Abstract
Kidney inflammation is a major contributor of progressive renal injury leading to glomerulonephritis and chronic kidney disease. Here, we review recent advances in our understanding of leukocyte accumulation in the kidney, emphasizing critical chemokines involved in glomerulonephritis. We discuss features of renal inflammation such as the evolving concept of immune cell plasticity. We also describe certain aspects of organ-specific tissue microenvironments in shaping immune cell responses, as well as the current knowledge of how regulatory T lymphocytes impact other immune effector cell populations to control inflammation. It is clear that present and future research in these areas may contribute to the development of novel targeted therapeutics, with the hope of alleviating the burden of end-stage renal disease.
Keywords: Leukocyte recruitment, Glomerulus, Local inflammation, Chemokines, Effector immune cells, Regulatory immune cells, Phenotype stability, Environmental triggers
Kidney Inflammation and End-Stage Renal Disease
The prevalence of end-stage renal disease (ESRD) is increasing worldwide and is associated with high mortality and morbidity. Glomerulonephritis (GN) (see Glossary), either in the context of a systemic condition (autoimmune disease, infections) or as a primary disease is the third cause of ESRD in the United States accounting for nearly 10% of cases [1], a figure that is probably much higher in developing countries. In addition, in diabetic and hypertensive nephropathy, the two primary causes of ESRD, as well as in renal ischemia-reperfusion injury, kidney inflammation contributes to progressive kidney damage that eventually leads to loss of glomeruli, tubular atrophy and fibrosis with a concomitant decrease in glomerular filtration rate. Therefore, an understanding of the molecular pathways driving persistent renal inflammation is essential to decipher the pathogenesis of various forms of kidney diseases and to develop novel and more efficient targeted therapeutics to prevent ESRD.
Kidney inflammation can be induced by a variety of triggers including infection, ischemia-reperfusion, immune-complex in situ formation or deposition as well as induction of pro-inflammatory pathways. Inflammation encompasses leukocyte recruitment, systemic and local regulation of leukocyte reactions and termination of these processes. The appropriate balance of these inflammatory responses allows defense against invading pathogens and/or tumor cells while limiting collateral damage. In contrast, the dysregulation of any of these responses sets the stage for inflammatory disease, as in the case of chronic GN.
In this article, we detail some recent advances in our understanding of mechanisms regulating leukocyte accumulation during renal inflammation, some which have been made possible using multiphoton intravital microscopy. We discuss new insights into the interactions of regulatory immune cells with effectors cells in the control of renal inflammation. Lastly, we conclude with a perspective on potential therapeutic targets that may recalibrate regulatory nodes and thus limit inflammation-induced kidney damage.
Leukocyte Accumulation in the Kidney: Patrolling and Adhesion
Leukocyte Recruitment in the Specialized Microvasculature of the Kidney
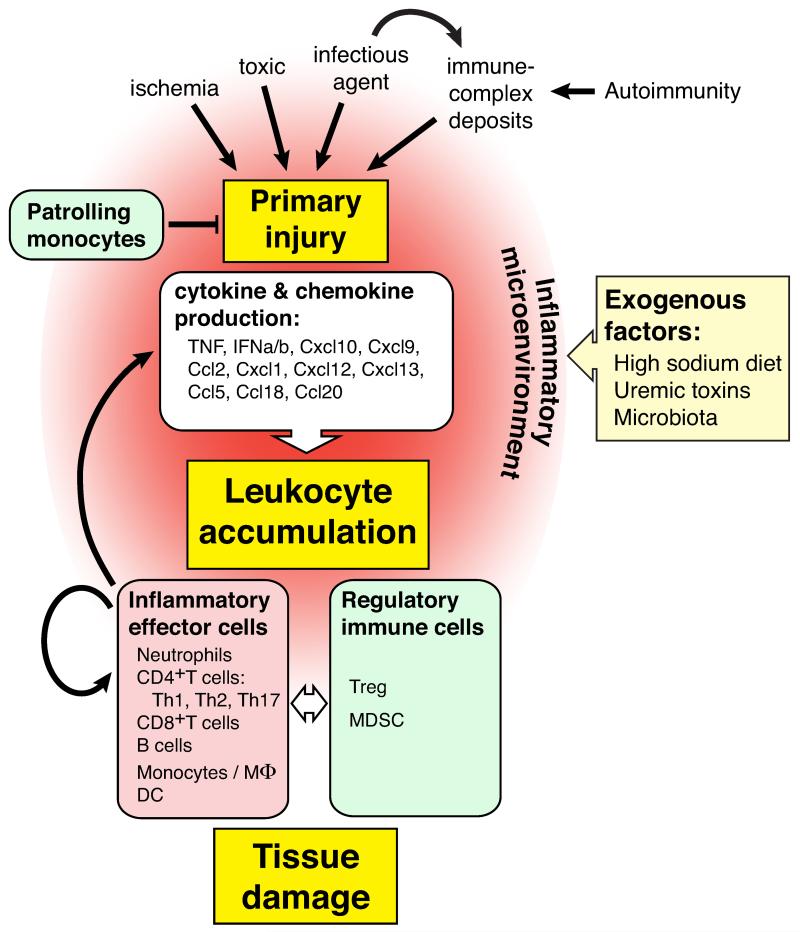
Leukocyte recruitment is the hallmark of inflammation. Local production of chemokines orchestrates their migration from the peripheral blood circulation into inflamed tissue, via a well-described complex cascade of events. These include leukocyte capture, rolling, slow rolling, arrest, adhesion, crawling and eventual transmigration via endothelial adhesion receptors and basement membrane constituents. The advent of advanced intravital microscopy (IVM) has permitted a better description and analysis of leukocyte recruitment in vivo, unveiling the complexity of leukocyte accumulation in the specialized microvasculature of the kidney. Patrolling leukocytes have been described in non-inflamed glomerular capillaries in the kidney[2]. Recent studies are beginning to uncover unique pathways of leukocyte accumulation in glomeruli, which may vary in response to different stimuli (Key Figure, Figure 1). Monocytes patrol the vascular endothelium removing damaged cells and debris, and thus help limit inflammation and maintain immune homeostasis [3]. Neutrophils also appear to be immunosurveillant, as they have been observed to crawl within the glomeruli of untreated animals. However, a caveat is that intravital microscopy requires the surgical exteriorization of the kidney in live animals, so the prevalence of leukocyte immunosurveillance in an unmanipulated kidney remains to be determined. Upon induction of acute, anti-glomerular basement membrane antibody (anti-GBM) mediated nephritis in mice, leukocytes don’t roll on the vessel wall via selectins but simply arrest. Neutrophils increase their dwell time within glomerular capillaries, and generate reactive oxygen species, but do not transmigrate, at least in this acute setting [4]. The mechanisms of glomerular leukocyte accumulation in a more chronic setting and the steps involved in T cell accumulation have not been investigated. Moreover, the mechanisms allowing T cells to detect cognate antigen in a setting where leukocytes appear to remain primarily intravascular remain to be elucidated.
Key Figure, Figure 1. Mechanisms Controlling Leukocyte Accumulation During Kidney Inflammation.
Various triggers induce primary renal tissue injury that subsequently lead to the production of cytokines and chemokines from intrinsic renal cells. These inflammatory cues, dictated by the nature and the site of the initiating injury, coordinate leukocyte accumulation and the temporal infiltration of leukocyte subsets. Recruited leukocytes, in turn, influence the recruitment and activation of other immune cells. As well as the responses from renal cells. Recruited immunoregulatory cells eventually terminate the inflammatory response. Patrolling monocytes may also control inflammatory responses by maintaining endothelial integrity. Finally, the inflammatory microenvironment, influenced by exogenous factors such as high sodium diet, uremic toxins and microbiota (see text), define the inflammatory landscape and thus, leukocyte influx.
The pathways of leukocyte accumulation in the kidney will likely depend on the primary site of damage (glomerulus versus tubulointerstitium) and the instigating stimulus. For example, unlike the glomerulus, selectin mediated rolling followed by arrest of leukocytes is observed in small vessels of the tubulointerstitium following kidney ischemia-reperfusion [5] or kidney graft rejection [6] induced damage of the tubulointerstitial tissue. Although, not yet directly evaluated in the kidney by intravital microscopy, the stimulus will dictate the molecular requirements for leukocyte accumulation. For example, in the liver, neutrophil sequestration in mouse liver sinusoids in response to LPS appears to rely on CD44 and hyaluronan, [7] representing an unusual pathway that is distinct from the classical ligand/receptor interactions of integrins. However, in response to sterile injury (Nlrp3 inflammasome), neutrophil accumulation is mediated by the classical leukocyte adhesion molecule Mac-1 [8]. In the kidney, in the context of glomerular IgG deposition, Fc-gamma-receptors on leukocytes may play a key role in recruitment as they may tether to the Fc portion of deposited IgG accessible to circulating blood through open endothelial fenestrae [9]. Direct evidence for this mechanism using multiphoton intravital microscopy is currently being investigated.
Chemokines and Cytokines: Major Determinants of Leukocyte Recruitment
The specific leukocyte subsets recruited to sites of renal inflammation are guided by the combination of chemokines and cytokines present, which all resident cells, including microvascular endothelial cells, podocytes, tubular and mesangial cells have the capacity to produce. Chemokines are associated with renal injury in human glomerulonephritis (GN), such as IgA nephropathy, membranoproliferative or crescentic GN [10] and in mouse models, appear to play a central role in disease pathogenesis (reviewed in [11]). The primary inflammatory trigger and nature of the initial damage dictates the type and extent of local cytokines produced and, in turn, the type of leukocytes recruited (e.g. ischemia, toxin exposure, pathogen invasion, immune complex deposition or formation, as well as dysregulation of proinflammatory pathways and complement, etc.).
Generally, chemokines binding CXCR3, CXCR6, CCR5, and CX3CR1 recruit Th1 cells that locally produce type 1 cytokines (IL-2, IFNγ), while chemokines engaging CCR3, CCR4, and CCR8 recruit Th2 cells and eosinophils. In chronic GN, CXCR3-binding chemokines, including CXCL10 (IP-10) and CXCL9 (Mig), are of special interest. They sustain the recruitment of CXCR3-bearing Th1 cells and macrophages in murine models of immune complex-induced nephrotoxic nephritis and lupus nephritis models. [12, 13, 14] In humans, high expression levels of CXCL10 and CXCL9 have been reported in resident glomerular cells in kidney biopsies of patients with membranoproliferative and crescentic GN. [15] Furthermore, high urinary excretion of CXCL9 and CXCL10 has been associated with systemic lupus erythematosus (SLE) kidney involvement. [16, 17] Th17 cells, elicited via CCR6 and Th1 cells, play a prominent role in kidney inflammation (reviewed in [18, 19]), and they also express CXCR3. Therefore, CXCR3-binding chemokines can recruit both Th1 and Th17 polarized cells. Th17 production of IL-17 can in turn induce the production of CCL2 by renal intrinsic cells leading to macrophage recruitment. [14] As both Th1 and Th17 play important roles in glomerular inflammation and share similar recruitment cues, their interactions have been further explored in the murine nephrotoxic nephritis model. Th17 cell accumulation is an early event during the adaptive immune response induced in the nephrotoxic nephritis mouse model (autologous phase) and these cells might be the major source of IL-17 responsible for neutrophil recruitment and renal damage. Th17 induction of local CXCL9 expression results in Th1 recruitment and IFNγ production, with subsequente macrophage and dendritic cell (DC) accumulation, thus extending kidney damage. IFNγ production inhibits CCL20 production and further enhances Th17 cell recruitment. [20] A recent report indicated that pathogenic Th17 cells recruited neutrophils through upregulation of CXCL5 expression in kidney tubular cells following induction of nephrotoxic nephritis. CXCL5 deficiency or blockade significantly reduced neutrophil accumulation and kidney damage. Notably, the Th17/IL-17-CXCL5 axis was not involved in bacterial clearance after pyelonephritis, suggesting that it was specifically engaged in sterile inflammation, as is the case in autoimmune pathology. [21] It is possible that initial Th17 recruitment comes from kidney DC chemokines, since their depletion prior to unilateral ureteral obstruction (UUO mouse model) attenuates both Th1 and Th17 cell accumulation. [22] CXCL13, locally produced by infiltrating DCs, appears to be another major pathogenic chemokine in (NZB/W) F1 mice, a murine model of lupus nephritis. [23] This may have relevance in humans, as high serum levels of CXCL13 have been associated with SLE nephritis. [24, 25] In vitro, CXCL13 engagement via CXCR5 on podocytes, leads to the secretion of proinflammatory molecules such as CXCL1, CXCL12 and macrophage inhibitory factor (MIF), which can prime oxidative bursts in isolated human neutrophils [23], a possible means of amplifying glomerular inflammation.
More recently, CCL18 was identified as a central chemokine in orchestrating inflammation and tissue damage specifically in human anti-neutrophil cytoplasmic antibodies (ANCA) associated crescentic GN. [26] High levels of CCL18 were found in patient biopsies, expressed by macrophages and myeloid DCs by immunohistochemistry. CCL18 serum levels were also significantly elevated in patients with ANCA-associated vasculitis (AAV), both at the time of diagnosis and during relapses. Engagement of CCL18 via CCR8 might promote lymphocyte and monocyte recruitment. However, CCL18 does not have a murine equivalent, precluding its study in murine models. CCR8 is expressed in mice and binds CCL1 and CCL8, the latter being a functional analog of CCL18. In the nephrotoxic nephritis murine model, CCR8 was shown to drive the recruitment of mononuclear phagocytes. [26] Besides CCL18, many other chemokines have also been identified in biopsies of AAV patients, including CCL5, CXCL9, CXCL10 and CCL20, [26] suggesting that CCL18 functions within a complex array of proinflammatory chemokines. Given the prominent role of CCL18 in AAV, it will be of interest to elucidate its importance in other forms of GN.
The role of TNF in kidney inflammation is particularly illustrative of the complex interactions between immune effector cells and kidney intrinsic cells driving local chemokine generation (Box 1). In the GN animal model, the main source of TNF comes from intrinsic renal cells, and TNF itself promotes kidney injury. [27, 28] In addition, TNF is a potent cytokine that further enhances the inflammatory response. Nevertheless, despite a prominent role for this pleiotropic cytokine in kidney inflammation, anti-TNF treatments have failed to show a clear benefit over standard immunosuppressive treatments of GN, and particularly, in ANCA-associated GN [28]. This may be explained by the various roles of TNF in promoting inflammation (e.g. inducing immune cell recruitment), but also, in resolving inflammation (e.g. apoptosis of immune cells), as well as in immunomodulation. The importance of TNF in immunomodulation is highlighted by findings in kidney disease patients where TNF blockade has been reported to induce antibodies against double-stranded DNA (dsDNA), and occasionally, in lupus-like syndromes [29-31]. Thus, a more targeted approach that specifically neutralizes TNF’s proinflammatory role while leaving its other functions intact may represent a more promising therapeutic strategy.
Box 1. TNF and Kidney Inflammation.
Soluble TNF, which favors engagement of the receptor TNFR1, has been shown to orchestrate a proinflammatory cascade in microvascular endothelial cells by inducing the generation of interferon beta (IFNβ) in an autocrine manner (binds the IFNα/β receptor (IFNAR)), thus triggering the production of CXCR3 chemokines (e.g. CXCL9 and CXCL10) and supporting monocyte recruitment. Both TNFR1 and TNFR2 are required for interferon regulated factor-1 (IRF1) dependent IFNβ production and, in vivo, support macrophage accumulation following soluble TNF-induced acute inflammation [32]. However, in a murine model of chronic immune-complex mediated GN (nephrotoxic nephritis), TNFR2 (but not TNFR1) was essential for macrophage accumulation. [32, 33] A central role for CXCR3-binding chemokines CXCL10 and CXCL9 has been demonstrated in the nephrotoxic nephritis model. [12, 13] Thus, although circulating soluble TNF is traditionally identified as a hallmark of systemic inflammation, its local production and subsequent generation of IFNβ likely shapes the inflammatory environment in the kidney. The pathogenic consequences are likely influenced by the relative abundance of TNFR1 versus TNFR2, and the extent of TNF sheddases present (e.g. ADAM17) [34], dictating the amount of membrane-bound versus soluble TNF available. Indeed, TNFR2 is significantly upregulated in endothelial cells and podocytes following glomerular inflammation induced by TNF injection or nephrotoxic nephritis in mice as well as in renal biopsies from patients with acute renal allograft rejection and ischemic kidney injury (acute tubular necrosis) [32, 33, 35, 36]. Inducible expression of TNFR2 at the transcriptional level might be regulated by mediators that increase intracellular cAMP levels, or that induce the transcriptional activation of NFkB, AP-1, IRF or GAS, as consensus sites for these factors are present within the TNFR2 promoter [32, 37]. Thus, control of TNFR2 expression may serve as an additional regulatory point for TNF-mediated leukocyte recruitment.
In addition to recruitment, retention of effector T cells at sites of inflammation appears to contribute to disease pathogenesis in different organs. For instance, recent mouse studies have shown that the antigen-dependent activation of effector T cells in situ by tissue resident antigen presenting cells can result in T cell accumulation in various inflamed tissues. An absence of such interactions led to increased T cell exit via afferent lymphatics and accumulation in draining lymph nodes, [38, 39] guided by CCR7-CCL19/21 chemokine receptor/ligand cues. [38, 40]. Deletion of a scaffold protein, AKAP9, important in TCR recycling has also been shown to impair in situ T cell reactivation and to increase effector T cell egress to draining lymph nodes, concomitant with reduced kidney or central nervous system injury in mouse models of nephrotoxic nephritis (NTN) and experimental autoimmune encephalitis (EAE), respectively. [41]
Roles of Leukocyte Subsets in Kidney Disease
Different subsets of leukocytes are involved in distinct and overlapping aspects of the immune response. First on site, neutrophils are able to quickly eliminate invading pathogens. They also communicate with other immune cells and participate in inflammation as is the case of chronic GN [42, 43]. There is mounting evidence that neutrophils are key players in SLE pathogenesis [44], associated with renal injury both in pediatric and adult SLE [43, 45-47], and in mouse models of lupus nephritis [48, 49]. Moreover, a distinct population of lupus patient neutrophils has been identified, and these low density granulocytes co-segregate with mononuclear cell fractions [50, 51]. Compared to normal neutrophils, this subset has a heightened capacity to induce vascular damage, synthesize granule proteins such as MPO and MMPs, express proinflammatory molecules and form neutrophil extracellular traps (NETs) [45, 52].
In terms of recruitment, phagocytic mononuclear cells (including monocytes, macrophages and DCs), B cells and different T cell subsets are likely playing important roles in the pathogenesis of renal diseases, but full supportive evidence remains to be accumulated in this arena. By the same token and warranting further validation, other than inflammatory molecules, the tissue environment itself might be impacting immune cell phenotypes (Box 2). Along these lines, a change in the intrinsic renal milieu by exogenous environmental stimuli to which the kidney is particularly susceptible may have a strong impact on immune cell phenotypes and thus, on the overall inflammatory landscape.
Box 2. Tissue Microenvironment Influences on Immune Cell Phenotypes.
In addition to the classical inflammatory molecules such as cytokines and chemokines, the tissue environment itself very likely impacts immune cell phenotypes. Gene transcription analyses of various subsets of resident macrophages (brain microglia and resident peritoneal macrophages) have revealed that the tissue microenvironment can have a large impact on the transcriptional profile of the cell. That is, a common enhancer landscape combined with tissue specific “superenhancers” has been shown to determine the phenotype of tissue-specific resident macrophages. [114] Although not yet fully supported, the transcriptional profile of immune cells recruited during an inflammatory response, could likewise be fundamentally influenced by tissue intrinsic factors in addition to inflammatory mediators. For example, infiltrating T cells following renal ischemia reperfusion injury (IRI) in mice have shown a marked change in gene transcription as early as 6 hours after injury [115]. As such, CCR5 upregulation has been suggested to be particularly prominent and functionally relevant to renal disease pathophysiology. [115]
Exogenous Factors Influencing the Inflammatory Microenvironment
Sodium
Sodium chloride (NaCl) levels, highly relevant to kidney disease, have recently been shown to modulate the inflammatory microenvironment. Intriguing data from two independent groups [53, 54] suggested that increasing concentrations of NaCl (10 to 40 mmol/L) induced a strong Th17 phenotype in naïve CD4+, cells in a dose-dependent manner. This effect was sodium-dependent as it was reproducible with sodium gluconate but not with MgCl2. Th17 induction was also dependent on SGK1, p38/MAPK and NFAT5 [53]. The biological significance of this observation was confirmed in vivo in a mouse model of EAE; mice on a high-salt diet exhibited an accelerated onset and increased severity of the disease [53, 54]. Similar results were observed in a murine model of chronic heart allograft rejection; a high-salt diet accelerated graft rejection in mice [55]. Thus, the mechanistic role of sodium in disease pathogenesis may represent a fruitful area of investigation.
Uremic toxins
Glomerulonephritis is the third cause of chronic kidney damage leading to reduced glomerular filtration rate and accumulation of uremic toxins, as in chronic kidney disease (CKD). CKD by itself is linked to some form of chronic systemic inflammation that is poorly characterized and might further contribute to kidney damage and ESRD. Enhanced oxidative stress in CKD due to uremic toxins can lead to tissue damage and secretion of damage-associated molecules activating pattern recognition receptors (DAMPs), such as Toll-like receptors (TLRs) during the innate immune response [56, 57]. In vitro, the uremic toxin indoxyl sulfate has been shown to increase LPS-induced macrophage activation (higher protein expression of COX2, iNOS, as well as production of NO, TNF and IL6). [58] In addition, analysis of stimulated peripheral T cell supernatants from CKD stage 4 patients has indicated a higher production of cytokines such as TNF, IL-10, IL-12, IL-15, IL-8, MCP-1, CXCL10, IFN-α2, IL-1α and eotaxin relative to controls. [59] Transcriptional profiling of circulating monocytes from CKD patients has also suggested dysregulated signaling in the Wnt/β-catenin pathway, possibly contributing to enhanced inflammation through increased monocyte adhesion and IL-6 production [60]. Uremic toxin accumulation has thereby the potential to alter significantly both innate and adaptive immune responses. Nonetheless, there is currently little mechanistic data available to elucidate the effect of the uremic environment per se on the immune system.
Microbiota
Alteration of the normal intestinal microbiome (or intestinal dysbiosis) has been previously associated with SLE [61]. This in turn may influence the development of IgA nephropathy [62]. In mice, transgenic overexpression of BAFF, a B cell stimulating factor, has been shown to lead to the development of high circulating levels of IgA which are associated with IgA mesangial deposition and nephritis [63]. Commensal bacterial-reactive IgA was produced in these mice, suggesting that the intestinal microbiota participates in autoimmune IgA nephropathy in this animal model [63]. Related to this, at steady state, the microbiota was shown to limit the transport of bacteria from the lumen to mesenteric lymph nodes (MLNs). However, upon breakdown of these conditions, (as in antibiotic-induced dysbiosis), non-invasive bacteria were transported to the MLNs via CX3CR1+ phagocytes, triggering both IgA production and T cell responses [64]. In humans, a genome-wide association study (GWAS) of IgA nephropathy patients identified new genomic loci, which included genes involved in intestinal epithelial barrier maintenance and in immune responses to mucosal pathogens (DEFA, TNFSF13, VAV3, ITGAM-ITGAX, PSMB8) [65]. Thus, alterations in host-intestinal pathogen interactions may increase susceptibility to IgA nephropathy.
Effector and Regulatory Immune Cells and the Renal Inflammatory Response
Regulatory Immune Cells
In the last two decades, significant strides have been made in understanding the mechanisms that restrict inflammation to maintain immune homeostasis. In particular, anti-inflammatory or immunoregulatory cell subsets belonging to the T cell and monocyte cell lineages have been demonstrated to play a major role in controlling autoimmunity and resolving inflammation.
Regulatory T cells
Regulatory T cells (Treg) are a subset of CD4+, T cells that express the transcription factor Foxp3, and through production of IL-10, exhibit numerous anti-inflammatory properties that fundamentally contribute to immune homeostasis [66]. Tregs have an important protective role in kidney inflammatory diseases. In mouse models, Treg impairment has been associated with exacerbated disease in cisplatin-induced nephrotoxicity, [66] nephrotoxic nephritis (NTN), [67-72] anti-myeloperoxidase GN, [73] and ischemic acute kidney injury. [74-76] Furthermore, adoptive transfer of Tregs prior to induction of nephrotoxic nephritis significantly decreased kidney damage. [77] In response to nephrotoxic nephritis, Treg expression of CCR6 and CCR7 was required for their proper recruitment to the kidney, and deficiency of either of these chemokine receptors was associated with worsened disease. [68, 69] Treg production of IL-10 appeared to be central in limiting the extent of GN disease, at least in part, by reducing the accumulation of IFNγ+, IL-17+, and double positive IFNγ+,/IL-17+, CD4+, T cells. [67] In an NTN model, Tregs were recruited to the kidney through Stat3-dependent expression of CCR6. Specific depletion of Tregs expressing Stat3 (Foxp3Cre/Stat3fl/fl mice) resulted in more severe glomerular and interstitial damage. [71] Indeed, these data highlight how Tregs can modulate a variable set of transcription factors and surface phenotypes to target disease-specific immune responses, including that of Th17-mediated kidney damage. [78]
The observed roles of Tregs in mouse models might extend to humans. For instance, decreased Treg numbers have been shown to be associated with human renal disease; as such, Foxp3+ T cells numbers in kidney biopsies might predict renal survival in patients with ANCA-associated vasculitis. [79] In SLE patients, fewer numbers of peripheral blood Tregs have been observed [80], and a lower ratio of peripheral blood Treg/Th17, has been associated with lupus nephritis and inversely correlated with the overall clinical severity of SLE. [81] In kidney biopsy samples from patients with active proliferative lupus nephritis and ANCA-associated vasculitis, the ratio of FoxP3+/CD3+ cells was significantly lower compared to hypertensive nephropathy, lupus membranous nephropathy (class V) and tubulointerstitial nephritis [82]. The change in Treg numbers in lupus may be a consequence of lower IL-2 levels observed in SLE patients because this cytokine plays an important role in Treg development and survival. [83] Indeed, the spontaneous autoimmune manifestations detected in IL-2-deficient mice might be associated with the large reduction in Tregs numbers found in the periphery [83].
Myeloid-derived Suppressor Cells (MDSC)
MDSCs, a heterogeneous population of myeloid-progenitor cells and immature myeloid cells contribute to immune homeostasis through their potent T cell suppressive abilities. In mice, they are characterized by the surface expression of integrin CD11b, together with mouse myeloid-cell lineage surface marker Ly6C+, (monocytic morphology) and Ly6G+, (granulocytic morphology), and in humans, by the expression of CD14+/CD16− or CD14+/CD16+. Their trafficking is mainly dictated by the chemokine receptor CCR2. [84, 85] They are found in mouse models of rheumatoid arthritis, diabetes and multiple sclerosis (EAE). Peripheral blood naïve monocytes (CD11b+,Ly6G−) also display in vitro intrinsic suppressive properties by inhibiting T cell proliferation through a mechanism that requires cell-contact and partially, nitric oxide (NO) production. [86].
The biological relevance of MDSC is inferred from murine models as well as clinical observations. In the kidney, MDSCs were found to accumulate in an allograft model in rats, inhibiting both proliferation and induced apoptosis of alloreactive T cell through a NO-dependent mechanism. [87]. More recent work has shown increased numbers of circulating MDSC in kidney allograft transplant recipients, correlating with higher numbers of Tregs in the circulation [88]. In addition, isolated MDSC from kidney transplant recipients were capable of inhibiting CD4+ T cell proliferation as well as inducing the expansion of Foxp3+ Tregs ex vivo. [88] Finally, glucocorticoid treatment of patients with focal segmental glomerulosclerosis (FSGS) induced a rapid increase in MDSCs in peripheral blood, which appeared to be predictive of the patients’ clinical responses. [89] In a corresponding proteinuria mouse model of doxorubicin-induced renal injury, MDSC depletion using an anti-Gr-1 antibody (although not fully specific) abolished the protective effect of glucocorticoids, while the adoptive transfer of MDSC appeared to be protective by inducing the proliferation of CD4+CD25+FoxP3+ and reducing the number of CD11c+ and F4/80+ cells in the kidneys [89].
Patrolling Monocytes
Patrolling monocytes exhibit a characteristic slow motion at the surface of endothelia, following complex tracks, including U-turns and spirals, as assessed by intravital microscopy. [90, 91] Their molecular phenotype is characterized by the high surface expression of the adhesion-related receptor CX3CR1, together with low expression of CD14 in human (CD14dim) and Ly6C− in mice. After phagocytosis of apoptotic cells, patrolling monocytes express high levels of PDL-1 and thereby suppress antigen-specific T cell responses in the spleen. [92]. In the context of inflammation, they can be retained in kidney capillaries and mediate neutrophil recruitment followed by focal and contained necrosis of endothelial cells and scavenging of cellular debris within the capillary lumen [96]. CD14dim monocytes also specifically target viruses through TLR7 and TLR8 and are able to produce high levels of proinflammatory cytokines such as TNF, IL-1β, CXCL10 and CCL5. [93] In lupus nephritis, their protective function may therefore be ambiguous as immune complex deposition containing self-nucleic acids in the glomeruli may trigger a proinflammatory response of patrolling monocytes by engaging TLRs. The human relevance of potentially protective CX3CR1-bearing cells is suggested by the reduced risk of acute kidney injury (AKI) during sepsis of patients expressing the I249 CX3CR1 polymorphism, which functionally increases CX3CR1-mediated monocyte binding to CX3CL1, its cognate ligand. [94] Thus, patrolling monocytes appear to play an important function in maintaining endothelium integrity and thus potentially, immune homeostasis. Of note, CX3CR1 is also expressed on CD11c+ DCs recruited to the kidney cortex during NTN, an immune-complex mediated disease. Hence, as opposed to DC-independent ischemic or obstructive kidney injury models, CX3CR1-expressing DCs appear to promote kidney inflammation and tissue damage in NTN. [95]
3.2 Plasticity of Regulatory Cells
IL-17 expressing Treg (Treg17) displaying a proinflammatory phenotype have also been reported in various inflammatory conditions. [96, 97] In view of the great diversity of CD4+ T cell and the various adaptive mechanisms that have been observed, certain aspects of lineage commitment and full determination are being questioned and the plasticity of Treg versus the paradigm of stable, terminally differentiated Th cells is now gaining acceptance. [98] The local microenvironment in which the CD4+ T cell is recruited may contribute to determine its fate as well as the stability of its immunophenotype. Indeed, there are many examples of transient or unstable expression of Th-specific transcription factors, notably Foxp3. [99, 100] A recent study demonstrated that TNF inhibition in patients with rheumatoid arthritis increased the number of peripheral Th17 cells with regulatory properties, as these cells expressed high levels of IL-10 in a Foxp3-independent manner. [101] This suggests that alterations in the proinflammatory milieu can affect the phenotype and function of Th17 effector cells in humans.
Further complexity is introduced by the recent identification of another subset of Tregs called bifunctional Treg or biTreg, expressing both Foxp3 and RORγt, a Th17 transcription factor; accordingly, the cells are able to produce IL-17. [102] Their role in renal inflammatory disease was recently explored in the murine NTN model. [103] In this study, biTregs expressing both IL-10 and IL-35, as well as proinflammatory IL-17 cytokines appeared to be distinct from Treg17 and did not derive from or transdifferentiate into either Tregs or Th17. On the one hand, adoptive transfer of biTregs suppressed renal proinflammatory cell infiltration, but on the other hand, biTregs produced RORγt-dependent-IL-17 and participated in glomerular damage [110]. Thus, biTregs appeared to contribute to both inflammatory damage as well as immunoregulation in this murine model. Although the specific function of this novel bifunctional T cell subpopulation in kidney inflammation remains elusive, the data exemplify the complex interchange between proinflammatory and regulatory immune cells.
Towards Better Targeted Anti-inflammatory Treatments
Cumulative knowledge of cytokines and chemokines in various inflammatory diseases has led to the development of new drugs in the last decade, predominantly blocking antibodies, to target key inflammatory mediators. Some have reached the clinic with great success but also with some disappointments. Given the multifaceted and/or redundant roles of cytokines, identifying the right targets in disease has proven to be difficult; modulating the immune response using biologics has produced unexpected results. For instance, potent anti-inflammatory drugs such as TNF inhibitors have been associated with adverse autoimmune manifestations such as drug-induced lupus. [28, 104, 105] Anti-TNF drugs induce a shift in production of anti-inflammatory cytokines (particularly IL-10), which may lead to cytokine imbalance, autoantibody production and/or autoimmune manifestations in susceptible individuals [104]. In addition, apoptosis of immune effector cells induced by such treatments may release auto-antigens that may further stimulate autoantibody production. [104] The use of the monoclonal anti-IL-6 receptor blocking antibody tocilizumab, has shown great efficacy in the treatment of severe RA [106], but exacerbated kidney damage in the NTN mouse model [107]. However, because IL-6 has been shown to inhibit the proliferation of spleen proinflammatory macrophages expressing high levels of IL-6Rα, an immunoregulatory function for IL-6 has been suggested [107], which may be clinically relevant for the treatment of psoriasis or of immune complex-mediated GN in patients receiving tocilizumab. [108, 109] Targeting IL-17 has also been proposed to treat many autoimmune diseases. However, in IL-17A-deficient MRL/lpr lupus mice, or in NZB/NZW lupus mice treated with anti-IL-17A, lupus-induced nephritis has not been successfully prevented. [110] Further studies are still needed to fully evaluate the benefit and safety of anti-IL-17 treatments in autoimmune diseases.
Novel therapeutic approaches are also aimed at enhancing Treg responses to control inflammation and prevent autoimmunity. Adoptive transfer of expanded Tregs ex vivo has shown some promising results in animal models and notably in a lupus nephritis model. [111] Another strategy has been to pharmacologically enhance Treg recruitment using N,N-dimethylsphingosine with some benefit in a mouse model of ischemia reperfusion kidney damage [112] Phase I clinical trials are currently ongoing to test the therapeutic potential of autologous adoptive transfer of ex vivo expanded Tregs in kidney transplantation [113], and lupus nephritis, among other conditions (can also see http://clinicaltrials.gov). Nevertheless, such strategies are challenging; for instance, the maintenance of a regulatory immunophenotype requires a specific molecular signature and a combination of transcriptional factors. A proinflammatory milieu may also influence the Treg suppressive phenotype towards a proinflammatory one. Therefore, in the clinic, one difficulty (among many) will be to sustain the regulatory phenotype of transferred Tregs. We speculate that this will require further pharmacological interventions or genetic reprogramming such that switching between regulatory and pro-inflammatory cellular phenotypes is enabled. This will clearly warrant further experimentation and insight.
Concluding Remarks
The changing landscape of renal inflammation is dictated by the secretion of proinflammatory molecules from tissue resident cells, amplified by the recruitment of immune effectors cells, and modulated by both regulatory and effector cell populations. This complex set of interactions and processes appears to continuously evolve during the course of an inflammatory response; the relative contribution of each results in either the escalation or resolution of inflammation. Although progress has been made in understanding these processes in the context of kidney inflammation, a greater understanding of the local renal-specific inflammatory microenvironment and its influence on the heterogeneity and plasticity of immune effector and regulatory cells is urgently needed (see Outstanding Questions). Moreover, the molecular pathways that promote leukocyte recruitment in the specialized microvasculature of the kidney in response to various inflammatory stimuli need to be further delineated. Future investigations into the conditions that enable an immunosuppressive microenvironment in the kidney are also warranted. These types of studies may lead to the identification of therapeutic targets to fine-tune the inflammatory microenvironment in the kidney such that the recruitment and function of effector cells may be suppressed while at the same time, enabling the participation of regulatory cells under a specific set of circumstances. This type of local immunoregulatory calibration may indeed represent the kind of scalpel approach needed to reduce the burden of ESRD due to inflammation, while ideally minimizing the risk of global immunosuppression.
Outstanding Questions.
How do inflammatory molecules interact with kidney intrinsic cells to generate chemokines?
What are the molecular cues and pathways that support recruitment of different immune cells in the glomeruli and interstitium, and do these differ depending on the stimulus?
What are the local renal factors that sustain the recruitment as well as maintain the regulatory phenotype of T regs?
Do ex vivo Treg conditioning and adoptive transfer methods represent an efficient therapeutic strategy for the treatment of kidney inflammation in humans?
What are some of the molecular pathways by which exogenous factors impact renal inflammation? Some of these factors could include changes in sodium levels, microbiota, uremic toxins or other.
Trends box.
Leukocyte recruitment in the specialized microvasculature of the renal glomerulus differs from the classical paradigm of leukocyte rolling, arrest and transmigration.
The local inflammatory microenvironment is largely defined by the local production of chemokines, which orchestrate leukocyte accumulation and can contribute to kidney dysfunction, as is the case in glomerulonephritis (GN).
Factors such as sodium levels, uremia or changes in the microbiota may reset homeostatic equilibria in the kidney, thus influencing the immune response.
Expanding subsets of immune regulatory cells help maintain immune homeostasis. Regulatory T cells are particularly important in limiting inflammatory kidney damage.
The concept of T cell plasticity has questioned certain aspects of lineage commitment and terminal differentiation of leukocyte subsets.
One of the challenges of targeted renal immunotherapy is attempting to balance and specifically elicit suppressive regulatory responses while inhibiting potentially damaging effector cell functions in the local kidney microenvironment.
Glossary
- ANCA-associated vasculitis (AAV)
vasculitis induced by the production of auto-antibodies directed against neutrophil granule proteins called anti-neutrophil cytoplasmic antibodies or ANCA.
- Crescentic glomerulonephritis (GN)
a form of severe glomerulonephritis observed in various forms of GN (e.g. lupus nephritis, ANCA-associated GN), morphologically characterized by crescent formation in the urinary space (Bowman’s space) resulting from the rupture of the glomerular capillary wall, plasma molecules effusion with subsequent fibrin formation and influx of macrophages and T cells, leading to cellular and/or fibrous proliferation inside the urinary space.
- Diabetic nephropathy
kidney disease caused by type 1 or type 2 diabetes, with characteristic glomerular changes including mesangial expansion and glomerular sclerosis.
- Focal segmental glomerulosclerosis (FSGS)
a form of glomerular disease characterized by a focal (involving only some glomeruli) and segmental (only a portion of the glomerulus) glomerular sclerosis, which can be idiopathic or secondary to infections, drugs or toxins.
- Glomerulonephritis
inflammatory disease of the kidney primarily involving the glomeruli.
- Hypertensive nephropathy
kidney disease caused by chronic hypertension characterized by vascular damage including glomerular arteries leading to glomerulosclerosis and tubulointersitial fibrosis.
- Lupus membranous nephropathy
a form of lupus nephritis (also called class V lupus nephritis) characterized by subepithelial immune-complex deposition leading to diffuse thickening of the glomerular basement membrane.
- NETs
nuclear chromatin fibers that contain immunostimulatory proteins and autoantigens
- Podocytes, mesangial and tubular cells
podocytes are specialized epithelial cells forming the visceral sheet of the urinary space (Bowman’s space) of the glomeruli and the main component of the glomerular barrier; mesangial cells are specialized smooth muscle cells surrounding the glomerular capillaries; tubular cells are specialized epithelial cells delineating the renal tubules.
- Pyelonephritis
infection of the kidney.
- Tubulointerstitial nephritis
kidney disease primarily characterized by inflammation of the tubulointerstitial compartment of the kidney.
- Uremic toxins
retained solutes accumulating when glomerular filtration rate is reduced in chronic kidney disease (CKD) and contributing to systemic symptoms of renal failure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saran R, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015;65(6 Suppl 1):A7. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Thomas G, et al. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol. 2015;35(6):1306–16. doi: 10.1161/ATVBAHA.114.304650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devi S, et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med. 2013;19(1):107–12. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- 5.Block H, et al. Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury. J Exp Med. 2012;209(2):407–21. doi: 10.1084/jem.20111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walch JM, et al. Cognate antigen directs CD8+ T cell migration to vascularized transplants. J Clin Invest. 2013;123(6):2663–71. doi: 10.1172/JCI66722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald B, et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J Exp Med. 2008;205(4):915–27. doi: 10.1084/jem.20071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–6. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 9.Mayadas TN, Tsokos GC, Tsuboi N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation. 2009;120(20):2012–24. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada T, Matsushima K, Kaneko S. The role of chemokines in glomerulonephritis. Front Biosci. 2008;13:3966–74. doi: 10.2741/2984. [DOI] [PubMed] [Google Scholar]
- 11.Chung AC, Lan HY. Chemokines in renal injury. J Am Soc Nephrol. 2011;22(5):802–9. doi: 10.1681/ASN.2010050510. [DOI] [PubMed] [Google Scholar]
- 12.Panzer U, et al. Chemokine receptor CXCR3 mediates T cell recruitment and tissue injury in nephrotoxic nephritis in mice. J Am Soc Nephrol. 2007;18(7):2071–84. doi: 10.1681/ASN.2006111237. [DOI] [PubMed] [Google Scholar]
- 13.Menke J, et al. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol. 2008;19(6):1177–89. doi: 10.1681/ASN.2007111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinmetz OM, et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol. 2009;183(7):4693–704. doi: 10.4049/jimmunol.0802626. [DOI] [PubMed] [Google Scholar]
- 15.Romagnani P, et al. IP-10 and Mig production by glomerular cells in human proliferative glomerulonephritis and regulation by nitric oxide. J Am Soc Nephrol. 2002;13(1):53–64. doi: 10.1681/ASN.V13153. [DOI] [PubMed] [Google Scholar]
- 16.Enghard P, et al. CXCR3+CD4+ T cells are enriched in inflamed kidneys and urine and provide a new biomarker for acute nephritis flares in systemic lupus erythematosus patients. Arthritis Rheum. 2009;60(1):199–206. doi: 10.1002/art.24136. [DOI] [PubMed] [Google Scholar]
- 17.Abujam B, Cheekatla S, Aggarwal A. Urinary CXCL-10/IP-10 and MCP-1 as markers to assess activity of lupus nephritis. Lupus. 2013;22(6):614–23. doi: 10.1177/0961203313484977. [DOI] [PubMed] [Google Scholar]
- 18.Turner JE, et al. The Th17 immune response in renal inflammation. Kidney Int. 2010;77(12):1070–5. doi: 10.1038/ki.2010.102. [DOI] [PubMed] [Google Scholar]
- 19.Azadegan-Dehkordi F, et al. The role of Th1 and Th17 cells in glomerulonephritis. J Nephropathol. 2015;4(2):32–7. doi: 10.12860/jnp.2015.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paust HJ, et al. Chemokines play a critical role in the cross-regulation of Th1 and Th17 immune responses in murine crescentic glomerulonephritis. Kidney Int. 2012;82(1):72–83. doi: 10.1038/ki.2012.101. [DOI] [PubMed] [Google Scholar]
- 21.Disteldorf EM, et al. CXCL5 drives neutrophil recruitment in TH17-mediated GN. J Am Soc Nephrol. 2015;26(1):55–66. doi: 10.1681/ASN.2013101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong X, et al. Dendritic cells facilitate accumulation of IL-17 T cells in the kidney following acute renal obstruction. Kidney Int. 2008;74(10):1294–309. doi: 10.1038/ki.2008.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worthmann K, et al. Pathogenetic role of glomerular CXCL13 expression in lupus nephritis. Clin Exp Immunol. 2014;178(1):20–7. doi: 10.1111/cei.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong CK, et al. Elevated production of B cell chemokine CXCL13 is correlated with systemic lupus erythematosus disease activity. J Clin Immunol. 2010;30(1):45–52. doi: 10.1007/s10875-009-9325-5. [DOI] [PubMed] [Google Scholar]
- 25.Schiffer L, et al. B-cell-attracting chemokine CXCL13 as a marker of disease activity and renal involvement in systemic lupus erythematosus (SLE) Nephrol Dial Transplant. 2009;24(12):3708–12. doi: 10.1093/ndt/gfp343. [DOI] [PubMed] [Google Scholar]
- 26.Brix SR, et al. CC Chemokine Ligand 18 in ANCA-Associated Crescentic GN. J Am Soc Nephrol. 2015;26(9):2105–17. doi: 10.1681/ASN.2014040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timoshanko JR, et al. Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14(7):1785–93. doi: 10.1097/01.asn.0000073902.38428.33. [DOI] [PubMed] [Google Scholar]
- 28.Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76(3):262–76. doi: 10.1038/ki.2009.142. [DOI] [PubMed] [Google Scholar]
- 29.Aringer M, Smolen JS. Efficacy and safety of TNF-blocker therapy in systemic lupus erythematosus. Expert Opin Drug Saf. 2008;7(4):411–9. doi: 10.1517/14740338.7.4.411. [DOI] [PubMed] [Google Scholar]
- 30.Charles PJ, et al. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: findings in open-label and randomized placebo-controlled trials. Arthritis Rheum. 2000;43(11):2383–90. doi: 10.1002/1529-0131(200011)43:11<2383::AID-ANR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.De Bandt M, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7(3):R545–51. doi: 10.1186/ar1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkatesh D, et al. Endothelial TNF receptor 2 induces IRF1 transcription factor-dependent interferon-beta autocrine signaling to promote monocyte recruitment. Immunity. 2013;38(5):1025–37. doi: 10.1016/j.immuni.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vielhauer V, Stavrakis G, Mayadas TN. Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest. 2005;115(5):1199–209. doi: 10.1172/JCI23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheller J, et al. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32(8):380–7. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Al-Lamki RS, et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J. 2005;19(12):1637–45. doi: 10.1096/fj.05-3841com. [DOI] [PubMed] [Google Scholar]
- 36.Taubitz A, et al. Distinct contributions of TNF receptor 1 and 2 to TNF-induced glomerular inflammation in mice. PLoS One. 2013;8(7):e68167. doi: 10.1371/journal.pone.0068167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santee SM, Owen-Schaub LB. Human tumor necrosis factor receptor p75/80 (CD120b) gene structure and promoter characterization. J Biol Chem. 1996;271(35):21151–9. doi: 10.1074/jbc.271.35.21151. [DOI] [PubMed] [Google Scholar]
- 38.Debes GF, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6(9):889–94. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6(9):895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 40.Hopken UE, et al. CCR7 regulates lymphocyte egress and recirculation through body cavities. J Leukoc Biol. 2010;87(4):671–82. doi: 10.1189/jlb.0709505. [DOI] [PubMed] [Google Scholar]
- 41.Herter JM, et al. AKAP9 regulates activation-induced T lymphocyte retention at sites of inflammation. Nature Communications. 2015 doi: 10.1038/ncomms10182. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 43.Mayadas TN, et al. Neutrophils: game changers in glomerulonephritis? Trends Mol Med. 2010;16(8):368–78. doi: 10.1016/j.molmed.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan MJ. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol. 2011;7(12):691–9. doi: 10.1038/nrrheum.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187(1):538–52. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camussi G, et al. The polymorphonuclear neutrophil (PMN) immunohistological technique: detection of immune complexes bound to the PMN membrane in acute poststreptococcal and lupus nephritis. Clin Nephrol. 1980;14(6):280–7. [PubMed] [Google Scholar]
- 47.Hakkim A, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–8. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosetti F, et al. Human lupus serum induces neutrophil-mediated organ damage in mice that is enabled by Mac-1 deficiency. J Immunol. 2012;189(7):3714–23. doi: 10.4049/jimmunol.1201594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teramoto K, et al. Microarray analysis of glomerular gene expression in murine lupus nephritis. J Pharmacol Sci. 2008;106(1):56–67. doi: 10.1254/jphs.fp0071337. [DOI] [PubMed] [Google Scholar]
- 50.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29(11):1334–42. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- 52.Denny MF, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184(6):3284–97. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kleinewietfeld M, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–22. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–7. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safa K, et al. Salt Accelerates Allograft Rejection through Serum- and Glucocorticoid-Regulated Kinase-1-Dependent Inhibition of Regulatory T Cells. J Am Soc Nephrol. 2015;26(10):2341–7. doi: 10.1681/ASN.2014090914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zewinger S, et al. Innate immunity in CKD-associated vascular diseases. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv358. [DOI] [PubMed] [Google Scholar]
- 57.Kurts C, et al. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13(10):738–53. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 58.Adesso S, et al. The uremic toxin indoxyl sulphate enhances macrophage response to LPS. PLoS One. 2013;8(9):e76778. doi: 10.1371/journal.pone.0076778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mansouri L, et al. Leukocyte proliferation and immune modulator production in patients with chronic kidney disease. PLoS One. 2013;8(8):e73141. doi: 10.1371/journal.pone.0073141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Chaqmaqchi HA, et al. Activation of Wnt/beta-catenin pathway in monocytes derived from chronic kidney disease patients. PLoS One. 2013;8(7):e68937. doi: 10.1371/journal.pone.0068937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hevia A, et al. Intestinal dysbiosis associated with systemic lupus erythematosus. MBio. 2014;5(5):e01548–14. doi: 10.1128/mBio.01548-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Angelis M, et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN) PLoS One. 2014;9(6):e99006. doi: 10.1371/journal.pone.0099006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCarthy DD, et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest. 2011;121(10):3991–4002. doi: 10.1172/JCI45563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diehl GE, et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494(7435):116–20. doi: 10.1038/nature11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiryluk K, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46(11):1187–96. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee H, et al. CD4+CD25+ regulatory T cells attenuate cisplatin-induced nephrotoxicity in mice. Kidney Int. 2010;78(11):1100–9. doi: 10.1038/ki.2010.139. [DOI] [PubMed] [Google Scholar]
- 67.Ostmann A, et al. Regulatory T cell-derived IL-10 ameliorates crescentic GN. J Am Soc Nephrol. 2013;24(6):930–42. doi: 10.1681/ASN.2012070684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner JE, et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol. 2010;21(6):974–85. doi: 10.1681/ASN.2009070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eller K, et al. CCR7 deficiency exacerbates injury in acute nephritis due to aberrant localization of regulatory T cells. J Am Soc Nephrol. 2010;21(1):42–52. doi: 10.1681/ASN.2009020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ooi JD, et al. Endogenous foxp3(+) T-regulatory cells suppress anti-glomerular basement membrane nephritis. Kidney Int. 2011;79(9):977–86. doi: 10.1038/ki.2010.541. [DOI] [PubMed] [Google Scholar]
- 71.Kluger MA, et al. Stat3 programs Th17-specific regulatory T cells to control GN. J Am Soc Nephrol. 2014;25(6):1291–302. doi: 10.1681/ASN.2013080904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paust HJ, et al. Regulatory T cells control the Th1 immune response in murine crescentic glomerulonephritis. Kidney Int. 2011;80(2):154–64. doi: 10.1038/ki.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan DS, et al. Thymic deletion and regulatory T cells prevent antimyeloperoxidase GN. J Am Soc Nephrol. 2013;24(4):573–85. doi: 10.1681/ASN.2012090898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gandolfo MT, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76(7):717–29. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 75.Kinsey GR, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20(8):1744–53. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kinsey GR, et al. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77(9):771–80. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolf D, et al. CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol. 2005;16(5):1360–70. doi: 10.1681/ASN.2004100837. [DOI] [PubMed] [Google Scholar]
- 78.Chow Z, Banerjee A, Hickey MJ. Controlling the fire--tissue-specific mechanisms of effector regulatory T-cell homing. Immunol Cell Biol. 2015;93(4):355–63. doi: 10.1038/icb.2014.117. [DOI] [PubMed] [Google Scholar]
- 79.Yoshimura J, et al. Interstitial Foxp3-positive T cells may predict renal survival in patients with myeroperoxidase anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Clin Exp Pharmacol Physiol. 2010;37(9):879–83. doi: 10.1111/j.1440-1681.2010.05412.x. [DOI] [PubMed] [Google Scholar]
- 80.Valencia X, et al. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178(4):2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 81.Xing Q, et al. Elevated Th17 cells are accompanied by FoxP3+ Treg cells decrease in patients with lupus nephritis. Rheumatol Int. 2012;32(4):949–58. doi: 10.1007/s00296-010-1771-0. [DOI] [PubMed] [Google Scholar]
- 82.Afeltra A, et al. The involvement of T regulatory lymphocytes in a cohort of lupus nephritis patients: a pilot study. Intern Emerg Med. 2015;10(6):677–83. doi: 10.1007/s11739-015-1212-x. [DOI] [PubMed] [Google Scholar]
- 83.Comte D, Karampetsou MP, Tsokos GC. T cells as a therapeutic target in SLE. Lupus. 2015;24(4-5):351–63. doi: 10.1177/0961203314556139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crook KR. Role of myeloid-derived suppressor cells in autoimmune disease. World Journal of Immunology. 2014;4(1):26. doi: 10.5411/wji.v4.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Slaney CY, et al. Naive blood monocytes suppress T-cell function. A possible mechanism for protection from autoimmunity. Immunol Cell Biol. 2011;89(1):7–13. doi: 10.1038/icb.2010.110. [DOI] [PubMed] [Google Scholar]
- 87.Dugast AS, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180(12):7898–906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 88.Luan Y, et al. Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4(+) Foxp3(+) Treg expansion. Am J Transplant. 2013;13(12):3123–31. doi: 10.1111/ajt.12461. [DOI] [PubMed] [Google Scholar]
- 89.Li L, et al. Role of Myeloid-Derived Suppressor Cells in Glucocorticoid-Mediated Amelioration of FSGS. J Am Soc Nephrol. 2015;26(9):2183–97. doi: 10.1681/ASN.2014050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Auffray C, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–70. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 91.Carlin LM, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153(2):362–75. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peng Y, Latchman Y, Elkon KB. Ly6C(low) monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. J Immunol. 2009;182(5):2777–85. doi: 10.4049/jimmunol.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cros J, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33(3):375–86. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chousterman BG, et al. Ly6Chigh Monocytes Protect against Kidney Damage during Sepsis via a CX3CR1-Dependent Adhesion Mechanism. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hochheiser K, et al. Exclusive CX3CR1 dependence of kidney DCs impacts glomerulonephritis progression. J Clin Invest. 2013;123(10):4242–54. doi: 10.1172/JCI70143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Okui T, et al. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J Dent Res. 2012;91(6):574–9. doi: 10.1177/0022034512446341. [DOI] [PubMed] [Google Scholar]
- 97.Koenen HJ, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112(6):2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 98.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bovenschen HJ, et al. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131(9):1853–60. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- 101.Evans HG, et al. TNFa blockade induces IL-10 expression in human CD4+ T cells. Nature Communications. 2014;5:1–12. doi: 10.1038/ncomms4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Voo KS, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106(12):4793–8. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kluger MA, et al. RORgammat+Foxp3+ Cells are an Independent Bifunctional Regulatory T Cell Lineage and Mediate Crescentic GN. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hogan JJ, Markowitz GS, Radhakrishnan J. Drug-induced glomerular disease: immune-mediated injury. Clin J Am Soc Nephrol. 2015;10(7):1300–10. doi: 10.2215/CJN.01910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramos-Casals M, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86(4):242–51. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 106.Smolen JS, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 107.Luig M, et al. Inflammation-Induced IL-6 Functions as a Natural Brake on Macrophages and Limits GN. J Am Soc Nephrol. 2015;26(7):1597–607. doi: 10.1681/ASN.2014060620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wendling D, et al. Psoriasis onset with tocilizumab treatment for rheumatoid arthritis. J Rheumatol. 2012;39(3):657. doi: 10.3899/jrheum.111166. [DOI] [PubMed] [Google Scholar]
- 109.Matsuo Y, et al. Tocilizumab-induced immune complex glomerulonephritis in a patient with rheumatoid arthritis. Rheumatology (Oxford) 2013;52(7):1341–3. doi: 10.1093/rheumatology/kes403. [DOI] [PubMed] [Google Scholar]
- 110.Schmidt T, et al. Function of the Th17/interleukin-17A immune response in murine lupus nephritis. Arthritis Rheumatol. 2015;67(2):475–87. doi: 10.1002/art.38955. [DOI] [PubMed] [Google Scholar]
- 111.Scalapino KJ, et al. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177(3):1451–9. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 112.Lai LW, Yong KC, Lien YH. Pharmacologic recruitment of regulatory T cells as a therapy for ischemic acute kidney injury. Kidney Int. 2012;81(10):983–92. doi: 10.1038/ki.2011.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Braza F, et al. Regulatory T Cells in Kidney Transplantation: New Directions? Am J Transplant. 2015;15(9):2288–300. doi: 10.1111/ajt.13395. [DOI] [PubMed] [Google Scholar]
- 114.Gosselin D, et al. Environment Drives Selection and Function of Enhancers Controlling Tissue-Specific Macrophage Identities. Cell. 2014;159(6):1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ko GJ, et al. Transcriptional analysis of infiltrating T cells in kidney ischemia-reperfusion injury reveals a pathophysiological role for CCR5. Am J Physiol Renal Physiol. 2012;302(6):F762–73. doi: 10.1152/ajprenal.00335.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]