Abstract
Objective
Childhood parental divorce is associated with an increased risk of behavioral and physical health problems. Alterations in adrenocortical activity may be a mechanism in this relation. Parent-child relationships have been linked to cortisol regulation in children exposed to adversity, but prospective research is lacking. We examined maternal warmth in adolescence as a predictor of young adults’ cortisol stress response 15 years after parental divorce.
Methods
Participants included 240 youth from recently divorced families. Mother and child reports of maternal warmth were assessed at 6 time points across childhood, adolescence, and young adulthood. Offspring salivary cortisol was measured in young adulthood before and after a social stress task. Structural equation modeling was used to predict cortisol response from maternal warmth across early and late adolescence.
Results
Higher child-reported maternal warmth in early adolescence predicted higher child-reported maternal warmth in late adolescence (std. regression = .45, SE = .065, p < .01), which predicted lower cortisol response to a challenging interpersonal task in young adulthood (std. regression = −.20, SE = .094, p = .031). Neither mother-reported warmth in early adolescence nor late adolescence was significantly related to offspring cortisol response in young adulthood.
Conclusions
Results suggest that for children from divorced families, a warm mother-child relationship post-divorce and across development, as perceived by the child, may promote efficient biological regulation later in life.
Keywords: parental divorce, cortisol, mother-child relationship, warmth
Mounting evidence demonstrates that adverse experiences early in life are associated with a number of health problems later in life. Stress that occurs during a developmentally sensitive period is theorized to promote dysregulation of biological stress responses systems, increasing vulnerability to stress-related illnesses during childhood and across the lifespan. The development and functioning of the neuroendocrine system is commonly posited as a mechanism by which childhood adversity can affect long-term health. For example, animal studies demonstrate that early life stress associated with low maternal care can alter hippocampal gene expression and result in exaggerated hypothalamic-pituitary-adrenal (HPA) reactivity to later stress (1). Dysregulation of components of the HPA axis, in particular the stress hormone cortisol, has been identified in children, adolescents, and adults exposed to childhood adversity (2–4) and can be evident in the form of exaggerated or attenuated reactivity to challenge, higher or lower basal levels, or flattened diurnal slopes (5–6). It has been proposed that severe adversity sustained across development may initially be associated with hypersecretion that downregulates over time to result in hyposecretion (5,7). Dysregulated HPA activity has been linked to a wide range of poor mental and physical health outcomes in childhood and later in life, including depression, anxiety disorders, inflammatory disorders, coronary heart disease, hypertension, and substance abuse (8–14).
Although the health risks of childhood adversity are well-documented, there are considerable individual differences in consequences, with many children demonstrating better-than-expected outcomes. Theory and empirical data consistently indicate that a supportive, warm relationship with the primary caregiver promotes psychosocial adaptation and may prevent or reduce hormonal dysregulation associated with adversity (2,9,15). Concurrent and short-term associations have been found between positive parenting and cortisol responses to stress in infancy, childhood, and adolescence (16–20). Retrospective studies indicate that a positive parent-child relationship may have a lasting protective effect. Maternal warmth predicts lower biological risk in adulthood following exposure to significant childhood adversities including maltreatment, poverty, and parental loss (9, 21–23). For example, Luecken (23) found that retrospectively reported warmth from the surviving parent buffered cortisol stress responses in young adults who experienced childhood parental loss. Retrospective reports of parental warmth and in childhood also predicted lower allostatic load in adults abused as children (24), and lower risk of metabolic syndrome at midlife (25).
Few prospective studies have examined whether the neuroendocrine benefits of positive parenting are observed at later developmental stages. In a small sample of predominately Caucasian, middle- or high-income families, Kuhlman et al. (26) found that mother-reported warmth when children were 5 years old predicted a lower child cortisol stress response at age 7. In contrast, a recent investigation by Hackman and colleagues (27) with a small sample of low-income African-American families found that low levels of parental responsivity, assessed when children were 4 years old, predicted blunted cortisol reactivity (increase from baseline) to a social stress task in late adolescence. In a sample of parentally-bereaved children (age 8–16), Hagan et al. (28) found that mother- and child-reported positive parenting in the first 2 years after the death moderated the impact of recent stressful events on cortisol output six years later: among youth with a history of low positive parenting, recent negative life events were associated with elevated cortisol. Despite small sample sizes, attrition, and/or the measurement of parenting at a single time point, these studies nonetheless suggest the potential for caregiver warmth in childhood to impact neuroendocrine regulation at a subsequent developmental stage.
Parental divorce is a stressful and emotionally challenging process for many affected children, who may experience a number of secondary adversities including economic stress, relocation, disrupted parenting, and conflict between parents and among extended family members. Although most children adapt well after parental divorce (29), and some even show improved well-being (30), numerous studies document that children from divorced families are at higher short-and long-term risk of internalizing and externalizing problems, poor health behaviors such as smoking and alcohol abuse, general health problems, and decreased longevity (29–34). Roustit et al. (35) reported that the relation of childhood parental divorce to poor self-rated health in adulthood was mediated by the quality of parent-child relationships. The wide-ranging stressors associated with parental divorce may make children particularly vulnerable to negative long-term biological consequences. Yet, stress response system functioning in children who experienced parental divorce has received little research attention relative to other adversities. Lower HPA stress responses have been documented in young adults from divorced families relative to those from two-parent families (36–37). Although not specific to parental divorce, studies have also noted exaggerated or attenuated patterns of cortisol activity following adversities commonly associated with parental divorce, including poor marital functioning (38), interparental conflict (39–41), poor parent-child relationships (42), and maternal depression (43).
There are a number of limitations in existing research that we sought to address in the current study. First, because much of the research on the long-term impact of childhood adversity has relied on retrospective reports of parenting and adversity, it is not known whether later biological indices are affected more by current perceptions or perceptions during childhood. Prospective longitudinal studies in which parental warmth is assessed at multiple time points during childhood as well as in adulthood, in a sample with documented childhood adversity, are critical for addressing this limitation. Second, existing longitudinal studies with children at risk due to a variety of adversities have not examined prospective relations between parental warmth and cortisol regulation over prolonged periods of time (e.g., into adulthood). Third, theoretical and empirical accounts suggest that parental divorce may pose a risk to developing biological regulation, and positive mother-child relationships are consistently associated with improved child psychological adjustment post-divorce (44), but research has not yet prospectively evaluated maternal warmth as a predictor of cortisol regulation in children from divorced families. Finally, studies of the impact of parental warmth on children’s health primarily rely on caregiver reports, while retrospective research in adulthood primarily relies on offspring ratings. A better understanding of the process may be achieved by separately examining child and caregiver reports of caregiver warmth.
The current study addresses these limitations with a prospective, longitudinal study that followed mothers and children at six time points across 15 years after parental divorce. Both mother and child reports of maternal warmth were assessed at each time point, and offspring cortisol response to a challenging speech task was assessed at the final time point (offspring age 23–27). We hypothesized that higher maternal warmth during childhood and adolescence would predict lower cortisol response to the task in adulthood. Because parenting quality during childhood has been shown to exert a unique impact on young adult functioning above and beyond parenting in young adulthood (45), we predicted that the effect of maternal warmth in childhood would be independent of current reports of maternal warmth. By separately evaluating mother and offspring reports of warmth, we also addressed whether mother’s or child’s perspective has a stronger effect on cortisol regulation following parental divorce.
Methods
Participants
Participants were 240 youth from divorced families who participated in a larger study of an experimental trial for families of divorce. Participants were 9–12 years old at the beginning of the study and 23–27 years old at the final time point. Most (80%) were recruited from randomly selected court records of divorce decrees granted within two years of the trial’s start; the remainder responded to media advertisements. The primary eligibility criteria included: 1) primary residential parent was female, 2) at least one 9–12 year-old child who resided at least 50% of the time with the mother, 3) neither mother nor any child was in treatment for mental health problems, 4) mother had not remarried, did not plan to remarry, and did not have a live-in boyfriend (46). Demographic information is displayed in Table 1. The study was approved by the Internal Review Board at Arizona State University and carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Children signed informed assent forms; parents and youths older than 18 signed informed consent.
Table 1.
Sample Characteristics
| Age at W1 (n=240; M, SD,) | 10.76 (1.4) |
| Age at W6 (n=194; M, SD) | 25.6 (1.2) |
| Age at parental divorce (n=240; M, SD) | 9.7 (1.3) |
| Sex (n, %) | |
| Male | 123 (51%) |
| Female | 117 (49%) |
| Maternal Ethnicity (n, %) | |
| Hispanic | 19 (8%) |
| Anglo/Caucasian | 210 (88%) |
| African American | 5 (2%) |
| Asian/Pacific Islander | 3 (1%) |
| Other | 3 (1%) |
| Hormonal contraceptive use (n, %) | 24 (31%) |
| Regular Smoker (n, %) | 50 (31%) |
| Use of medications (n, %)1 | 37 (23%) |
| Pre-task cortisol (n = 160; μg/dL; M, SD)2 | .088 (.05) |
| Post-task cortisol (n = 159; μg/dL; M, SD) 2 | .086 (.06) |
| 20-min post cortisol (n = 161; μg/dL; M, SD)2 | .091 (.07) |
| 40-min post cortisol (n = 159; μg/dL; M, SD)2 | .074 (.05) |
| Cortisol AUCg (n = 155; μg/dL; M, SD)2 | .258 (.16) |
includes allergy, antidepressant, anti-anxiety, or other medications that could potentially affect cortisol
non-transformed data
Assessments were conducted in participants’ homes at 6 time points across 15 years: W1 (baseline; 5/1992-2/1994) was conducted when children were 9–12 years old, Waves 2-4 were conducted approximately 3, 6, and 9 months after W1; W5 was conducted six years later (ages 15–19; 1998–2000), and W6 was conducted 15-years later (ages 23–27; 4/2007-2/2009). At W1, families were randomly assigned to an intervention (New Beginnings Program; n = 164) or Literature Control group (n = 76). Waves 2-6 were conducted post-intervention. Because intervention effects are not the focus of the current analyses, and prior analyses indicated that participation in the intervention did not predict cortisol output at the 15-year follow-up (47), the groups are combined for the current analyses.
Of the 240 mother-child dyads who participated at W1, 194 (89.6% of the families) completed the 15-year follow-up. Cortisol samples were not obtained from 12 youth (8 refused, 4 were living outside the country). Two participants had cortisol levels outside normal physiological levels (i.e., > 50 nmol/L), indicating interference in the assay. Nineteen were excluded a priori from analyses due to pregnancy (n= 8), current use of thyroid or other medications known to affect glucocorticoids (n = 9), or cortisol levels > 4 SD’s from the mean (n=2) 1. Five participants did not have at least 3 viable samples for calculation of AUCg. Thus cortisol AUCg was available from 155 participants.
Internal and external validity was evaluated by testing if attriters differed from nonattriters on baseline demographics or maternal warmth at waves 1-5. Attrition status was not associated with participant age (p =.75), ethnicity (p =.82), gender (p =.41), months since parental divorce (p =.53), or family income (p =.51). Attrition status also was not associated with child- or mother-reported maternal warmth at any of the first five waves (p’s = .21 – .85).
Procedure
To aid sample retention, interviews were scheduled at the participants’ convenience, between 1 PM and 8 PM. Saliva samples were collected before, immediately after, and 20 and 40-minutes after a modified Trier Social Stress task (TSST; 48; described below) which began approximately 30 minutes after arrival at the home.
Measures
Maternal warmth
Maternal warmth was assessed with the acceptance subscale of the Child Report of Parental Behavior Inventory (49), completed by mothers and children at W1-W6. The full Acceptance subscale (16 items; α’s = .83 – .94) was completed by youth at W1, W2, W5, and W6. To reduce participant burden, a shortened Acceptance subscale (10 items) was completed by youth at W3 and W4 (α= .86 & α= .86, respectively). Mothers completed the full Acceptance subscale at all waves (α’s = .81–.88).
Cortisol response to the TSST
Saliva samples were collected from participants before, immediately after, and 20 and 40-minutes after a video-recorded modified TSST. Three one-minute trials of mental arithmetic (difficulty adjusted based on performance, and conducted under time pressure) were followed by two minutes of preparation and four minutes of a speech about personal strengths and weaknesses. To increase the social-evaluative aspect of the task, participants were told would the speech would be graded and evaluated by a panel of psychologists. Participants rated their moods before and after the task with the following items: 1) How angry, irritable, or disgusted do you feel? 2) How nervous, scared, or jittery do you feel? 3) How sad, blue, or lonely do you feel? Response choices were from 1 = not at all to 10 = extremely. A paired samples t-test comparing negative mood states before (M= 4.62, SD = 2.65) and after (M= 6.92, SD = 4.68) the task was significant, t(161) = −6.8, p = < .001, indicating negative emotional response to the task.
Saliva samples were obtained with the Salivette (Sarstedt, Rommelsdorf, Germany) and shipped on dry ice to Salimetrics (State College, PA) for analysis of free cortisol using high-sensitive enzyme immunoassay. The test has a range of sensitivity from .007 to 1.8 μg/dl, and mean intra-and inter-assay coefficients of variation 4.13% and 8.89%. Cortisol values were log-transformed to correct for deviations from normality. Total cortisol across the task was the primary outcome variable, quantified by area under the curve with respect to ground (AUCg), a summary measure reflective of the magnitude of cortisol response over a specified time (50).
Data analyses
Preliminary Analyses
T-tests and correlations were used to evaluate potential covariates for cortisol AUCg, including participant demographics (e.g., age, sex, W1 family income, ethnicity, time since parental divorce) and information from the day of testing (time of day, recent meals, exercise, caffeine, medications, or hormonal contraceptive use). Only time of day of testing was a statistically significant correlate of cortisol AUCg (r = −.17, p = .035).
Primary analyses
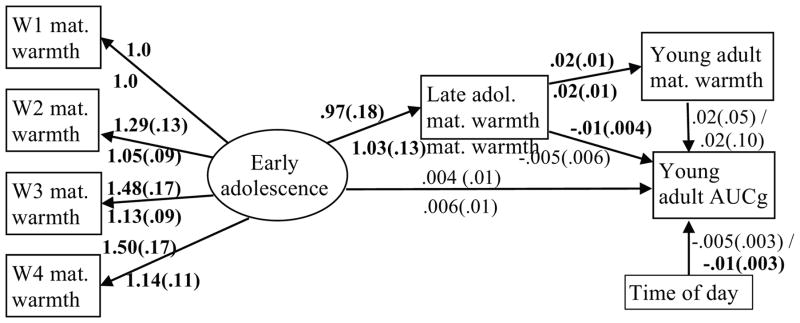
The primary study analyses were conducted using the general analysis program MPlus 7.0 (51), which uses full information maximum likelihood to manage missing data. Reports of maternal warmth from W1-W4 were modeled with a latent variable to capture warmth during childhood/early adolescence, which was used to predict warmth in late adolescence (W5) and cortisol AUCg in young adulthood (W6; see Figure 1). Model fit was examined using Comparative Fit Index (CFI), Root Mean Square Error of Approximation (RMSEA), and Standardized Root Mean Square Residual (SRMR), using criteria by Hu and Bentler (52) to interpret fit indices. The statistical significance of the indirect path from maternal warmth in early adolescence to cortisol AUCg in young adulthood via maternal warmth in late adolescence was evaluated using the multivariate delta method (53, 54). The proposed model was evaluated separately for child- and mother- reported maternal warmth. Time of day was covaried with cortisol AUCg. The models were repeated adjusting for W6 child- and mother-reported maternal warmth to address the hypothesis that the effects would be independent of current maternal warmth. 2
Figure 1.
Prediction of offspring cortisol in young adulthood by maternal warmth in early and late adolescence, controlling for current maternal warmth1
1 Unstandardized model results shown; estimates and standard errors. Error terms and covariances and not shown. “W1-W4” = waves 1-4. “mat. warmth” = maternal warmth. “adol” = adolescence. “AUCg” = cortisol AUCg. Results for child report are shown above the lines; results for mother report are shown below the lines. Significant estimates are in bold font.
Results
Preliminary Results
Pearson correlations were computed for mother and child reports of maternal warmth at each wave (see Table 2). Across waves 1-5, child reports were significantly and positively correlated with each other and mother self-reports of warmth were significantly correlated with each other. Correlations between child and mother reports, however, ranged from non-significant to significant (p’s = .001–.69).
Table 2.
Zero-order correlations between study variables (Pearson r/N)
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. W1 Warmth – Child report | 1.0 | |||||||||||
| 2. W2 Warmth – Child report | .56** 233 |
1.0 | ||||||||||
| 3. W3 Warmth – Child report | .48** 232 |
.53** 231 |
1.0 | |||||||||
| 4. W4 Warmth – Child report | .42** 229 |
.58** 228 |
.60** 229 |
1.0 | ||||||||
| 5. W5 Warmth – Child report | .31** 199 |
.31** 193 |
.35** 193 |
.33** 193 |
1.0 | |||||||
| 6. W6 Warmth – Child report | .20** 183 |
.12 177 |
.07 177 |
.23** 177 |
.29** 167 |
1.0 | ||||||
| 7. W1 Warmth – mother report | .20** 240 |
.20** 233 |
.19** 232 |
.16* 229 |
.23** 199 |
.24** 183 |
1.0 | |||||
| 8. W2 Warmth – mother report | .11 236 |
.15* 233 |
.10 231 |
.13* 228 |
.18* 196 |
.11 180 |
.58** 236 |
1.0 | ||||
| 9. W3 Warmth – mother report | .15 236 |
.22** 232 |
.19* 232 |
.22** 229 |
.24** 197 |
.13 181 |
.64** 236 |
.70** 235 |
1.0 | |||
| 10. W4 Warmth – mother report | .04 234 |
.15* 230 |
.13 230 |
.26** 229 |
.08 197 |
.17* 181 |
.49** 234 |
.63** 233 |
.63** 234 |
1.0 | ||
| 11. W5 Warmth – mother report | .04 207 |
.01 201 |
.09 201 |
.07 201 |
.35** 194 |
.07 172 |
.47** 207 |
.46** 204 |
.44** 205 |
.51** 205 |
1.0 | |
| 12. W6 Warmth – mother report | .12 198 |
.15* 192 |
.19** 192 |
.24* 192 |
.13 181 |
.45** 178 |
.25** 198 |
.31** 195 |
.32** 196 |
.32** 196 |
.36** 186 |
1.0 |
| 13. W6 Cortisol AUCg | −.01 155 |
−.01 151 |
−.06 151 |
−.06 151 |
−.20* 135 |
−.03 147 |
−.002 155 |
−.03 152 |
−.04 153 |
.08 153 |
−.01 139 |
−.01 143 |
p < .05
p < .01
Primary Results
Child report of maternal warmth
The first model evaluated child reports of maternal warmth across early (W1-W4) and late (W5) adolescence in the prediction of cortisol AUCg in young adulthood (W6). The model was of good fit, CFI = .986, χ2 (11) =15.8, p = .15, RMSEA = 0.043, {0.0; 0.086}, SRMR = .025. Maternal warmth in early adolescence positively predicted warmth in late adolescence (p < .01). Higher warmth in late adolescence significantly predicted lower cortisol AUCg in young adulthood, p = .031. Warmth in early adolescence did not directly predict cortisol AUCg (p = .58), but the indirect path to AUCg from early adolescence through late adolescence was significant, standardized estimate = −.092, SE = .045, p = .041. When warmth in young adulthood (W6) was included in the model, overall model fit declined slightly CFI=.970, χ2 (15) = 26.1, p =.04, RMSEA = 0.056, {0.014; 0.090}, SRMR = .031, but the path from late adolescent maternal warmth to AUCg remained significant, p = .036 (see Figure 1).
Mother report of maternal warmth
The second model evaluated mother reports of maternal warmth in the prediction of offspring cortisol AUCg in young adulthood. Although the overall model was of adequate fit, CFI=.98, χ2 (11)=20.1, p =.04, RMSEA=0.059, {0.01; 0.10}, SRMR = .030, neither of the paths predicting cortisol AUCg from mother-reported maternal warmth was statistically significant (early adolescence, p= .58; late adolescence, p = .42). Inclusion of mother-reported warmth at W6 improved model fit, CFI=.987, χ2 (15) =22.24, p =.11, RMSEA=0.045, {0.0; 0.082}, SRMR = .030, but W6 mother-reported maternal warmth was not significantly associated with AUCg, p = .84 (see Figure 1).
Discussion
Although rates are decreasing, parental divorce remains a common stressor for children, with nearly 50% of first marriages in the United States ending in divorce (55). Parental divorce is a well-established risk factor for a number of short-and long-term mental and physical health problems. However, the presence of a responsive, supportive caregiver has consistently emerged as a protective influence for children exposed to varied forms of adversity, including children from divorced families (44). The current longitudinal study evaluated maternal warmth as a protective influence on biological regulation among young adults who experienced parental divorce during childhood. Results suggested that higher child-reported maternal warmth, assessed at multiple time-points in late childhood and adolescence, was associated with lower cortisol response (AUCg) to a challenging interpersonal task in young adulthood. Mother-reported maternal warmth, however, did not predict cortisol response in offspring.
This study advances research on the long-term impact of childhood adversity on cortisol regulation in several respects. First, although other forms of adversity (e.g., maltreatment) are well-studied, limited research has evaluated long-term biological correlates of childhood parental divorce. Second, rather than relying on retrospective reports of maternal warmth or a single self-report of maternal warmth, the current study prospectively examined both mother and child reports of maternal warmth at five time points across late childhood and adolescence. Children’s perceptions of higher maternal warmth in early adolescence predicted higher maternal warmth in late adolescence, which predicted lower cortisol response in young adulthood, even after including maternal warmth in young adulthood in the model. These findings contribute to a growing literature documenting the critical influence of warm, sensitive caregiving during childhood on biological regulation later in development. The results also extend previous research utilizing retrospective reports to demonstrate relations of childhood caregiver warmth with adult cortisol stress responses.
Third, this study assessed both child and mother-reports of caregiver warmth. Only child-reported maternal warmth predicted later life cortisol response. In combination with a relatively low degree of correlation between mother and child reports of warmth, not uncommon in developmental research (56), these results suggest that children’s perceptions of the quality of their relationships with their mothers may be better predictors of long-term biological consequences than maternal perceptions. The potential for social desirability bias in caregiver self-reports of their parenting behavior calls for research with a broader perspective that can be obtained by collecting child reports (57). The current results support the importance of considering the child’s perspective when evaluating the long-term biological correlates of childhood adversity.
There are several limitations of the study. First, we did not assess cortisol activity at study entry, and we cannot determine the developmental timing or changing pattern of influence of maternal warmth on HPA axis regulation. Second, assessments were conducted in the home, which likely reduced the magnitude of cortisol reactivity to the task relative to lab-based protocols. Third, because all participants experienced parental divorce, we cannot determine whether the prospective influence of maternal warmth on cortisol in adulthood applies broadly to childhood adversity or is unique to divorce. Finally, we did not evaluate the impact of paternal warmth on offspring cortisol regulation. The father-child relationship is typically more disrupted following divorce than the mother-child relationship (58) and the quality of paternal parenting is independently related to children’s post-divorce adjustment (44). A growing research literature recognizes the importance of father-child relationships for developing biological regulatory systems as well (e.g., 59), and it will be important to consider paternal warmth in future research.
Conclusions
Compelling evidence demonstrates the potential mental and physical health risks associated with childhood exposure to parental divorce. However, there are considerable individual differences in outcomes, with many children demonstrating resilience following parental divorce. Consistent research has suggested the power of positive parenting to promote child mental and physical health in the face of adversity, but how positive parenting promotes resilience has not been fully identified (60). The current study provides support for biological regulation as a pathway by which caregiver warmth promotes positive offspring adjustment following parental divorce. This study advances the literature by prospectively examining both mother and child reports of maternal warmth at multiple time points across development in a sample of recently divorced families. Child reports of maternal warmth in adolescence predicted lower cortisol response across a challenging interpersonal task in young adulthood, but mother reports were not significantly associated with later offspring cortisol response. The results demonstrate the importance of children’s perceptions of parenting, and suggest that for children, the presence of a supportive caregiver during a stressful period in development may promote efficient biological regulation during moderately challenging situations later in life.
Acknowledgments
Funding: This research was supported by the National Institute of Mental Health (5R01MH071707; 5P30MH068685, 5P30MH039246). Trial Registration: clinicaltrials.gov Identifier: NCT01407120. The funding source had no role in study design, collection, analysis, interpretation, or writing of the report; or in the decision to submit the article for publication. Sharlene Wolchik and Irwin Sandler declare the following competing financial interest: partnership in Family Transitions - Programs that Work LLC, which trains and supports providers to deliver the New Beginnings Program. The remaining authors have no financial interest in the application of this research.
Abbreviations
- AUCg
Area Under the Curve -Ground
- W1-6
Waves 1-6
- SD
Standard Deviation
- CFI
Comparative Fit Index
- RMSEA
Root Mean Square Error of Approximation
- SRMR
Standardized Root Mean Square Residual
- TSST
Trier Social Stress task
Footnotes
Primary analyses were repeated without excluding these 19 participants. The pattern was similar and results retained statistical significance. However, because the identified factors are known to alter cortisol levels, these cases were excluded from analyses. Additionally, examination of Cook’s D identified one case as potentially influential on the model. The case was examined and noted to have begun the task earlier than all other participants. When analyses were repeated excluding the case, model results and statistical significance were consistent. Therefore the case was retained for analyses.
Although intervention effects are not a focus of the current analyses, models were evaluated that also included group assignment, age, and the group by age interaction, shown to predict cortisol reactivity (change from baseline) in prior analyses with this sample (36). Model fit remained good (CFI=.974, χ2 (22)=30.3, p =.11, RMSEA=0.042, {0.0; 0.076}, SRMR = .032) and higher child-reported maternal warmth in late adolescence significantly predicted lower AUCg, std est = −.20, p = .032. Neither group (p = .52) nor the group by age interaction (p = .09) significantly predicted AUCg, and thus were not included in the final model.
References
- 1.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 2.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–66. [PubMed] [Google Scholar]
- 3.Gunnar MR, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 4.Luecken LJ, Lemery KS. Early caregiving and physiological stress responses. Clin Psychol Rev. 2004;24(2):171–91. doi: 10.1016/j.cpr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 6.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 7.Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol. 2010;22(1):165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 9.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160(9):1554–65. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 11.Hamer M, Endrighi R, Venuraju SM, Lahiri A, Steptoe A. Cortisol responses to mental stress and the progression of coronary artery calcification in healthy men and women. PLoS One. 2012;7:e31356. doi: 10.1371/journal.pone.0031356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vreeburg S, Zitman FG, van Pelt J, DeRijk RH, Verhagen JC, van Dyck R, Hoogendijk JG, Smit JH, Penninx BW. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom Med. 2010;72:340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- 13.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroenrocrinol. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 15.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–46. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Albers EM, Riksen-Walraven JM, Sweep FCGJ, de Weerth C. Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. J Child Psychol Psychiatry. 2008;49(1):97–103. doi: 10.1111/j.1469-7610.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 17.Bernard K, Dozier M. Examining infants’ cortisol responses to laboratory tasks among children varying in attachment disorganization: Stress reactivity or return to baseline? Dev Psychol. 2010;46:1771–78. doi: 10.1037/a0020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant K-A, McMahon C, Austin M-P, Reilly N, Leader L, Ali S. Maternal prenatal anxiety, postnatal caregiving and infants’ cortisol responses to the still-face procedure. Dev Psychobiol. 2009;51:625–37. doi: 10.1002/dev.20397. [DOI] [PubMed] [Google Scholar]
- 19.Hastings PD, Ruttle PL, Serbin LA, Mills RSL, Stack DM, Schwartzman AE. Adrenocortical responses to strangers in preschoolers: Relations with parenting, temperament, and psychopathology. Dev Psychobiol. 2011;53:694–710. doi: 10.1002/dev.20545. [DOI] [PubMed] [Google Scholar]
- 20.Marsman R, Nederhof E, Rosmalen JGM, Oldehinkel AJ, Ormel J, Buitelaar Family environment is associated with HPA-axis activity in adolescents. Biol Psychol. 2012;89:460–6. doi: 10.1016/j.biopsycho.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proc Natl Acad Sci. 2013;110(42):17149–53. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2010;16(7):729–37. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luecken LJ. Parental caring and loss during childhood and adult cortisol responses to stress. Psychol Health. 2000;15:841–51. [Google Scholar]
- 24.Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proc Natl Acad Sci USA. 2013;110(42):17149–17153. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011;22(12):1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhlman KR, Olson SL, Lopez-Duran NL. Predicting developmental changes in internalizing symptoms: Examining the interplay between parenting and neuroendocrine stress reactivity. Dev Psychobiol. 2014;56(5):908–23. doi: 10.1002/dev.21166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackman DA, Betancourt LM, Brodsky NL, Kobrin L, Hurt H, Farah MJ. Selective impact of early parental responsivity on adolescent stress reactivity. PloS One. 2013;8(3):e58250. doi: 10.1371/journal.pone.0058250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagan M, Roubinov D, Gress J, Luecken LJ, Sandler I, Wolchik S. Positive parenting during childhood moderates the impact of recent negative events on cortisol activity in parentally bereaved youth. Psychopharmacol. 2011;214:231–8. doi: 10.1007/s00213-010-1889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amato PR, Sobolewski JM. The effects of divorce and marital discord on adult children’s psychological well-being. Am Sociol Rev. 2001;66:900–21. [Google Scholar]
- 30.Amato PR. Research on divorce: Continuing trends and new developments. J Marriage Fam. 2010;72(3):650–66. [Google Scholar]
- 31.Fuller-Thomson E, Dalton AD. Gender differences in the association between parental divorce during childhood and stroke in adulthood: findings from a population-based survey. Int J Stroke. 2012 doi: 10.1111/j.1747-4949.2012.00935.x. [DOI] [PubMed] [Google Scholar]
- 32.Larson K, Halfon N. Parental divorce and adult longevity. Int J Pub Health. 2013;58:89–97. doi: 10.1007/s00038-012-0373-x. [DOI] [PubMed] [Google Scholar]
- 33.Martin LR, Friedman HS, Clark KM, Tucker JS. Longevity following the experience of parental divorce. Soc Sci Med. 2005;61:2177–89. doi: 10.1016/j.socscimed.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 34.Troxel WM, Matthews KA. What are the costs of marital conflict and dissolution to children’s physical health? Clin Child Fam Psychol Rev. 2004;7:29–57. doi: 10.1023/b:ccfp.0000020191.73542.b0. [DOI] [PubMed] [Google Scholar]
- 35.Roustit C, Campoy E, Renahy E, King G, Parizot I, Chauvin P. Family social environment in childhood and self-rated health in young adulthood. BMC Public Health. 2011;11:949. doi: 10.1186/1471-2458-11-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloch M, Peleg I, Koren D, Aner H, Klein E. Long-term effects of early parental loss due to divorce on the HPA axis. Horm Behav. 2007;51:516–23. doi: 10.1016/j.yhbeh.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Kraft AJ, Luecken LJ. Childhood parental divorce and cortisol in young adulthood: evidence for mediation by family income. Psychoneuroendocrinology. 2009;34:1363–9. doi: 10.1016/j.psyneuen.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Pendry P, Adam EK. Associations between parents’ marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. Int J Behav Dev. 2007;31(3):218–31. [Google Scholar]
- 39.Davies PT, Sturge-Apple ML, Cicchetti D. Interparental aggression and children’s adrenocortical reactivity: Testing an evolutionary model of allostatic load. Dev Psychopathol. 2011;23(03):801–14. doi: 10.1017/S0954579411000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koss KJ, George MRW, Davies PT, Cicchetti D, Cummings EM, Sturge-Apple ML. Patterns of children’s adrenocortical reactivity to interparental conflict and associations with child adjustment: A growth mixture modeling approach. Dev Psychol. 2013;49:317–26. doi: 10.1037/a0028246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagan MJ, Roubinov DS, Purdom Marreiro CL, Luecken LJ. Childhood interparental conflict and HPA axis activity in young adulthood: Examining nonlinear relations. Dev Psychobiol. 2014;56(4):871–80. doi: 10.1002/dev.21157. [DOI] [PubMed] [Google Scholar]
- 42.Luecken LJ, Kraft AJ, Hagan M. Negative relationships in the family-of-origin predict attenuated cortisol in emerging adults. Horm Behav. 2009;55:412–17. doi: 10.1016/j.yhbeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Dev Psychopathol. 2002;14(02):333–49. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- 44.Sandler I, Wolchik S, Winslow E, Mahrer N, Moran J, Weinstock D. Quality of maternal and paternal parenting following separation and divorce. In: Kuehnle K, Drozd L, editors. Parenting plan evaluations: Applied research for the Family Court. New York, NY: Oxford University Press; 2012. pp. 85–123. [Google Scholar]
- 45.Masten AS, Burt KB, Roisman GI, Obradović J, Long JD, Tellegen A. Resources and resilience in the transition to adulthood: Continuity and change. Dev Psychopathol. 2004;16(4):1071–94. doi: 10.1017/s0954579404040143. [DOI] [PubMed] [Google Scholar]
- 46.Wolchik SA, West SG, Sandler IN, Tein J-Y, Coatsworth JD, Lengua L, Weiss L, Anderson ER, Greene SM, Griffin WA. An experimental evaluation of theory-based mother and mother-child programs for children of divorce. J Consult Clin Psychol. 2000;68:843–56. [PubMed] [Google Scholar]
- 47.Luecken LJ, Hagan M, Mahrer N, Wolchik SA, Sandler IN, Tein JY. Effects of a prevention program for divorced families on youth cortisol reactivity 15-years later. Psychol Health. 2014 doi: 10.1080/08870446.2014.983924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”: a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiol. 1993;28:76–8. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 49.Schaefer ES. Children’s reports of parental behavior: An inventory. Child Dev. 1965:413–24. [PubMed] [Google Scholar]
- 50.Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69(7):651–9. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- 51.Muthén LK, Muthén BO. Mplus (Version 7.0) [Computer Software] Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- 52.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. [Google Scholar]
- 53.MacKinnon DP. Introduction to statistical mediation analysis. NY: Erlbaum; 2008. [Google Scholar]
- 54.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 55.National Center for Health Statistics. First marriages in the United States: Data from the 2006–2012 National Survey of Family Growth. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 56.Tein J-Y, Roosa MW, Michaels M. Agreement between parent and child reports on parental behaviors. J Marriage Fam. 1994;56:341–55. [Google Scholar]
- 57.Tucker MC, Rodriguez CM. Utility of child report: Correspondence with parent-reported child abuse risk. Child Abuse Rev. 2014;23:334–41. [Google Scholar]
- 58.Schwartz SJ, Finley GE. Mothering, fathering, and divorce: The influence of divorce on reports of and desires for maternal and paternal involvement. Fam Court Rev. 2009;47(3):506–22. [Google Scholar]
- 59.Boyce WT, Essex MJ, Alkon A, Goldsmith H, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. J Am Acad Child Adol Psychiatry. 2006;45(12):1510–20. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- 60.Sandler I, Schoenfelder E, Wolchik SA, MacKinnon DP. Long-term impact of prevention programs to promote effective parenting: Lasting effects but uncertain processes. Annu Rev Psychol. 2011;62:299–329. doi: 10.1146/annurev.psych.121208.131619. [DOI] [PMC free article] [PubMed] [Google Scholar]