Abstract
As serotonin reuptake inhibitor (SRI) use may decrease platelet function, previous research has shown a relationship between SRI use and an increased risk for bruising and bleeding. The literature regarding the association between SRI use during pregnancy and increased bleeding at delivery, referred to as postpartum hemorrhage (PPH), is mixed. In secondary analyses from two prospective observational studies of pregnant women with mood disorders, 263 women were exposed to an SRI (n=51) or not (n=212) in the third trimester. To be precise, we used the terminology estimated blood loss (EBL) > 600 cc rather than the term PPH because the current definition of PPH differs. The occurrence of EBL > 600 cc was determined using the Peripartum Events Scale (PES) completed from obstetrical records by a blinded medically trained member of the study team. EBL > 600 cc occurred in 8.7% of women in this cohort. There was no statistically significant difference in the rates of EBL > 600 cc in the 24 hours after delivery in women taking SRIs during the third trimester (9.8%) compared to non-exposed women (8.5%). Utilizing generalizing estimating equations, the odds of EBL > 600 cc in each group were not significantly different (OR 1.17, CI-0.41-3.32, p=0.77). When the SRI group was limited to women with exposure at the time of delivery, the difference in the odds of EBL > 600 cc was unchanged (OR 1.16, CI=0.37-3.64, p=0.79). In population, both third trimester and use at delivery of SRIs during pregnancy was not associated with an increased risk of excessive blood loss.
Introduction
Major depressive disorder occurs in 8-13% of pregnant women (Bennett et al., 2004, Gavin et al., 2005, Vesga-Lopez et al., 2008), and approximately 4-8% of pregnant women are exposed to serotonin reuptake inhibitor antidepressants (SRIs) (Alwan et al., 2011, Andrade et al., 2008, Mitchell et al., 2011). Antidepressant use during pregnancy increased from less than 1% prior to 1990 to 7.5% in 2006-08 (Alwan et al., 2011). Some investigations (Grzeskowiak et al., 2015, Lindqvist et al., 2014, Palmsten et al., 2013) but not all (Lupattelli et al., 2014, Salkeld et al., 2008) have shown an association between third trimester SRI or antidepressant use and postpartum hemorrhage (PPH). In some non-pregnant populations SRI use is associated with an increased bleeding risk (Anglin et al., 2014, Hankey, 2014, Harirchian et al., 2012, Jiang et al., 2015, Tavakoli et al., 2012). The impact of SRIs on increased bleeding risk is inconsistent in non-pregnant populations as well. For example, these drugs have been associated with spontaneous gastrointestinal bleeding (Andrade et al., 2010, Lee et al., 2012) but not hemorrhage during surgical procedures (Tavakoli et al., 2012).
PPH is a primary cause of maternal morbidity and mortality (Rath, 2011, World Health Organization, 2007) and occurs in 4-6% of births (Combs et al., 1991, Salkeld et al., 2008). PPH is classified as primary (occurring within 24 hours of delivery) or secondary (occurring from 24 hours after delivery to 12 weeks postpartum), also referred to as acute or delayed PPH (American College of Obstetricians and Gynecologists, 2006). Almost 50% of all postpartum deaths are attributed to acute PPH (Rath, 2011). Risk factors for PPH include placental abruption or previa, multiple gestation, gestational hypertension, pre-eclampsia, prolonged or induced labor, infection, obesity, use of forces, vaginal trauma and multiple previous births; however, each individual risk factor is a poor predictor of PPH (Mathai et al., 2007). PPH is generally related to uterine atony but can be caused by defects in coagulation. Antidepressants that affect serotonergic pathways have the potential to decrease platelet aggregation and increase bleeding; thus the question of whether third trimester SRI use is associated with an increased risk of PPH is clinically relevant.
Six investigators have examined the association between SRI use during pregnancy and PPH with two reporting no significant association, three finding a significant positive relationship ,and one reporting results that are difficult to interpret due to a non-specific definition of PPH (Reis and Kallen, 2010). Given these disparate findings in the literature we used our well characterized cohort to conduct a secondary analysis to evaluate whether women taking SRIs in the third trimester differed with respect to the incidence of acute postpartum blood loss from women not taking SRIs.
Methods
Subjects
The sample is comprised of women from two National Institute of Mental Health-supported observational studies (Antidepressant Use during Pregnancy, R01-MH60335; and Antimanic Use During Pregnancy, R01-MH07592; Principal investigator: K.L.W.) with similar designs that followed women throughout pregnancy and 12 months after birth. Details of the study procedures have been reported elsewhere (Wisner et al., 2009). Briefly, women with a lifetime history of major depressive disorder (MDD) (n=173) or bipolar disorder (n=151) and a comparison group of women with neither psychiatric disorder (n=140) were enrolled in Cleveland, Ohio and Pittsburgh, Pennsylvania from 2000 to 2011. Pregnant women between 18-44 years old were recruited by self-referral, physician referral, advertising, and screening in obstetric ultrasound suites. Main exclusion criteria included active substance abuse disorders or gestational exposure to benzodiazepines or prescription drugs defined as category D or X by the U.S. Food and Drug Administration (FDA). In addition, for this analysis, women on medications that could affect bleeding (e.g. Omega-3 fatty acids, aspirin, etc.) were excluded (for a complete list see Appendix I (UW Health)). All procedures and consents were approved by the institutional review boards from Case Western Reserve University and University of Pittsburgh. All women provided written informed consent prior to study entry.
A total of 263 women with singleton gestations were divided into exposed (n=51) and unexposed (n=212) groups, based on whether they took an SRI in the third trimester. Women were classified as exposed if they were taking an SRI in the third trimester (defined as >27 weeks gestation). Unexposed women were not taking an SRI during pregnancy at any time. The exposure timeframe was determined by adding one week to the start date and two weeks to the stop date for the SRI to account for time needed to develop meaningful levels following SRI initiation and to achieve clearance following SRI cessation. For all women, baseline assessments (prior to or at week 20 of pregnancy) included a clinical interview, physical measurements, a urine drug screen, a blood draw for drug levels (if an antidepressant or anti-manic drug was being taken), and completion of questionnaires. Women were assessed again at 30 and 36 weeks gestation and at 2, 12, 26 and 52 weeks after birth. Follow-up assessments included the same measurements (excluding the urine drug screen) as those administered at baseline.
Measures
Demographic characteristics were assessed at baseline via standard instruments and included: age, race, employment status (categorized as employed/unemployed), and education (dichotomized by college completion). Psychiatric diagnosis was established at baseline via the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1994). Pre-pregnancy body mass index (BMI) was calculated using the woman’s reported pre-pregnancy weight and measured height. The Peripartum Events Scale (PES) (O'Hara, 1986) is an 11-category scale which records obstetric history, medical history, indication for labor and delivery, method of delivery, duration of labor and infant outcome. It also assesses delivery complications which include estimated blood loss > 600 cc, which we used as our primary outcome. Because this definition is differs from the current definition of PPH, which is > 500 cc blood loss after vaginal delivery and >1000 cc blood loss after cesarean delivery (American College of Obstetricians and Gynecologists, 2006), our primary outcome is specifically named estimated blood loss (EBL) > 600 cc. The PES was completed through abstraction from obstetrical records by a blinded medically trained member of the study team (an obstetrician, a nurse doctoral student, or a nurse family practitioner).
For interviews after the baseline assessment, the Longitudinal Interval Follow-up Evaluation (LIFE) was used in conjunction with the SCID to assess for diagnostic status change. Depressive symptoms were assessed with the 29-item Structured Interview Guide for the Hamilton Depression Rating Scale with the Atypical Depression Supplement (SIGH-ADS) (Williams and Terman, 2003). Psychotropic drug exposure was documented by recording the subject’s drug doses at each visit and documenting any changes that occurred between visits (Wisner et al., 2009). Use of tobacco, alcohol, and illicit drugs was assessed at each visit.
Data Analysis
Women with third-trimester SRI exposure were compared to women with no SRI exposure during pregnancy on sociodemographic, behavioral, clinical, and delivery measures. Descriptive statistics were calculated as means and standard deviations for continuous measures and as frequencies and percentage distributions for categorical measures. Tests of association included Student’s t or Mann-Whitney U for continuous measures (depending on whether distributional assumptions were met) and Chi-Square and Fisher’s exact for categorical measures (depending on expected cell frequencies). Due to some mothers having more than one pregnancy while participating in the study, hemorrhaging during delivery was modeled using generalized estimating equations to account for the possibility of correlation among siblings. Three models were estimated: Model I included SRI exposure alone; in Model II we added imbalanced baseline measures including age, race, education, parity, and comorbid anxiety to the SRI exposure variable; Because Lupattelli et al. (2014) reported that depression was independently associated with vaginal bleeding, Model III added the baselineSIGH-ADS29 to the measures in Model II.
Results
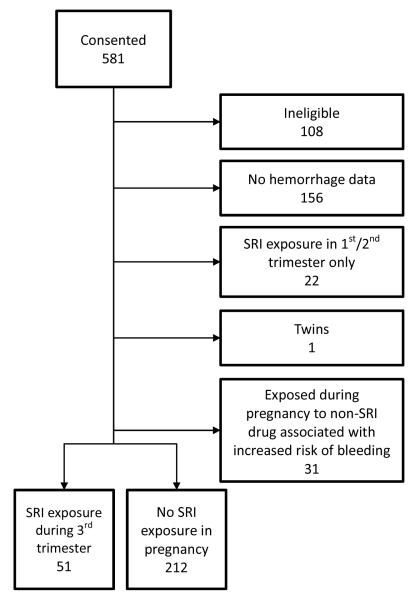
Of the 581 women screened, 473 were eligible for study entry (Figure 1). For the purpose of this analysis, women were initially excluded if: a) hemorrhage data were not available (usually due to delivery at an outside hospital, n=156), b) they were exposed to an SRI prior to the third trimester but not during the third trimester (n=22), c) they had a twin gestation (n=1), or d) they reported taking a drug that could increase the risk of bleeding such as a non-steroidal anti-inflammatory, anticoagulant, or anti-platelet agent as identified in reference 27 (n=31). Secondarily, we assessed whether including women exposed to SRIs and drugs that increased bleeding impacted our results.
Figure 1.
CONSORT Chart
EBL > 600 cc occurred in 8.7% of women in this cohort (Table 3). There was no statistically significant difference in rates of acute excessive blood loss in women taking SRI during the third trimester (9.8%) versus women not exposed (8.5%). The women taking an antidepressant in the third trimester were significantly older (p = 0.0002), Caucasian (p=0.0008) and had more pregnancies (p=0.0337) (Table 1). Women with SRI exposure were more likely to have a secondary anxiety disorder diagnosis by SCID criteria (37.3% vs 23.6%, p=0.0463) including obsessive compulsive disorder (9.8% vs 2.4%, p=0.0263) (Table 2). In addition, women with third trimester SRI use had lower scores on the Global Assessment of Functioning Scale (GAS) (p=0.0036), and the SF12 Mental Scale (p=0.0001), and higher depression scores as measured by the SIGH-ADS (p=0.0268).There was no difference in cesarean section rates between groups but women with SRI exposure were less likely to have developed secondary arrest of labor (p=0.0314). Breastfeeding rates were not statistically different between groups.
Table 3.
Delivery measures by group
| Group |
Analysis |
||||
|---|---|---|---|---|---|
| Measure | All (N=263) |
SRI Exposed (N=51) |
Unexposed (N=212) |
Test statistic |
p |
| Secondary arrest of labor | χ2(1) = 4.63 | 0.0314 | |||
| Requiring Oxytocis | 53 (21.1) | 5 (10.0) | 48 (23.9) | ||
| Not Requiring Oxytocis | 198 (78.9) | 45 (90.0) | 153 (76.1) | ||
| Caesarean section | 54 (20.5) | 12 (23.5) | 42 (19.8) | χ2(1) = 0.35 | 0.5551 |
| Other method of delivery | 142 (54.0) | 27 (52.9) | 115 (54.2) | χ2(1) = 0.03 | 0.8668 |
| Blood loss > 600 cc | 23 (8.7) | 5 (9.8) | 18 (8.5) | TP(0) = 0.20 | 0.7833 |
| Significant lacerations | 35 (13.3) | 5 (9.8) | 30 (14.2) | χ2(1) = 0.67 | 0.4119 |
Abbreviations TP table probability from Fisher’s exact test.
Table 1.
Demographic and behavioral measures by group
| Group |
Analysis |
||||
|---|---|---|---|---|---|
| Measure | All (N=263) |
SRI Exposed (N=51) |
Unexposed (N=212) |
Test statistic |
p |
| Age | 28.5 ± 5.94 | 31.3 ± 5.08 | 27.8 ± 5.96 | t(261) = 3.81 | 0.0002 |
| Race | χ2(2) = 11.7 | 0.0029 | |||
| White | 187 (71.1) | 46 (90.2) | 141 (66.5) | ||
| Black | 63 (24.0) | 5 (9.8) | 58 (27.4) | ||
| Other | 13 (4.9) | 0 (0.0) | 13 (6.1) | ||
| White race | 187 (71.1) | 46 (90.2) | 141 (66.5) | χ2(1) = 11.2 | 0.0008 |
| Education level | χ2(4) = 9.77 | 0.0444 | |||
| <High school | 29 (11.1) | 4 (7.8) | 25 (11.8) | ||
| High school | 45 (17.2) | 2 (3.9) | 43 (20.4) | ||
| Some college | 62 (23.7) | 14 (27.5) | 48 (22.7) | ||
| College | 69 (26.3) | 16 (31.4) | 53 (25.1) | ||
| Graduate school | 57 (21.8) | 15 (29.4) | 42 (19.9) | ||
| Completed college | 126 (48.1) | 31 (60.8) | 95 (45.0) | χ2(1) = 4.09 | 0.0432 |
| Employed | 133 (51.8) | 22 (44.9) | 111 (53.4) | χ2(1) = 1.14 | 0.2859 |
| Marital status | TP(0) < 0.01 | 0.0285 | |||
| Single | 98 (37.3) | 12 (23.5) | 86 (40.6) | ||
| Married/cohabiting | 155 (58.9) | 35 (68.6) | 120 (56.6) | ||
| Divorced/separated | 9 (3.4) | 4 (7.8) | 5 (2.4) | ||
| Widowed | 1 (0.4) | 0 (0.0) | 1 (0.5) | ||
| Married/cohabiting | 155 (58.9) | 35 (68.6) | 120 (56.6) | χ2(1) = 2.46 | 0.1171 |
| Parity | 2.08 ± 1.15 | 2.41 ± 1.33 | 2.00 ± 1.09 | U(1) = 4.51 | 0.0337 |
| Parity | TP(0) < 0.01 | 0.1351 | |||
| 1 | 89 (34.6) | 13 (25.5) | 76 (36.9) | ||
| 2 | 99 (38.5) | 19 (37.3) | 80 (38.8) | ||
| 3+ | 69 (26.8) | 19 (37.3) | 50 (24.3) | ||
| Pre-pregnancy BMI | 27.2 ± 7.25 | 28.1 ± 6.82 | 27.0 ± 7.35 | U(1) = 2.29 | 0.1303 |
| Pre-pregnancy BMI 30+ | 74 (29.7) | 16 (31.4) | 58 (29.3) | χ2(1) = 0.08 | 0.7720 |
| Smoked tobacco | 52 (19.9) | 12 (23.5) | 40 (19.0) | χ2(1) = 0.52 | 0.4723 |
| Drank alcohol | 63 (24.0) | 11 (21.6) | 52 (24.6) | χ2(1) = 0.21 | 0.6446 |
| Used illicit drugs | 33 (12.6) | 3 (5.9) | 30 (14.2) | χ2(1) = 2.59 | 0.1074 |
| Ever breastfed | 154 (69.4) | 30 (65.2) | 124 (70.5) | χ2(1) = 0.47 | 0.4926 |
Abbreviations BMI body mass index; TP table probability from Fisher’s exact test.
Table 2.
Clinical measures by group
| Group |
Analysis |
||||
|---|---|---|---|---|---|
| Measure | All (N=263) |
SRI Exposed (N=51) |
Unexposed (N=212) |
Test statistic |
p |
| Alcohol abuse | 7 (2.7) | 3 (5.9) | 4 (1.9) | TP(0) = 0.11 | 0.1350 |
| Anxiety | 69 (26.2) | 19 (37.3) | 50 (23.6) | χ2(1) = 3.97 | 0.0463 |
| Drug abuse | 9 (3.4) | 2 (3.9) | 7 (3.3) | TP(0) = 0.30 | 0.6874 |
| Generalized anxiety | 13 (4.9) | 1 (2.0) | 12 (5.7) | TP(0) = 0.19 | 0.4733 |
| Obsessive-compulsive | 10 (3.8) | 5 (9.8) | 5 (2.4) | TP(0) = 0.02 | 0.0263 |
| Panic | 15 (5.7) | 3 (5.9) | 12 (5.7) | TP(0) = 0.26 | 1.0000 |
| Post-traumatic stress | 18 (6.8) | 5 (9.8) | 13 (6.1) | TP(0) = 0.15 | 0.3575 |
| Tetrahydrocannabinol | 9 (3.4) | 2 (3.9) | 7 (3.3) | TP(0) = 0.30 | 0.6874 |
| GAS | 79.0 ± 12.9 | 75.3 ± 11.5 | 80.0 ± 13.1 | U(1) = 8.48 | 0.0036 |
| SF12 Mental scale | 49.9 ± 11.6 | 43.2 ± 13.1 | 51.4 ± 10.6 | U(1) = 14.9 | 0.0001 |
| SF12 Physical scale | 45.5 ± 9.28 | 45.7 ± 9.14 | 45.4 ± 9.35 | t(192) = 0.16 | 0.8735 |
| SIGH-ADS29 | 12.3 ± 7.55 | 14.5 ± 7.90 | 11.7 ± 7.37 | t(215) = 2.23 | 0.0268 |
| SIGH-ADS29 ⩾ 18 | 49 (22.6) | 16 (35.6) | 33 (19.2) | χ2(1) = 5.47 | 0.0194 |
Abbreviations GAS Global assessment scale; SF Short form health survey; SIGH-ADS Structured interview guide for the Hamilton rating scale for depression with atypical depressive symptoms scale; TP table probability from Fisher’s exact test.
Utilizing generalized estimating equations, we found that the difference in the odds of EBL > 600 cc between exposure groups was not significant (OR=1.17, CI=0.41-3.32, p=0.77). In the adjusted model, group differences remained non-significant (OR=1.63, CI=0.52-5.12, p=0.41). Group differences in the odds of EBL > 600 cc also remained non-significant when depression scores were added to the adjusted model (OR=0.88, CI=0.22-3.57, p=0.86). When the SRI-exposed group was limited to women with SRI exposure at the time of delivery, group differences remained non-significant in the unadjusted models (OR=1.16, CI=0.37-3.64, p=0.79) and adjusted models with (OR=0.93, CI=0.23-3.78, p=0.92) and without (OR=1.28, CI=0.38-4.33, p=0.69) depression scores. Finally, when we included women taking other drugs that might increase the risk for bleeding in the SRI-exposed group, there was again no significant difference (OR=1.06, CI=0.37-2.98, p=0.92).
Discussion
In this cohort of women followed prospectively during pregnancy and postpartum, third trimester SRI use was not significantly associated with an increased risk of EBL > 600 cc. These findings are in agreement with two prior studies (Lupattelli et al., 2014, Salkeld et al., 2008). SRIs significantly decrease platelet serotonin which can result in impaired platelet aggregation (Javors et al., 2000); however, a recent study did not confirm a change in platelet aggregation or flow-mediated vascular dilatation over a four week period in a healthy group of young women (Hantsoo et al., 2014). Maayan-Metzger et al. (2006) examined platelet function in newly postpartum mothers taking SSRI compared to controls. They found no significant correlations between SSRI use and surface coverage or average size (indicators of platelet function).
Because the effect of SRI on platelet aggregation is an acute effect, the timing of the consumption of the medication and the evaluation of the risk for bleeding is critical (Andrade et al., 2010).
Compared to previous studies, ours had the most comprehensive patient assessments. In a prospective cohort study by Lupattelli et al. (2014) (n=57,279), self-report of SRI use (n=123) from gestational week 30 through the end of pregnancy did not significantly increase the risk of acute PPH after vaginal (aOR, 0.90; 95% CI, 0.47-1.74) or cesarean (aOR, 1.47; 95% CI, 0.51-4.22) delivery. PPH, defined as > 500 cc of blood loss and medically confirmed, occurred in 14.4% of the whole cohort. Neither depressive symptoms nor mode of delivery was associated with PPH. However, women with depressive symptoms had an increased rate of vaginal bleeding during pregnancy. Salkeld et al. (2008) conducted a case-control analysis of women who had an antidepressant prescription filled in the 180 days before delivery between 1999 and 2005. In the 59 cases of women who had filled an SRI prescription in the 90 days prior to delivery and 442 matched controls, there was no statistically significant increase in acute or delayed PPH (OR 1.30, 95% CI 0.98-1.72). PPH was broadly defined by 10 International Classification of Diseases (ICD) 9 and 10 codes. Women with PPH were more likely to have known PPH risk factors such as hypertension during pregnancy, placental problems, prolonged or obstructed labor, and previous PPH. The diagnosis for which the SRI was prescribed was not reported.
Three investigators reported an association between SRI use during pregnancy and PPH. Using Medicaid data from a cohort of women who delivered between 2000-07 and had a diagnosis of a mood or anxiety disorder (n=106,000), Palmsten et al. (2013) found that SRI use at the time of delivery (n=12,710) was associated with an increased risk for an ICD diagnosis of PPH (OR 1.47, 95% CI 1.33-1.62) while antidepressants taken at other times during gestation were not. The risk of PPH when taking an SRI was 4%, which was similar to the general population risk. Of note, the risk was increased with both serotonergic and non-serotonergic antidepressants. There was no difference in PPH rate among unexposed women with (2.8%) or without a mood or anxiety diagnosis (2.4%). The investigators were unable to control for BMI, smoking, or alcohol use in this cohort. Lindqvist et al. (2014) used a Swedish university hospital’s records from 2007-2011 to compare women who reported SRI use at their first prenatal visit (n=500) to those without SRI exposure (n=39,594). They found that women exposed to an SRI in pregnancy with vaginal non-surgical deliveries had significantly higher rates of PPH (OR, 2.6; 95% CI, 2.0-3.5), greater blood loss (484 mL vs. 398 mL, p<0.001), and longer hospitalizations (3.8 days vs. 2.4 days, p<0.001). The risk of PPH with SRI use was 18.0% vs. 8.7% in non-users. SRI-exposed women also had higher BMIs, were more likely to be smokers, and more often received epidural analgesia. In Australia, Grzeskowiak et al. (2015) examined records from 28,348 unexposed women, 1,292 women who had unspecified psychiatric illness but no antidepressant use, and 558 women who used antidepressants in late gestation. They found that women exposed to antidepressants had an increased risk of PPH compared to controls (aRR 1.53; 95% CI 1.25-1.86), while women with psychiatric illness but no antidepressant exposure had no increase in risk (aRR 1.04; 95% CI 0.89-1.23). The investigators did not evaluate exposure to other medications associated with increased bleeding or differentiate among various types of psychiatric illness or antidepressants in their analyses. Other investigations have demonstrated that restricting the study cohort to women with only major depression compared to non-specified psychiatric disorders substantially reduces the association between drug exposure and reproductive outcomes (such as birth defects (Huybrechts et al., 2014) and persistent pulmonary hypertension of the newborn (Huybrechts et al., 2015)). Restriction of the cohort to women with a depression diagnosis (treated with SSRI and non-treated with SSRI) controls for the potential effect of the underlying illness or factors associated with it.
Reis and Kallen (2010) analyzed data from the Swedish Medical Birth Register from 1995-2007, and found that bleeding during delivery was significantly more common in women who took antidepressants only early in gestation (OR, 1.33, CI, 1.20-1.46), only late in gestation (OR, 1.45, CI, 1.27-1.65), and both early and late in gestation (OR, 1.58, CI, 1.36-1.84). Women using antidepressants were older, had lower parity, smoked more and had higher BMIs. Information on dose and exact timing of antidepressant use was incomplete. In addition, this study was designed to examine the rate of congenital malformations and so increased bleeding was defined as “during partus” and “after partus” which makes the data difficult to compare to other studies.
Our study is unique in several ways. We had the most extensive list of co-medications that could add additional risk to bleeding from SRI exposure. Bleeding may be more likely to occur if antidepressants are taken concurrently with non-steroidal anti-inflammatory drugs (NSAIDs), anticoagulants, or antiplatelet agents because the mechanism of anticoagulation for antidepressants is different than for each of these medications, which results in additive risk (Andrade et al., 2010, Juurlink, 2011). To examine this possibility we conducted an additional analysis where women who were on an SRI and a medication associated with bleeding, but otherwise met inclusion criteria for this analysis, were included. With these women included we still did not observe a significant difference between the SRI-exposed and non-exposed groups. We collected antidepressant data prospectively throughout the study and adherence was confirmed by plasma sampling (Wisner et al., 2009). Only the Palmsten et al. (2013) study evaluated antidepressant use at delivery as a separate variable from use during the third trimester. When we limited our exposed cohort to those with SRI exposure at delivery, we, unlike Palmsten et al. (2013), did not find increased risk of PPH.
PPH was three times more common in our cohort compared to the one reported by Palmsten et al. (2013) but less prevalent than in the Lupattelli et al. (2014), Grzeskowiak et al. (2015), and Reis and Kallen (2010) studies. One might expect that if a woman is known to be exposed to an SRI, the obstetrician might overestimate blood loss, thereby increasing the incidence of EBL > 600 cc in the SRI-exposed group, which we did not find.
Reis and Kallen (2010) and Lupattelli et al. (2014) assessed depression with rating scales, Palmsten et al. (2013) used medical record diagnoses of depression, Grzeskowiak et al. (2015) used medical record diagnoses of any psychiatric illness, and Salkeld et al. (2008) and Lindqvist et al. (2014) did not assess psychiatric diagnosis or depressive symptoms. It is plausible that the association between antidepressants and increased risk of PPH or increased EBL at delivery may be due to factors related to depression, based on the observation that positive associations were reported with non-serotonergic antidepressants. This may be the reason many of the studies reported increased rates of PPH compared to the general population rates. Elevated platelet reactivity has been seen in a small cohort of depressed subjects compared to non-depressed subjects such that enhanced platelet activity is thought to contribute to the increased risk for coronary artery disease in depression (Musselman et al., 1996)). Mean platelet volume and platelet activating factors are also increased in depressed cohorts (Mazereeuw et al., 2013).
PPH diagnosis is difficult because visual estimation of blood loss is often incorrect (Rath, 2011). Estimation of blood loss by calibrated bags or a decline in hematocrit of at least 10% increases the accuracy of measurement (American College of Obstetricians and Gynecologists, 2006). Among the six reviewed previously published cohorts, Grzeskowiak et al. (2015) used data recorded in the Perinatal Statistics Collection where PPH was defined as blood loss >500 mL following vaginal delivery and >1000 mL following a caesarean section, Palmsten et al. (2013) defined PPH by International Classification of Diseases, Ninth Revision (ICD-9) coding, Salkeld et al. (2008) and Lindqvist et al. (2014) used PPH as defined by ICD-10 coding, Lupattelli et al. (2014) used medical records to obtain information on PPH defined as > 500 ml blood loss, and Reis and Kallen (2010) referred to “bleeding during partus” without further definition. Our definition was set as > 600 ml blood loss according to the PES and was visually estimated by the attending obstetrician.
Since PPH is most likely due to a failure of uterine muscle contraction, and because serotonergic agents increase uterine tone, antidepressant-induced platelet dysfunction may not cause sufficient perturbation to result in PPH (Andrade et al., 2010). Therefore, we considered whether other medications that would increase bleeding would increase the association of third trimester SRI use and PPH, but we did not observe a significant association.
In summary, we did not find a positive association with third trimester SRI use and EBL > 600 cc. The most rigorous study to find an association found an excess of 1 PPH case for every 80-97 women treated with an antidepressant, and the risk was not associated with serotonergic antidepressants alone (Palmsten et al., 2013). This brings into question the biological plausibility based on the impact of these drugs on platelet serotonin. For the vast majority of women, SRIs are unlikely to be associated with PPH. However, women rarely may have pharmacogenetic or hematologic vulnerability. Inter-individual variability in both adverse reactions and drug response is a major problem in medical practice. Factors that influence drug response include age, race, ethnicity, concomitant diseases, smoking, alcohol and other drug use, and diet (Samer et al., 2013). Pharmacogenetic research has demonstrated dramatic differences in plasma concentrations due to polymorphisms, such as in cytochrome P4502D6, which is involved in the metabolism of all SRI. Poor metabolizers may develop unusually high plasma concentrations in pregnancy, particularly of paroxetine (Ververs et al., 2009). For example, if a woman is a poor CYP2C9 metabolizer, the use of a non-steroidal anti-inflammatory drug plus an SRI could theoretically increase her risk of bleeding (Samer et al., 2013). Hematologic abnormalities such as severe gestational thrombocytopenia (Gernsheimer et al., 2013) or hemoglobinopathies (Samuels, 2012) would also potentially increase bleeding risk for a woman taking an SRI.
Limitations
We did not examine SRI dose as a predictor of EBL > 600 cc because there is wide inter-individual variation in metabolism of antidepressants, particularly during pregnancy. In addition, the PES used for this retrospective study included only one item for EBL defined as >600 cc. This does not incorporate the traditional obstetrical cutoffs (American College of Obstetricians and Gynecologists, 2006) that define PPH as >500 cc for vaginal delivery and >1000 cc for cesarean delivery. Further, we were unable to confirm EBL by measuring blood loss, and needed to rely on the obstetrician’s report of the volume of blood loss. Approximately one third of our cohort did not have PPH data and although there were few demographic differences between groups, and missingness was not related to SRI exposure (data not shown), a larger sample would have been preferable. In addition, we were only able to examine acute EBL, as we did not collect data on delayed EBL. We could not control for all PPH risk factors due to the limited statistical power based on our N that we obtained.
In conclusion, this study adds to the literature suggesting that EBL > 600 cc within the first 24 hours following delivery is not affected by SRI use in the third trimester or at delivery. While additional studies that can control for more risk factors would be welcomed, at this time, there is no support to recommend tapering an SRI prior to delivery to prevent increased bleeding risk at delivery.
Highlights.
Estimated blood loss (EBL) > 600 cc occurred in 8.7% of women in this cohort
We found no association between third trimester SRI use and EBL > 600 cc
We found no association between SRI use at delivery and EBL > 600 cc
Acknowledgments
This research was supported by NIMH grant 5R01 MH60335 (PI: K.L.W), NIHM grant R01 MH07592 (PI: K.L.W.), and NIMH grant 5K23 MH092399-05 (D.R.K).
Dr. Wisniewski has current grant funding from Johnson and Johnson
Appendix I. Excluded Exposures: Medications and Herbs that May Affect Bleeding (UW Health)
Anticoagulants
Anisindione
Enoxaparin injection
Heparin injection
Pentosan polysulfate
Warfarin
Anti-platelet Agents
Anagrelide
Aspirin
Cilostazol
Clopidogrel
Dipyradamole
Dipyridamole/aspirin
Enteric coated aspirin
Ticlopidine
Non-steroidal anti-inflammatory drugs
Diclofenac
Diflunisal
Etodolac
Fenoprofen
Flurbiprogen
Ibuprofen
Indomethacin
Ketoprofen
Ketorlac
Meclofenamate
Meloxican
Nabumeton
Naproxen
Oxaprozin
Piroxicam
Salsalate
Sulindac
Sulfinpyrazone
Herbs/Vitamins
Ajoene
Birch bark
Cayenne
Chinese black tree fungus
Cumin
Evening primrose oil
Feverfew
Garlic
Ginger
Ginkgo biloba
Ginseng
Grapeseed extract
Milk Thistle
Omega -3-fatty acid
Onion extract
St. John’s Wort
Tumeric
Vitamins C and E
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: The Department of Psychiatry at Northwestern University receives contractual fees for Dr. Wisner's consultation to Quinn Emanuel Urquhart & Sullivan, LLP (New York City), who represent Pfizer Pharmaceutical Company.
Conflict of Interest:
The Department of Psychiatry at Northwestern University receives contractual fees for Dr. Wisner's consultation to Quinn Emanuel Urquhart & Sullivan, LLP (New York City), who represent Pfizer Pharmaceutical Company.
The remaining authors have no conflicts to report.
References
- Alwan S, Reefhuis J, Rasmussen SA, Friedman JM. Patterns of antidepressant medication use among pregnant women in a United States population. J Clin Pharmacol. 2011;51:264–270. doi: 10.1177/0091270010373928. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039–1047. doi: 10.1097/00006250-200610000-00046. [DOI] [PubMed] [Google Scholar]
- Andrade C, Sandarsh S, Chethan KB, Nagesh KS. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71:1565–1575. doi: 10.4088/JCP.09r05786blu. [DOI] [PubMed] [Google Scholar]
- Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198:194.e191–195. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Anglin R, Yuan Y, Moayyedi P, Tse F, Armstrong D, Leontiadis GI. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811–819. doi: 10.1038/ajg.2014.82. [DOI] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- Combs CA, Murphy EL, Laros RK., Jr. Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol. 1991;77:69–76. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for Axis I DSM-IV disorders. 1994. Patient Edition (SCID-I/P, vs 20) [DOI] [PubMed]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gernsheimer T, James AH, Stasi R. How I treat thrombocytopenia in pregnancy. Blood. 2013;121:38–47. doi: 10.1182/blood-2012-08-448944. [DOI] [PubMed] [Google Scholar]
- Grzeskowiak LE, McBain R, Dekker GA, Clifton VL. Antidepressant use in late gestation and risk of postpartum haemorrhage: a retrospective cohort study. BJOG. 2015 doi: 10.1111/1471-0528.13612. [DOI] [PubMed] [Google Scholar]
- Hankey GJ. Selective serotonin reuptake inhibitors and risk of cerebral bleeding. Stroke. 2014;45:1917–1918. doi: 10.1161/STROKEAHA.114.005844. [DOI] [PubMed] [Google Scholar]
- Hantsoo L, Czarkowski KA, Child J, Howes C, Epperson CN. Selective serotonin reuptake inhibitors and endothelial function in women. Journal of women's health (2002) 2014;23:613–618. doi: 10.1089/jwh.2013.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harirchian S, Zoumalan RA, Rosenberg DB. Antidepressants and bleeding risk after face-lift surgery. Arch Facial Plast Surg. 2012;14:248–252. doi: 10.1001/archfacial.2012.2. [DOI] [PubMed] [Google Scholar]
- Huybrechts KF, Bateman BT, Palmsten K, Desai RJ, Patorno E, Gopalakrishnan C, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142–2151. doi: 10.1001/jama.2015.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts KF, Palmsten K, Avorn J, Cohen LS, Holmes LB, Franklin JM, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397–2407. doi: 10.1056/NEJMoa1312828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors MA, Houston JP, Tekell JL, Brannan SK, Frazer A. Reduction of platelet serotonin content in depressed patients treated with either paroxetine or desipramine. Int J Neuropsychopharmacol. 2000;3:229–235. doi: 10.1017/S146114570000198X. [DOI] [PubMed] [Google Scholar]
- Jiang HY, Chen HZ, Hu XJ, Yu ZH, Yang W, Deng M, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13:42–50. e43. doi: 10.1016/j.cgh.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Juurlink DN. Antidepressants, antiplatelets and bleeding: one more thing to worry about? CMAJ. 2011;183:1819–1820. doi: 10.1503/cmaj.111576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Shau WY, Chang CH, Chen ST, Lin MS, Lai MS. Antidepressant use and the risk of upper gastrointestinal bleeding in psychiatric patients: a nationwide cohort study in Taiwan. J Clin Psychopharmacol. 2012;32:518–524. doi: 10.1097/JCP.0b013e31825ccd5a. [DOI] [PubMed] [Google Scholar]
- Lindqvist PG, Nasiell J, Gustafsson LL, Nordstrom L. Selective serotonin reuptake inhibitor use during pregnancy increases the risk of postpartum hemorrhage and anemia: a hospital-based cohort study. J Thromb Haemost. 2014;12:1986–1992. doi: 10.1111/jth.12757. [DOI] [PubMed] [Google Scholar]
- Lupattelli A, Spigset O, Koren G, Nordeng H. Risk of vaginal bleeding and postpartum hemorrhage after use of antidepressants in pregnancy: a study from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2014;34:143–148. doi: 10.1097/JCP.0000000000000036. [DOI] [PubMed] [Google Scholar]
- Maayan-Metzger A, Kuint J, Lubetsky A, Shenkman B, Mazkereth R, Kenet G. Maternal selective serotonin reuptake inhibitor intake does not seem to affect neonatal platelet function tests. Acta Haematol. 2006;115:157–161. doi: 10.1159/000090929. [DOI] [PubMed] [Google Scholar]
- Mathai M, Gulmezoglu AM, Hill S. Saving womens lives: evidence-based recommendations for the prevention of postpartum haemorrhage. Bull World Health Organ. 2007;85:322–323. doi: 10.2471/BLT.07.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazereeuw G, Herrmann N, Bennett SA, Swardfager W, Xu H, Valenzuela N, et al. Platelet activating factors in depression and coronary artery disease: a potential biomarker related to inflammatory mechanisms and neurodegeneration. Neurosci Biobehav Rev. 2013;37:1611–1621. doi: 10.1016/j.neubiorev.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 2011;205:51.e51–58. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Tomer A, Manatunga AK, Knight BT, Porter MR, Kasey S, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry. 1996;153:1313–1317. doi: 10.1176/ajp.153.10.1313. [DOI] [PubMed] [Google Scholar]
- O'Hara MW. Social support, life events, and depression during pregnancy and the puerperium. Arch Gen Psychiatry. 1986;43:569–573. doi: 10.1001/archpsyc.1986.01800060063008. [DOI] [PubMed] [Google Scholar]
- Palmsten K, Hernandez-Diaz S, Huybrechts KF, Williams PL, Michels KB, Achtyes ED, et al. Use of antidepressants near delivery and risk of postpartum hemorrhage: cohort study of low income women in the United States. BMJ (Clinical research ed) 2013;347:f4877. doi: 10.1136/bmj.f4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath WH. Postpartum hemorrhage--update on problems of definitions and diagnosis. Acta Obstet Gynecol Scand. 2011;90:421–428. doi: 10.1111/j.1600-0412.2011.01107.x. [DOI] [PubMed] [Google Scholar]
- Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med. 2010;40:1723–1733. doi: 10.1017/S0033291709992194. [DOI] [PubMed] [Google Scholar]
- Salkeld E, Ferris LE, Juurlink DN. The risk of postpartum hemorrhage with selective serotonin reuptake inhibitors and other antidepressants. J Clin Psychopharmacol. 2008;28:230–234. doi: 10.1097/JCP.0b013e318166c52e. [DOI] [PubMed] [Google Scholar]
- Samer CF, Lorenzini KI, Rollason V, Daali Y, Desmeules JA. Applications of CYP450 testing in the clinical setting. Mol Diagn Ther. 2013;17:165–184. doi: 10.1007/s40291-013-0028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels P. Hematologic Complications of Pregnancy. In: Gabbe SG, Niebyl JR, Galan HL, Jauniaux ER, Landon MB, Simpson JL, et al., editors. Obstetrics: Normal and Problem Pregnancies. 6 ed Elsevier; Philadelphia: 2012. [Google Scholar]
- Tavakoli HR, DeMaio M, Wingert NC, Rieg TS, Cohn JA, Balmer RP, et al. Serotonin reuptake inhibitors and bleeding risks in major orthopedic procedures. Psychosomatics. 2012;53:559–565. doi: 10.1016/j.psym.2012.05.001. [DOI] [PubMed] [Google Scholar]
- UW Health Medications and Herbs which Affect Bleeding
- Ververs FF, Voorbij HA, Zwarts P, Belitser SV, Egberts TC, Visser GH, et al. Effect of cytochrome P450 2D6 genotype on maternal paroxetine plasma concentrations during pregnancy. Clin Pharmacokinet. 2009;48:677–683. doi: 10.2165/11318050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65:805–815. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JBW, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS) New York State Psychiatric Institute; New York: 2003. [Google Scholar]
- Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166:557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO recommendations for the prevention of postpartum haemorrhage. World Health Organization: Department of Making Pregnancy Safer; Geneva: 2007. [Google Scholar]