorylation at Thr308 by promoting Akt-PP2AB56β interaction
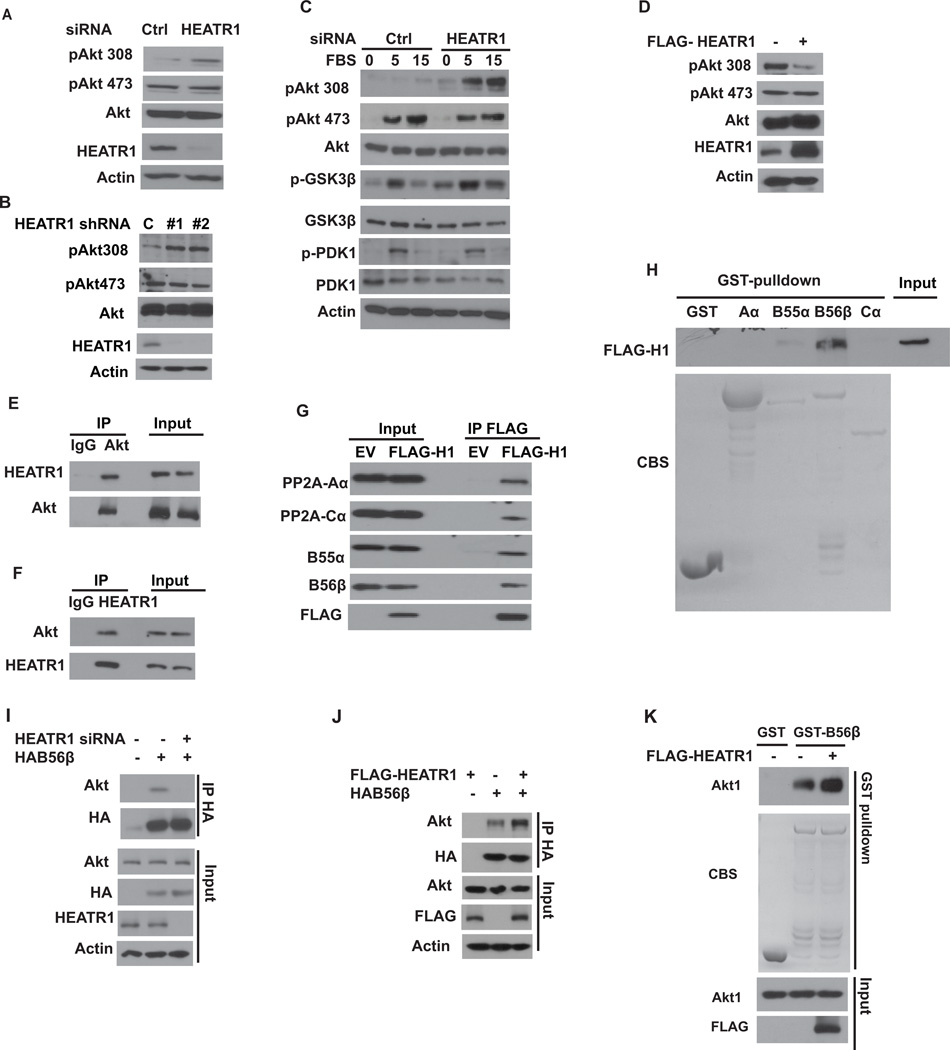
Since HEATR1 affects cellular response to a broad range of chemotherapeutic agents, it is likely that HEATR1 affects a common pathway such as apoptosis. Gemcitabine induced apparently stronger apoptosis in control cells than in cells transfected with HEATR1 shRNA (Supplementary Fig.S2A). Next, we investigated how HEATR1 affects chemotherapy response by probing several pathways regulating cell death. We found that downregulation of HEATR1 led to an increased phosphorylation of Akt at Thr308, but not at Ser473 (Figure 2A–B). These results suggest that depletion of HEATR1 affects steady state Akt phosphorylation, which is consistent with an increase in cell growth (Supplementary Fig.S2B). We next examined how HEATR1 affects acute Akt activation. Cells were starved and stimulated with serum. Phosphorylation of Akt Thr308 and the Akt substrate GSK-3β were significantly increased in cells transfected with HEATR1 siRNA (Figure 2C). Furthermore, overexpression of HEATR1 decreased phosphorylation of Akt at Thr308, but not Ser473 (Figure 2D). We also found that HEATR1 interacted with Akt in cells (Figure 2E–F). The intracellular co-localization of HEATR1 and Akt were detected by confocal microscopy. Akt and HEATR1 colocalize in the cytoplasm and occasionally at the cell membrane in most cells examined (Supplementary Fig.S3A).
Figure 2. HEATR1 regulates Akt phosphorylation at Thr308 by promoting Akt-B56β interaction.
(A) Cells were transfected with indicated siRNA and the phosphorylation of Akt were detected. (B) Cells were transfected with indicated shRNA and the phosphorylation of Akt were detected. (C) Cells transfected with indicated siRNA were serum starved for 16 hours and then serum was added. Whole-cell lysates were harvested and western blotting was performed. (D) Cells were transfected with indicated constructs and Akt phosphorylation were detected. (E–F) Cell lysates were subjected to immunoprecipitation with control IgG, anti-Akt (E) or anti-HEATR1 (F) antibodies. The immunoprecipitates were blotted with indicated antibodies. (G) Cells were transfected with vector or FLAG-HEATR1 and immunoprecipitated with anti-FLAG beads, followed by western analysis. (H) Cells were transfected with FLAG-HEATR1. Immunoprecipitates with anti-FLAG beads were washed extensively and eluted with FLAG peptide. Elutes were incubated indicated GST or GST-fusion protein and western blotting were performed. (I) Cells were transfected with control or HEATR1 siRNA, and the interaction between Akt and B56β was examined. (J) Cells were transfected with indicated constructs, and the interaction between Akt and B56β was examined. (K) Purified recombinant Akt, GST-B56β and FLAG-HEATR1 were incubated in vitro as indicated. The interaction between Akt and B56β was then examined.
Since HEATR1 specifically regulates Thr308, it is likely that HEATR1 regulates signaling events that directly control Akt phosphorylation at Thr308. Thr308 of Akt is phosphorylated by PDK1 and dephosphorylated by PP2A (16, 18, 27). No apparent difference of PDK1 phosphorylation was observed in control and HEATR1-depleted cells (Figure 2C). HEATR1 neither interacted with PDK1 nor affected the interaction between Akt and PDK1 (Supplementary Fig.S3B–C), indicating HEATR1 regulates Akt activity independent of PDK1. Next, we examined whether HEATR1 regulates Thr308 phosphorylation through PP2A. Treatment of PP2A inhibitor okadaic acid significantly increased Akt phosphorylation at Thr308, and HEATR1 knockdown did not further increase Thr308 phosphorylation (Supplementary Fig.S3D). As shown in Figure 2G, HEATR1 interacted with PP2A scaffolding subunit Aα, catalytic subunit Cα and regulatory subunits B55α and B56β, which have been reported to specifically target Akt Thr308 dephosphorylation (16–18, 27). Furthermore, purified HEATR1 only interacted with recombinant GST-B56β, suggesting a direct interaction between HEATR1 and B56β (Figure 2H). Because HEATR1 interacts with both Akt and B56β and regulates Akt phosphorylation, we hypothesized that HEATR1 may function as a scaffolding protein to facilitate Akt dephosphorylation by PP2A. Downregulation of HEATR1 decreased the interaction between B56β and Akt (Figure 2I), while HEATR1 overexpression increased it (Figure 2J). Furthermore, purified FLAG-HEATR1 increased the interaction between recombinant His-Akt1 and GST-B56β (Figure 2K). These results suggest that HEATR1 promotes the interaction between Akt and PP2A, facilitating the dephosphorylation of Akt at Thr308.
HEATR1 regulates Akt phosphorylation and cell response to gemcitabine through its scaffolding function
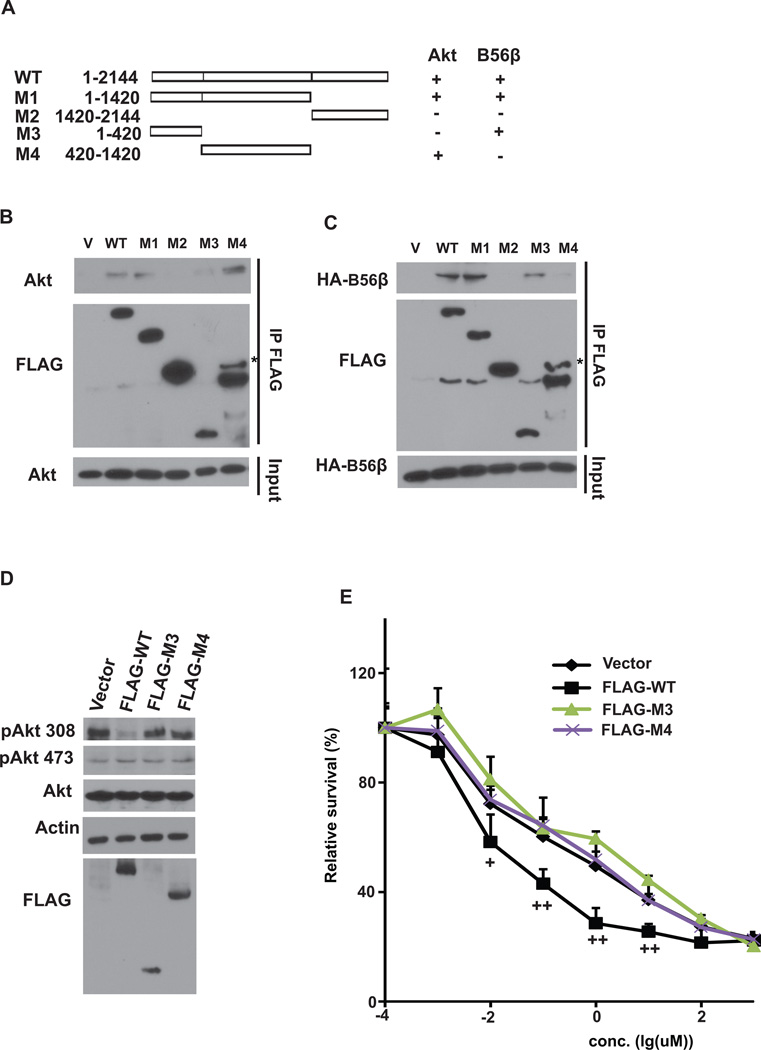
To investigate specific regions of HEATR1 for Akt and B56β interaction, we generated deletion mutants of HEATR1 (Figure 3A). N-terminal region (aa 1–420) was essential for the binding of HEATR1 with B56β; while the middle region (420–1420) was responsible for the binding of HEATR1 with Akt (Figure 3B–C). These results suggest that HEATR1 binds Akt and B56β using different regions. To further confirm that the scaffolding function of HEATR1 is important for the regulation of Akt phosphorylation, we overexpressed WT and mutants in cells (Figure 3D). WT decreased Akt phosphorylation at Thr308 and cellular sensitivity to gemcitabine, while HEATR1 mutants that abolish Akt or B56β interaction failed to do so (Figure 3D–E). These results suggest that HEATR1 regulates Akt phosphorylation and cell response to gemcitabine through its scaffolding function.
Figure 3. HEATR1 scaffolding function regulates Akt phosphorylation and cell survival.
(A) Schematic diagram of WT and deletion mutants of HEATR1 used in the study. (B–C) Cells were transfected with indicated constructs. Precipitation reactions were conducted with S-protein agarose beads and subjected to western blotting. (D) Cells were transfected with indicated constructs. Western blotting was performed. (E) Cells were transfected as in (D) and gemcitabine sensitivity was examined. The data presented are mean ± SD (n=6). +P<0.05, ++ P<0.01 (Ctrl vs HEATR1 KD)
Akt inhibitor sensitizes pancreatic cells with HEATR1 knockdown to gemcitabine
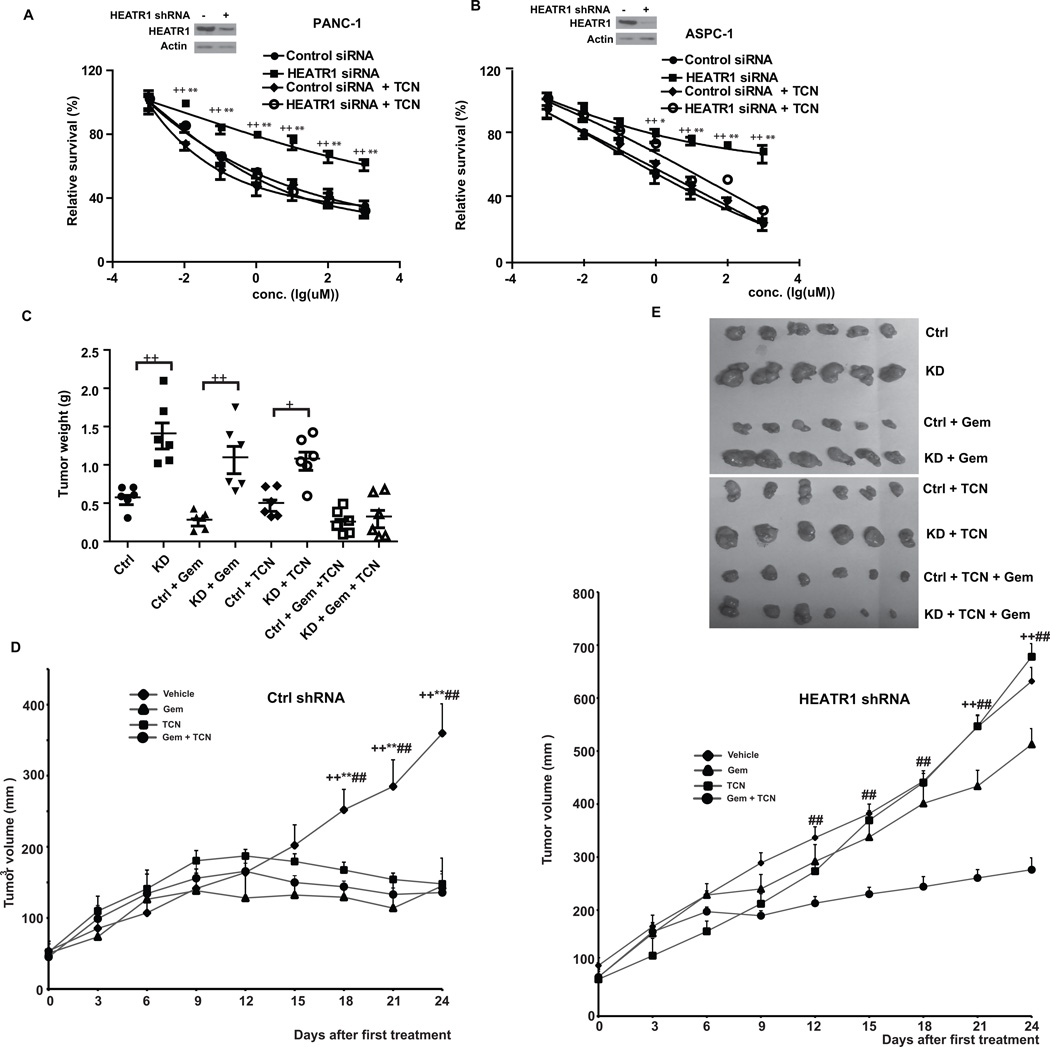
The results described above suggest that HEATR1 regulates gemcitabine sensitivity at least partly through regulating Akt and hyperactivation of Akt in cells with low HEATR1 level might be responsible for increased chemoresistance. If this was the case, treating cells with an Akt inhibitor should reverse chemoresistance in cells depleted of HEATR1. TCN-P, the active metabolite of Akt inhibitor triciribine (TCN), binds to PH domain of Akt and prevents its recruitment to cell membrane, where Akt is phosphorylated by PDK1 at Thr308 (28). When TCN was used alone, no significant difference of cytotoxicity and apoptosis was observed in different cell lines transfected with control and HEATR1 siRNA (Supplementary Fig.S4A–E). In addition, TCN did not significantly affect gemcitabine sensitivity and apoptosis in control cells. However, TCN sensitized cells depleted of HEATR1 to gemcitabine (Figure 4A–B, Supplementary Fig.S4B–E). We next tested whether addition of TCN would reverse chemoresistance of tumors with low HEATR1 level in vivo. Xenograft experiments showed that downregulation of HEATR1 promoted tumor growth and resistance to gemcitabine (Figure 4C–E and Supplementary Fig.S4F). TCN treatment resensitized these cells’ response to gemcitabine. This is consistent with in vitro results using pancreatic cancer cell lines and indicates that HEATR1 affects chemosensitivity through regulating Akt activity in vivo.
Figure 4. Akt inhibitor sensitizes pancreatic tumors with HEATR1 knockdown to gemcitabine.
(A–B) PANC-1 (A) or ASPC-1 (B) cells were transfected with indicated siRNA. Gemcitabine sensitivity was examined in the presence of vehicle or 10µM TCN. Data represent means ± SD (n=6). ++ P<0.01 (control vs HEATR1 siRNA), ** P<0.01 (HEATR1 siRNA vs HEATR1 siRNA + TCN). (C–E) Mice with subcutaneously established tumors from PANC-1 cell stably expressing indicated shRNA were treated with PBS, TCN (0.5 mg/kg), gemcitabine (50 mg/kg) or combination. Xenograft tumors were dissected and tumor weights were measured after mice were sacrificed (C). ANOVA analysis was performed (Mann-Whitney test) +P<0.05, ++ P<0.01 (Ctrl vs KD). Tumor volumes were measured every three days (D). ++ P<0.01 (Vehicle vs gemcitabine), ** P<0.01 (Vehicle vs TCN) and ## P<0.01 (Vehicle vs gemcitabine plus TCN). Representative photographs of indicated xenograft tumors were shown in (E).
Association of HEATR1 expression in pancreatic cancer patients with survival and response to chemotherapy
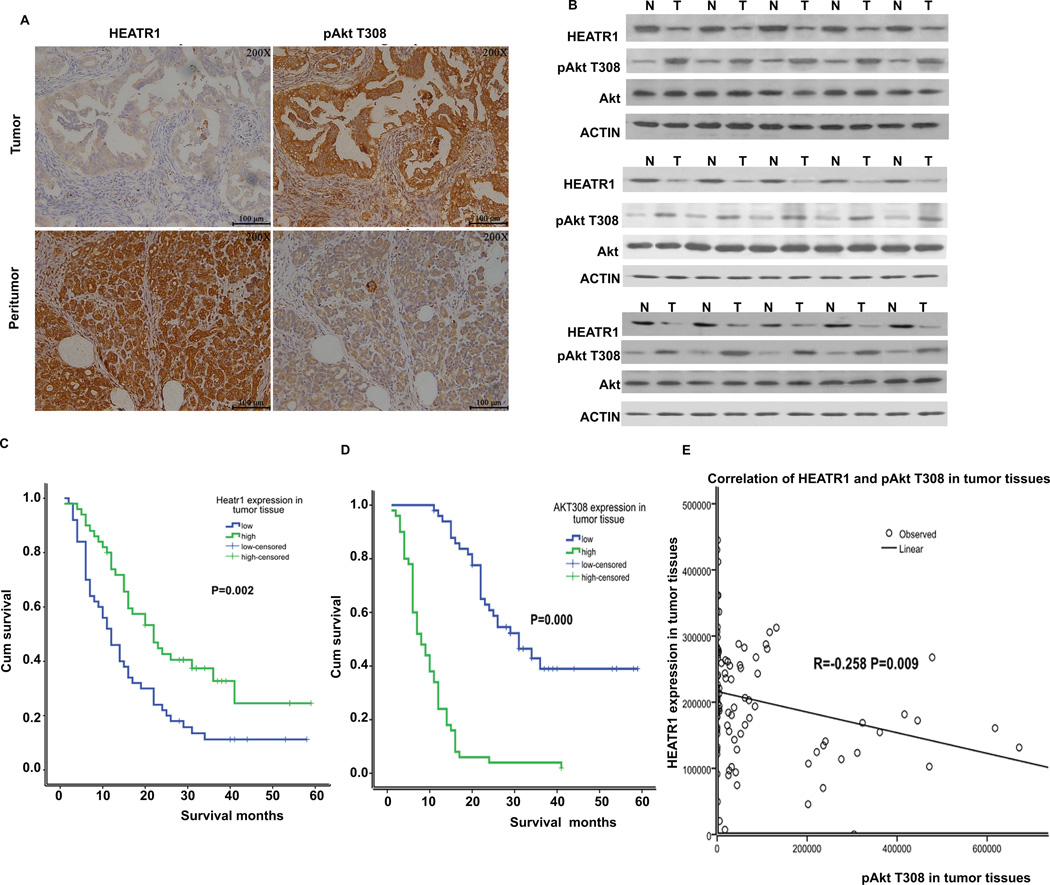
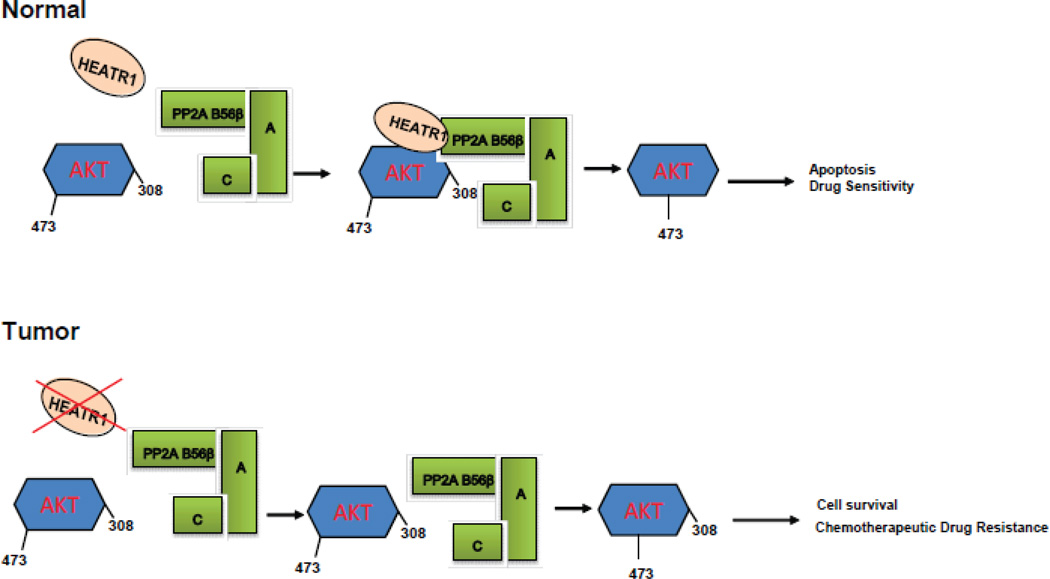
We next used clinical samples to examine the role of HEATR1 in clinical response. Among 100 patients with PDAC, 76 patients died and 24 patients survived, with the median survival time of 18 months. Pathological analysis using pancreatic ductal adenocarcinoma and peritumoral tissues from patients were performed. Statistical analysis revealed that expression of HEATR1 was significantly lower in pancreatic tumor tissue than in normal pancreatic tissues (Figure 5A). The staining of Akt phosphorylation at Thr308 was significantly higher in pancreatic tumor tissue than in normal pancreatic tissue (Figure 5A). Furthermore, low HEATR1 proteins levels correlates with increased Akt phosphorylation at Thr308 in representative pancreatic cancer samples (Figure 5B). We next evaluated whether HEATR1 expression was associated with the response of patients to standardized gemcitabine chemotherapy. As shown in Figure 5C–D, Supplementary Fig.S5 and Supplementary Table 1, PDAC patients with high HEATR1 or low AktT308 expression in tumors had a significant improvement in overall survival. Thus, determination of HEATR1 expression in PDAC tissues may be useful as an independent predictor for gemcitabine response. Based on IOD value, we observed a negative correlation between HEATR1 expression and Akt phosphorylation at Thr308 in tumor tissues (Figure 5E). Meanwhile, we observed a significant correlation between the Akt308 phosphorylation and TNM staging, lymph node metastasis, whereas we did not find the correlation between HEATR1 expression and clinical pathological feature (Supplementary Table 2). From multivariate survival analysis, HEATR1 expression and Akt308 phosphorylation might be independent prognostic factors among these variables (Supplementary Table 3). Overall, our results showed that HEATR1 negatively regulates Akt activation and upregulation of Akt activity by loss of HEATR1 in pancreatic cancers might give rise to the resistance to chemotherapy (Figure 6).
Figure 5. Association of HEATR1 expressions in pancreatic cancer patients with survival and response to chemotherapy.
(A) Representative staining of HEATR1 and pAkt T308 in peritumoral pancreatic tissues and pancreatic ductal adenocarcinoma. (B) Western blot of lysates from a subset of tumor and normal tissues. (C–D) Univariate survival of HEATR1 and pAkt T308. (E) Correlation of expression of HEATR1 and pAkt T308 in tumor tissues.
Figure 6.
Experimental Model
Discussion
Despite continuous efforts, effective early detection markers of PDAC are currently not available and approximately 80% of patients are diagnosed at locally advanced or metastatic stages (29). In these patients at advanced stage, limited response to current treatments results in an extremely poor prognosis (30). Thus, improvements in diagnosis and predictability of patient response to existing therapies are urgently needed for PDAC. Cancer treatment by chemotherapy mainly induces apoptosis to kill cancer cells. However, failure to activate apoptosis causes resistance to therapy, which is an important clinical problem in the treatment of cancers.
The Akt pathway, one of the most frequently hyperactivated signaling pathways in human cancers, plays an important role in both tumorigenesis and chemo-resistance. Half of pancreatic adenocarcinomas tumors demonstrated activation of Akt by immunohistochemical staining of pAkt (31). Significant correlation between activation of Akt and poor survival suggests an important role of Akt activation in pancreatic cancer (32). Thus, targeted inhibition of Akt shows promise in the treatment of pancreatic cancers given its role in tumorigenesis and chemo-resistance. Currently, several small molecule inhibitors of Akt have been developed and are in various stages of clinical testing (33). For instance, in combination with a cyclin-dependent kinase inhibitor (dinaciclib), the oral pan-AKT Inhibitor MK-2206 can dramatically block pancreatic tumor growth and metastases in patient-derived xenograft models (34). Phase I trial results of MK-2206 in patients with advanced solid tumors indicate that MK-2206 was well tolerated, with evidence of Akt signaling blockade (35). RX-0201, an antisense oligonucleotide to mRNA encoding Akt1, in combination with gemcitabine were investigated in a phase II study and the safety and efficacy results are awaited (36). Thus, these AKT inhibitors alone or in combination with other clinically approved anticancer agents should be further explored in clinical studies as potential novel therapeutic agents.
Here we found that depletion of HEATR1 in pancreatic cancer cells causes resistance to gemcitabine and other chemotherapeutic agents (Figure 1 and Supplementary Fig.S1).We propose that HEATR1 functions in gemcitabine resistance through inhibiting Akt activity. The evidences we observed are as follows. First, the interaction between Akt and B56β was decreased when HEATR1 was depleted, indicating that HEATR1 acts as a scaffolding protein for Akt and B56β, theraby enhancing the phosphatase activity of B56β toward Thr308 (Figure 2). Second, HEATR1 mutants that abolish Akt or B56β interaction failed to affect Akt Thr308 phosphorylation and chemosensitity (Figure 3). Third, addition of triciribine (TCN) sensitizes gemcitabine treatment in cells with low HEATR1 level both in vitro (Figure 4A) and in animal models (Figure 3B–D). Finally, we demonstrated that expression of HEATR1 is positively correlated with overall survival in patients with pancreatic cancer (Figure 5).
Identification of diagnostic and prognostic biomarkers for PADC patients has attracted much attention (29). However, existing biomarkers such as CA19-9 are not adequate as early detection markers of pancreatic cancer and predictor to treatment response because of low sensitivity and specificity (37). The biological function of HEATR1 in sensitizing pancreatic cancer cell to gemcitabine is potentially important. We report here that HEATR1 expression level in PDAC significantly correlates with patient survival (Figure 5), suggesting a potentially prognostic value. Because gemcitabine represents the standard of care for PDAC, therapeutic interventions targeting HEATR1 may improve outcome in combination with existing therapies. HEATR1 contains 2144 amino acids, thus making it difficult to restore HEATR1 expression in PADC cancer cells. In the future, after we are able to narrowdown small motifs of HEATR1 responsible Akt or PP2A binding, we might be able to engineer a polypeptide to restore the regulation of Akt by PP2A. In addition, if we are able to identify the mechanism of HEATR1 downregulation in PDAC, we might be able to restore HEATR1 expression. However, how the expression of HEATR1 is deregulated in PDAC is unclear. From public databases, we did not found that HEATR1 mRNA to be downregulated in PDAC (TCGA and Oncomine). The HEATR1 gene is mutated in a small percentage of pancreatic cancer cases (3.4% TCGA), however, this could not account for all pancreatic cancers showing weak IHC staining. We speculate that HEATR1 is downregulated in pancreatic cancer at posttranscriptional level. Possible mechanisms include decreased translation or protein turnover. Thus, further investigation is essential to understand how HEATR1 is dysregulated in cancers. As an alternative strategy, combination therapy using an Akt inhibitor and gemcitabine was able to overcome gemcitabine resistance in PDAC.
Our study provides evidence of HEATR1 as a potential biomarker for predicting chemoresistance, although clinic outcomes of the combination of therapies targeting Akt plus gemcitabine should be further tested. It would also be interesting to investigate whether HEATR1 is potentially implicated in cancer development considering the role of Akt in cancers. Furthermore, although our study mostly focused on gemcitabine and PDAC, our findings that HEATR1 affects the response to other chemotherapeutic agents would have broader impact for the treatment of other cancers and should be validated in the future. In summary, we identify HEATR1 as a negative regulator of the Akt pathway in PADC and a potential biomarker for predicting chemo-resistance.
Supplementary Material
Acknowledgements
We thank all colleagues in Dr. Lou lab for insightful discussions. We thank the flow cytometry and confocal core facility in Mayo Clinic for their expert technical assistance.
Financial Support
This work was supported by grants from the National Institutes of Health (CA130996 and CA151329, Z.Lou) and Special Research Fund for public welfare from Ministry of Health of China (201202007, W.Lou).
Footnotes
Conflict of Interest statement
No author has conflict of interest with the contents of this article.
References
- 1.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Advances in cancer research. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 2.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 4.Jazirehi AR, Wenn PB, Damavand M. Therapeutic implications of targeting the PI3Kinase/AKT/mTOR signaling module in melanoma therapy. American journal of cancer research. 2012;2:178–191. [PMC free article] [PubMed] [Google Scholar]
- 5.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 6.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 7.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 8.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 9.Happo L, Cragg MS, Phipson B, Haga JM, Jansen ES, Herold MJ, et al. Maximal killing of lymphoma cells by DNA damage-inducing therapy requires not only the p53 targets Puma and Noxa, but also Bim. Blood. 2010;116:5256–5267. doi: 10.1182/blood-2010-04-280818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 11.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends in biochemical sciences. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 12.Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell. 2012;149:1098–1111. doi: 10.1016/j.cell.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–1138. doi: 10.1126/science.1175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Current biology : CB. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 15.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 16.Padmanabhan S, Mukhopadhyay A, Narasimhan SD, Tesz G, Czech MP, Tissenbaum HA. A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell. 2009;136:939–951. doi: 10.1016/j.cell.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodgers JT, Vogel RO, Puigserver P. Clk2 and B56beta mediate insulin-regulated assembly of the PP2A phosphatase holoenzyme complex on Akt. Molecular cell. 2011;41:471–479. doi: 10.1016/j.molcel.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. The Journal of biological chemistry. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 19.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Molecular cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Molecular cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groves MR, Hanlon N, Turowski P, Hemmings BA, Barford D. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell. 1999;96:99–110. doi: 10.1016/s0092-8674(00)80963-0. [DOI] [PubMed] [Google Scholar]
- 23.Wu ZB, Qiu C, Zhang AL, Cai L, Lin SJ, Yao Y, et al. Glioma-associated antigen HEATR1 induces functional cytotoxic T lymphocytes in patients with glioma. Journal of immunology research. 2014;2014:131494. doi: 10.1155/2014/131494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azuma M, Toyama R, Laver E, Dawid IB. Perturbation of rRNA synthesis in the bap28 mutation leads to apoptosis mediated by p53 in the zebrafish central nervous system. J Biol Chem. 2006;281:13309–13316. doi: 10.1074/jbc.M601892200. [DOI] [PubMed] [Google Scholar]
- 25.Dima SO, Tanase C, Albulescu R, Herlea V, Chivu-Economescu M, Purnichescu-Purtan R, et al. An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas. 2012;41:1001–1007. doi: 10.1097/MPA.0b013e3182546e13. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Fridley B, Kalari K, Jenkins G, Batzler A, Safgren S, et al. Gemcitabine and cytosine arabinoside cytotoxicity: association with lymphoblastoid cell expression. Cancer Res. 2008;68:7050–7058. doi: 10.1158/0008-5472.CAN-08-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruvolo PP, Qui YH, Coombes KR, Zhang N, Ruvolo VR, Borthakur G, et al. Low expression of PP2A regulatory subunit B55alpha is associated with T308 phosphorylation of AKT and shorter complete remission duration in acute myeloid leukemia patients. Leukemia. 2011;25:1711–1717. doi: 10.1038/leu.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berndt N, Yang H, Trinczek B, Betzi S, Zhang Z, Wu B, et al. The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane. Cell Death Differ. 2010;17:1795–1804. doi: 10.1038/cdd.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costello E, Greenhalf W, Neoptolemos JP. New biomarkers and targets in pancreatic cancer and their application to treatment. Nat Rev Gastroenterol Hepatol. 2012;9:435–444. doi: 10.1038/nrgastro.2012.119. [DOI] [PubMed] [Google Scholar]
- 30.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nature reviews Clinical oncology. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 31.Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89:2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846–2850. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 33.Pal SK, Reckamp K, Yu H, Figlin RA. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2010;19:1355–1366. doi: 10.1517/13543784.2010.520701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu C, Dadon T, Chenna V, Yabuuchi S, Bannerji R, Booher R, et al. Combined Inhibition of Cyclin-Dependent Kinases (Dinaciclib) and AKT (MK-2206) Blocks Pancreatic Tumor Growth and Metastases in Patient-Derived Xenograft Models. Mol Cancer Ther. 2015;14:1532–1539. doi: 10.1158/1535-7163.MCT-15-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 36.A Safety and Efficacy Study of RX-0201 Plus Gemcitabine in Metastatic Pancreatic Cancer. NCT01028495 [Google Scholar]
- 37.Misek DE, Patwa TH, Lubman DM, Simeone DM. Early detection and biomarkers in pancreatic cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2007;5:1034–1041. doi: 10.6004/jnccn.2007.0086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.