Abstract
We aimed to evaluate the effects of the natural compounds embelin and piperine on the biofilm-formation property of Streptococcus mutans. A total of 30 clinical isolates were identified as S. mutans and screened for biofilm formation using the microtiter plate method. The strongest biofilm producer (SM03) was used for identifying both minimum inhibitory concentration (MIC) and minimum biofilm inhibitory concentration (MBIC). We subsequently used this concentration against each of the strong biofilm producer isolates at A492 < 0.5 optical density (OD). Of the 30 isolates screened for biofilm formation, 18 isolates showed strong biofilm formation, 09 isolates showed moderate formation, and 03 isolates showed poor/nonbiofilm formation. The MIC of embelin for the strongest biofilm producer (SM03) was 0.55 ± 0.02, whereas that of piperine was 0.33 ± 0.02. The MBIC of embelin was 0.0620 ± 0.03, whereas that of piperine was 0.0407 ± 0.03, which was lower than that of embelin. At OD492 < 0.5, the MBIC of both compounds significantly inhibited biofilm formation of all the 18 strong biofilm-forming isolates. The results of this study demonstrate a significant antibiofilm effect of the natural compounds embelin and piperine, which can contribute towards the development of a database for novel drug candidates for treating oral infections caused by S. mutans.
Keywords: antibiofilm activity, biofilm, natural compound, Streptococcus mutans, embelin and piperine
Graphical abstract
1. Introduction
Microorganisms exist as free-floating cells or, more often, in a community of cells attached to a substrate. This sessile form of life is referred to as a biofilm. By definition, a biofilm is a community of cells attached to either a biotic or an abiotic surface enclosed in a complex exopolymeric substance.1 Biofilms allow microorganisms to trap nutrients and withstand hostile environmental conditions by quorum sensing (QS). QS is a widespread and well-known cell-to-cell communication phenomena that regulates biofilm formation and virulence behaviors.2, 3, 4 QS also involves chemical communication among bacteria including formation, secretion, detection, and reaction to molecules known as autoinducers. Several serious infections are reported to be a result of biofilm formation, which leads to chronic diseases in most cases. These persistent infections are a challenge for public health on a global scale, because they reduce the effectiveness of treatments and increase morbidity, mortality, and health-care costs.5
Streptococcus mutans is an impotent pathogen and is a common cause of oral infections such as dental caries. S. mutans effectively utilizes dietary sucrose to synthesize large amounts of exopolysaccharides, which play an important role in the accumulation, adhesion, and plaque matrix formation of microorganisms. These processes in most cases lead to serious infections. The ability of a microorganism to form a biofilm on a host tissue surface is an important step in the development of infection.6 Because of poor hygiene, various pathogenic microorganisms cause infections. At present, a number of antibiotics are used for treating these infections. However, because these antibiotics are associated with significant side effects, there is increased attention toward using natural, biologically active herbal compounds as an alternative medicine.7, 8 Both embelin and piperine are natural compounds that are found in Embelia ribes and Piper longum, respectively. E. ribes and P. longum species are widely distributed across India. They are reputed medicinal herbs and their various parts have been used as a traditional cure in the Asian system of medicine (Indian, Chinese, and Malaysian) for treating a variety of disease conditions.9, 10, 11, 12
The objective of this study was to identify the effects of natural compounds such as embelin and piperine on biofilm formation.
2. Material and methods
2.1. Isolation and identification of bacteria
S. mutans was isolated from dental caries or plaque from patients in the Outpatient Department of Peoples Dental Academy (Bhopal, Madhya Pradesh, India). The bacterium was isolated from both male and female patients (mean age, 20 years). The standard strain isolated was S. mutans ATCC 25175. Bacterial samples were cultured on the following media: brain heart infusion (BHI) agar medium (HiMedia Laboratories, India) in a 5% CO2-enriched atmosphere and Mutans-Sanguis agar medium (HiMedia Laboratories, India). Biochemical tests were performed to identify the bacterial strains. Among the 20 samples from patients having dental caries, 30 isolates were identified as S. mutans.
2.2. Preparation of natural compounds
The natural compounds embelin and piperine were purchased from Natural Remedies (India). The compounds were dissolved in dimethyl sulfoxide (DMSO, Mark, Germany) at a concentration of 10 mg/mL.
2.3. Screening of S. mutans for biofilm formation
2.3.1. Microtiter plate method
Quantification of S. mutans isolates' biofilm formation was carried out using the microtiter plate method. To assay biofilm formation of the S. mutans isolates, an overnight culture of each isolate was grown in BHI broth (HiMedia Laboratories, India) for 18–20 hours at 37°C. Approximately 1 mL of each overnight culture was transferred to 10 mL of sterile BHI broth with the addition of 1% sucrose for biofilm production. The suspensions were adjusted using the same BHI medium to 0.5 on the McFarland turbidity standard as measured by absorbance (0.08–0.1 at 625 nm) in a spectrophotometer (Shimadzu, Australia), corresponding to approximately 102 CFU/mL. Then, from each culture, a 250-μL volume was transferred into the wells of a microtiter plate (HiMedia Laboratories).13 Blank wells contained only the broth. Plates were made in triplicate and incubated at 37°C for 24 hours. After 24 hours, the planktonic suspension and nutrient solution were aspirated and each well was washed three times with 300 μL of sterile physiological saline. The plates were strongly shaken to remove all nonadherent bacteria. The remaining attached bacteria were fixed with 250 μL of 96% ethanol/well and, after 15 minutes, the plates were emptied and left to dry. Each well was then stained for 5 minutes with 200 μL of 2% crystal violet (CV Gram stain, Merck, Germany). The stain was rinsed off by placing the plates under running tap water. After drying the stained plates, biofilms were visible as purple rings on the sides of each well. The quantitative analysis of biofilm formation was performed by adding 200 μL of 33% (v/v) glacial acetic acid (Merck) per well. The optical density (OD) of the stain was then measured at 492 nm using an enzyme-linked immunosorbent assay reader (Lisa, Germany) as described previously.13 Biofilm formation was scored as follows: nonbiofilm forming (A492 ≤ 1); +, weak (1 ≤ A492 ≤ 2); ++, moderate (2 < A492 ≤ 3); and +++, strong (A492 > 3). Microtiter assay was performed in triplicate.
2.4. Microscopic analysis using the coverslip method
The biofilm of S. mutans clinical isolates was grown as follows: individual sterile culture dishes were filled with 2.5 mL of BHI broth with 1% sucrose. A sterile 18-mm diameter glass coverslip was added to cover each culture dish. Each sample was inoculated with a defined volume of overnight culture. The dishes were incubated microaerobically at 37°C for 48 hours. Glass cover slips containing the attached biofilm were removed from the dishes, rinsed briefly with phosphate-buffered saline, and stained for 5 minutes with 0.5% crystal violet. The stained biofilms were observed under a microscope.14
2.5. Determination of the minimum inhibitory concentration of embelin and piperine
The minimum inhibitory concentration (MIC) of the natural compounds embelin and piperine was evaluated on all the isolates by the broth dilution method. The natural compounds were dissolved in DMSO (initial concentration, 2–0.0078 mg/mL). The initial test concentration was serially diluted twofold. Each well was inoculated with 5 μL of suspension containing 108 CFU/mL of bacteria. The plates with bacteria were incubated at 37°C for 24 hours. After incubation, 5 μL of tested broth was placed on the sterile BHI plates and incubated at respective temperature (37C). The MIC for bacterial isolates was determined as the lowest concentration of the extracts inhibiting the visual growth of the test cultures on the agar plate. Triplicates were maintained.15, 16
2.6. Biofilm-inhibition assay in the presence of embelin and piperine
Only those isolates of S. mutans that were classified as strong biofilm producers were used in the biofilm-inhibition assay. Test compounds were dissolved in DMSO (10 mg/mL), and twofold dilutions were prepared to obtain a final concentration ranging from 2 mg/mL to 0.0078 mg/mL in the wells after the addition of the freshly diluted BHI broth culture containing 106 CFU of the strong biofilm-forming isolates per well. After incubation at 37°C for 24 hours, the microtiter plate was washed, fixed, and biofilms were stained and visualized as explained earlier. The inhibitory effect of the compounds on biofilm production was calculated by subtracting the media control. The biofilm inhibitory concentration (BIC) is the concentration of the natural compound at which the biofilm formation was reduced to an absorbance (A492) < 0.5 OD. Each assay for BIC determination was performed in triplicate.
2.7. Statistical analysis
Calculations and statistics were performed using GraphPad 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). The results were analyzed using one-way analysis of variance. Significance was defined as p < 0.05. Results are presented as mean ± the standard error of the mean.
3. Results
3.1. Screening of S. mutans for biofilm formation
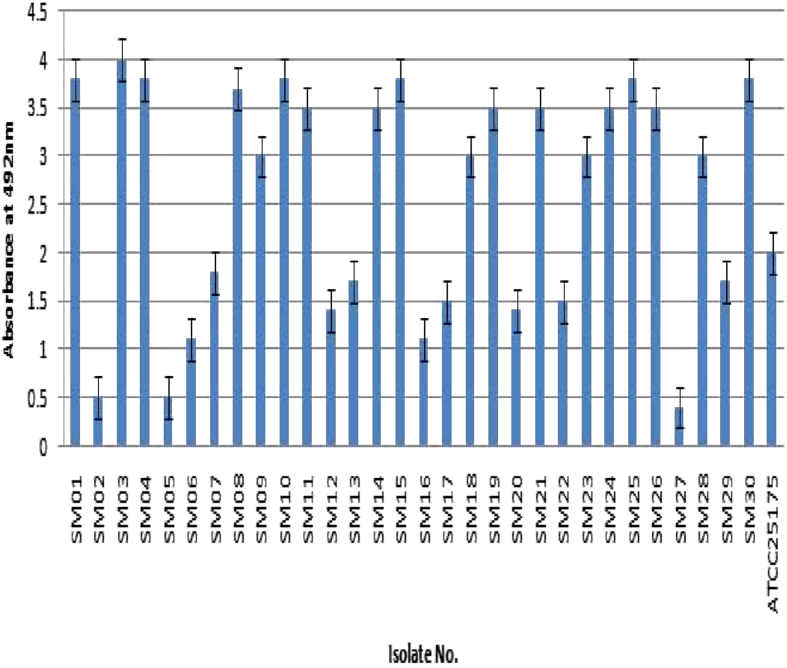
The 30 S. mutans isolates were screened for biofilm formation by the microtiter plate method and the results are shown in Fig. 1. All 30 isolates were classified based on their biofilm-forming potential as follows: 18 were strong biofilm producers, nine isolates were moderate producers, and three were poor/nonbiofilm producers. The S. mutans ATCC 25175 was included as an assay control and identified to be a moderate biofilm producer. This observation confirms that the magnitude and intensity of biofilm formation of the 18 isolates were significantly greater than those of the poor/nonbiofilm producers. Fig. 1 also shows the quantitative evaluation for identifying and demarcating strong biofilm-producing S. mutans isolates from moderate- and poor/nonbiofilm-producing isolates. The biofilm-forming potential of the strong producers at OD492 was greater than 1, whereas that of nonbiofilm-producing isolates at OD492 was less than 1. The isolate SM03 was identified as the strongest biofilm producer, whereas SM06 and SM27 were identified as moderate and poor/nonbiofilm producers, respectively.
Fig. 1.
Screening of Streptococcus mutans isolates (n = 30) for biofilm formation using microtiter plate assay. Poor/nonbiofilm forming (A492 ≤ 1); weak (1 ≤ A492 ≤ 2); moderate (2 < A492 ≤ 3); strong (A492 > 3).
3.2. Microscopic analysis of biofilm formation
To visualize biofilm formation by the three categories of S. mutans isolates (strong, moderate, and poor/nonbiofilm producers), the microscopic slide assay was performed and the results are shown in Fig. 2. The biofilm formation was clearly visible for the strong biofilm producer SM03, followed by the moderate biofilm producer SM06 at 48 hours. However, even after 48 hours, the poor/nonbiofilm producer SM27 did not form the biofilm. The strong biofilm producers also showed strong adherence to the slide, and therefore, we selected one of the strongest biofilm producers (SM03 isolate) for evaluating the MIC and BIC of the natural compounds.
Fig. 2.
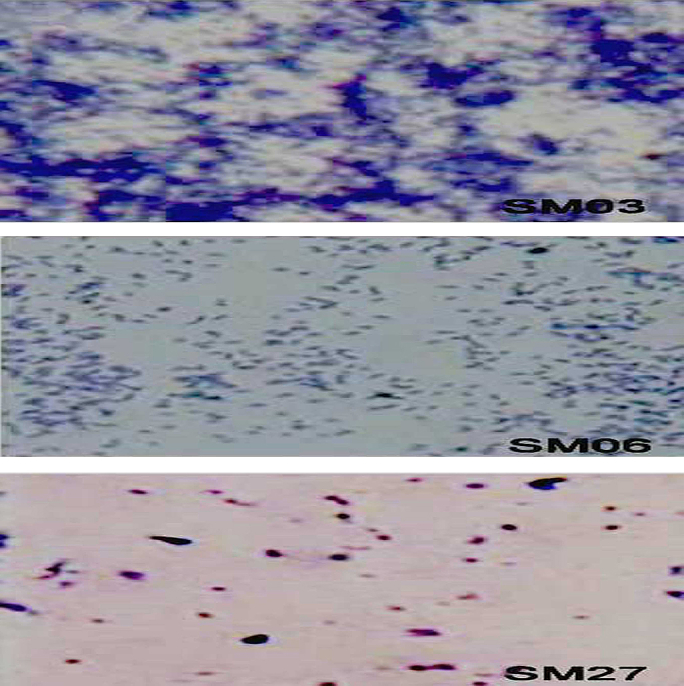
Microscopic visualization of biofilm production. SM03 = strong biofilm producer; SM06 = moderate biofilm producer; and SM27 = poor/nonbiofilm producer.
3.3. Determination of MIC and BIC of embelin and piperine
The results of MIC and minimum biofilm inhibitory concentration (MBIC) of the natural compounds analyzed for the strongest biofilm producer isolate SM03 are presented in Table 1. A significant difference in the MIC and BIC of the natural compounds was noted: the MIC of embelin was 0.55 ± 0.02, whereas that of piperine was 0.33 ± 0.02, (MIC, piperine < embelin). The MBIC of embelin was 0.0620 ± 0.03, whereas that of piperine was 0.0407 ± 0.03. These results confirm that piperine is the most potent antimicrobial and antibiofilm compound (piperine > embelin).
Table 1.
Comparative effect of two different natural compounds on growth inhibition and biofilm inhibition. The values were determined for the strong biofilm-forming isolate SM03. Values are mean ± standard error of the mean of three replicates.
| S. No. | Compound | Minimum inhibitory concentration (MIC) mg/mL | Biofilm inhibition concentration (BIC) mg/mL |
|---|---|---|---|
| 1 | Embelin | 0.55 ± 0.02 | 0.0620 ± 0.03 |
| 2 | Piperine | 0.33 ± 0.02 | 0.0407 ± 0.03 |
Values are mean ± S.E.M. of three replicates.
3.4. Biofilm inhibition of strong biofilm producers
The results of biofilm inhibition by embelin and piperine in each strong biofilm-forming S. mutans isolate are presented in Table 2. These results clearly confirm that the MBIC of embelin and piperine had reproducible biofilm inhibitory activity against each of the strong biofilm-producing isolates of S. mutans. The comparative effect of minimum biofilm-inhibition concentrations of embelin (0.0620 ± 0.03) and piperine (0.0407 ± 0.03) at OD492 < 0.5 shows the significant inhibition of biofilm by these compounds. The MBIC and MIC values indicate that piperine has better ability to inhibit biofilm formation than embelin.
Table 2.
Biofilm inhibition by embelin and piperine in strong biofilm-forming isolates of Streptococcus mutans. Values are mean ± standard error of the mean of three replicates *p < 0.05.
| Isolate No. | Absorbance at 492 nm for biofilm inhibition in the presence of natural compounds |
|
|---|---|---|
| Embelin 0.0620 mg/mL | Piperine 0.0407 mg/mL | |
| SM01 | 0.19 ± 02 | 0.14 ± 02 |
| SM03 | 0.21 ± 02 | 0.21 ± 02 |
| SM04 | 0.22 ± 02 | 0.18 ± 02 |
| SM08 | 0.20 ± 02 | 0.22 ± 02 |
| SM09 | 0.26 ± 02 | 0.21 ± 02 |
| SM10 | 0.23 ± 02 | 0.17 ± 02 |
| SM11 | 0.21 ± 02 | 0.14 ± 02 |
| SM14 | 0.19 ± 02 | 0.11 ± 02 |
| SM15 | 0.17 ± 02 | 0.16 ± 02 |
| SM18 | 0.20 ± 02 | 0.13 ± 02 |
| SM19 | 0.18 ± 02 | 0.23 ± 02 |
| SM21 | 0.21 ± 02 | 0.19 ± 02 |
| SM23 | 0.24 ± 02 | 0.13 ± 02 |
| SM24 | 0.19 ± 02 | 0.11 ± 02 |
| SM25 | 0.18 ± 02 | 0.16 ± 02 |
| SM26 | 0.14 ± 02 | 0.10 ± 02 |
| SM28 | 0.23 ± 02 | 0.15 ± 02 |
| SM30 | 0.19 ± 02 | 0.13 ± 02 |
| Control ATTCC 25175 | 0.21 ± 02 | 0.23 ± 02 |
4. Discussion
S. mutans plays a significant role in dental infection by effectively utilizing sugars and synthesizing large amounts of exopolysaccharides, which plays a vital role in adhesion of bacteria and accumulation of biofilm. Thus, the organized structure and mechanisms of biofilm are responsible for the emergence of drug-resistant bacteria.17 The present allopathic formulations used in oral care contain antibiotics, antimicrobial agents, surfactants, and alcohol, but these are not efficient in completely eradicating oral pathogens; in addition, they were found to be cytotoxic.18, 19 Therefore, there is a growing interest in using plant-derived products against oral pathogens. Thus, in this study, the natural compounds embelin, which is commonly found in E. ribes, and piperine, which is commonly found in P. longum—both are widely distributed plant species in India—were chosen for the evaluation of the biofilm-inhibition effect.20, 21
The objective of this study was to evaluate the biofilm-inhibition effect of natural compounds on clinical isolates of S. mutans. Thirty clinical isolates were screened; of these, 18 strong biofilm producers were identified (Fig. 1). To determine the minimal concentration required for biofilm inhibition, we evaluated the inhibitory effects of embelin and piperine on bacterial growth and biofilm formation by SM03, which was identified to be the strongest biofilm-forming isolate (Table 1). We subsequently used this concentration against each of the strong biofilm producer isolates.
The comparative effects of embelin and piperine compounds on growth inhibition and biofilm inhibition are summarized in Table 1. The biofilm-inhibition concentration was significantly lower than the concentration required for inhibition of bacterial growth. These data reflect the fact that the MBIC used has the potential to inhibit biofilm formation by S. mutans. Our results also showed that the MBIC of piperine was lower than that of embelin. The compounds might have exhibited these effects by inhibiting the activity of receptors and molecules involved in the quorum sensing pathway, which is required for biofilm formation.16, 22, 23 Absorbance at 492 nm for biofilm inhibition in the presence of natural compounds suggested the significant potential of biofilm inhibition by piperine and embelin (piperine > embelin; Table 2).
5. Conclusion
We have presented a comprehensive analysis of the effects (inhibitory) of embelin and piperine on strong biofilm-forming isolates of S. mutans. The results show that both compounds have potential biofilm-inhibition capabilities. It would be interesting to find out the mode of action or cell-to-cell inhibition action behind this effect. We conclude that these compounds would be very useful in controlling the biofilm-forming infection of S. mutans.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Sandasi M., Leonard C.M., Viljoen A.M. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett Appl Microbiol. 2010;50:30–35. doi: 10.1111/j.1472-765X.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 2.Waters C.M., Bassler B.L. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 3.Reading N.C., Sperandio V. Quorum sensing: the many languages of bacteria. FEMS Microbiol Lett. 2006;254:1–11. doi: 10.1111/j.1574-6968.2005.00001.x. [DOI] [PubMed] [Google Scholar]
- 4.Taga M.E. Bacterial signal destruction. ACS Chem Biol. 2007;2:89–92. doi: 10.1021/cb7000186. [DOI] [PubMed] [Google Scholar]
- 5.Filoche S., Wong L., Sissons C.H. Oral biofilms: emerging concepts in microbial ecology. J Dent Res. 2010;89:8–18. doi: 10.1177/0022034509351812. [DOI] [PubMed] [Google Scholar]
- 6.Goulhen F., Grenier D., Mayrand D. Oral microbial heat-shock proteins and their potential contributions to infections. Crit Rev Oral Biol Med. 2003;14:399–412. doi: 10.1177/154411130301400603. [DOI] [PubMed] [Google Scholar]
- 7.Al-Haroni M., Skaug N. Incidence of antibiotic prescribing in dental practice in Norway and its contribution to national consumption. J Antimicrob Chemother. 2007;59:1161–1166. doi: 10.1093/jac/dkm090. [DOI] [PubMed] [Google Scholar]
- 8.Raghunath D. Emerging antibiotic resistance in bacteria with special reference to India. J Biosci. 2008;33:593–603. doi: 10.1007/s12038-008-0077-9. [DOI] [PubMed] [Google Scholar]
- 9.Indian Council of Medicinal Research . vol. V. Indian Council of Medicinal Research (ICMR); New Delhi, India: 2008. (Quality Standards of Indian Medicinal Plants). [Google Scholar]
- 10.Ministry of Health and Family Welfare . vol. II. Department of AYUSH, Government of India; New Delhi, India: 2008. (The Ayurvedic Pharmacopoeia of India (Part I)). [Google Scholar]
- 11.Regional Research Laboratory (Council of Scientific & Industrial Research)/Indian Drug Manufacturers' Association . Indian Drug Manufacturers Association; Mumbai, India: 2002. Indian Herbal Pharmacopoeia; pp. 206–313. [Google Scholar]
- 12.Ahmad N., Fazal H., Abbasi B.H., Farooq S., Ali M., Khan M.A. Biological role of Piper nigrum L. (black pepper): a review. Asian Pac J Trop Biomed. 2012;2:S1945–S1953. [Google Scholar]
- 13.O'Toole G.A., Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 14.Merritt J.H., Kadouri D.E., O'Toole G.A. Growing and analyzing static biofilms. Curr Protoc Microbiol. 2011;22 doi: 10.1002/9780471729259.mc01b01s00. 1B.1.1–1B.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dwivedi D., Patidar R.K., Singh V. Antioxidant and antibacterial potential of Murraya koenigii against human cariogenic pathogens. Int J Pharm Sci Res. 2012;3:3399–3406. [Google Scholar]
- 16.Al-Dhabi N.A., Balachandran C., Raj M.K. Antimicrobial, antimycobacterial and antibiofilm properties of Couroupita guianensis Aubl. fruit extract. BMC Complement Altern Med. 2012;12:242. doi: 10.1186/1472-6882-12-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flemingson, Emmadi P., Ambalavanan N., Ramakrishnan T., Vijayalakshmi R. Effect of three commercial mouth rinses on cultured human gingival fibroblast: an in vitro study. Indian J Dent Res. 2008;19:29–35. doi: 10.4103/0970-9290.38929. [DOI] [PubMed] [Google Scholar]
- 19.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 20.Truchado P., Gil-Izquierdo A., Tomás-Barberán F., Allende A. Inhibition by chestnut honey of N-acyl-l-homoserine lactones and biofilm formation in Erwinia carotovora, Yersinia enterocolitica, and Aeromonas hydrophila. J Agric Food Chem. 2009;57:11186–11193. doi: 10.1021/jf9029139. [DOI] [PubMed] [Google Scholar]
- 21.Tan L.Y., Yin W.F., Chan K.G. Piper nigrum, Piper betle and Gnetum gnemon—natural food sources with anti-quorum sensing properties. Sensors (Basel) 2013;13:3975–3985. doi: 10.3390/s130303975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Issac Abraham S.V., Palani A., Ramaswamy B.R., Shunmugiah K.P., Arumugam V.R. Antiquorum sensing and antibiofilm potential of Capparis spinosa. Arch Med Res. 2011;42:658–668. doi: 10.1016/j.arcmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Chenia H.Y. Anti-quorum sensing potential of crude Kigelia Africana fruit extracts. Sensors (Basel) 2013;13:2802–2817. doi: 10.3390/s130302802. [DOI] [PMC free article] [PubMed] [Google Scholar]