Abstract
Objective
The goal of the present investigation was to develop and test a brief therapist-guided manualized treatment for problematic caffeine use including cognitive-behavioral strategies and 5-weeks of progressively decreased consumption.
Methods
Individuals seeking treatment for problematic caffeine use (mean daily caffeine consumption of 666.0 mg at baseline) were randomized using a waitlist-control design to receive immediate (N = 33) treatment or delayed (N = 34) treatment (∼6 weeks later). A one-hour long treatment session designed to help individuals quit or reduce caffeine consumption was provided by a trained counselor along with a take-home booklet. After the treatment session, participants completed daily diaries of caffeine consumption for 5 weeks. They returned for follow-up assessments at 6, 12, and 26 weeks and had a telephone interview at 52-weeks post-treatment.
Results
Treatment resulted in a significant reduction in self reported caffeine use and salivary caffeine levels. No significant post-treatment increases in caffeine use were observed for up to one year follow-up. Comparisons to the waitlist control condition revealed that reductions in caffeine consumption were due to treatment and not the passing of time, with a treatment effect size of R2 = .35 for the model.
Conclusions
A brief one-session manualized intervention with follow-up was efficacious at reducing caffeine consumption. Future research should replicate and extend these findings, as well as consider factors affecting dissemination of treatment for problematic caffeine use to those in need.
Caffeine is the most widely used psychoactive drug in the world. In the United States, more than 85% of adults and children regularly consume caffeinated foods and beverages (Frary et al., 2005). The widespread popularity of caffeine is likely due to its mild positive stimulating effects, presence in a wide variety of products, and integration into cultural customs and routines. In general, when consumed at low to moderate daily doses (e.g., < 400 mg) caffeine is a relatively safe drug that offers some functional (e.g., staying awake during a long drive) and perhaps health protective effects (e.g., Parkinson's Disease) (Juliano, Ferre & Griffiths, 2014). However, for some individuals, caffeine is capable of causing various undesirable effects and disorders across a wide range of doses, which may warrant limiting its consumption (James, 2011).
Caffeine can produce various negative subjective and somatic effects including anxiety, jitteriness, upset stomach, and tense mood, especially at higher acute doses (e.g., ≥ 200 mg). Caffeine also has negative effects on planned sleep including delaying sleep onset and reducing total sleep time (Roehrs & Roth, 2008). Physical dependence on caffeine, observed at doses as low as 100 mg per day, is well-documented and caffeine withdrawal symptoms (e.g., headache, fatigue, flu-like symptoms) may produce significant distress and impairment of normal functioning (e.g., missing work) (Juliano & Griffiths, 2004). Withdrawal symptoms alone may not be deemed problematic in the absence of significant negative consequences and when the caffeine user is able and willing to consume caffeine on a regular basis. However, a growing number of investigations have found that in addition to withdrawal symptoms, some caffeine users report symptoms including, but not limited to, continued use despite psychological or physical harm, difficulty stopping caffeine use, and using more caffeine than intended (Meredith, Juliano, Hughes, & Griffiths, 2013). As a result caffeine use disorder was added to the DSM-5 as a condition for further study (APA, 2013). Caffeine use disorder has a set of diagnostic criteria different from other drug use disorders, which requires the endorsement of three specific criteria relating to withdrawal, use despite harm, and difficulty stopping use. Additional DSM-5 generic drug use disorder criteria that apply to other drugs may also be present with problematic caffeine use (e.g., tolerance, using more caffeine than intended) (APA, 2013).
In addition to negative psychological effects there are medical conditions that may be worsened by caffeine. Caffeine use is associated with some negative pregnancy outcomes (e.g., spontaneous abortion) and thus pregnant women are advised to restrict caffeine use (Anderson, Juliano & Shulkin, 2009). Caffeine also appears to have adverse effects on glucose metabolism among individuals with Type 2 Diabetes (Lane, Feinglos, & Surwit, 2008). High levels of caffeine use (i.e., > 450 mg versus < 150 mg daily) increase the incidence of urinary incontinence (Jura et al., 2011) and caffeine reduction is associated with improved urinary symptoms (Bryant, Dowell, & Fairbrother, 2002). Furthermore, some individuals report wanting to limit caffeine to improve their general health, eliminate their physical dependence on caffeine, and/or as a means to reduce their consumption of sugary soft drinks (Juliano, Evatt, Richards, & Griffiths, 2012).
As reviewed above, there are a number of reasons why caffeine reduction or cessation may be warranted or desired. However, many individuals are unable to stop or cut down caffeine consumption despite their desire to do so (Hughes, Oliveto, Liguori, Carpenter, & Howard, 1998; Juliano et al., 2012). In one population based study, 56% of individuals reported being unable to stop or cut down caffeine (Hughes et al., 1998). In another study, nearly 90% of individuals seeking assistance to modify caffeine use reported being unable to stop or cut down caffeine on their own, including 40% who had been advised to do so by a medical professional (Juliano et al., 2012).
Thus, there is a clear need for treatment approaches to help individuals who would like to stop or reduce caffeine. However, only a handful of prior studies have investigated controlled approaches for reducing caffeine consumption. Following up on a caffeine fading paradigm (i.e., slowly reducing consumption over time) that produced favorable pilot study results (Foxx & Rubinoff, 1979), James et al. (1985) examined the effects of four weeks of caffeine fading on attempts to reduce caffeine consumption among 27 heavy caffeine users. The group instructed to use caffeine fading had greater reductions in caffeine use relative to the group instructed to initiate caffeine reduction on their own (James, Stirling, & Hamton, 1985). Caffeine fading was similarly effective in a follow-up investigation of 12 subjects that included biochemical verification of self-reported caffeine use (James et al., 1988). Another investigation provided caffeine reduction education with caffeine fading instructions (reduce by one drink each day until < 100 mg is achieved) among 48 patients with urinary symptoms (Bryant, Dowell, & Fairbrother, 2002). Participants in the active condition of this study reported a 58% reduction in caffeine consumption compared to an 11% reduction in the control group. An important limitation of these previous investigations was that while the interventions were tested on individuals who were heavy caffeine users, it is unknown whether their use was problematic as defined by DSM diagnostic criteria. With the exception of James et al. (1998), additional limitations include relatively small sample sizes and nonspecific measures of caffeine consumption (e.g., “cups” of caffeine per day).
The goal of the present investigation was to develop and test a brief caffeine intervention for individuals seeking treatment for problematic caffeine use. Importantly, participants for this study were drawn from a sample of caffeine users who were previously demonstrated to have problematic caffeine use as determined by diagnostic clinical interview (see xxxx, 2012). Using a waitlist-control design, participants were randomly assigned to receive immediate treatment (1 to 2 weeks after Assessment Session) or delayed treatment (5 to 6 weeks after Assessment Session) to control for the effects of time. Treatment consisted of a one-hour long in-person counseling session in which participants were instructed to follow a 5-week program of structured caffeine fading. Participants returned to the clinic after 6, 12, and 26 weeks for follow-up assessments and participated in a telephone interview at 52 weeks. It was hypothesized that caffeine treatment would reduce caffeine consumption.
Methods
Participant Recruitment
Participants were recruited from the Baltimore metropolitan area with newspaper advertisements that offered treatment assistance for people who feel that they “are psychologically or physically dependent on caffeine” or “have tried unsuccessfully to quit using caffeinated products in the past.” Two hundred and seventy-five men and women responded to advertisements and were administered a telephone screening interview that served as the first level of exclusionary requirements for this study. Respondents to the telephone screening who reported consuming at least 100 mg of caffeine per day and endorsed an interest in receiving assistance to modify their caffeine use were invited to the on-site Assessment Session (N = 94). The Assessment Session served two purposes: 1) to characterize individuals who were seeking treatment for problematic caffeine use, and 2) to permit additional screening of participants for the study. Participants were paid $20 for participating in the Assessment Session. The findings characterizing the treatment seekers at the Assessment Session are presented elsewhere (XXXX et al., 2012). Institutional review board approval was obtained for this study.
Treatment study participants
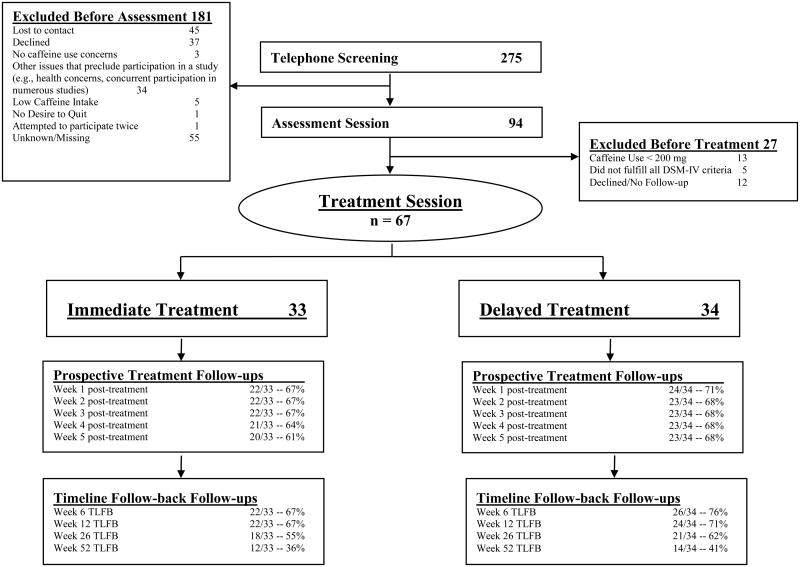
A consort flow diagram is presented in Figure 1. Sixty-six percent of telephone responders did not participate in the subsequent Assessment Session or Treatment Session. Common reasons for exclusion after the telephone screening included loss of contact or other issues that precluded participation (e.g., concurrent participation in other studies). It is likely that many participants lost interest immediately after the level of monetary reimbursement was indicated, which was purposefully kept low to attract only those interested in treatment.
Figure 1.
Consort Flow Diagram. TLFB = Timeline Follow-back
The second level of screening for the study occurred at the Assessment Session. Inclusion criteria were: 1). interest in receiving assistance to reduce or quit caffeine; 2). consumption of at least 200 mg of caffeine per day; and 3). demonstrating problematic caffeine use as per DSM-IV-TR substance dependence criteria as applied to caffeine. One exception to DSM-IV-TR diagnostic inclusion criteria was made for a participant who, despite only endorsing one caffeine use disorder symptom, had extremely high caffeine use (> 2000mg per day), was interested in treatment, and was advised by his physician to reduce caffeine consumption. Eighty-four percent of the sample met criteria for DSM-5 caffeine use disorder (APA, 2013).
Sixty-seven eligible volunteers participated in the Treatment Session (50.7% female). The mean age of the sample was 42 years (SD = 12.7, range 18-65). Among the sample, 83.6% identified as Caucasian, 7.5% as African American, 6% as Asian, and 3% as other. One participant additionally identified as Hispanic/Latino. All participants had completed High School. Moreover, 70.1% of the sample had a Bachelors-level degree and 32.8% had a graduate level degree.
Study participants were randomized into one of two groups: Immediate Treatment (n = 33) or Delayed Treatment (n = 34). Participants in the Immediate Treatment group were scheduled to receive caffeine treatment 1 to 2 weeks after the Assessment Session. Participants randomized to the Delayed Treatment group were scheduled to receive caffeine treatment 5 to 6 weeks after the Assessment Session. The purpose of the waitlist-control design was to control for the effects of time on caffeine consumption (i.e., spontaneous improvement) and establish that all participants who were interested in receiving treatment would be offered caffeine treatment. Participants were paid up to $70 for participation ($60 for returning for several follow-up assessments and $10 for completing self-report and diary assessments).
Measures
Demographics and medical history
During the Assessment Session, participants completed several self-report questionnaires that were developed for the study to assess demographics, smoking history, alcohol history, and medical history.
Caffeine patterns and caffeine history
Patterns of caffeine use and types of caffeine consumed prior to treatment were assessed with the Caffeine Exposure Questionnaire (CEQ; see Harrell & Juliano, 2009; Svikis et al., 2005) during the Assessment Session. Participants also completed a caffeine modification history questionnaire that was developed for the study and were queried about past attempts to reduce or quit caffeine use.
Self-monitoring of caffeine use
At the end of the Assessment Session, participants who were eligible for and agreed to participate in the treatment study were given instructions for completing a daily caffeine diary of caffeine consumption. Participants in both the Immediate Treatment and Delayed Treatment groups were instructed to keep a caffeine diary for the one week prior to the Treatment Session and for five weeks following the treatment. The primary outcome of caffeine consumption was calculated from daily diary assessments of caffeine use for the weeks following the Treatment Session (described in the Treatment subsection of the Methods below).
Biochemical caffeine assessment
Saliva samples were collected during the Assessment Session and at the 6-week follow-up in order to corroborate participant self-reported changes in caffeine consumption. Samples were sent to Labstat International ULC (Kitchener, Ontario) for gas chromatography analysis. Caffeine has a relatively short half-life of four to six hours and small quantities of caffeine can be incidentally consumed in everyday foods (e.g., chocolate). As such, salivary caffeine levels should only be considered rough estimates of daily caffeine use and were not considered biochemical verification of abstinence. A prior study found evidence that biochemical measurement of caffeine generally coincides with self-reported reductions in caffeine (James, Paul, & Cameron-Traub, 1988). Thus, salivary caffeine was expected to corroborate mean changes in caffeine consumption, but was not used as a verification of caffeine abstinence.
Caffeine use disorder
The Composite International Diagnostic Interview, Substance Abuse Module, Section E, Version 4.1 (SAM–Section E; Cottler, Robins, & Helzer, 1989) and additional questions were administered during the Assessment Session that address both the DSM-IV-TR substance dependence “as applied to caffeine” criteria and the DSM-5 definition of caffeine use disorder. In total, ten caffeine use disorder criteria were queried to assess DSM-IV-TR and DSM-5 caffeine use disorder criteria. For endorsement of DSM-IV-TR and DSM-5 caffeine use disorder criteria, see Table 1.
Table 1. Caffeine Use Disorder Criteria.
| Criteria | Description | DSM-IV-TR Criteria | DSM-5 Criteria | Endorsed |
|---|---|---|---|---|
| Tolerance |
|
X | X | 75% |
| Withdrawal |
|
X | X | 97% |
| Caffeine is taken in larger amounts or over longer periods of time than intended | Using caffeine in larger amounts or over longer periods of time than wanted or intended, for example, intending to consume only one cup of coffee but consuming three or more; or intending to consume four cups but consuming six. | X | X | 43% |
| Persistent desire or unsuccessful efforts to cut down |
|
X | X | 91% |
| Spending a great deal of time obtaining, using, or recovering from caffeine effects | Spending at least 20 minutes per day obtaining, using, or recovering from the effects of caffeine. | X | X | 70% |
| Important social, occupational, or recreational activities are given up or reduced because of caffeine | Caffeine consumption resulted in a clinically significant reduction or loss in important social, occupational, or recreational activities. | X | 10% | |
| Caffeine is used despite knowledge of persistent or recurrent physical or psychological problems | Caffeine use continues despite physical or psychological problems believed to be caused or exacerbated by caffeine. | X | X | 91% |
| Caffeine use persisted despite social or interpersonal problems | Caffeine use continues despite social or interpersonal problems believed to be caused or exacerbated by caffeine. | X | 1.5% | |
| Caffeine use resulting in a failure to fulfill major role obligations | Caffeine use continues despite a failure to fulfill major role obligations at work home or school. | X | 0% | |
| Craving | Craving for caffeine. | X | 87% |
Note: Criteria endorsement was determined by a trained test administrator who took into account the severity and frequency of the problem and the relationship between caffeine use and the problem.
Caffeine Treatment
A trained study team counselor with at least a Bachelor level education administered the one-hour manual-based treatment to assist in reducing or quitting caffeine consumption. The manual was provided to the participant and served as both a step-by-step guide for the counselor and participant during the Treatment Session, as well as a self-help resource for the participant following the treatment session. The manual consisted of several treatment components designed to assist the participant to commit to a treatment goal, outline a plan for reducing caffeine over time, and use tools to cope with issues that may arise when reducing caffeine consumption (e.g., withdrawal symptoms, cravings). Many of the treatment components were modeled after strategies used in Relapse Prevention (Marlatt & Gordon, 1985). The manual is available upon request from the authors.
At the beginning of the Treatment Session, the counselor presented basic background and pharmacological information about caffeine. The counselor then described reasons that many people quit caffeine and supported the participant in exploring the pros and cons of modifying caffeine use. Participants were then given instructions for gradually reducing their caffeine use over five weeks. Gradually reducing caffeine over time, otherwise known as caffeine fading, is a strategy used to decrease unpleasant withdrawal symptoms known to coincide with abrupt cessation of caffeine consumption (James, et al., 1988). Participants were instructed to consume no more than 75% of their pre-treatment daily caffeine level in the week after treatment, 50% during the 2nd week post-treatment, 25% during the 3rd week post-treatment, 12.5% during the 4th week post-treatment, and, in the 5th week post-treatment, to abstain from products containing more than 15 mg caffeine and consume less than 50 mg of caffeine per day.
During the Treatment Session, participants were trained to complete daily caffeine diaries for the five weeks following treatment including caffeine content (in mg) for caffeine products. An appendix in the treatment manual provided caffeine contents for more than 70 food, beverage, energy, and medicinal products for reference. Participants were also provided with a graph format and instructions for monitoring (i.e. plotting) changes in their daily caffeine use over time.
The Treatment Session also addressed caffeine dependence and caffeine withdrawal symptoms. Suggestions were offered for making dietary and exercise changes to make it easier to start the day without caffeine. Participants were also instructed in behavioral and cognitive coping strategies for dealing with stress and the desire to consume caffeine. Finally, tips for maintaining caffeine abstinence or reduction were discussed including continued caffeine monitoring, re-reviewing the information in the treatment manual, and rewarding oneself for continued success.
Participants were instructed to complete daily caffeine diaries for 5 weeks following treatment. Timeline followback assessments (Sobell & Sobell, 1992) of caffeine consumption were completed at 6, 12, 26, and 52 weeks post-treatment.
Data Analyses
Data analyses were conducted in IBM SPSS Version 22.0. Descriptive statistics were conducted on baseline variables and independent samples t-tests were conducted to test for group differences (Immediate versus Delayed) in baseline variables. Baseline analyses revealed between-condition differences in age. As such, age was entered as a covariate in all models.
Time course data were analyzed using linear mixed models procedures with Time and Treatment Condition (Immediate Treatment versus Delayed Treatment) included as fixed factors. A Toeplitz covariance structure was chosen based on comparisons of Information Criteria between models for the analysis of changes in caffeine consumption from Treatment Session to Week 5 Follow-up. Significant interactions between independent variables were analyzed in the model. Significant effects were followed-up with estimated marginal means comparisons using a Bonferroni adjustment. The effect size R2 of the primary hypothesized interaction between treatment condition and time of caffeine consumption was calculated by comparing the model parameter estimates obtained including the treatment condition relative to the model parameter estimates obtained when treatment condition was not added to the model (Feng et al., 2001).
To determine if the waitlist significantly affected caffeine consumption before treatment, group differences in caffeine consumption were examined from Assessment Session to Treatment Session (1 to 2 weeks for the Immediate Treatment condition compared to 5 to 6 weeks for the Delayed Treatment condition). The effects of treatment on caffeine consumption were analyzed using two approaches. The first approach examined changes in caffeine consumption between treatment groups for 6 weeks after the Assessment Session (Assessment Session to Treatment Session for the Delayed Treatment condition compared to Assessment Session to Week 5 Follow-up for the Immediate Treatment condition). Analyses also examined the effects of treatment at each week in both treatment groups by examining combined post-treatment data from both treatment groups from the Treatment Session to Week 5 follow-up (6 repeated measures of caffeine consumption). Separate analyses were conducted to examine changes in caffeine between timeline followback (TLFB) follow-up sessions from 6 Week TLFB to 52 Week TLFB (4 repeated measures). The TLFB sessions were analyzed separately from the Week 1 to Week 5 Follow-up measures, because each assessment used different approaches for collecting caffeine consumption (daily diary for week 1 to week 5 follow-up compared to TLFB for week 6 to week 52). Daily diaries were not used for all follow-ups, because it was deemed excessive to have participants who were receiving modest remuneration complete daily diaries for up to 52-weeks follow-up.
Salivary caffeine data from one participant was removed from analysis as an outlier. This participant had very low caffeine salivary assessments likely due to assessment occurring before her typical caffeine consumption, as verified by examination of her daily caffeine diaries. Similar to caffeine self-report results, salivary caffeine assessments were positively skewed with an abnormal distribution and were therefore ln (10) transformed in all analyses. Changes in salivary caffeine were examined with paired samples t-tests conducted between Assessment Session and Week 6 Follow-up.
Treatment goal outcomes were examined to determine the proportion of participants who met or exceeded their treatment goal. The proportion of participants who met their treatment goal was calculated as a proportion of change score of caffeine consumption from Assessment Session to Week 5 post-treatment. Descriptive analyses were conducted to examine caffeine relapse in participants who achieved treatment success during 5 weeks of caffeine fading, but endorsed increased caffeine use during follow-up assessments.
Exploratory follow-up analyses were conducted with caffeine modification history and caffeine consumption patterns entered as covariates in the model to assess the influence of these variables on changes in caffeine consumption over time.
Results
Baseline Descriptive Statistics
Mean caffeine consumption at the Assessment Session was 733.4 mg per day (SD = 559.5) and 600.7 mg (SD = 448.8) in the Immediate Treatment and Delayed Treatment groups respectively and ranged from 200 mg to 2667 mg. There were no significant differences between treatment groups in caffeine consumption at the Assessment Session. The caffeine consumption variable was positively skewed with an abnormal distribution. Therefore, milligrams per day caffeine consumption variables were ln(10) transformed in all subsequent analyses. The Immediate Treatment group mean age was higher (45.1, SD = 12.5) than the Delayed Treatment group (38.9, SD = 12.3). No other statistically significant differences were observed between treatment groups in baseline variables. Observed caffeine consumption means over the course of the study are presented in Table 2 for descriptive purposes. Statistical conclusions should not be drawn from observed means, such that observed means have not been log transformed or adjusted for relevant covariates in the statistical model. All subsequent analyses used log transformed and model adjusted means.
Table 2. Observed Caffeine Consumption.
| Immediate Treatment (N = 33, 17 women) | Delay Treatment (N = 34, 17 women) | |||||
|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | |
| Assessment | 733.4 | 559.5 | 200 – 2666.7 | 600.7 | 448.8 | 207 - 2032.6 |
| Treatment | 512.2 | 348.5 | 47.6 – 1650.2 | 408.4 | 326.6 | 68.4 – 1794.4 |
| Week 1 | 364.8 | 331.2 | 3.7 – 1600 | 291.2 | 255 | 56.9 – 1377.8 |
| Week 2 | 255.7 | 279.4 | 0 - 1300 | 230.9 | 187.3 | 45.4 – 923.1 |
| Week 3 | 182.4 | 244.4 | 0 - 1071 | 199.8 | 194.5 | 3.3 – 891.7 |
| Week 4 | 169.5 | 280.5 | 0 – 1209.5 | 192.6 | 198.3 | 0 – 847.6 |
| Week 5 | 162.6 | 301.5 | 0 – 1261.9 | 153.8 | 173.5 | 0 - 723.8 |
| Week 6 TLFB | 171.4 | 311.2 | 0 – 1333.3 | 172.8 | 208.2 | 0 - 800 |
| Week 12 TLFB | 135.9 | 182.7 | 0 – 642.9 | 168.2 | 199.4 | 0 – 923.8 |
| Week 26 TLFB | 122.3 | 174.5 | 0 - 710 | 200 | 261.3 | 0 - 1200 |
| Week 52 TLFB | 137.9 | 175.7 | 0 – 642.9 | 227 | 241.6 | 10.7 – 983.3 |
Note: All values indicate caffeine consumption measured in milligrams. Assessment, Treatment, and Week 6 TLFB – Week 52 TLFB were measured via retrospective self-report. Treatment and Week 1 – Week 5 were measured via daily diary. Values represent observed caffeine consumption and do not indicate log transformed and model adjusted caffeine estimates that were used in longitudinal analyses.
TLFB = timeline follow-back; Assessment = caffeine measured at Assessment Session; Treatment = caffeine measured via daily diary at Treatment Session prior to delivery of treatment.
Pre-treatment changes in caffeine consumption
Caffeine consumption was significantly lower at the Treatment Session than the Assessment Session, F(1, 65) = 37.56, p < 001. There was no significant difference between Immediate Treatment and Delayed Treatment groups in caffeine use per day at either the Assessment Session or the Treatment Session. The duration between the Assessment Session and the Treatment Session was longer in the Delayed Treatment group (5-6 weeks) than the Immediate Treatment group (1-2 weeks). Thus reductions in caffeine between the Assessment and Treatment Sessions were not significantly different between those participants who waited 1-2 weeks and those participants who waited 5-6 weeks.
Effects of Caffeine Treatment on Caffeine Consumption
The effects of treatment condition on caffeine consumption from the Assessment Session to six weeks after the assessment session were examined. In this analysis, six weeks represented caffeine consumption from the Assessment Session to the Treatment Session in the Delayed Treatment group and the Assessment Session to Week 5 follow-up in the Immediate Treatment Group. Thus, in this analysis, the Immediate Treatment group had received the treatment, but the Delayed Treatment group had not. Results revealed a significant interaction of Time and Treatment Condition on caffeine consumption, F(1, 63.04) = 57.65, p <.001, R2 = .35. The interaction revealed a significantly greater reduction in caffeine consumption in the Immediate Treatment group that had received caffeine treatment during this time period, relative to the Delayed Treatment group that had not received caffeine treatment during this period (see Figure 2).
Figure 2.
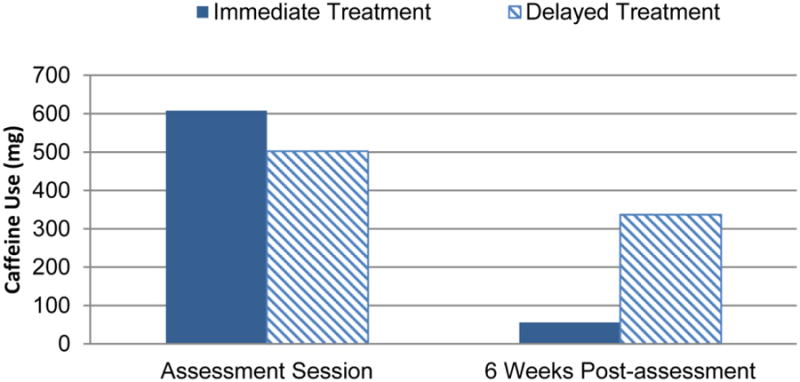
Caffeine use in milligrams (mg) per day from Assessment Session to six weeks after the Assessment Session. Caffeine use was ln transformed for analysis and then reverse ln transformed for the purposes of displaying data. Bars show estimated marginal mean caffeine use produced from the model. Six weeks following the Assessment Session, the Immediate Treatment group (solid bar, n = 33) had received treatment, but the Delayed Treatment group (striped bar, n = 34) had not. A significant interaction, p < .001, reveals a greater reduction in caffeine in the Immediate Treatment group than the Delayed Treatment group.
Next, analyses examined changes in combined caffeine use from both treatment conditions from the Treatment Session to Week 5 post-treatment (Table 3). Results revealed a significant change in caffeine consumption over time, F(5, 67.43) = 17.54, p < .001, such that caffeine use declined over time. Follow-up pairwise comparisons of caffeine consumption estimated marginal means with a Bonferroni adjustment revealed significant reductions in caffeine from Treatment Session to Week 1, Week 1 to Week 2, Week 2 to Week 3, and Week 3 to Week 4, p < .05 (see Table 3). No significant changes in caffeine use were observed from Week 4 to Week 5. No significant effects of Treatment Condition were observed in the model, suggesting that similar caffeine reductions were observed in both groups in the five weeks after Treatment.
Table 3. Caffeine use (mg/day) over the five week period post treatment.
| Assessments | Pre-Assessment Caffeine Use (mg/day) | Post-Assessment Caffeine Use (mg/day) | p-value | 95% Confidence Interval for Difference | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Treatment to Week 1 | 365 | 230.4 | < .001* | 52.7 | 216.5 |
| Week 1 to Week 2 | 230.4 | 152.9 | < .001* | 22.7 | 130.4 |
| Week 2 to Week 3 | 152.9 | 90.9 | < .001* | 27.4 | 95.4 |
| Week 3 to Week 4 | 90.9 | 67.4 | < .05* | .8 | 47 |
| Week 4 to Week 5 | 67.4 | 58.6 | 1.0 | ns | ns |
Significant pre vs post assessment difference p < .05 after a Bonferonni adjustment. ns = not significant. Model adjusted means are combined for both treatment groups after receiving treatment. Analyses were conducted with an (ln) transformed caffeine consumption milligrams per day (mg/day) variable but was reverse transformed for table presentation.
Salivary Caffeine
Mean salivary caffeine levels at the Assessment Session and Week 6 follow-up were 2026.70 ng/ml and 1063.53 ng/ml respectively. Paired samples t-tests revealed a significant reduction in salivary caffeine between the Assessment Session (M = 7.27, SD = 1.2) and Week 6 follow-up (M = 5.83, SD = 1.9); t (46) = 4.66, p = <.001, d = .68.
Caffeine Consumption from 6 Week Follow-up to 52 Week Follow-up
Results did not reveal significant changes in caffeine consumption from TLFB assessments collected at 6, 12, 26, and 52 week follow-ups. Model adjusted caffeine consumption means at TLFB assessments were 83.9 mg, 91.8 mg, 102.5 mg, and 121.5 mg respectively. No significant effects of Time or Treatment Condition on caffeine consumption were observed in the model. The results indicate that there were no significant changes in caffeine consumption in the year after treatment. Nevertheless, considering the clinical importance of characterizing potential relapse patterns, descriptive analyses were conducted to determine how many participants relapsed to previous caffeine consumption behaviors at 6, 12, 26, and 52-week follow-up. Relapse was defined as consuming more than the individual caffeine goal level at any of the TLFB follow-ups after achieving the treatment goal at Week 5 follow-up. Among the 27 participants who achieved their individual goal, nine (33%) relapsed to levels above their treatment goal at one of four follow-up assessments. Increases in caffeine consumption were typically modest with only one of the nine relapsing to pre-treatment caffeine consumption levels. Three of the nine participants who relapsed later achieved their treatment goal at the most recent TLFB. Altogether, 5 of 27 participants (19%) who had achieved their goal at 5 weeks post-treatment had relapsed without complete recovery at most recent follow-up.
Treatment Goals
Forty percent of participants reached the study-defined treatment goal of 50 mg or less of caffeine consumption per day at Week 5 follow-up. Participants were also given the opportunity to self-report personal goals for reducing or quitting caffeine consumption during the Assessment Session. The majority of the sample (71%) set a goal to reduce, rather than quit, caffeine consumption. Participants who wanted to reduce caffeine consumption set a mean goal to reduce caffeine use by 71%. Sixty-one percent of the sample achieved their goal for reduction or quitting of caffeine consumption. Participants reduced their caffeine consumption by an average of 77%. Furthermore, 77% of responders reported consuming less than 200 mg of caffeine at Week 5 post-treatment. Data were also examined making the more conservative assumption that participants who did not provide complete follow-up data did not reduce caffeine consumption at all. Using these criteria, 69% of the sample reported a reduction in daily caffeine on their daily diaries at Week 5 post-treatment. More than half (54%) reported consuming less than 200 mg of caffeine per day and 39% consumed less than or equal to 100 mg of caffeine per day.
Exploratory Analyses
For purposes of exploratory analyses, Treatment Condition was removed from the model because it was found to be a non-significant predictor in analyses in which groups were matched for time after receiving treatment. No significant effects of total number of past caffeine reduction or quit attempts were observed on the model. There was a main effect of using caffeine in the first 15 minutes of the day on caffeine consumption, F (1, 70.67) = 8.53, p = .005, such that those participants who used caffeine in the first 15 minutes of the day consumed more caffeine, relative to those participants who did not consume caffeine in the first 15 minutes of the day. However, there was no statistical interaction, thus indicating that using caffeine in the first 15 minutes of the day was not associated with observed changes in caffeine consumption.
Discussion
The purpose of this study was to investigate a brief intervention to reduce caffeine consumption in individuals who had problematic caffeine use and were interested in reducing or quitting caffeine consumption. Regardless of whether treatment was immediate or delayed, participants reported significant reductions in caffeine use in the first four weeks following treatment. These reductions were maintained, such that no significant increases in mean caffeine consumption were observed at subsequent follow-up assessments. Significant reductions in salivary caffeine were also observed from pre- to post-treatment that corroborated self-reported reductions in caffeine. Medium effect sizes (Cohen, 1988) of the treatment produced from changes in self-report (R2 = .35) and salivary caffeine (d = .68) were observed.
Overall, the reductions in caffeine consumption in the study sample were of considerable clinical significance. Few official general guidelines for daily caffeine consumption exist, but Health Canada, as an example, recommends that healthy adults consume no more than 400 mg per day (Health Canada, 2010). The Food Standards Agency of the United Kingdom advises that pregnant women keep their daily intake of caffeine below 200 mg (FSA News, 2008). In the present study, all participants reported daily caffeine intake ≥ 200 mg at the Assessment Session, but only 23% of responders reported caffeine intake ≥ 200 mg at Week 5 follow-up.
Findings also revealed a significant decrease in caffeine consumption before receiving treatment. Pre-treatment reductions in caffeine intake may have been the result of overestimation of caffeine use by retrospective self-report when entering the study compared to the more systematic assessment via daily diary during the study. A second, non-exclusive, possibility is that participants began to reduce caffeine consumption before the Treatment Session as a consequence of keeping track of daily caffeine use. Daily dietary tracking may positively influence health behaviors. Weight loss studies have shown that individuals who keep a food journal lose more weight than individuals who do not consistently keep a food journal (Hollis, et al., 2008; Kong, et al., 2012).
Repeated follow-up TLFB assessments up to one year did not reveal significant increases in caffeine consumption after treatment. Nevertheless, relapse cannot be ruled out. Previous research suggests that caffeine relapse may only be observed when biochemical verification is employed at long-term follow-up (James, 2011). Long-term follow-up may have been confounded by retrospective reporting and acquiescence biases.
The findings of this study can be viewed in the framework of a harm reduction strategy (Logan and Marlatt, 2010) for problematic caffeine consumption patterns. Similar to harm reduction approaches for alcohol and illicit drug problems, the intervention used in the present study encouraged participants to explore reasons for change and to weigh the positive and negative effects of their substance use (e.g., Miller & Rollnick, 2002; Velicer et al., 1985). Moreover, although participants were encouraged to set a goal to quit caffeine, individual goals to reduce caffeine use were also encouraged and inability to remain completely abstinent from caffeine was not considered failure. Considering the possible positive effects of caffeine at low to moderate doses, reduction of caffeine may be a reasonable and healthy goal for individuals who are having problems associated with excessive caffeine consumption.
Study Limitations
There may be some controversy in the field regarding the clinical meaningfulness of caffeine use disorder, as it was only recently added to the DSM-5 as a condition for further study (Budney, Lee & Juliano, 2015). Importantly, the participants in this study were drawn from a larger sample of participants who were shown to have clinically meaningful consequences associated with their caffeine consumption (see xxxx, 2012). The majority of participants had also previously tried to modify their caffeine use with over half failing to maintain reductions for more than 30 days (see xxxx, 2012). All of the participants in this study met DSM-IV-TR criteria for substance dependence as applied to caffeine and 84% met the much more restrictive DSM-5 caffeine use disorder criteria. As such, this sample did appear to have clinically significant symptoms worthy of intervention, although further research examining the characteristics of those caffeine users who would most benefit from treatment are warranted.
Retention of participants steadily declined over the course of the study, although study drop-out is not representative of treatment retention. All of the treatment components were provided on the day of the Treatment Session. Modest monetary reimbursement may have contributed to drop-out and also prohibited collection of long-term prospective daily diary data.
Future Directions
Future studies should consider offering increased monetary compensation to improve study retention and collect improved follow-up data. The findings of this study would be strengthened by replication, as well as examination of the effectiveness of the treatment in different settings such as outpatient behavioral health or primary care (e.g., Katon et al., 2012), or in different patient populations (e.g, adolescents; Bernstein, Carroll, & Thuras, 2002). Future studies should also examine if more extensive and potentially potent treatments are appropriate for subgroups of caffeine users with more complex and significant consequences associated with caffeine use. Caffeine withdrawal is the most common criterion for caffeine use disorder endorsed by treatment-seeking caffeine users (Juliano et al., 2012) and evidence suggests caffeine withdrawal symptoms plays a prominent role in caffeine reinforcement (Juliano & Griffiths, 2004; Yeomans, Spetch & Rogers, 1998). Prospective time-course measurements of withdrawal symptoms were beyond the scope of this investigation, but future studies should examine the effects of withdrawal symptoms on treatment success.
Public Health Significance Statement.
This study suggests that a brief manualized treatment is efficacious at reducing caffeine consumption in individuals seeking treatment for problematic caffeine use.
Acknowledgments
This research was funded by NIDA Grant R01 DA 03890, awarded to Roland R. Griffiths.
Contributor Information
Daniel P. Evatt, Department of Psychiatry and Behavioral Sciences, The Johns Hopkins University School of Medicine
Laura M. Juliano, Department of Psychology, American University
Roland R. Griffiths, Department of Psychiatry and Behavioral Sciences, The Johns Hopkins University School of Medicine
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th., Text rev. Washington, D.C; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Anderson BL, Juliano LM, Schulkin J. Caffeine's implications for women's health and survey of obstetrician-gynecologists' caffeine knowledge and assessment practices. Journal of Women's Health. 2009;18(9):1457–1466. doi: 10.1089/jwh.2008.1186. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford; 1979. [Google Scholar]
- Bernstein GA, Carroll ME, Thuras PD, Cosgrove KP, Roth ME. Caffeine dependence in teenagers. Drug and alcohol dependence. 2002;66(1):1–6. doi: 10.1016/s0376-8716(01)00181-8. [DOI] [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Niaura R, Abrams DB, Sales SD, Ramsey SE, Goldstein MG, Burgess ES, Miller IW. Cognitive–Behavioral Treatment for Depression in Smoking Cessation. Journal of Consulting and Clinical Psychology. 2001;69(3):471–480. doi: 10.1037//0022-006x.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CM, Dowell CJ, Fairbrother G. Caffeine reduction education to improve urinary symptoms. British Journal of Nursing. 2002;11(8):560–565. doi: 10.12968/bjon.2002.11.8.10165. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Lee DC, Juliano LM. Evaluating the validity of caffeine use disorder. Current Psychiatry Reports. 2015;17(9) doi: 10.1007/s11920-015-0611-z. [DOI] [PubMed] [Google Scholar]
- Chambers L, Mobini S, Yeomans MR. Caffeine deprivation state modulates expression of acquired liking for caffeine-paired flavors. The Quarterly Journal of Experimental Psychology. 2007;60:1356–1366. doi: 10.1080/17470210601154545. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA: the Journal of the American Medical Association. 2006;295(10):1135–1141. doi: 10.1001/jama.295.10.1135. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The Reliability of the CIDI-SAM: a comprehensive substance abuse interview. British Journal of Addiction. 1989;84(7):801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Feng Z, Diehr P, Peterson A, McLerran D. Selected statistical issues in group randomized trials. Annual Review of Public Health. 2001;22(1):167–187. doi: 10.1146/annurev.publhealth.22.1.167. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J. Research Version, Patient Edition. New York: New York State Psychiatric Institute; 1991. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. [Google Scholar]
- Food Standards Agency. Pregnancy Women Advised to Limit Caffeine Consumption. Food Standards Agency. 2008 Retrieved June, 2012, from http://webarchive.nationalarchives.gov.uk/20120206100416/http:/food.gov.uk/news/newsarchive/2008/nov/caffeinenov08.
- Foxx RM, Brown RA. Nicotine fading and self-monitoring for cigarette abstinence or controlled smoking. Journal of Applied Behavior Analysis. 1979;12:111–125. doi: 10.1901/jaba.1979.12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxx RM, Rubinoff A. Behavioral treatment of caffeinism: Reducing excessive coffee drinking. Journal of Applied Behavior Analysis. 1979;12(3):335–344. doi: 10.1901/jaba.1979.12-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. Journal of the American Dietetic Association. 2005;105:110–113. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Evans SM, Heishman SJ, Preston KL, Sannerud CA, Wolf B, Woodson PP. Low-dose caffeine physical dependence in humans. Journal of Pharmacology and Experimental Therapeutics. 1990;255:1123–1132. [PubMed] [Google Scholar]
- Hollis JF, Gullion CM, Stevens VJ, Brantley DPJ, Appel DLJ, Ard DJD, et al. Svetkey LP. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. American journal of preventive medicine. 2008;35(2):118. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Oliveto AH, Liguori A, Carpenter J, Howard T. Endorsement of DSM-IV dependence criteria among caffeine users. Drug and Alcohol Dependence. 1998;52:99–107. doi: 10.1016/s0376-8716(98)00083-0. [DOI] [PubMed] [Google Scholar]
- James JE. Addiction Medicine. Springer; New York: 2011. Caffeine; pp. 551–583. [Google Scholar]
- James JE, Stirling KP, May Hampton BA. Caffeine fading: behavioral treatment of caffeine abuse. Behavior Therapy. 1985;16(1):15–27. [Google Scholar]
- James JE, Paull I, Cameron-Traub E, Miners JO, Lelo A, Birkett DJ. Biochemical validation of self-reported caffeine consumption during caffeine fading. Journal of behavioral medicine. 1988;11(1):15–30. doi: 10.1007/BF00846166. [DOI] [PubMed] [Google Scholar]
- Jones HA, Lejuez CW. Personality correlates of caffeine dependence: the role of sensation seeking, impulsivity, and risk taking. Experimental and clinical psychopharmacology. 2005;13:259. doi: 10.1037/1064-1297.13.3.259. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Evatt DP, Richards BD, Griffiths RR. Characterization of Individuals Seeking Treatment for Caffeine Dependence. Psychology of Addictive Behaviors. 2012 doi: 10.1037/a0027246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Ferre S, Griffiths RR. The pharmacology of caffeine. In: Ries RK, Fiellin DA, Miller SC, Saitz R, editors. ASAM Principles of Addiction Medicine. Fifth. Baltimore: Lippincott Williams & Wilkins; 2014. pp. 180–200. [Google Scholar]
- Juliano LM, Griffiths RR. A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology. 2004;176:1–29. doi: 10.1007/s00213-004-2000-x. [DOI] [PubMed] [Google Scholar]
- Jura YH, Townsend MK, Curhan GC, Resnick NM, Grodstein F. Caffeine intake, and the risk of stress, urgency and mixed urinary incontinence. The Journal of Urology. 2011;185(5):1775–1780. doi: 10.1016/j.juro.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karacan I, Thornby JI, Anch M, Booth GH, Williams RL, Salis PJ. Dose-related sleep disturbances induced by coffee and caffeine. Clinical Pharmacology and therapeutics. 1976;20(6):682. doi: 10.1002/cpt1976206682. [DOI] [PubMed] [Google Scholar]
- Katon W. Collaborative depression care models: from development to dissimenation. American Journal of Preventive Medicine. 2012;42(5):550–552. doi: 10.1016/j.amepre.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Kong A, Beresford SA, Alfano CM, Foster-Schubert KE, Neuhouser ML, Johnson DB, et al. McTiernan A. Self-Monitoring and Eating-Related Behaviors Are Associated with 12-Month Weight Loss in Postmenopausal Overweight-to-Obese Women. Journal of the Academy of Nutrition and Dietetics. 2012 doi: 10.1016/j.jand.2012.05.014. Volume and pagination not yet specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JD, Feinglos MN, Surwit RS. Caffeine increases ambulatory glucose and postprandial responses in coffee drinkers with type 2 diabetes. Diabetes Care. 2008;31(2):221–222. doi: 10.2337/dc07-1112. [DOI] [PubMed] [Google Scholar]
- Lieberman HR, Wurtman RJ, Emde GG, Roberts C, Coviella ILG. The effects of low doses of caffeine on human performance and mood. Psychopharmacology. 1987;3:308–312. doi: 10.1007/BF00210835. [DOI] [PubMed] [Google Scholar]
- Logan DE, Marlatt GA. Harm reduction therapy: a practice-friendly review of research. Journal of Clinical Psychology. 2010;66(2):201–214. doi: 10.1002/jclp.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR EDS. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York: Guilford Press; 1985. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States, POMS. EdiTS, Educational and Industrial Testing Service; 1992. [Google Scholar]
- Meredith SE, Juliano LM, Hughes JR, Griffiths RR. Caffeine use disorder: a comprehensive review and research agenda. Journal of caffeine research. 2013;3(3):114–130. doi: 10.1089/jcr.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesas AE, Leon-Muñoz LM, Rodriguez-Artalejo F, Lopez-Garcia E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. The American journal of clinical nutrition. 2011;94(4):1113–1126. doi: 10.3945/ajcn.111.016667. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. Guilford; New York: 2002. [Google Scholar]
- Oberstar JV, Bernstein GA, Thuras PD. Caffeine use and dependence in adolescents: one-year follow-up. Journal of Child and Adolescent Psychopharmacology. 2002;12:127–135. doi: 10.1089/104454602760219162. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Caffeine: sleep and daytime sleepiness. Sleep Medicine Reviews. 2008;12(2):153. doi: 10.1016/j.smrv.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Shi J, Benowitz NL, Denaro CP, Sheiner LB. Pharmacokinetic-pharmacodynamic modeling of caffeine: Tolerance to pressor effects. Clinical Pharmacology and Therapeutics. 1993;53:6–14. doi: 10.1038/clpt.1993.3. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Herning RI, Better W, Cadet JL, Griffiths RR. Caffeine withdrawal, acute effects, tolerance, and absence of net beneficial effects of chronic administration: cerebral blood flow velocity, quantitative EEG, and subjective effects. Psychopharmacology. 2009;204(4):573–585. doi: 10.1007/s00213-009-1489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption: Psychosocial and biological methods. New Jersey: Humana Press; 1992. [Google Scholar]
- Speilberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- Steffen M, Kuhle C, Hensrud D, Erwin PJ, Murad MH. The effect of coffee consumption on blood pressure and the development of hypertension: a systematic review and meta-analysis. Journal of hypertension. 2012;30(12):2245–2254. doi: 10.1097/HJH.0b013e3283588d73. [DOI] [PubMed] [Google Scholar]
- Strain EC, Mumford GK, Silverman K, Griffiths RR. Caffeine dependence syndrome. JAMA: the journal of the American Medical Association. 1994;272:1043–1048. [PubMed] [Google Scholar]
- Svikis DS, Berger N, Haug NA, Griffiths RR. Caffeine dependence in combination with a family history of alcoholism as a predictor of continued use of caffeine during pregnancy. American Journal of Psychiatry. 2005;162:2344–2351. doi: 10.1176/appi.ajp.162.12.2344. [DOI] [PubMed] [Google Scholar]
- Health Canada. Caffeine: It's Your Health. Health Canada. 2010 Mar; Retrieved June, 2012, from http://www.hc-sc.gc.ca/hl-vs/iyh-vsv/food-aliment/caffeine-eng.php.
- Velicer WF, DiClemente CC, Prochaska JO, Brandenburg N. Decisional balance measure for assessing and predicting smoking status. Journal of personality and social psychology. 1985;48(5):1279. doi: 10.1037//0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. World Health Organization; 1993. [Google Scholar]
- Yeomans MR, Spetch H, Rogers PJ. Conditioned flavour preference negatively reinforced by caffeine in human volunteers. Psychopharmacology. 1998;137:401–419. doi: 10.1007/s002130050636. [DOI] [PubMed] [Google Scholar]