Abstract
Introduction
The National HIV & Tuberculosis Health Care Worker (HCW) Hotline provides advice on the management of suspected adverse drug reactions (ADRs). We describe suspected ADRs reported to the hotline by HCWs, concordance with advice, and patient outcomes.
Methods
We reviewed suspected ADRs in HIV-infected patients, patients taking antiretrovirals and patients taking anti-tuberculosis therapy reported from May 2013 to October 2014. We performed causality assessment using the World Health Organization Uppsala Monitoring Centre (WHO-UMC) criteria. We included suspected ADRs categorized as certain, probable or possible in further analysis.
Results
We received 772 ADR reports, of which 87/772 (11.3 %) were classified as certain, 176/772 (22.8 %) as probable, 361/772 (46.8 %) as possible, and 148/772 (19.2 %) as unlikely or unassessable. The most frequent ADRs were rash, drug-induced liver injury (DILI) and kidney injury, comprising 110/624 (17.6 %), 87/624 (13.9 %), and 77/624 (12.3 %), respectively. The ADR was severe in 27.3 % of rashes, 36.4 % of kidney injury reports and 88.5 % of DILI reports. Most frequently implicated drugs, either alone or in combination with other potentially causative drugs, were efavirenz (rashes), efavirenz and anti-tuberculosis drugs (DILI) and tenofovir (kidney injury). In 383 cases with HCW follow-up, 254 (66.3 %) improved, 9 (2.3 %) had complete resolution, 32 (8.4 %) remained unchanged, 6 (1.6 %) deteriorated, 10 (2.6 %) died and 72 (18.8 %) had unknown outcome. Advice provided was followed in 93.2 % of these cases. Of 223 ADRs with preventability data, 40 (17.9 %) were preventable.
Conclusion
Queries about rashes, DILIs and kidney injuries were common. Detection and management of these ADRs should be included in HCW training. In cases with follow-up, concordance with advice was high, and HCWs reported improvement in the majority.
1 Introduction
Medicine information centres (MICs) support pharmacovigilance activities by providing information that aids in assessing, managing and reporting adverse drug reactions (ADRs) [1, 2]. ADRs account for a large proportion of MIC queries, ranging from 14 to 42 % [3–6]. The MIC at the University of Cape Town, South Africa, an independent drug information centre, was established in 1980. In 2008, a national HIV health care worker (HCW) hotline was established within the MIC to support the public sector antiretroviral roll out in South Africa. The hotline service was expanded to include tuberculosis in 2012. The National HIV & Tuberculosis HCW Hotline supports the clinical management of people living with HIV and/or tuberculosis by answering telephonic queries about HIV and tuberculosis-related topics, including diagnosis, clinical management, laboratory and clinical monitoring, post-exposure prophylaxis and adverse drug reactions. Queries come from a range of HCWs, predominantly doctors, nurses and pharmacists. Queries are handled by the MIC team of drug information pharmacists, supported by an established network of experienced clinicians. The details of the hotline have been described previously [7].
Previous descriptions of HIV hotline services do not provide details of reported ADRs, causality, severity, preventability, implicated drugs or patient outcomes [7–11]. In addition, literature from resource-limited settings has been restricted to general descriptions of queries received by MICs [6, 12, 13]. Analysis of ADR queries received by the HIV & Tuberculosis HCW Hotline could identify common ADRs, which can be used to select priority topics for HCW training, to inform treatment guidelines and improve patient safety and clinical outcomes.
We analysed ADR queries received by the HIV & Tuberculosis HCW Hotline. Our objectives were (1) to describe the pattern, causality, severity and preventability of ADRs; (2) to identify commonly implicated drugs; (3) to determine HCW concordance with advice given; and (4) to report on patient outcomes.
2 Methods
2.1 Setting
In this study, we analysed ADR queries received by the National HIV & Tuberculosis HCW Hotline at the MIC, located in the Division of Clinical Pharmacology, University of Cape Town, South Africa. The MIC receives calls from both the private and the public healthcare sector.
2.2 Study Design and Population
We included all queries received by the HIV & Tuberculosis HCW Hotline about suspected ADRs in HIV-infected patients, and/or patients taking antiretrovirals and/or anti-tuberculosis therapy during the first 18 months (1 May 2013 to 31 October 2014) of implementing an enhanced HIV and tuberculosis ADR surveillance system within the hotline. HIV and tuberculosis queries were received by a team of drug information pharmacists supported by a team of clinicians with expertise in HIV and tuberculosis management. A dedicated information pharmacist contacted reporting HCWs telephonically for follow-up information on the ADRs. Data required for preventability assessment was included in ADR follow up from 1 June 2014.
2.3 Data Collection
We prospectively collected patient demographics, drug history, details of the suspected ADR and advice provided by the HIV & Tuberculosis HCW Hotline at the time of the initial query. We collected data regarding the HCW's actions and the patient's outcome during a follow-up call, approximately 1 month after the initial query. Both baseline and follow-up data were collected using standardized data collection forms (see Electronic Supplementary Material 1). A database of all HIV and tuberculosis-specific queries is maintained.
We performed causality assessment using World Health Organization Uppsala Monitoring Centre (WHO-UMC) causality criteria [14], and included ADRs classified as certain, probable or possible in our analysis. ADR severity was determined using the Division of AIDS (DAIDS) classification system [15]. We categorized ADR severity into one of two categories: mild/moderate if the ADR was graded 1 or 2 or severe if the ADR was graded 3 or 4. We used a dual severity categorization to reflect drug discontinuations: grades 1 and 2 generally do not require drug discontinuations, whilst grades 3 and 4 required drug discontinuations in most cases. We assessed preventability using a modified version of the Schumock and Thornton criteria [16] in a subset of queries from 1 June 2014 onwards, when preventability assessment questions were incorporated in the standardized data capture forms. The modification was to split the first Schumock and Thornton question into two, to distinguish between inappropriate initiation and inappropriate continuation of a drug. Preventability categories used were as follows: drug inappropriately initiated, drug inappropriately continued, history of allergy to drug, inappropriate dose/route of administration, laboratory monitoring not performed, compliance contributed to ADR, drug interaction involved, serum concentration above therapeutic range. We categorized the ADR as preventable if one or more of these categories was fulfilled. Causality, severity, and preventability assessments were performed by a pharmacist, who discussed reports with a clinical pharmacologist and/or a physician where necessary. When reports were assessed by more than one person, decisions were made by consensus.
2.4 Ethical Approval
The study was approved by the Faculty of Health Sciences Human Research Ethics Committee, University of Cape Town (UCT). The UCT Human Research Ethics Committee has Federal Wide Assurance (FWA) for the Protection of Human Subjects accreditation with the US Department of Health and Human Services.
2.5 Data Analysis
We used STATA version 13 for data analysis. We calculated frequencies and proportions to describe categorical data.
3 Results
We received 772 patient-specific queries concerning suspected ADRs involving 734 patients. This comprised 8.8 % of total calls (n = 8364) received by the National HIV & Tuberculosis Hotline between 1 May 2013 and 31 October 2014. The majority of ADR queries, 521/734 (71.0 %), were from doctors. 189/734 (25.7 %) ADR queries were from nurses, 13/734 (1.8 %) from pharmacists, and 2/734 (0.27 %) from other healthcare professionals. The profession of the caller was not documented in 9/734 queries (1.23 %). Most of the ADR queries, 626/734 (85.3 %) came from the public sector.
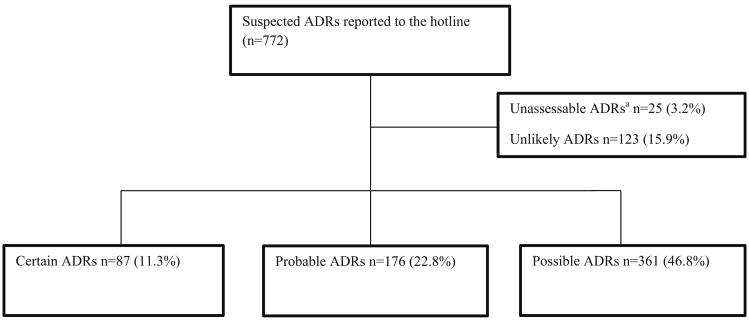
Using the WHO-UMC causality assessment categories, we classified the 772 suspected ADRs as shown in Fig. 1. We included the 624 ADRs categorized as certain, probable or possible, in 601 patients, in further analysis. 578/601 patients (96 %) had a single ADR reported, while 23/601 patients (3.8 %) each had two ADRs reported. There were a total of 950 drug suspects for the 624 ADRs categorized as certain, probable or possible: 440 (70.5 %) ADRs had one drug suspect, 143 (22.9 %) ADRs had two to three drug suspects and 41 (6.6 %) ADRs had four or more drug suspects.
Fig. 1.
Flow diagram of suspected adverse drug reactions (ADRs) received by the South African National HIV & Tuberculosis Health Care Worker Hotline, May 2013–October 2014. aReports were categorized as unassessable when causality could not be assessed because of insufficient or contradictory information, and the information could not be supplemented or verified
3.1 Patient Characteristics
The characteristics of the 601 patients with 624 ADRs are described in Table 1.
Table 1. Characteristics of patients with ADRs received by the South African National HIV & Tuberculosis Health Care Worker Hotline, May 2013– October 2014 (n = 601).
| Characteristic | Number of patients (%) (n = 601) |
|---|---|
| Age categories (years) | |
| <18 | 47 (7.8) |
| 18–34 | 187 (31.1) |
| 35–50 | 208 (34.6) |
| >50 | 52 (8.7) |
| Unknown | 107 (17.8) |
| Sex | |
| Female | 330 (54.9) |
| Male | 222 (36.9) |
| Unknown | 49 (8.2) |
| HIV status | |
| Seropositive | 576 (95.8) |
| Seronegative | 18 (3.0) |
| Unknown | 7 (1.2) |
| ART status | |
| On ART for treatment | 549 (91.3) |
| On nevirapine monotherapy as PMTCT | 3 (0.5) |
| On ART as PEP | 12 (2.0) |
| Not on ART | 37 (6.2) |
| Anti-tuberculosis treatment | |
| On anti-tuberculosis treatment | 141 (23.5) |
| On isoniazid prophylaxis | 42 (7.0) |
| Not on anti-tuberculosis treatment | 323 (53.7) |
| Unknown | 95 (15.8) |
| On ART and anti-tuberculosis treatment | 148 (24.6) |
| On co-trimoxazole | |
| Yes | 142 (23.6) |
| No | 306 (50.9) |
| Unknown | 153 (25.5) |
ADRs adverse drug reaction, ART antiretroviral therapy, PEP post-exposure prophylaxis, PMTCT prevention of mother-to-child transmission
In patients taking antiretroviral therapy (ART) for treatment of HIV infection (n = 549), nucleoside reverse transcriptase inhibitors (NRTIs) were combined with efavirenz in 411 (74.9 %), nevirapine in 24 (4.4 %) and ritonavir-boosted regimens in 113 (20.6 %).
The NRTI backbone was tenofovir and emtricitabine/lamivudine in 384 (69.9 %), zidovudine and lamivudine in 73 (13.3 %), stavudine and lamivudine in 42 (7.7 %) and abacavir and lamivudine in 38 (6.9 %). Less common ART backbones were zidovudine and didanosine (three patients), abacavir and zidovudine (one patient) and a triple-ART backbone consisting of zidovudine, tenofovir, and lamivudine (one patient).
3.2 Adverse Drug Reactions (ADRs) and Corresponding Drug Suspects
The four most common ADRs were rash in 110/624 (17.6 %), drug-induced liver injury (DILI) in 87/624 (13.9 %), kidney injury in 77/624 (12.3 %) and gynaecomastia in 66 (10.6 %) (Table 2). The severity grading of the three most common ADRs and implicated drugs are listed in Table 3.
Table 2. ADRs reported to the South African National HIV & Tuberculosis Health Care Worker Hotline and the corresponding drug suspects.
| Name of ADR | Number of ADR reports (%) (n = 624) | Drug suspectsa (n = 950) |
|---|---|---|
| Allergy/immunology | 24 (3.8) | |
| Allergic reaction/hypersensitivity | 11 (1.8) | EFV (7), ABC (3), TDF (2), co-trimoxazole (1), FTC (1), ritonavir (1), rifampicin (1), ampicillin (1) |
| Angioedema | 8 (1.3) | TDF (5), LPV/r (3), EFV (2), enalapril (1), 3TC (1), FTC (1), AZT (1), amlodipine (1) |
| Urticaria | 5 (0.8) | EFV (3), TDF (2), co-trimoxazole (1), 3TC (1), FTC (1), LPV/r (1), rifampicin (1), isoniazid (1) |
| Auditory | 4 (0.6) | |
| Hearing loss | 3 (0.5) | 3TC (2), D4T (1), kanamycin (1) |
| Vertigo | 1 (0.2) | Kanamycin (1), moxifloxacin (1), terizidone (1) |
| Cardiovascular | 2 (0.3) | |
| Left ventricular hypertrophy | 1 (0.2) | AZT (1) |
| Palpitations | 1 (0.2) | EFV (1) |
| Dermatology | 129 (20.7) | |
| Alopecia | 2 (0.3) | EFV (1), LPV/r (1) |
| Hyperpigmentation | 3 (0.5) | FTC (3) |
| Pruritus | 3 (0.5) | EFV (1), ethambutol (1), isoniazid (1), rifampicin (1), terizidone (1) |
| Rash | 110 (17.6) | EFV (74), co-trimoxazole (34), isoniazid (21), rifampicin (15), pyrazinamide (14), ethambutol (11), NVP (7), LPV/r (6), ABC (6), TDF (5), FTC (3), 3TC (2), moxifloxacin (2), AZT (1), ATV/r (1), captopril (1), dapsone (1), diclofenac (1), ethionamide (1), furosemide (1), griseofulvin (1), ibuprofen (1), streptomycin (1) |
| Rash with liver involvement | 8 (1.3) | NVP (5), co-trimoxazole (2), isoniazid (2), rifampicin (2), EFV (1), ethambutol (1), LPV/r (1), pyrazinamide (1) |
| Dermatology (other)b | 3 (0.5) | Co-trimoxazole (2), 3TC (1), AZT (1), penicillin (1), ibuprofen (1) |
| Endocrine/metabolic | 112 (17.9) | |
| Dyslipidaemia | 13 (2.1) | LPV/r (11), ATV/r (1), DDI (1), EFV (1) |
| Electrolyte disturbances | 2 (0.3) | Co-trimoxazole (2) |
| Gynaecomastia | 66 (10.6) | EFV (66), isoniazid (14) |
| Hyperlactataemia | 5 (0.8) | TDF (3), D4T (2), 3TC (1), FTC (1), metformin (1) |
| Lactic acidosis | 1 (0.2) | D4T (1) |
| Lipoatrophy | 22 (3.5) | AZT (11), D4T (11), TDF (2), DDI (1) |
| Lipohypertrophy | 3 (0.5) | D4T (1), EFV (2) |
| Gastrointestinal | 59 (9.5) | |
| Abdominal pain | 1 (0.2) | LPV/r (1) |
| Epigastric pain | 1 (0.2) | Amiodarone (2) |
| GIT intolerancec | 55 (8.8) | LPV/r (42), EFV (7), TDF (6), AZT (5), FTC (4), isoniazid (4), pyrazinamide (4), rifampicin (4), ABC (3), ethambutol (3), 3TC (1), doxycycline (1), ethionamide (1), HCTZ (1), indomethacin (1), moxifloxacin (1), omeprazole (1) |
| Pancreatitis | 1 (0.2) | D4T (1) |
| Taste alteration | 1 (0.2) | EFV (1) |
| Haematological | 32 (5.1) | |
| Anaemia | 17 (2.7) | AZT (16), co-trimoxazole (1), FTC (1) |
| Bicytopaenia | 2 (0.3) | AZT (2), EFV (1) |
| Neutropaenia | 6 (1.0) | AZT (6), co-trimoxazole (3), ofloxacin (1) |
| Pure red cell aplasia | 4 (0.6) | 3TC (4) |
| Thrombocytopaenia | 3 (0.5) | Co-trimoxazole (3), rifampicin (3), ceftriaxone (1), ibuprofen (1) |
| Hepatic | 93 (14.9) | |
| Hyperbilirubinaemia | 6 (1.0) | ATV/r (6), isoniazid (1) |
| Drug-induced liver injury | 87 (13.9) | EFV (37), isoniazid (34), rifampicin (32), co-trimoxazole (24), pyrazinamide (22), LPV/r (16), NVP (7), fluconazole (2), amitriptyline (1), fluoxetine (1), paracetamol (1), simvastatin (1) |
| Musculoskeletal/soft tissue | 6 (1.0) | |
| Arthralgia | 3 (0.5) | Pyrazinamide (3), levofloxacin (1), rifampicin (1), TDF (1) |
| Myalgia | 3 (0.5) | TDF (3), 3TC (1), FTC (1), rifampicin (1), pyrazinamide (1) |
| Neurology | 59 (9.5) | |
| Ataxia | 1 (0.2) | EFV (1) |
| Dizziness | 15 (2.4) | EFV (15), amitriptyline (1), HCTZ (1), nifedipine (1), omeprazole (1), perindopril (1) |
| Headaches | 7 (1.1) | TDF (6), EFV (2), AZT (1), 3TC (1) |
| Memory impairment | 2 (0.3) | EFV (1), isoniazid (1) |
| Multiple neurological effects | 6 (1.0) | EFV (5), AZT (1) |
| Peripheral neuropathy | 8 (1.3) | Isoniazid (3), D4T (2), EFV (2), FTC (2), TDF (2), co-trimoxazole (1), AZT (1) |
| Seizure | 1 (0.2) | EFV (1) |
| Sleep disturbances | 18 (2.9) | EFV (16), isoniazid (1), LPV/r (1) |
| Tremor | 1 (0.2) | Isoniazid (1) |
| Ocular/vision | 4 (0.6) | |
| Conjunctivitis | 1 (0.2) | Co-trimoxazole (1) |
| Vision deterioration | 3 (0.5) | Ethambutol (3), isoniazid (2) |
| Psychiatric | 19 (3.0) | |
| Psychosis | 19 (3.0) | EFV (18), isoniazid (1), moxifloxacin (1), NVP (1), terizidone (1) |
| Renal impairment | 77 (12.3) | |
| Kidney injury | 77 (12.3) | TDF (72), rifampicin (4), kanamycin (3), amphotericin B (1), HCTZ (1), LPV/r (1), streptomycin (1) |
| Sexual reproductive function | 1 (0.2) | |
| Erectile dysfunction | 1 (0.2) | EFV (1) |
| Other | 3 (0.5) | |
| Otherd | 3 (0.5) | D4T (1), EFV (1), FTC (1), isoniazid (1), rifampicin (1), TDF (1) |
ABC abacavir, ADR adverse drug reaction, ATV/r atazanavir/ritonavir, AZT zidovudine, D4T stavudine, DDI didanosine, EFV efavirenz, FTC emtricitabine, GIT gastrointestinal tract, HCTZ hydrochlorothiazide, LPV/r lopinavir/ritonavir, NVP nevirapine, TDF tenofovir, 3TC lamivudine
More than one drug suspect may be implicated for each ADR
Dermatology (other) includes one report each of skin warts, scleroderma and erythema nodosum
GIT intolerance includes nausea, vomiting, diarrhoea
Other includes one report each of fever, vaginal pain and weight loss
Table 3. ADR severity of the three commonest ADRs using DAIDS severity criteria and corresponding drug suspects.
| ADR | Grade 1/2 (%) | Drug suspectsa (number of times implicated/number of times recommended to stop) | Grade 3/4 (%) | Drug suspectsa (number of times implicated/number of times recommended to stop) |
|---|---|---|---|---|
| Rash (n = 110)b | 65 (59.1) | EFV (46/9), co-trimoxazole (17/11), isoniazid (12/6), pyrazinamide (7/3), rifampicin (5/2), ethambutol (5/2), ABC (5/2), atazanavir (1/1), captopril (1/0), emtricitabine (2/1), ethionamide (1/1), furosemide (1/0), lamivudine (2/1), LPV/r (4/2), moxifloxacin (2/0), streptomycin (1/1), tenofovir (2/0), AZT (1/1) | 30 (27.3) | EFV (21/20), co-trimoxazole (11/11), isoniazid (5/5), rifampicin (5/5), pyrazinamide (4/4), ethambutol (3/3), NVP (3/3), TDF (2/2), ABC (1/1), dapsone (1/1), emtricitabine (1/1), LPV/r (1/1) |
| Drug-induced liver injury (n = 87)c | 7 (8.0) | Co-trimoxazole (4/4), isoniazid (4/2), LPV/r (3/0), rifampicin (2/0), EFV (1/1), pyrazinamide (1/0), paracetamol (1/0) | 77 (88.5) | EFV (36/36), isoniazid (29/22), rifampicin (28/22), pyrazinamide (20/15), co-trimoxazole (19/17), LPV/r (13/5), NVP (7/7), fluconazole (2/2), amitryptyline (1/1), fluoxetine (1/1), simvastatin (1/1) |
| Kidney injury (n = 77)d | 45 (58.4) | TDF (44/39), amphotericin B (1/0), hydrocholorothiazide (1/0), kanamycin (1/0), streptomycin (1/1) | 28 (36.4) | TDF (25/25), kanamycin (2/2), rifampicin (4/4) |
ABC abacavir, ADR adverse drug reaction, AZT zidovudine, DAIDS Division of AIDS classification system, EFV efavirenz, LPV/r lopinavir/ritonavir, NVP nevirapine, TDF tenofovir
More than one drug suspect may be implicated for each ADR
Fifteen reports of skin rash could not be assessed with the DAIDS severity criteria
Three reports of drug-induced liver injury could not be assessed with the DAIDs severity criteria
Four reports of kidney injury could not be assessed with the DAIDS severity criteria
Efavirenz was implicated in 74/110 (67.3 %) reports of rashes, either alone or in combination with other potentially causative drugs: co-trimoxazole in 21 reports, anti-tuberculosis treatment (rifampicin, isoniazid, pyrazinamide or ethambutol) in 14 reports, tenofovir and/or emtricitabine in four reports, abacavir in three reports, and captopril/furosemide in one report each.
Efavirenz and anti-tuberculosis drugs (rifampicin, isoniazid or pyrazinamide) were the most commonly implicated drugs in DILI reports. Efavirenz was implicated in 37/87 (42.5 %) reports of DILI, either alone or in combination with other drugs: anti-tuberculosis treatment (rifampicin, isoniazid or pyrazinamide) in six reports, co-trimoxazole in nine reports, lopinavir/ritonavir in one report, simvastatin in one report and fluconazole in one report.
Tenofovir was implicated in 72/77 (93.5 %) reports of kidney injury, either alone or in combination with other drugs: rifampicin in two reports, amphotericin B, kanamycin, streptomycin and hydrochlorothiazide (in one report each).
Efavirenz was implicated in all 66 reports of gynaecomastia, either alone or in combination with isoniazid in 14 reports.
Mitochondrial toxicities (hyperlactataemia, lactic acidosis, lipoatrophy, and peripheral neuropathy) occurred less frequently, accounting for 36 (5.8 %) of the total ADRs. Stavudine was implicated in 16/36 (44.4 %) reports (Table 2).
3.3 Common Drug Suspects with Corresponding ADRs and Severity Assessment
Overall, efavirenz was the most commonly implicated drug suspect, implicated in 42.9 % (268/624) of ADRs reported (Table 4). In particular, efavirenz was associated with all reports of gynaecomastia (n = 66), 94.7 % (18/19) of reports of psychosis, 67.3 % (74/110) of reports of rash and 72.9 % (43/59) of reports of neurological ADRs.
Table 4. Most common drug suspects with corresponding ADRs, categorized by severity.
| Drug suspect | ADR | n | ADR severity grading | ||
|---|---|---|---|---|---|
|
|
|||||
| Grade 1 or 2 | Grade 3 or 4 | Unassessable | |||
| Efavirenz | 268 | ||||
| In 66/66 (100 %) reports of gynaecomastia | 66 | 1 | 3 | 62 | |
| In 18/19 (94.7 %) reports of psychosis | 18 | 0 | 0 | 18 | |
| In 43/59 (72.9 %) reports of neurological ADRsa | 43 | 0 | 3 | 40 | |
| In 74/110 (67.3 %) reports of rash | 74 | 46 | 21 | 7 | |
| In 37/87 (42.5 %) reports of drug-induced liver injury | 37 | 1 | 36 | 0 | |
| In 30 other ADRs | 30 | 5 | 8 | 17 | |
| Tenofovir | 110 | ||||
| In 72/77 (93.5 %) reports of kidney injury | 72 | 44 | 25 | 3 | |
| In 6/7 (85.7 %) reports of headache | 6 | 0 | 1 | 5 | |
| In 9/24 (37.5 %) reports of hypersensitivity reactionb | 9 | 2 | 5 | 2 | |
| In 5/110 (4.5 %) reports of rash | 5 | 2 | 2 | 1 | |
| In 18 other ADRs | 18 | 2 | 0 | 16 | |
| Isoniazid | 88 | ||||
| In 34/87 (39.1 %) reports of drug-induced liver injury | 34 | 4 | 29 | 1 | |
| In 14/66 (21.2 %) reports of gynaecomastia | 14 | 0 | 0 | 14 | |
| In 6/59 (10.2 %) reports of neurological ADRsa | 6 | 0 | 0 | 6 | |
| In 21/110 (19.1 %) reports of rash | 21 | 12 | 5 | 4 | |
| In 13 other ADRs | 13 | 0 | 5 | 8 | |
| Lopinavir/ritonavir | 84 | ||||
| In 11/13 (84.6 %) reports of dyslipidaemia | 11 | 1 | 10 | 0 | |
| In 42/55 (76.4 %) reports of GIT intolerance | 42 | 0 | 3 | 39 | |
| In 16/87 (18.4 %) reports of drug-induced liver injury | 16 | 3 | 13 | 0 | |
| In 6/110 (5.5 %) reports of rash | 6 | 4 | 1 | 1 | |
| In 9 other ADRs | 9 | 2 | 3 | 4 | |
| Co-trimoxazole | 75 | ||||
| In 3/3 (100 %) reports of thrombocytopaenia | 3 | 0 | 3 | 0 | |
| In 3/6 (50.0 %) reports of neutropaenia | 3 | 1 | 2 | 0 | |
| In 34/110 (30.9 %) reports of rash | 34 | 17 | 11 | 6 | |
| In 24/87 (27.6 %) reports of drug-induced liver injury | 24 | 4 | 19 | 1 | |
| In 11 other ADRs | 11 | 0 | 6 | 5 | |
ADR adverse drug reaction, GIT gastrointestinal tract
Includes reports of ataxia, dizziness, headache, memory impairment, multiple neurological effects, peripheral neuropathy, seizure, sleep disturbances, and tremor
Includes allergic reactions, angioedema and urticaria
3.4 Preventability of ADRs
This analysis is restricted to a sub-group of ADRs reported between 1 June 2014 (when preventability questions were incorporated into the standardized data collection forms) and 31 October 2014. We included a total of 223 ADRs in 211 patients. Forty (17.9 %) of the ADRs were preventable, and 183 (82.1 %) ADRs were not preventable. The top three preventable ADRs were DILI, kidney injury and rash, comprising 11 (27.5 %), 8 (20.0 %) and 3 (7.5 %) of the 40 preventable ADRs, respectively. Of the 11 DILI reports considered to be preventable, nine reports were also classified as severe/life threatening.
The most frequent reasons for preventability were continuation of drug therapy despite clinical features or laboratory markers of toxicity where drug therapy should have been stopped, inappropriate drug therapy and previous history of allergy or drug reaction (Table 5). The drug suspects associated with these preventability criteria are listed in Table 5.
Table 5. Preventable ADRs categorized using the modified Schumock and Thornton [16] preventability criteria (n = 40).
| Preventability criteria | Frequency (n = 51)a | Drug suspects (n = 51) |
|---|---|---|
| Drug continued despite symptoms and/or laboratory signs of toxicity | 22 | TDF (5), EFV (4), co-trimoxazole (3), rifampicin (3), other (7) |
| Drug inappropriate for patient's clinical condition | 12 | Co-trimoxazole (4), ATV/r (3), TDF (3), other (2) |
| History of allergy or previous reaction to the drug | 7 | EFV (4), co-trimoxazole (1), LPV/r (1), AZT (1) |
| Dose/route of administration not appropriate for patient | 3 | LPV/r (2), EFV (1) |
| Laboratory monitoring not performed | 3 | TDF (2), AZT (1) |
| Compliance contributed to the reaction | 3 | EFV (3) |
| Drug interaction involved | 1 | Amiodarone (1)—interaction with LPV/r |
| Serum concentration above the therapeutic range | 0 | N/A |
ADR adverse drug reaction, ATV/r atazanavir/ritonavir, AZT zidovudine, EFV efavirenz, LPV/r lopinavir/ritonavir, TDF tenofovir
More than one preventability criteria may be applicable to each preventable ADR
3.5 National HIV & Tuberculosis HCW Hotline Recommendations and HCW Actions Taken
There were 596 hotline recommendations and HCW actions taken corresponding to 596 drug suspects. Drug substitution or continuation of the suspected drug, with supportive care, were the most common recommendations and actions taken (Table 6).
Table 6. Hotline recommendations and HCW actions taken for each drug suspect (n = 596) in patients with follow-up (n = 383).
| Actions recommendedb | Actions takena | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Continue drugc | Decrease dose | Other actionsd | Stop and rechallenge | Stop and refer | Drug substitutione | Stop drug indefinitelyf | Unknown actions | Total | |
| Continue drugc | 133 (92 %) | 0 | 1 | 1 | 0 | 2 | 7 | 1 | 145 |
| Decrease dose | 2 | 10 (83 %) | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| Other actionsd | 5 | 0 | 32 (78 %) | 0 | 0 | 4 | 0 | 0 | 41 |
| Stop and rechallenge | 3 | 0 | 0 | 64 (93 %) | 0 | 2 | 0 | 0 | 69 |
| Stop and refer | 0 | 0 | 0 | 0 | 13 (100 %) | 0 | 0 | 0 | 13 |
| Drug substitutione | 15 | 0 | 3 | 4 | 0 | 203 (88 %) | 6 | 1 | 232 |
| Stop drug indefinitelyf | 3 | 0 | 0 | 1 | 0 | 1 | 76 (90 %) | 3 | 84 |
| Total | 161 | 10 | 36 | 70 | 13 | 212 | 89 | 5 | 596 |
Bold values indicate concordance between actions recommended by the hotline and actions taken by HCWs
ADR adverse drug reaction, HCW health care worker
More than one HCW action may have been taken per ADR
More than one recommendation may have been given per ADR
Continue drug with/without symptomatic treatment
Other drug actions (e.g. switch if test normal)
Including stop, cover tail, substitute
Stop drug indefinitely (including already stopped)
3.6 HCW Concordance with Advice and Patient Outcomes
We obtained follow-up information for 383 (63.7 %) patients. Of these, 275 (71.8 %) were managed as outpatients and 83 (21.7 %) as inpatients. Level of management was not recorded in 25 (6.5 %). Reasons for the MIC's inability to get follow-up information, where documented, included queries for which HCWs did not consent to be contacted for follow-up (n = 28), where HCWs moved to other facilities (n = 6), HCWs could not remember the patient about whom they had called (n = 3), or where the patient had been lost to follow-up (n = 3). In most cases, HCWs could simply not be reached for telephonic follow-up despite all our attempts (n = 78).
HCWs followed all advice given in 314/399 (78.7 %) ADRs, followed some advice in 58/399 (14.5 %) ADRs, and did not follow any advice in 24/399 (6.0 %) ADRs. Concordance could not be determined in three (0.8 %) ADRs, as in these cases, management of the ADR was unknown. At follow-up, patient outcomes for the ADR were mostly favourable, with 254 (66.3 %) patients recording improvement, nine (2.3 %) patients with complete resolution, six (1.6 %) patients with deterioration, 32 (8.4 %) were unchanged, 10 (2.6 %) died and 72 (18.8 %) patients had unknown status at follow-up.
3.7 Deaths
Ten patients with suspected ADRs died during the study period (drug suspects listed in brackets): five with acute kidney injury (tenofovir and rifampicin), three with DILI (efavirenz, co-trimoxazole and rifampicin) one with both DILI and acute kidney injury (tenofovir and rifampicin) and one with thrombocytopaenia (rifampicin). In three patients, the ADR was assessed as having contributed directly to the death: cholestatic DILI due to either rifampicin or co-trimoxazole, kidney injury due to tenofovir, and DILI with acute kidney injury due to rifampicin and/or tenofovir. In the patient with cholestatic DILI due to either rifampicin or co-trimoxazole, the hotline recommended rechallenge with rifampicin once the cholestasis had resolved. In this patient, liver injury recurred after rechallenge.
4 Discussion
Over an 18-month period, the MIC responded to 624 ADR-related queries from healthcare workers contacting the HIV/tuberculosis hotline. About one in six ADRs were considered preventable. The most common ADRs about which healthcare workers requested advice were rashes, DILI, and kidney injury, which correspond to the known ADR profiles of antiretrovirals and antituberculosis drugs used in standardized regimens in South Africa. About nine out of every ten reports of DILI, about one in three reports of kidney injuries, and about one in four reports of rash were rated as severe. Three patients had died with an ADR considered to have contributed directly to the death.
To our knowledge, this is the first study from a resource-limited setting that describes the pattern, severity, preventability, causality and outcomes of ADRs reported to an MIC in patients with HIV and/or tuberculosis. Patients being treated for HIV and/or tuberculosis usually take multiple drugs, with shared and sometimes additive or synergistic side effects. Causality assessment of ADRs with multiple drug suspects and overlap in presentation with underlying disease is challenging. Decisions regarding management of ADRs due to antiretrovirals and tuberculosis treatment are also challenging. Therapeutic options are often limited, and there is frequently an urgent need to treat the underlying disease. This results in consideration of rechallenge even when causality is likely. For example, in cases of DILI due to first-line antituberculosis treatment, patients frequently require rechallenge with rifampicin and isoniazid because of limited treatment options and inferior tuberculosis outcomes if rifampicin and isoniazid are not included in the treatment regimen [17, 18]. These challenges may be reflected by HCWs' use of the telephonic hotline service for assistance.
The majority of ADR queries came from doctors. This finding is similar to another HIV hotline service from a resource-limited setting [10]. The proportion of ADR queries coming from nurses (25.7 %) was approximately double that seen in an analysis of queries to our hotline service in 2011 [7], which may reflect both the scale-up of nurse initiation of ART in South Africa and the increasing awareness of our service among nurses. There are ongoing efforts to increase awareness of the hotline service, including monthly HCW newsletters and distribution of educational material about important HIV and TB-related topics.
Queries regarding rashes, DILI and kidney injury were the most frequent. Efavirenz was the most commonly implicated drug associated with rashes, efavirenz together with first-line anti-tuberculosis drugs (rifampicin, isoniazid or pyrazinamide) were the most frequent causes of DILI, and tenofovir was the most frequent cause of kidney injury. These findings reflect the known ADR profiles of antiretrovirals used in standardized regimens in the public sector treatment programme in South Africa [19] and the high burden of tuberculosis in this setting [20]. Conversely, queries regarding mitochondrial toxicities such as peripheral neuropathy, lipoatrophy, hyperlactataemia and lactic acidosis were infrequent, and most cases were caused by stavudine. Stavudine has largely been replaced by tenofovir following new recommendations in the 2010 South African adult ART guidelines, because of its toxicity [19].
The ADR was severe in the majority (88.5 %) of reports of DILI. Anti-tuberculosis treatment was implicated in 39.1 % of DILI reports, often in combination with other drugs. DILI due to anti-tuberculosis treatment was recently identified as the second most common cause of ADR-related deaths in medical wards in South Africa [21]. The frequency of queries regarding severe DILI highlights the need for training of HCWs in diagnosis and management of this ADR.
In the sub-group analysis, we found that continuation of drug therapy despite symptoms or laboratory markers of toxicity was the most frequent preventability criterion reported. This finding may be a reflection of lack of knowledge on the prompt recognition and appropriate management of ADRs, a result of overburdened HCWs failing to respond to laboratory results in a timely manner, or system failures resulting in HCWs not receiving results from the laboratory timeously. Each of these possibilities warrants further investigation in our setting.
HCW concordance with advice was high. This finding is comparable with studies from high-income countries, although these studies included ADR queries in addition to other types of queries (e.g. rational drug selection queries, questions about indications and contraindications, the administration or dose, drug interactions, queries about the availability or supply of drugs, drug identification queries, and non-clinical queries, such as drug costs or legal issues [4, 22, 23]).
Our study has limitations. First, it is an analysis of telephonic ADR queries with no denominator, and does not reflect ADR prevalence in South Africa. However, it gives insight into the ADRs that HCWs encounter that require assistance and additional information with respect to diagnosis and/or management. Second, we determined patient outcomes based on telephonic follow-up with HCWs and had no access to patient records to confirm outcomes. Third, drug exposure histories may not be complete, as they rely on information available to the HCW making the query. In particular, exposure to over-the-counter drugs and complementary and traditional remedies may not be completely captured and/or recorded. Fourth, we did not obtain complete follow-up data on all patients, which may have introduced bias in our observed follow-up analysis, as HCWs who could be contacted may have been more likely to adhere to our recommendations. Finally, we were only able to assess ADR severity and preventability in a sub-set of reports due to insufficient data, and we did not validate our modification of the preventability assessment tool.
5 Conclusion
Rashes, DILI and kidney injury were the most frequent ADRs about which HCWs sought advice from the National HIV & Tuberculosis HCW Hotline. Guidance on diagnosis and management of these ADRs should be prioritized for inclusion in HCW training. In cases with follow-up information, concordance with advice was good and HCWs reported improvement in the majority of patients. As the HIV treatment programme expands in South Africa, it will be essential to enhance the national hotline's outreach and strengthen its utility both for ADR surveillance and clinical decision support for HCWs. Furthermore, further studies are needed to assess the preventability of ADRs in a larger sample of patients.
Supplementary Material
Key Points.
The National HIV & Tuberculosis Health Care Worker Hotline identified rashes, drug-induced liver injuries (DILIs) and kidney injuries as the most frequently reported adverse drug reactions (ADRs). These ADRs should be included in ongoing healthcare worker training.
The management of ADRs resulted in favourable patient outcomes in the majority, highlighting the utility of the hotline service.
Acknowledgments
The authors would like to acknowledge all the drug information pharmacists at the Medicines Information Centre who were involved in the data collection process.
Funding: This research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the US Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement Number GGH000371.
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s40264-015-0359-8) contains supplementary material, which is available to authorized users.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Compliance with Ethical Standards: Conflicts of interest Christine Njuguna, Annemie Stewart, Johannes Mouton, Marc Blockman, Gary Maartens, Annoesjka Swart, Briony Chisholm, Jackie Jones, Mukesh Dheda, Ehimario Igumbor and Karen Cohen have no conflicts of interest that are directly relevant to the content of this study.
Ethical approval The study was approved by the Faculty of Health Sciences Human Research Ethics Committee, University of Cape Town (UCT) and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. The UCT Human Research Ethics Committee has Federal Wide Assurance (FWA) for the Protection of Human Subjects accreditation with the US Department of Health and Human Services.
References
- 1.Medicines Information Centre [Internet] [Cited 20 Aug 2015]; Available from: http://www.mic.uct.ac.za/
- 2.Müllerová H, Vlcek J. European drug information centres—survey of activities. Pharm World Sci. 1998;20(3):131–5. doi: 10.1023/a:1008672311674. [DOI] [PubMed] [Google Scholar]
- 3.Stubbington C, Bowey J, Hands D, Brown D. Drug information replies to queries involving adverse events: impact on clinical practice. Hosp Pharm. 1998;5(3):81–4. [Google Scholar]
- 4.McEntee JE, Henderson SL, Rutter PM, Rutter J, Davis HJ. Utility and value of a medicines information service provided by pharmacists: a survey of health professionals. Int J Pharm Pract. 2010;18(6):353–61. doi: 10.1111/j.2042-7174.2010.00068.x. [DOI] [PubMed] [Google Scholar]
- 5.Jimmy B, Jose J, Rao PG. Short communication: pattern of adverse drug reaction related queries received by the drug information centre of a tertiary care teaching hospital. Pak J Pharm Sci. 2007;20(4):333–9. [PubMed] [Google Scholar]
- 6.Palaian S, Mishra P, Shankar PR, Bista D, Purwar B. Contribution of the regional drug information center towards drug safety. JNMA J Nepal Med Assoc. 2006;45(161):216–8. [PubMed] [Google Scholar]
- 7.Chisholm BS, Cohen K, Blockman M, Kinkel HF, Kredo TJ, Swart AM. The impact of the National HIV Health Care Worker Hotline on patient care in South Africa. AIDS Res Ther. 2011;8(1):4. doi: 10.1186/1742-6405-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang LW, Kagaayi J, Nakigozi G, Galiwango R, Mulamba J, Ludigo J, et al. Telecommunications and health care: an HIV/AIDS warmline for communication and consultation in Rakai, Uganda. J Int Assoc Physicians AIDS Care (Chic) 2008;7(3):130–2. doi: 10.1177/1545109708318525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldschmidt RH, Graves DW. The National HIV Telephone Consultation Service (Warmline): a clinical resource for physicians caring for African–Americans. J Natl Med Assoc. 2003;95(2 Suppl 2):8S–11S. [PMC free article] [PubMed] [Google Scholar]
- 10.Karari C, Tittle R, Penner J, Kulzer J, Bukusi EA, Marima R, et al. Evaluating the uptake, acceptability, and effectiveness of Uliza! clinicians' HIV hotline: a telephone consultation service in Kenya. Telemed J E Health. 2011;17(6):420–6. doi: 10.1089/tmj.2010.0220. [DOI] [PubMed] [Google Scholar]
- 11.Swart AM, Chisholm BS, Cohen K, Workman L, Cameron D, Blockman M. Analysis of queries from nurses to the South African National HIV & TB Health Care Worker Hotline. S Afr J HIV Med. 2013;14(4):179–82. [Google Scholar]
- 12.Ball DE, Tagwireyi D, Maponga CC. Drug information in Zimbabwe: 1990–1999. Pharm World Sci. 2007;29(3):131–6. doi: 10.1007/s11096-007-9110-6. [DOI] [PubMed] [Google Scholar]
- 13.Tumwikirize AW, Ogwal-Okeng JW, Vernby A, Anokbonggo WW, Gustafsson LL, Lundborg CS. Use of a pilot drug information centre. Afr Health Sci. 2011;11(3):493–8. [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organisation. The use of the WHO-UMC system for standardized case causality assessment [Internet] [Cited 20 Aug 2015];The Uppsala Monitoring Centre. Available from: http://who-umc.org/Graphics/24734.pdf.
- 15.US Department of Health and Human Services. Division of AIDS table for grading the severity of adult and pediatric adverse events, Version 1.0. [Updated August 2009] [Cited 20 Aug 2015]; Available from: http://rsc-beta.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf.
- 16.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27(6):538. [PubMed] [Google Scholar]
- 17.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: Hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174(8):935–52. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 18.Sharma SK, Singla R, Sarda P, Mohan A, Makharia G, Jayaswal A, et al. Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin Infect Dis. 2010;50(6):833–9. doi: 10.1086/650576. [DOI] [PubMed] [Google Scholar]
- 19.Department of Health. The South African Antiretroviral Treatment Guidelines. Pretoria: Department of Health; 2013. [Google Scholar]
- 20.World Health Organisation. Global tuberculosis report. Geneva: World Health Organisation; 2013. [Google Scholar]
- 21.Mouton JP, Mehta U, Parrish AG, Wilson DPK, Stewart A, Njuguna CW, et al. Mortality from adverse drug reactions in adult medical inpatients at four hospitals in South Africa: a cross-sectional survey. Br J Clin Pharm. 2015;80(4):818–26. doi: 10.1111/bcp.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melnyk PS, Shevchuk YM, Remillard AJ. Impact of the dial access drug information service on patient outcome. Ann Pharmacother. 2000;34(5):585–92. doi: 10.1345/aph.19173. [DOI] [PubMed] [Google Scholar]
- 23.Repchinsky CA, Masuhara EJ. Quality assurance program for a drug information center. Drug Intell Clin Pharm. 1987;21(10):816–20. doi: 10.1177/106002808702101011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.