Abstract
Molecular genetic testing on formalin fixed, paraffin embedded (FFPE) tumors frequently requires dissection of tumor from tissue sections mounted on glass slides. In a process referred to as “manual macrodissection,” the pathologist reviews an H&E stained slide at the light microscope and marks areas for dissection, and then the laboratory performs manual dissection from adjacent sections without the aid of a microscope, using the marked reference H&E slide as a guide. Manual macrodissection may be inadequate for tissue sections with low tumor content. We compared manual macrodissection to a new method, digitally guided microdissection, on a series of 32 FFPE pancreatic cancer samples. KRAS hotspot mutation profiling was performed using the Sequenom MassARRAY system (Agena Bioscience). Digitally guided microdissection was performed on multiple smaller areas of high tumor content selected from within the larger areas marked for manual macrodissection. The KRAS mutant allele fraction and estimated neoplastic cellularity were significantly higher in samples obtained by digitally guided microdissection (p <0.01), and 7 of the 32 samples (22%) showed a detectable mutation only with digitally guided microdissection. DNA quality and yield per cubic millimeter of dissected tissue were similar for both dissection methods. These results indicate a significant improvement in tumor content achievable with digitally guided microdissection.
Keywords: microdissection, macrodissection, digital pathology, formalin fixed paraffin embedded
Introduction
Molecular genetic testing is increasingly performed on material extracted from formalin fixed, paraffin embedded (FFPE) tissue sections to aid histopathologic diagnosis, to define molecular subtypes for treatment planning, and to provide relevant prognostic information. Most current molecular assays on solid tumors are designed to tolerate a mixture of tumor and non-tumor cells, but higher neoplastic cellularity maximizes analytical sensitivity. Histologic examination of FFPE tissue sections is essential to confirm sufficient neoplastic cellularity prior to testing.
Different molecular testing approaches have different thresholds for mutation detection. Sanger sequencing is unreliable for cases with a neoplastic cellularity of 40% or lower, since somatic mutations are often heterozygous and the lower threshold for mutation detection is a mutant allele fraction of about 20% [1, 2]. Targeted pyrosequencing can detect lower frequency mutations, with some assays reliably detecting a mutant allele fraction as low as 5% [2]. The analytical sensitivity of mass spectrometry and next generation sequencing (as currently used for multigene panels) is similar to that of targeted pyrosequencing [1, 3]. Ultrasensitive technologies such as allele specific PCR or digital droplet PCR can detect even lower frequency mutations. The clinical relevance of low frequency mutations present in minor subpopulations of tumor cells is not completely understood, however, even though the importance of tumor heterogeneity is increasingly recognized [4, 5]. Additionally, spurious low level mutations can occur as a result of the formalin fixation process [6]. Regardless of the testing strategy, high tumor content is desirable for molecular testing because the presence of non-tumor cells can hinder the detection of clinically relevant somatic mutations relative to the detection thresholds of various molecular technologies.
Whole sections or scrolls from paraffin blocks are not always acceptable for molecular testing due to variable tumor content. Therefore, some form of tumor enrichment is often necessary when using FFPE tissue. In clinical molecular oncology testing, the most frequently utilized tumor enrichment method is manual tissue dissection from glass slides. Areas of tumor are selected by microscopic examination, manually circling, under microscopic guidance, areas on a hematoxylin and eosin (H&E) stained glass slide with a slide marking pen. Dissection is carried out on stained or unstained serial sections from the same block, using the marked H&E slide as a guide [7]. A variety of manual dissection methods have been adopted, including the use of a scalpel, pipette tip, razor blade, or needle manually guided with a micromanipulator [7-13]. In a commonly used method called manual macrodissection, a scalpel blade or similar scraping tool is used to remove microscopically marked areas off of the dissection slide without the aid of a microscope. Manual macrodissection is adequate for the majority of cases submitted for clinical testing, but it may be inadequate in cases with low tumor content. Because molecular testing can be an essential part of treatment planning, some cancer patients may have to undergo an additional clinical procedure in order to obtain an adequate sample for molecular testing. In cases where there is sufficient tumor on the slide but it cannot be adequately dissected by hand, a more precise dissection method is desirable. Laser capture microdissection (LCM) was introduced about two decades ago [14], but it has not been widely adopted in the clinical laboratory setting. LCM has been noted in multiple studies to diminish the yield and quality of retrieved material [9, 15-17], although others report that sample recovery is not impaired by the LCM process [18]. Even so, the precise dissection of single tumor cells from FFPE slides is seldom necessary for clinical molecular testing, whereas the rapid and cost effective retrieval of a sufficient DNA or RNA sample is paramount; therefore, lower resolution microdissection methods can be substituted for LCM.
A new microdissection method utilizing a modified computer numerical control (CNC) milling machine was recently introduced as an intermediate resolution method for dissecting tissue from glass slides. Current milling microdissection technology can dissect regions with a diameter of 200 microns or greater, and early experiments have been promising [19]. This new microdissection method can be performed on any glass surface and therefore the method integrates easily into clinical laboratory workflow. When used in combination with digital slide marking (“digitally guided microdissection”), the technology can achieve a much greater resolution than manual dissection methods. We compared digitally guided microdissection to our traditional dissection method, manual macrodissection, on a series of FFPE pancreatic adenocarcinoma specimens. Pancreatic adenocarcinomas usually have an infiltrative growth pattern with small clusters of tumor cells surrounded by non-tumor cells, and so these cancers can be difficult to dissect manually. Pancreatic adenocarcinomas also have a high prevalence of KRAS mutations [20, 21], and the mutant allele fraction can be used as a surrogate measure of the tumor purity in comparing the same area of a tumor dissected both ways. We used both the estimated neoplastic cellularity and the KRAS mutant allele fraction to compare dissection methods on 32 FFPE pancreatic adenocarcinoma samples.
Materials and Methods
Case Selection, Slide Marking for Dissection, and Evaluation of Tumor Content
The University of Utah Institutional Review Board approved the study protocol. Pancreatic adenocarcinoma cases from the University of Utah Department of Pathology archives were evaluated for tumor content. Thirty two paraffin embedded tissue blocks from eighteen different cases of pancreatic adenocarcinoma were selected for this comparison study. Tumor characteristics are shown in Table 1. Tissue blocks were serially sectioned, deparaffinized, and stained with either hematoxylin and eosin (H&E) or aniline blue, a water soluble collagen stain similar to toluidine blue that permits easy visualization of the tissue on the slide for dissection. For each tissue block, a coverslipped H&E section was used for microscopic evaluation, and additional adjacent aniline blue stained serial sections (5 microns thick) were used for dissection. Regions for manual macrodissection were identified microscopically and marked directly on the H&E slide with a slide marking pen. The marked H&E slides were then digitally scanned using the Aperio ScanScope® XT system (Leica Biosystems, Vista, CA, USA). Regions measuring at least 200 microns in diameter were chosen for digitally guided microdissection within the manually marked areas and were marked digitally using the pen tool in Aperio ImageScope® software (Leica Biosystems). Digital slide annotations were typically performed at 40×-100× resolution in ImageScope software (equivalent to use of the 4× and 10× objective lenses on a standard light microscope with 10× ocular lens magnification). Images exported from ImageScope® software to guide milling microdissection were typically acquired at 10× resolution.
Table 1.
Samples used for manual macrodissection and digitally guided microdissection.
| Sample ID | Description |
|---|---|
| 1 | moderately to poorly differentiated adenocarcinoma, pancreas |
| 2 | moderately differentiated adenocarcinoma, pancreas |
| 3 | metastatic pancreatic carcinoma, peritoneum |
| 4 | moderately to poorly differentiated adenocarcinoma, pancreas |
| 5 | moderately differentiated adenocarcinoma, pancreas |
| 6 | moderately differentiated adenocarcinoma, pancreas |
| 7 | metastatic pancreatic carcinoma, liver |
| 8 | moderately to poorly differentiated adenocarcinoma, pancreas |
| 9 | moderately to poorly differentiated adenocarcinoma, pancreas |
| 10 | moderately to poorly differentiated adenocarcinoma, pancreas |
| 11 | moderately to poorly differentiated adenocarcinoma, pancreas |
| 12 | moderately to poorly differentiated adenocarcinoma, pancreas |
| 13 | moderately to poorly differentiated adenocarcinoma, pancreas |
| 14 | well-differentiated adenocarcinoma, pancreas |
| 15 | well-differentiated adenocarcinoma, pancreas |
| 16 | panIN-3* in pancreas with well-differentiated adenocarcinoma |
| 17 | poorly differentiated adenocarcinoma, pancreas |
| 18 | poorly differentiated adenocarcinoma, pancreas |
| 19 | moderately differentiated adenocarcinoma, pancreas |
| 20 | metastatic invasive extrahepatic distal bile duct carcinoma, pancreas |
| 21 | metastatic invasive extrahepatic distal bile duct carcinoma, pancreas |
| 22 | metastatic invasive extrahepatic distal bile duct carcinoma, pancreas |
| 23 | poorly differentiated adenocarcinoma, pancreas |
| 24 | poorly differentiated adenocarcinoma, pancreas |
| 25 | poorly differentiated adenocarcinoma, pancreas |
| 26 | poorly differentiated adenocarcinoma, pancreas |
| 27 | poorly differentiated adenocarcinoma, pancreas |
| 28 | moderately to poorly differentiated adenocarcinoma, pancreas |
| 29 | moderately differentiated adenocarcinoma, pancreas |
| 30 | moderately differentiated adenocarcinoma, pancreas |
| 31 | moderately differentiated adenocarcinoma, pancreas |
| 32 | poorly differentiated adenocarcinoma, pancreas |
panIN, pancreatic intraductal carcinoma
Three pathologists (KG, ED-K, and MB) independently estimated the neoplastic cellularity of the regions designated for manual macrodissection and digitally guided microdissection. Neoplastic cellularity, defined as the percentage of cells that are neoplastic, was estimated on a semi-quantitative scale and stratified into groups of <1%, 1-5%, 5-10%, 10%, 20%, 30%, and so on up to 100%.
Slide Dissection
Serial sections used for manual macrodissection or digitally guided microdissection were distributed equally in distance from the reference slide. For manual macrodissection, the marked H&E reference slide was manually aligned to the corresponding aniline blue stained dissection slide, the marked area was manually traced onto the dissection slide, the area to be scraped was soaked briefly in molecular biology grade water to make it easier to scrape, and the marked area was scraped by hand with a surgical steel scalpel. The MilliSect™ instrument (AvanSci Bio LLC, Salt Lake City, UT, USA) was used for milling microdissection. Digitally marked reference images in tagged image file (tif) format were imported into 2iD software (AvanSci Bio) and were manually aligned and resized to match the image of the dissection slide on the stage. Areas for dissection were transferred automatically from the reference slide to the dissection slide using a color selection tool in 2iD software, and a milling path was automatically generated. Milling microdissection was performed using xScisor milling bits (AvanSci Bio) loaded with milling buffer (2 mM Tris, 0.2 mM EDTA, 0.1% Tween-20, pH 8.5). xScisors with either 200 micron or 400 micron blades were used, based on the size and shape of the area to be dissected.
Preparation of Tissue Crude Lysate
All dissected material was recovered in sterile microfuge tubes containing buffer solution (2 mM Tris, 0.2 mM EDTA, 0.1% Tween-20, pH 8.5). The volume of buffer solution was adjusted to match area dissected, with approximately 2 microliters (μl) per square millimeter of tissue dissected. For samples less than 150 μl , an equal volume of Crystal Plus 70T light mineral oil (STE Oil Company, San Marcos, TX, USA) was added to prevent evaporation. The tubes were incubated on a heated shaker for one hour at 92°C at 1200 cycles per minute. A one tenth volume of Proteinase K solution (5 μg/μl) was then added to each tube and tubes were incubated for one hour at 56°C at 1000 cycles per minute, followed by 15 minutes at 92°C at 1200 cycles per minute to heat inactivate the Proteinase K. Tubes were then centrifuged at 4000 revolutions per minute (rpm) for two minutes, and mineral oil was removed with hydrophobic paper wicking strips (AvanSci Bio).
Mutation Profiling by Mass Spectrometry
Specimens were prepared for mass spectrometry analysis utilizing iPlex® chemistry, which consists of three steps: multiplexed PCR, shrimp alkaline phosphatase (SAP) treatment, and iPlex® single nucleotide extension (Sequenom MassARRAY® System, Agena BioScience, Inc., San Diego, CA, USA). Multiplexed PCR was performed using 4 wells per sample to amplify hotspots in KRAS codons 12, 13, 61, and 146 using the following primer sequences:
Well 1: forward primer 5’-ACGTTGGATGAGGCCTGCTGAAAATGACTG-3’ and reverse primer 5’-ACGTTGGATGGCTGTATCGTCAAGGCACTC-3’ (codon 13, position 1); forward primer 5’-ACGTTGGATGTTCAGTGTTACTTACCTGTC-3’ and reverse primer 5’-ACGTTGGATGCAGGCTCAGGACTTAGCAAG-3’ (codon 146, position 2)
Well 2: forward primer 5’- ACGTTGGATGCAGGCTCAGGACTTAGCAAG-3’ and reverse primer 5’-ACGTTGGATGTTCAGTGTTACTTACCTGTC-3’ (codon 146, position 1); forward primer 5’-ACGTTGGATGCATGTACTGGTCCCTCATTG-3’ and reverse primer 5’-ACGTTGGATGTGGAGAAACCTGTCTCTTGG-3’ (codon 61, position 1); forward primer 5’-ACGTTGGATGAGGCCTGCTGAAAATGACTG-3’ and reverse primer 5’-ACGTTGGATGGCTGTATCGTCAAGGCACTC-3’ (codon 13, position 2)
Well 3: forward primer 5’- ACGTTGGATGGCTGTATCGTCAAGGCACTC-3’ and reverse primer 5’-ACGTTGGATGAGGCCTGCTGAAAATGACTG-3’ (codon 12, position 1); forward primer 5’-ACGTTGGATGCATGTACTGGTCCCTCATTG-3’ and reverse primer 5’-ACGTTGGATGTGGAGAAACCTGTCTCTTGG-3’ (codon 61, position 3)
Well 4: forward primer 5’- ACGTTGGATGAGGCCTGCTGAAAATGACTG-3’ and reverse primer 5’-ACGTTGGATGGCTGTATCGTCAAGGCACTC-3’ (codon 12, position 2); forward primer 5’- ACGTTGGATGCATGTACTGGTCCCTCATTG-3’ and reverse primer 5’-ACGTTGGATGTGGAGAAACCTGTCTCTTGG-3’ (codon 61, position 2).
All primers had a 5’ tail (5’-ACGTTGGATG-3’) and amplicon sizes ranged from 76 to 81 base pairs. PCR reactions contained 1.3 μL molecular biology grade water, 0.5 μL PCR buffer, 0.4 μL 25 mM MgCl2, 0.1 μL 25 mM dNTPs, 0.2 μL PCR enzyme (5 U/μL), 1 μL multiplexed primer mix (0.5 μM), 0.5 μL PCR QA control primer, and 1 μL tissue crude lysate. For SAP treatment, a cocktail of 1.53 μL water, 0.17 μL 10X SAP Buffer, 0.5 μL SAP QA control primer, and 0.3 μL SAP (1.7 U/μL) was added to the PCR products and incubated at 37 °C for 40 min followed by 85 °C for 5 min in a thermal cycler. The iPlex® single nucleotide extension reaction contained 7 μL SAP treated PCR products, 0.24 μL H2O, 0.2 μL 10× iPlex® buffer, 0.1 μL 10× termination mix, 0.02 μL Thermo Sequenase (5 U/μL), 0.5 μL extension QA ctrl primer (Sequenom), and 0.94 μL of the multiplexed extend primer mix (10-14 μM). The extension reaction was performed in a thermal cycler using the following conditions: 95 °C for 30 seconds followed by 40 cycles of 95 °C for 5 seconds, 52 °C for 5 seconds, 80 °C for 5 seconds (the 52 °C and 80 °C steps were repeated 5 times), followed by 72 °C for 3 minutes. Following extension, 41 μL of molecular grade water and ion exchange resin (Sequenom) was added to each sample. The plate was rotated for approximately 30 minutes at room temperature and centrifuged at 4000 rpm for 5 minutes. All samples were spotted on the SpectroCHIP II G96 using the MassARRAY® Nanodispenser (Agena BioScience). Data was collected on the MassARRAY® analyzer 4 system using autorun settings. Data was analyzed using Sequenom Typer version 4.0 where peak heights were used to determine the relative mutant allele fractions in each sample. Based on limit of detection studies performed during test validation, samples with a mutant allele fraction between 5-10% were repeated for confirmation, and samples with a mutant allele fraction of 5% or lower were reported as negative.
DNA Yield Assessment
In order to directly compare the DNA yield per mm3 of dissected tissue, identical areas were dissected by digitally guided microdissection or manual macrodissection on adjacent sections. Crude lysate preparation was performed as described above, and samples were stored at 4°C. DNA yield assessment was performed simultaneously for all samples. DNA concentrations of unpurified crude lysate samples were determined using PicoGreen fluorescence according to the manufacturer's instructions (Molecular Probes®, Thermo Fisher Scientific, Waltham, MA, USA). DNA quality was also determined by fluorescence resonance energy transfer (FRET) real time PCR amplification of a 200 base pair sequence from the beta globin gene (HBB) (forward primer 5’ - ACA CAA CTG TGT TCA CTA GC – 3’ and reverse primer 5’ - CAA CTT CAT CCA CGT TCA CC – 3’ with a fluorescein labeled probe: 5′ - GGA GAA GTC TGC CGT TAC TGC C/dye - 3′ and LC Red 640 probe: 5′- dye/AGA CTT CTC CTC AGG AGT CAG GTG CAC CAT G - 3′) (LightCycler®, Roche Diagnostics, Indianapolis, IN, USA).
Statistical Analysis
Appropriate t test calculations (one-sided or two-sided) were performed to compare KRAS mutant allele fractions, to determine if the digitally guided microdissection method yielded mutant allele fractions that were consistently higher than those obtained with manual macrodissection, and to compare the DNA yield as measured by PicoGreen fluorescence and quantitative PCR. All statistical calculations were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC, USA).
Results
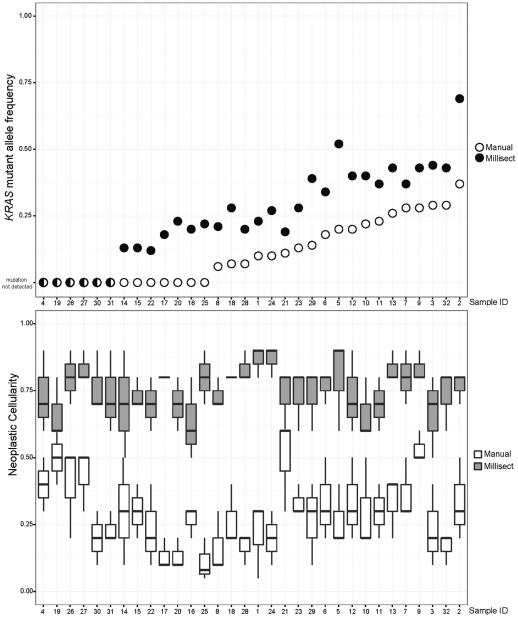
KRAS mutations were detected in 26 of the 32 (81%) FFPE blocks tested. Nineteen samples had detectable KRAS mutations using both methods, and for these cases, the mutant allele fraction in the samples obtained using digitally guided microdissection was significantly higher (p < 0.01). Five of the 32 samples obtained by manual macrodissection showed a KRAS mutant allele fraction below 10%, whereas the mutant allele fraction was above 10% for all 26 KRAS-mutated samples obtained by digitally guided microdissection. Seven FFPE blocks (22%) showed a detectable KRAS mutation only in the samples obtained by digitally guided microdissection. The comparative neoplastic cellularity and mutant allele fractions are shown in Table 2 and are also depicted graphically in Figure 1.
Table 2.
Neoplastic cellularity, mutant allele fraction, and genotype for each sample pair.
| Sample ID | Neoplastic cellularity | Mutant allele fraction (%) | KRAS Genotype | ||
|---|---|---|---|---|---|
| Manual | DGM | Manual | DGM | ||
| 1 | 5-30% | 80-90% | 10 | 23 | c.35G>T, p.Gly12Val |
| 2 | 20-50% | 70-80% | 37 | 69 | c.35G>A, p.Gly12Asp |
| 3 | 10-40% | 50-80% | 29 | 44 | c.35G>C, p.Gly12Ala |
| 4 | 30-50% | 60-90% | <5 | <5 | wild type |
| 5 | 20-40% | 60-90% | 20 | 52 | c.34G>C, p.Gly12Arg |
| 6 | 20-50% | 70-80% | 18 | 34 | c.34G>C, p.Gly12Arg |
| 7 | 30-50% | 70-90% | 28 | 37 | c.35G>A, p.Gly12Asp |
| 8 | 10-30% | 70-80% | 6 | 21 | c.35G>T, p.Gly12Val |
| 9 | 50-60% | 80-90% | 28 | 43 | c.35G>T, p.Gly12Val |
| 10 | 20-50% | 60-80% | 22 | 40 | c.35G>A, p.Gly12Asp |
| 11 | 20-40% | 60-80% | 23 | 37 | c.35G>A, p.Gly12Asp |
| 12 | 20-50% | 60-90% | 20 | 40 | c.35G>A, p.Gly12Asp |
| 13 | 20-40% | 80-90% | 26 | 43 | c.35G>A, p.Gly12Asp |
| 14 | 10-50% | 50-90% | <5 | 13 | c.35G>T, p.Gly12Val |
| 15 | 20-40% | 70-80% | <5 | 13 | c.35G>T, p.Gly12Val |
| 16 | 20-30% | 50-80% | <5 | 20 | c.35G>T, p.Gly12Val |
| 17 | 10-20% | 80% | <5 | 18 | c.35G>T, p.Gly12Val |
| 18 | 20-40% | 80% | 7 | 28 | c.35G>T, p.Gly12Val |
| 19 | 40-60% | 60-80% | <5 | <5 | wild type |
| 20 | 10-20% | 60-80% | <5 | 23 | c.35G>T, p.Gly12Val |
| 21 | 30-60% | 60-80% | 11 | 19 | c.35G>T, p.Gly12Val |
| 22 | 10-40% | 60-80% | <5 | 12 | c.35G>T, p.Gly12Val |
| 23 | 30-40% | 60-80% | 13 | 28 | c.35G>A, p.Gly12Asp |
| 24 | 10-30% | 80-90% | 10 | 27 | c.35G>A, p.Gly12Asp |
| 25 | 5-20% | 70-90% | <5 | 22 | c.35G>A, p.Gly12Asp |
| 26 | 20-50% | 70-90% | <5 | <5 | wild type |
| 27 | 30-50% | 80-90% | <5 | <5 | wild type |
| 28 | 10-20% | 80-90% | 7 | 20 | c.35G>A, p.Gly12Asp |
| 29 | 10-40% | 60-80% | 14 | 39 | c.34G>C, p.Gly12Arg |
| 30 | 10-30% | 70-90% | <5 | <5 | wild type |
| 31 | 20-30% | 60-90% | <5 | <5 | wild type |
| 32 | <10-20% | 60-80% | 29 | 43 | c.35G>T, p.Gly12Val |
DGM, digitally guided microdissection.
Figure 1.
KRAS mutant allele frequencies (upper panel) and estimated neoplastic cellularity (lower panel) for samples obtained by manual macrodissection or digitally guided microdissection (MilliSect).
Determination of neoplastic cellularity was highly subjective and varied by up to 40% between pathologists. The KRAS mutant allele fraction showed an average variation of only 5% and a maximum variation of 7% between replicates. Replicates and different FFPE blocks from the same tumor showed 100% concordance for KRAS genotype. Mutation detection was unreliable for samples where at least two of the three pathologists estimated the neoplastic cellularity to be below 50%. In all of the samples submitted for digitally guided microdissection, the estimated neoplastic cellularity was at least 50% for all three pathologists, and the average estimated neoplastic cellularity was 70-80%. The increased resolution of digitally guided microdissection allowed dissection of small clusters of tumor cells as shown in Figure 2.
Figure 2.
Example case (sample 16) showing a high grade pancreatic intraductal neoplasm in a patient with concurrent invasive pancreatic adenocarcinoma. The estimated neoplastic cellularity was 20% - 30% for the area circled with a slide marking pen for manual macrodissection and 50% - 80% for the areas circled digitally for milling microdissection. Although a KRAS mutation was undetectable in the manually macrodissected sample, a mutation (KRAS c.35G>T, p.Gly12Val, mutant allele frequency 20%) was detected in the sample obtained by digitally guided microdissection. The top image shows the intraductal neoplasm on the H&E slide at high (20×) magnification. The bottom two images are the aligned H&E digital image and the dissection slide (stained with aniline blue) with the region for milling dissection marked digitally in green.
The median total volume of tissue for manual macrodissection was 0.26 mm3 per slide (range 0.03-1.41 mm3), and the median total volume of tissue for digitally guided microdissection was 0.01 mm3 per slide (range 0.003-0.06 mm3). Despite the use of smaller tissue volumes for digitally guided microdissection, all of the extracted samples were sufficient for mutation profiling. In order to directly compare DNA yield and amplifiability per mm3 of extracted tissue, the manually marked areas were also dissected from additional serial sections using digitally guided microdissection; the average yield per mm3 was 0.017 ng for manual macrodissection, and 0.0185 ng for digitally guided microdissection. In this direct comparison of the same area dissected both ways, there was no statistically significant difference in the DNA yield by either PicoGreen fluorescence (p = 0.14) or quantitative PCR (p = 0.39). All dissection samples yielded a crossing threshold below 35 cycles, and the proportion of samples with a crossing threshold below 30 cycles was similar (56% for digitally guided microdissection and 63% for manual macrodissection).
The use of digital microscopy allowed for maximum flexibility in the slide annotation process. Pathologists did not need to have the physical slides but could instead circle tumor remotely through computer access. This simplified the workflow for slide annotation, alignment with unstained sections, and finally dissection. Using the MilliSect instrument to dissect tissue did take longer than manual macrodissection: on average, 5 minutes and 24 seconds per slide, versus 53 seconds per slide for manual macrodissection of the same area. Although more time consuming, digitally guided microdissection provided process documentation, including images of the reference slide and the dissection slide before and after dissection, the dimensions of the area dissected, and the time spent on each step of the process.
Discussion
We compared manual macrodissection to digitally guided microdissection for somatic mutation detection in difficult to dissect FFPE tumors. Pancreatic adenocarcinomas are a useful model of difficult to dissect tumors; these cancers typically infiltrate the surrounding non-tumor tissue and are often composed at least in part of single tumor cells and small clusters of tumor cells admixed with non-tumor stromal and inflammatory cells. By comparing dissection methods on adjacent sections from the same paraffin embedded tissue block, we were able to mitigate potential confounding factors such as mutant allele specific imbalance and intra-tumor genetic heterogeneity.
Not surprisingly, we found that the KRAS mutant allele fraction was a more precise measurement of tumor content than the estimated neoplastic cellularity. Several studies have documented the imprecision of pathologists’ estimation of tumor content, with overestimation errors being regarded as the most concerning for potential false negative results in molecular testing [22, 23]. In an attempt to mitigate the subjectivity of neoplastic cellularity determinations in our study, three pathologists independently and blindly evaluated each case. As has been shown in previous studies, the mean value of multiple independent estimates of neoplastic cellularity is more accurate than a single estimate generated by one pathologist [22].
Several samples yielded KRAS mutant allele fractions that were inconsistent with the estimated neoplastic cellularity and the expected heterozygous state. Two samples (2 and 5) showed a higher than expected mutant allele frequency. The most likely cause for this finding is loss of heterozygosity or copy number gain favoring the mutant allele. Mutant allele-specific imbalance has been reported in pancreatic adenocarcinomas and has been associated with progression to undifferentiated carcinoma [24]. Other samples showed mutant allele fractions that were lower than expected for a heterozygous mutation. Likely contributing factors for this discrepancy include the subjectivity of neoplastic cellularity estimation and the use of separate slides for histologic evaluation and dissection. There is always some variability in tissue composition between adjacent sections, and this variability can only be eliminated if microscopic review and tissue dissection are performed on the same slide. While a coverslip could be applied to a dissection slide and removed immediately prior to dissection, this approach is cumbersome and not adaptable to a high volume clinical laboratory. The use of a coverslipped H&E slide facilitates detailed morphologic review needed to discriminate tumor from non-tumor cells. Unfortunately, while more convenient, marking an H&E slide to guide dissection from adjacent sections can introduce extra variability in the tumor content. For our cases where the observed mutant allele fraction was lower than expected, it is possible that the alignment of the H&E slide to the dissection slides was impaired by a lack of tissue landmarks or by use of a reference image that was not of sufficiently high magnification for aligning small areas.
The quality assessment of the extracted DNA samples obtained by the two dissection methods showed that digitally guided microdissection does not appear to diminish the yield or the quality of retrieved DNA. This is an important finding, because the samples that would benefit the most from a more precise dissection technique are usually limited in overall tumor content, and efficient retrieval of nucleic acids is critical. Often there are only one or two sections available for molecular testing after the primary diagnostic workup using H&E, special stains, and immunohistochemistry.
In evaluating the application of digitally guided microdissection to clinical testing, laboratories must consider the costs and benefits of the technology. After the capital equipment cost of the instrumentation, the cost of increased technologist time plus the cost of the consumables must be considered. These costs may not be recoverable in the current laboratory test billing environment, and a specific CPT (Current Procedural Terminology) code does not yet exist for digitally guided microdissection. If use of the technology is restricted to samples deemed inadequate for manual macrodissection, the additional revenue from testing performed on such challenging samples could be useful in determining the return on investment (ROI). Laboratories connected with a hospital system may also consider the avoidance of additional costs for procuring an adequate sample for molecular testing as part of the overall ROI. While not all samples submitted for molecular testing are salvageable, a fraction of samples that could not be tested otherwise will be testable following digitally guided microdissection. Anecodotally, we have observed a slight decline in the percentage of samples that are rejected due to inadequate tumor content, from 2.9% to 2.8%, in the year following the adoption of digitally guided microdissection for difficult specimens.
The most salient finding in this study is that digitally guided microdissection can enrich tumor content well beyond manual macrodissection, thereby reducing the risk of false negative results in somatic mutation testing on difficult to dissect cases. This study also demonstrates the subjectivity of neoplastic cellularity estimations by pathologists and underscores the need to maximize tumor content, because samples approaching the low end of acceptability may in fact be inadequate for clinical testing. A recent summary of external quality assessment for KRAS mutation testing in colorectal cancer has demonstrated that erroneous results are more likely when tumor content is on the borderline for acceptability [25].
Maximizing the tumor burden prior to molecular testing is useful not only for eliminating potential false negative results, but also for limiting the need for additional biopsies to procure a sample for molecular testing. In the diagnostic workup of solid tumors, samples (especially biopsies) are frequently all but exhausted for immunohistochemistry testing and other ancillary studies prior to receipt in the molecular oncology lab; in these cases, an additional biopsy might be necessary for molecular testing. Another frequent scenario is that the tumor is scant and is so difficult to dissect manually that it cannot be used for testing. In this latter scenario, digitally guided microdissection can be performed on the sample to ensure adequate neoplastic cellularity for molecular testing. Samples such as cytological cell block preparations and needle biopsies with small populations of tumor cells can be very challenging to dissect manually because tumor cells are frequently in small clusters surrounded by non-tumor cells. Many difficult to dissect specimens are also challenging due to their small size, and it is important to preserve as much sample as possible during the dissection and extraction process. We have found that digitally guided microdissection is a highly effective method for enriching tumor content while preserving DNA yield.
Acknowledgements
This study was supported by grant funding from the Small Business Innovation Research Program at the National Institutes of Health (Grant numbers 1R43GM100645-01, 1R43GM101790-01, and 2R44GM100645-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Molinari F, Felicioni L, Buscarino M, De Dosso S, Buttitta F, Malatesta S, Movilia A, Luoni M, Boldorini R, Alabiso O, Girlando S, Soini B, Spitale A, Di Nicolantonio F, Saletti P, Crippa S, Mazzucchelli L, Marchetti A, Bardelli A, Frattini M. Increased detection sensitivity for KRAS mutations enhances the prediction of anti-EGFR monoclonal antibody resistance in metastatic colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4901–14. doi: 10.1158/1078-0432.CCR-10-3137. [DOI] [PubMed] [Google Scholar]
- 2.Monzon FA, Ogino S, Hammond ME, Halling KC, Bloom KJ, Nikiforova MN. The role of KRAS mutation testing in the management of patients with metastatic colorectal cancer. Archives of pathology & laboratory medicine. 2009;133:1600–6. doi: 10.5858/133.10.1600. [DOI] [PubMed] [Google Scholar]
- 3.Hadd AG, Houghton J, Choudhary A, Sah S, Chen L, Marko AC, Sanford T, Buddavarapu K, Krosting J, Garmire L, Wylie D, Shinde R, Beaudenon S, Alexander EK, Mambo E, Adai AT, Latham GJ. Targeted, high-depth, next-generation sequencing of cancer genes in formalin-fixed, paraffin-embedded and fine-needle aspiration tumor specimens. J Mol Diagn. 2013;15:234–47. doi: 10.1016/j.jmoldx.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, Fisher R, McGranahan N, Matthews N, Santos CR, Martinez P, Phillimore B, Begum S, Rabinowitz A, Spencer-Dene B, Gulati S, Bates PA, Stamp G, Pickering L, Gore M, Nicol DL, Hazell S, Futreal PA, Stewart A, Swanton C. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nature genetics. 2014;46:225–33. doi: 10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye X, Zhu ZZ, Zhong L, Lu Y, Sun Y, Yin X, Yang Z, Zhu G, Ji Q. High T790M detection rate in TKI- naive NSCLC with EGFR sensitive mutation: truth or artifact? Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:1118–20. doi: 10.1097/JTO.0b013e31829f691f. [DOI] [PubMed] [Google Scholar]
- 7.Morikawa T, Shima K, Kuchiba A, Yamauchi M, Tanaka N, Imamura Y, Liao X, Qian ZR, Brahmandam M, Longtine JA, Lindeman NI, Fuchs CS, Ogino S. No evidence for interference of h&e staining in DNA testing: usefulness of DNA extraction from H&E-stained archival tissue sections. American journal of clinical pathology. 2012;138:122–9. doi: 10.1309/AJCP28LAOOKSZSVW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabjani G, Kucera E, Schuster E, Minai-Pour M, Czerwenka K, Sliutz G, Leodolter S, Reiner A, Zeillinger R. Genetic alterations in endometrial hyperplasia and cancer. Cancer letters. 2002;175:205–11. doi: 10.1016/s0304-3835(01)00714-5. [DOI] [PubMed] [Google Scholar]
- 9.Going JJ. Histological microdissection in diagnostic and investigative pathology. Diagnostic Histopathology. 2009;16:43–8. [Google Scholar]
- 10.Beltinger CP, Debatin KM. A simple combined microdissection and aspiration device for the rapid procurement of single cells from clinical peripheral blood smears. Molecular pathology : MP. 1998;51:233–6. doi: 10.1136/mp.51.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JY, Dong SM, Kim SY, Yoo NJ, Lee SH, Park WS. A simple, precise and economical microdissection technique for analysis of genomic DNA from archival tissue sections. Virchows Archiv : an international journal of pathology. 1998;433:305–9. doi: 10.1007/s004280050253. [DOI] [PubMed] [Google Scholar]
- 12.Nzula S, Going JJ, Stott DI. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer research. 2003;63:3275–80. [PubMed] [Google Scholar]
- 13.Lukish JR, Muro K, DeNobile J, Katz R, Williams J, Cruess DF, Drucker W, Kirsch I, Hamilton SR. Prognostic significance of DNA replication errors in young patients with colorectal cancer. Annals of surgery. 1998;227:51–6. doi: 10.1097/00000658-199801000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA. Laser capture microdissection. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 15.Michel C, Desdouets C, Sacre-Salem B, Gautier JC, Roberts R, Boitier E. Liver gene expression profiles of rats treated with clofibric acid: comparison of whole liver and laser capture microdissected liver. The American journal of pathology. 2003;163:2191–9. doi: 10.1016/S0002-9440(10)63577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HK, Kim J, Korolevich S, Choi IJ, Kim CH, Munroe DJ, Green JE. Distinctions in gastric cancer gene expression signatures derived from laser capture microdissection versus histologic macrodissection. BMC medical genomics. 2011;4:48. doi: 10.1186/1755-8794-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Bruin EC, van de Pas S, Lips EH, van Eijk R, van der Zee MM, Lombaerts M, van Wezel T, Marijnen CA, van Krieken JH, Medema JP, van de Velde CJ, Eilers PH, Peltenburg LT. Macrodissection versus microdissection of rectal carcinoma: minor influence of stroma cells to tumor cell gene expression profiles. BMC genomics. 2005;6:142. doi: 10.1186/1471-2164-6-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez S, Lloreta J. Manual versus laser micro-dissection in molecular biology. Ultrastructural pathology. 2006;30:221–8. doi: 10.1080/01913120500521018. [DOI] [PubMed] [Google Scholar]
- 19.Adey N, Emery D, Bosh D, Callahan S, Schreiner J, Chen Y, Greig A, Geiersbach K, Parry R. A mill based instrument and software system for dissecting slide-mounted tissue that provides digital guidance and documentation. BMC clinical pathology. 2013;13:29. doi: 10.1186/1472-6890-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–54. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 21.Reid MD, Saka B, Balci S, Goldblum AS, Adsay NV. Molecular genetics of pancreatic neoplasms and their morphologic correlates: an update on recent advances and potential diagnostic applications. American journal of clinical pathology. 2014;141:168–80. doi: 10.1309/AJCP0FKDP7ENVKEV. [DOI] [PubMed] [Google Scholar]
- 22.Viray H, Li K, Long TA, Vasalos P, Bridge JA, Jennings LJ, Halling KC, Hameed M, Rimm DL. A prospective, multi-institutional diagnostic trial to determine pathologist accuracy in estimation of percentage of malignant cells. Archives of pathology & laboratory medicine. 2013;137:1545–9. doi: 10.5858/arpa.2012-0561-CP. [DOI] [PubMed] [Google Scholar]
- 23.Smits AJ, Kummer JA, de Bruin PC, Bol M, van den Tweel JG, Seldenrijk KA, Willems SM, Offerhaus GJ, de Weger RA, van Diest PJ, Vink A. The estimation of tumor cell percentage for molecular testing by pathologists is not accurate. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014;27:168–74. doi: 10.1038/modpathol.2013.134. [DOI] [PubMed] [Google Scholar]
- 24.Krasinskas AM, Moser AJ, Saka B, Adsay NV, Chiosea SI. KRAS mutant allele-specific imbalance is associated with worse prognosis in pancreatic cancer and progression to undifferentiated carcinoma of the pancreas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26:1346–54. doi: 10.1038/modpathol.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tembuyser L, Ligtenberg MJ, Normanno N, Delen S, van Krieken JH, Dequeker EM. Higher Quality of Molecular Testing, an Unfulfilled Priority: Results from External Quality Assessment for KRAS Mutation Testing in Colorectal Cancer. J Mol Diagn. 2014;16:371–7. doi: 10.1016/j.jmoldx.2014.01.003. [DOI] [PubMed] [Google Scholar]