Abstract
BACKGROUND
Poor zinc nutritional status is suspected as a risk factor for coronary heart disease (CHD). Since zinc absorption may be influenced by some nutritional and physiologic factors, it would be better to investigate zinc status through biochemical measurements. The objective of the present study was to review recent studies investigating the association of zinc biomarkers with CHD, systematically.
METHODS
The MEDLINE database was used for relevant studies published from January 2009 to December 2013 with appropriate keywords. Articles were included in this study if they were human studies, original articles, and published in English.
RESULTS
Six case-control studies and two prospective cohort studies that measured zinc biomarkers were included in the study. Almost all case-control studies suggest that decreased plasma zinc was associated with increased CHD risk. Cohort studies did not support this relationship.
CONCLUSION
The majority of the evidence for this theory is extracted from case-control studies, which might have bias. Prospective studies and randomized clinical trials are needed to investigate whether poor zinc status is associated with increased CHD risk. Consequently, a protective role of zinc in CHD could not be still established.
Keywords: Zinc, Coronary Heart Disease, Minerals, Systematic Review, Cardiovascular, Myocardial Infarction
Introduction
Cardiovascular disorders are the most common cause of mortality in the world.1 Mortality due to cardiovascular diseases has increased from 31.9% in 1990 to 46.8% in 2010, in Iran.2 There are several risk factors have been considered to be effective in the pathogenesis of cardiovascular disorders. Among them, nutrition has an important role.3,4 High intake of calorie, total fat, cholesterol and processed foods and low intake of fruits, vegetables, and dietary fiber have been associated with higher risk of coronary heart disease (CHD).4-6 When micronutrients were investigated, the protective effect of folate, vitamin B6, vitamin B12, and vitamin E has been shown.7 However, the role of minerals is not well-known.
Prior studies indicate that some dietary minerals,8-10 such as selenium intake may affect the risk of CHD and related mortality.11-13 Although, the prevalence of zinc deficiency is estimated to be high among all population worldwide,14 and its role in developing some chronic diseases has been shown recently,15 the role of zinc in developing CHD is not clear.8,16,17
There are different methods for evaluating zinc status in nutritional epidemiology such as nutrient intake assessment through questionnaire and biochemical measurement. Biochemical measures or biomarkers are attractive because they are objective and less suspicious to forgetful and biased human answers to the questionnaire.18 In addition, within-food variation may occur due to geographical difference in soil zinc content. Consequently, since the concentration of Zn in most foods is not inherent and more important, Zn absorption may be affected by some physiologic and dietary factors such as phytate,19 observational studies of Zn status may benefit from the use of biochemical measures of zinc levels in hair, nail, serum or plasma Zn concentrations more than its dietary intake.20 While, serum and urine zinc usually inhibits recent intake,14 nails and hairs slow growth have been shown to be a reliable biomarker for trace element status, especially reflecting past year exposure.21
Since the role of zinc is not clear in such a common disease and dietary recommendations are cost-effective and safe22 and usually work even in developing countries,23 the objective of this study was to review the results from studies of the association of Zn biomarkers with CHD, systematically.
Materials and Methods
Database MEDLINE was searched for observational studies and randomized trials investigating the relationship between Zn biochemical measurement and CHD. The following Medical Subject Headings (MeSH) terms were applied (cardiovascular OR myocardial infarction OR peripheral arterial disease OR stroke OR mortality OR coronary); and were combined with each of the terms (“zinc,” “Zn,” “zinc gluconate,” “zinc sulfate,” “zinc acetate,” “zinc oxide”). The articles published in recent 5 years were included. The potentially relevant articles were included if the full paper had been obtained. Studies were restricted to human studies and publications in English. References of identified articles and reviews were also searched for additional relevant articles.
We aimed to identify all observational and randomized trials studies that assessed the association of Zn with CHD. Articles met the following criteria were excluded:
Not original research (reviews, editorials, non-research letters);
Case reports or case series;
Ecologic studies;
Studies lacking a biochemical measurement of Zn status;
Cellular or molecular studies;
Studies which their outcomes were a specific risk factor of CHD (for example lipid profile) or total mortality.
Data extraction and quality assessment
One investigator reviewed search results. Abstract of 209 retrieved articles were studied, and articles which met exclusion criteria were excluded. Although the stringent criteria were used for inclusion and exclusion, the studies which were finally included in the study, had different methodology and biochemical measurements. Therefore, the results were summarized and tabulated. For the included articles, some important information were extracted and tabulated including study design, first author, year of publication, country, patient characteristics (gender and mean age), sample size, case and control definition and the reported Zn status from studies (Tables 1 and 2). All biochemical measurement including hair, nail, urine, serum and plasma were included in the study. Case-control studies were concluded in table 1 and cohort studies were concluded in table 2. The quality of observational studies was assessed according to the criteria used by Flores-Mateo et al.11 to minimize including of articles which have bias (Appendix 1).
Table 1.
Case-control studies of biochemical measurement of zinc and coronary heart disease (CHD)
| Author | Country | Men among control (%) | Mean age of case subjects (years) | Mean age of control subjects (years) | Type of case subjects | Source of control subjects | Number of case subjects/control subjects | Zinc assessment (technique) | Zinc concentration |
P | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case subjects | Control subjects | ||||||||||
| Tan et al.24 | China | 50 | 36-81 | 24-72 | ACS | Healthy volunteers | 24/100 | Hair (ICP-AES) | 168.12 ± 69.74 ng/ml | 166.60 ± 68.24 | NR |
| Giannoglou et al.28 | Greece | 53 | 66 | 61 | ACS (diagnosed with angiography) | Healthy volunteers (R/O ACS with angiography) | 40/32 | Serum, urine (AAS) | Serum = 626.5 μg/l | Serum = 628.5 | 0.830 0.014 |
| Urine = 620 μg/24 h | Urine = 469.4 | ||||||||||
| Afridi et al.25 | Pakistan | 100 | 31-60 | 31-60 | ACS (diagnosed with angiography) | Healthy volunteers | 457/536 | Serum, hair, urine (AAS) | NR | NR | < 0.050 |
| Cebi et al.29 | Turkey | NR | 59.1 ± 11.0 | 57.5 ± 10.0 | ACS (diagnosed with angiography) | Healthy volunteers (R/O ACS with angiography) | 30/20 | Serum (AAS) | 0.85 μg/dl | 0.90 μg/dl | 0.650 |
| Islamoglu et al.26 | Turkey | 73 | 58 ± 12.0 | 53 ± 12 | ACS (diagnosed with angiography) | Healthy volunteer (R/O ACS with angiography) | 67/26 | Serum (AAS) | 0.61 ng/l | 0.96 ng/l | < 0.010 |
| Bayir et al.27 | Turkey | 56 | 61.4 ± 12.0 | 61.6 ± 18.0 | ACS | Healthy volunteers | 100/100 | Serum (AAS) | 0.72 ppm | 1.3 ppm | < 0.010 |
ACS: Acute coronary syndrome; AAS: Atomic absorption spectrometry; ICP-AES : Inductively coupled plasma atomic emission spectroscopy; NR: Not reported
Table 2.
Prospective studies of biochemical measurement of zinc and coronary heart disease
| Author | Country | Population | Men (%) | Mean age (year) | Endpoint ascertainment | Follow-up (years) | Outcome | Number of case subjects/non-case subjects | Zinc assessment (technique) | Unadjusted HR (95%CI)/P | Adjusted HR (95%CI)/P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bates et al.31 | Britain | British National diet and nutrition survey | 51 | 76.6 ± 7.4 | Death certificate | 14 | CHD mortality | 1054 | Plasma (colorimetric assays) | 0.73 (0.61-0.88)/ 0.001 | 0.83 (0.65-1.07)/ 0.150 |
| Lobo et al.30 | Brazil | Brazilian cohort | 62 | 54.6 ± 12.7 | Death certificate | 2 | CHD mortality of hemodialysis patients | 45 | Plasma (AAS) | NR | NS |
HR: Hazard ratio; CHD: Coronary heart diseases; AAS: Atomic absorption spectrometry; NR: Not reported; NS: Not significant; CI: Confidence interval
Results
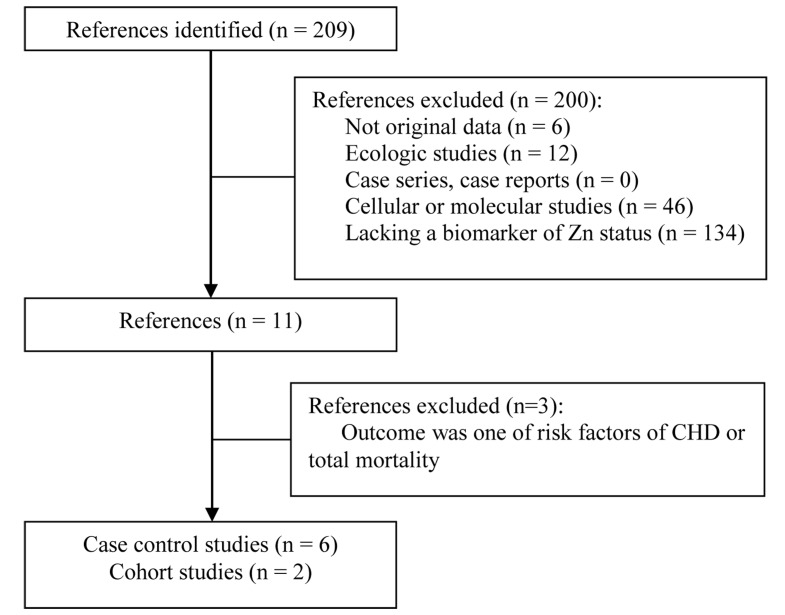
Six case-control studies and two prospective cohort studies were included in the study (Figure 1). The studies were published between 2009 and 2013 (Table 1). The number of case subjects varied between 2424 and 457.25 The quality scores varied widely. In almost all studies, serum Zn concentration was less in CHD patients as compared to control subjects, but this difference was not significant in all studies (Table 1). In the study of Islamoglu et al.26 67 patients with CHD were compared with 26 clinically healthy individuals. The serum Zn was found to be significantly lower in patients than in healthy control (P < 0.010). In the study of Bayir et al.27 patients with diagnosed acute coronary syndrome (ACS) (n = 100) were compared with their age-matched controls (n = 100). Serum Zn concentration was significantly less in the CHD group compared to the control group (P < 0.010).
Figure 1.
Flow of study selection process CHD: Coronary heart diseases
However, in the study of Giannoglou et al.28 40 patients with diagnosed CHD were compared with 32 controls. Serum Zn was not significantly associated with CHD risk and severity (P = 0.320). However, urinary Zn concentration was significantly higher in patients with CHD (P = 0.030). In the study of Cebi et al.29 which compared 30 patients with diagnosed CHD with 20 healthy subjects, serum Zn was not statistically different in two groups (P = 0.650).
Hair Zn was assessed in two studies with contradictory results.24,25 In the study of Afridi et al.25 who investigated Zn status in hair, urine, and blood, 457 male CHD patients were enrolled and compared with 536 healthy individuals.
The results showed that the concentrations of Zn were lower in CHD patients in both blood and hair samples (P > 0.001). However, the excretion of Zn was higher in CHD patients. Tan et al.24 enrolled 24 CHD patients and 100 healthy persons aged 24-72. The concentration of hair Zn was not different significantly in case and control groups. Urine Zn was reported in two studies, and it was significantly higher in CHD patients than controls.25,28
In cohort studies included into this review study, serum Zn was not associated with CHD. Lobo et al.30 followed 45 hemodialysis patients for 2 years to investigate the risks for CHD mortality. During the 24 months, 24.4% of the patients died, all due to CHD. In Lobo et al.30 study, decreased plasma Zn was associated with increased tumor necrosis factor alpha (TNF-α) levels and oxidized low-density lipoprotein (LDL) in patients, but analysis by the Cox model showed that plasma Zn was not a significant predictor of mortality. In Bates et al.31 prospective study, mortality status and its underlying causes were studied in 1054 subjects aged more than 65 years in the British National Diet and Nutrition Survey in 1994-2008. Primary vascular disease mortality comprised about 26% of all mortality. Model adjustment for sex and age, plasma Zn was suggested to be protective against vascular mortality, but after adjusting for all known risk factors (body mass index, systolic blood pressure, smoking, number of prescribed drugs, health score, physical activity score, and poverty) it was not significant.
Discussion
According to our knowledge, this study is the first systematic review evaluating the association between Zn status and CHD. Eight studies were included in this study, and the majority of the case-control studies showed an inverse association between Zn status through different indicators and CHD. This inverse association was observed in populations with different baseline Zn concentrations and in persons from different countries. Prospective cohort studies did not protect this association. However, we found no intervention studies to summarize.
In almost all studies, serum Zn concentration was less in CHD patients as compared to control subjects, but this difference was not significant in all studies (Table 1). Zn deficiency may have a roll in CHD risk. This theory is consistent with cellular and molecular findings. There are evidences that Zn may suppress apoptosis.28,32-34 Therefore, Zn depletion may affect myocardial reperfusion injury by induction of apoptosis.35 Zn deficiency can alter angiotensin-conversion enzyme activity adversely and cause hypertension due to vasoconstriction.36 In addition, Zn may affect inflammatory response in humans thorough affecting the production of inflammatory cytokines such as interleukin-1 (IL-1) and TNF-α.37 Poor Zn status has been shown to be associated with inflammation.38,39 Inflammation has been shown to play a role in the pathogenesis of myocardial ischemia40,41 and anti-inflammatory strategies which reduce myocardial tissue damage are challenges in interventional cardiology.42-46 Inflammation response will be initiated after releasing of inflammatory cytokines and absorbing neutrophils to the injury site, which lead to injury to the endothelial cells by the production of reactive oxygen species.41,47 Moreover, the gene IL-6 which regulates the amount of circulating proteins involved in inflammatory responses, may be influenced by zinc status.48 This gene has been shown to be associated with CHD.49
Although Zn deficiency could be a risk factor for CHD, decreased serum Zn in these patients could be caused by CHD. Evidence has shown that plasma Zn is a negative acute-phase reactant and after ACS plasma Zn may decrease in response to inflammation. Plasma Zn is affected by metallothioneins homeostasis, which is itself influenced by proinflammatory cytokines. Metallothionein which is expected to be increased in chronic inflammation causes low Zn availability in inflammatory conditions.37 Thus, impaired Zn homeostasis in CHD patients may be due to chronic inflammation in these patients versus a risk factor of CHD. Therefore we could not determine which one comes first based on case control studies which is a limitation of case control studies. However, ignoring which comes first, CHD patients have poor Zn status and it is recommended to investigate if improving Zn status in these patients could improve their survival.
Urine Zn was reported in two studies and it was significantly higher in CHD patients than controls25,28 which indicate zinc excretion and it is in consistent with studies shows lower level of zinc in serum. Hair Zn was assessed in two studies with contradictory results.24,25 The Afridi et al. study is limited to men and this difference may be related to sex.25 Tan et al. study might have not enough power to detect the difference between two groups because of low sample size in this study (only 24 cases).24
Careful consideration of choice of biochemical indicators will be valuable. All biochemical indicators of Zn, such as hair, urine, nails or plasma may reflect Zn exposure to some degree.14,50,51 However, the interpretation of biomarkers is not simple because circulating plasma or serum Zn concentrations respond to conditions such as inflammation, infection, and time of last meal. Nails are susceptible to soil contamination. Contamination by coloring dyes and anti-dandruff shampoos may limit the suitability of hair.
In cohort studies included into this study, serum Zn was not associated with CHD. Lobo et al. have evaluated the association of plasma Zn and oxidized LDL and TNF-α.30 It was concluded that decreased plasma Zn were associated with increased TNF-α levels and oxidized LDL in patients who undergone hemodialysis. It can be concluded that there is a relationship between Zn deficiency with inflammation and lipid peroxidation in hemodialysis patients, but serum zinc was not a predictor of CHD mortality. It should be mentioned that the follow-up for mortality was 2 years in this study, and it could be not enough for evaluating the effect of this mineral. Small sample size (45 hemodialysis patients) could be another reason for failure in finding significant relationship. Large prospective studies with enough sample size are needed for strong conclusion.
In another prospective study, it was suggested plasma Zn could be protective against vascular mortality, but after adjusting for all known risk factors it was not significant.31 In this study, four model were used, and the protective effect of Zn was significant in all models except the full model which all confounders were entered into the model. However, the hazard ratio was very similar to other three models and paying attention to a confidence interval (0.65-1.07) shows the protective effect of zinc. The Bates et al. study investigated risk factors in elderly. However, the predictive value of risk factors for disease and mortality appears to decrease with age.52 Moreover, the association between Zn and CHD might be underestimated in this study.
The discrepancy between most case-control studies and prospective studies may be related to the fact that this is inflammation of post-CHD which causes low plasma Zn which could be detected in case-control studies.
In assessing the association between Zn and CHD, different confounders should be considered. Zinc depletion could be due to a strict vegetarian diet (a diet which is limited to plant products)53 or alcohol or drug addiction which all of them have relation with CHD and may confound the relation between Zn and CHD. Moreover, physical activity and age may affect Zn absorption and Zn loss.14 They should be also taken into account because they may also confound this relationship.
The competency for absorption among nutrients and such interactions should be considered too, as they may affect Zn utilization.
Conclusion
In conclusion, the results from observational studies evaluating the association between Zn status and risk of CHD are controversial; however, case-control studies with sufficient sample size could find a significant inverse association between serum Zn concentration and CHD risk. None of the prospective studies confirmed this association. More cohort studies with CHD mortality endpoint and appropriate biologic samples which have collected in the beginning of the study are needed to be able to conclude about this association in future.
Acknowledgments
The authors thank Dr. Reza Malekzadeh and Dr Nizal Sarrafzadegan for their critical review of this paper.
Appendix 1. Quality criteria for observational studies on zinc and CHD (coronary heart disease).
| Reference number | 24 | 28 | 25 | 31 | 29 | 26 | 30 | 27 |
|---|---|---|---|---|---|---|---|---|
| All observational studies | ||||||||
| Exposure was assessed at the individual level | √ | √ | √ | √ | √ | √ | √ | √ |
| Outcomes were based on objective tests or standard criteria in 90% of study participants | √ | √ | √ | √ | √ | √ | √ | √ |
| The authors presented internal comparisons within study participants | √ | √ | √ | √ | ||||
| The authors controlled for potential confounding risk factors in addition to age | √ | √ | √ | √ | ||||
| Prospective cohort studies | ||||||||
| Loss to follow-up was independent of exposure | ||||||||
| The intensity of search of disease was independent of exposure status | √ | |||||||
| Case-control studies | ||||||||
| Data were collected in a similar manner for all participants | √ | √ | √ | √ | √ | |||
| The same exclusion criteria were applied to all participants | √ | √ | √ | |||||
| The selection process for Non-cases was described | √ | |||||||
| Samples were collected ≤ 24 hour after the onset of symptoms for all cases | √ | √ | ||||||
| The study was based on incident cases of disease | √ | √ | ||||||
| Non cases were persons who would have been excluded if they had developed CAD |
CAD: Coronary artery diseases
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Nichols M, Townsend N, Scarborough P, Rayner M. Trends in age-specific coronary heart disease mortality in the European Union over three decades: 1980-2009. Eur Heart J. 2013;34(39):3017–27. doi: 10.1093/eurheartj/eht159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, Sepanlou SG, Shahraz S, Dicker D, Naghavi P, Pourmalek F, et al. Evaluating causes of death and morbidity in Iran, global burden of diseases, injuries, and risk factors study 2010. Arch Iran Med. 2014;17(5):304–20. [PubMed] [Google Scholar]
- 3.Haghighatdoost F, Sarrafzadegan N, Mohammadifard N, Sajjadi F, Maghroon M, Boshtam M, et al. Healthy eating index and cardiovascular risk factors among Iranians. J Am Coll Nutr. 2013;32(2):111–21. doi: 10.1080/07315724.2013.767590. [DOI] [PubMed] [Google Scholar]
- 4.Forman D, Bulwer BE. Cardiovascular disease: optimal approaches to risk factor modification of diet and lifestyle. Curr Treat Options Cardiovasc Med. 2006;8(1):47–57. doi: 10.1007/s11936-006-0025-7. [DOI] [PubMed] [Google Scholar]
- 5.Slavin JL, Martini MC, Jacobs DR, Marquart L. Plausible mechanisms for the protectiveness of whole grains. Am J Clin Nutr. 1999;70(3 Suppl):459S–63S. doi: 10.1093/ajcn/70.3.459s. [DOI] [PubMed] [Google Scholar]
- 6.Slavin J. Why whole grains are protective: biological mechanisms. Proc Nutr Soc. 2003;62(1):129–34. doi: 10.1079/PNS2002221. [DOI] [PubMed] [Google Scholar]
- 7.Olthof MR, van Vliet T, Verhoef P, Zock PL, Katan MB. Effect of homocysteine-lowering nutrients on blood lipids: results from four randomised, placebo-controlled studies in healthy humans. PLoS Med. 2005;2(5):e135. doi: 10.1371/journal.pmed.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reunanen A, Knekt P, Marniemi J, Maki J, Maatela J, Aromaa A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr. 1996;50(7):431–7. [PubMed] [Google Scholar]
- 9.Klevay LM. Interactions of copper and zinc in cardiovascular disease. Annals of the New York Academy of Sciences. 1980;355:140–51. doi: 10.1111/j.1749-6632.1980.tb21334.x. [DOI] [PubMed] [Google Scholar]
- 10.Jain VK, Mohan G. Serum zinc and copper in myocardial infarction with particular reference to prognosis. Biol Trace Elem Res. 1991;31(3):317–22. doi: 10.1007/BF02990200. [DOI] [PubMed] [Google Scholar]
- 11.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 2006;84(4):762–73. doi: 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stranges S, Navas-Acien A, Rayman MP, Guallar E. Selenium status and cardiometabolic health: state of the evidence. Nutr Metab Cardiovasc Dis. 2010;20(10):754–60. doi: 10.1016/j.numecd.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Eaton CB, Abdul Baki AR, Waring ME, Roberts MB, Lu B. The association of low selenium and renal insufficiency with coronary heart disease and all-cause mortality: NHANES III follow-up study. Atherosclerosis. 2010;212(2):689–94. doi: 10.1016/j.atherosclerosis.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Lowe NM, Dykes FC, Skinner AL, Patel S, Warthon-Medina M, Decsi T, et al. EURRECA-Estimating zinc requirements for deriving dietary reference values. Crit Rev Food Sci Nutr. 2013;53(10):1110–23. doi: 10.1080/10408398.2012.742863. [DOI] [PubMed] [Google Scholar]
- 15.Hashemian M, Poustchi H, Abnet CC, Boffetta P, Dawsey SM, Brennan PJ, et al. Dietary intake of minerals and risk of esophageal squamous cell carcinoma: results from the Golestan Cohort Study. Am J Clin Nutr. 2015;102(1):102–8. doi: 10.3945/ajcn.115.107847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghayour-Mobarhan M, Taylor A, New SA, Lamb DJ, Ferns GA. Determinants of serum copper, zinc and selenium in healthy subjects. Ann Clin Biochem. 2005;42(Pt 5):364–75. doi: 10.1258/0004563054889990. [DOI] [PubMed] [Google Scholar]
- 17.Leone N, Courbon D, Ducimetiere P, Zureik M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17(3):308–14. doi: 10.1097/01.ede.0000209454.41466.b7. [DOI] [PubMed] [Google Scholar]
- 18.Willett W. Nutritional epidemiology. In: Rothman KJ, Greenland S, Lash TL, editors. Modern epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 19.Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF. Zinc bioavailability and homeostasis. Am J Clin Nutr. 2010;91(5):1478S–83S. doi: 10.3945/ajcn.2010.28674I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashemian M, Hekmatdoost A, Poustchi H, Mohammadi NF, Abnet CC, Malekzadeh R. Systematic review of zinc biomarkers and esophageal cancer risk. Middle East J Dig Dis. 2014;6(4):177–85. [PMC free article] [PubMed] [Google Scholar]
- 21.He K. Trace elements in nails as biomarkers in clinical research. Eur J Clin Invest. 2011;41(1):98–102. doi: 10.1111/j.1365-2362.2010.02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288(20):2569–78. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 23.Sarrafzadegan N, Kelishadi R, Esmaillzadeh A, Mohammadifard N, Rabiei K, Roohafza H, et al. Do lifestyle interventions work in developing countries? Findings from the Isfahan Healthy Heart Program in the Islamic Republic of Iran. Bull World Health Organ. 2009;87(1):39–50. doi: 10.2471/BLT.07.049841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan C, Chen H, Xia C. The prediction of cardiovascular disease based on trace element contents in hair and a classifier of boosting decision stumps. Biol Trace Elem Res. 2009;129(1-3):9–19. doi: 10.1007/s12011-008-8279-4. [DOI] [PubMed] [Google Scholar]
- 25.Afridi HI, Kazi TG, Kazi N, Kandhro GA, Baig JA, Jamali MK, et al. Interactions between cadmium and zinc in the biological samples of Pakistani smokers and nonsmokers cardiovascular disease patients. Biol Trace Elem Res. 2011;139(3):257–68. doi: 10.1007/s12011-009-8607-3. [DOI] [PubMed] [Google Scholar]
- 26.Islamoglu Y, Evliyaoglu O, Tekbas E, Cil H, Elbey MA, Atilgan Z, et al. The relationship between serum levels of Zn and Cu and severity of coronary atherosclerosis. Biol Trace Elem Res. 2011;144(1-3):436–44. doi: 10.1007/s12011-011-9123-9. [DOI] [PubMed] [Google Scholar]
- 27.Bayir A, Kara H, Kiyici A, Ozturk B, Akyurek F. Levels of selenium, zinc, copper, and cardiac troponin I in serum of patients with acute coronary syndrome. Biol Trace Elem Res. 2013;154(3):352–6. doi: 10.1007/s12011-013-9754-0. [DOI] [PubMed] [Google Scholar]
- 28.Giannoglou GD, Konstantinou DM, Kovatsi L, Chatzizisis YS, Mikhailidis DP. Association of reduced zinc status with angiographically severe coronary atherosclerosis: a pilot study. Angiology. 2010;61(5):449–55. doi: 10.1177/0003319710366702. [DOI] [PubMed] [Google Scholar]
- 29.Cebi A, Kaya Y, Gungor H, Demir H, Yoruk I, Soylemez N, et al. Trace elements, heavy metals and vitamin levels in patients with coronary artery disease. Int J Med Sci. 2011;8(6):456–60. doi: 10.7150/ijms.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobo JC, Stockler-Pinto MB, Farage NE, Faulin TE, Abdalla DS, Torres JP, et al. Reduced plasma zinc levels, lipid peroxidation, and inflammation biomarkers levels in hemodialysis patients: implications to cardiovascular mortality. Ren Fail. 2013;35(5):680–5. doi: 10.3109/0886022X.2013.789960. [DOI] [PubMed] [Google Scholar]
- 31.Bates CJ, Hamer M, Mishra GD. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. Br J Nutr. 2011;105(1):123–32. doi: 10.1017/S0007114510003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. 2001;14(3-4):315–30. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- 33.Cao J, Bobo JA, Liuzzi JP, Cousins RJ. Effects of intracellular zinc depletion on metallothionein and ZIP2 transporter expression and apoptosis. J Leukoc Biol. 2001;70(4):559–66. [PubMed] [Google Scholar]
- 34.Zhou Z, Liu J, Song Z, McClain CJ, Kang YJ. Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long-term ethanol exposure. Exp Biol Med (Maywood) 2008;233(5):540–8. doi: 10.3181/0710-RM-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viswanath K, Bodiga S, Balogun V, Zhang A, Bodiga VL. Cardioprotective effect of zinc requires ErbB2 and Akt during hypoxia/reoxygenation. Biometals. 2011;24(1):171–80. doi: 10.1007/s10534-010-9371-8. [DOI] [PubMed] [Google Scholar]
- 36.Tamura T, Johanning GL, Goldenberg RL, Johnston KE, DuBard MB. Effect of angiotensin-converting enzyme gene polymorphism on pregnancy outcome, enzyme activity, and zinc concentration. Obstet Gynecol. 1996;88(4 Pt 1):497–502. doi: 10.1016/0029-7844(96)00239-6. [DOI] [PubMed] [Google Scholar]
- 37.Vasto S, Mocchegiani E, Malavolta M, Cuppari I, Listi F, Nuzzo D, et al. Zinc and inflammatory/immune response in aging. Ann N Y Acad Sci. 2007;1100:111–22. doi: 10.1196/annals.1395.009. [DOI] [PubMed] [Google Scholar]
- 38.Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4(7):676–94. doi: 10.3390/nu4070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li B, Tan Y, Sun W, Fu Y, Miao L, Cai L. The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy. Toxicol Mech Methods. 2013;23(1):27–33. doi: 10.3109/15376516.2012.735277. [DOI] [PubMed] [Google Scholar]
- 40.Mehta JL, Li DY. Inflammation in ischemic heart disease: response to tissue injury or a pathogenetic villain? Cardiovasc Res. 1999;43(2):291–9. doi: 10.1016/s0008-6363(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 41.Timmers L, Pasterkamp G, de Hoog DC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94(2):276–83. doi: 10.1093/cvr/cvs018. [DOI] [PubMed] [Google Scholar]
- 42.Garg M, Khanna D. Exploration of pharmacological interventions to prevent isoproterenol-induced myocardial infarction in experimental models. Ther Adv Cardiovasc Dis. 2014;8(4):155–69. doi: 10.1177/1753944714531638. [DOI] [PubMed] [Google Scholar]
- 43.Dinicolantonio JJ, Niazi AK, McCarty MF, Lavie CJ, Liberopoulos E, O'Keefe JH. L-carnitine for the treatment of acute myocardial infarction. Rev Cardiovasc Med. 2014;15(1):52–62. doi: 10.3909/ricm0710. [DOI] [PubMed] [Google Scholar]
- 44.Hashemian M, Vakili AR, Akaberi A. Effect of glucose? insulin? potassium on plasma concentrations of C-reactive protein in acute ST- elevation myocardial infarction; a randomized clinical trial. Pak J Med Sci. 2011;27(3):673–6. [Google Scholar]
- 45.Rajaie S, Esmaillzadeh A. Dietary choline and betaine intakes and risk of cardiovascular diseases: review of epidemiological evidence. ARYA Atheroscler. 2011;7(2):78–86. [PMC free article] [PubMed] [Google Scholar]
- 46.Eftekhari MH, Akbarzadeh M, Dabbaghmanesh MH, Hassanzadeh J. The effect of calcitriol on lipid profile and oxidative stress in hyperlipidemic patients with type 2 diabetes mellitus. ARYA Atheroscler. 2014;10(2):82–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61(3):481–97. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Giacconi R, Cipriano C, Muti E, Costarelli L, Malavolta M, Caruso C, et al. Involvement of -308 TNF-alpha and 1267 Hsp70-2 polymorphisms and zinc status in the susceptibility of coronary artery disease (CAD) in old patients. Biogerontology. 2006;7(5-6):347–56. doi: 10.1007/s10522-006-9049-3. [DOI] [PubMed] [Google Scholar]
- 49.Giacconi R, Cipriano C, Muti E, Costarelli L, Maurizio C, Saba V, et al. Novel -209A/G MT2A polymorphism in old patients with type 2 diabetes and atherosclerosis: relationship with inflammation (IL-6) and zinc. Biogerontology. 2005;6(6):407–13. doi: 10.1007/s10522-005-4907-y. [DOI] [PubMed] [Google Scholar]
- 50.Wolowiec P, Michalak I, Chojnacka K, Mikulewicz M. Hair analysis in health assessment. Clin Chim Acta. 2013;419:139–71. doi: 10.1016/j.cca.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Lowe NM, Medina MW, Stammers AL, Patel S, Souverein OW, Dullemeijer C, et al. The relationship between zinc intake and serum/plasma zinc concentration in adults: a systematic review and dose-response meta-analysis by the EURRECA Network. Br J Nutr. 2012;108(11):1962–71. doi: 10.1017/S0007114512004382. [DOI] [PubMed] [Google Scholar]
- 52.Kannel WB. Coronary heart disease risk factors in the elderly. Am J Geriatr Cardiol. 2002;11(2):101–7. doi: 10.1111/j.1076-7460.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- 53.Solomons N, Leader DG, Rapporteur JLS. What impact does stage of physiological development and/or physiological state have on the bioavailability of dietary supplements? Summary of Workshop Discussion. J Nutr. 2001;131(4):1392S–5S. doi: 10.1093/jn/131.4.1392S. [DOI] [PubMed] [Google Scholar]