Abstract
Fear regulation is impaired in anxiety and trauma-related disorders. Patients experience heightened fear expression and reduced ability to extinguish fear memories. Because fear regulation is abnormal in these disorders and extinction recapitulates current treatment strategies, understanding the underlying mechanisms is vital for developing new treatments. This is critical because although extinction-based exposure therapy is a mainstay of treatment, relapse is common. We examine recent findings describing changes in network activity and functional connectivity within limbic circuits during fear regulation, and explore how activity-dependent signaling contributes to the neural activity patterns that control fear and anxiety. We review the role of the prototypical activity-dependent molecule, brain-derived neurotrophic factor (BDNF), whose signaling has been critically linked to regulation of fear behavior.
Introduction
Anxiety disorders are common, with an up to 28% lifetime prevalence rate [1]. Post-traumatic stress disorder (PTSD) is a specific anxiety disorder that develops following trauma exposure. Hallmarks of PTSD include re-living the trauma, avoidance of situations resembling the event and hyperarousal. Deficits in fear regulation, including enhanced reactivity to cues linked with the trauma and the inability to reduce those fear responses, are common in PTSD [2]. Given that many people are exposed to trauma while only a small proportion develop PTSD, understanding the biological risk factors is important [3••]. To understand and better treat fear-related disorders, identifying the processes occurring during association of contextual and sensory cues with trauma is also critical [4,5]. Importantly, this learning can be modeled in the laboratory with Pavlovian fear-conditioning, a paradigm in which an aversive unconditioned stimulus (US) footshock is paired with a neutral conditioned stimulus (CS) (Figure 1a). The learned association is evaluated in rodents by measuring the time spent freezing, a behavior indicating high levels of fear. Freezing behavior is used to assess fear learning, recall and extinction. During extinction training, animals are re-exposed to the CS and/or conditioning context in the absence of the US. Repeated exposure results in decreased freezing, indicating successful extinction, that is learning that the CS or context no longer predicts the US. Specificity for the CS–US association is probed by exposing an animal to the original CS (CS+) or a CS never paired with the US (CS−). Animals showing heightened fear towards both CS+ and CS− demonstrate non-specific, or generalized fear. Fear acquisition, recall and extinction represent distinct learning events, which are linked to specific patterns of neural activity and functional connectivity between brain regions in the fear circuitry. We discuss how molecules that sense and respond to changes in neural activity are in a powerful position to control these processes. We specifically focus on the prototypical activity-dependent molecule, brain-derived neurotrophic factor (BDNF), which has been extensively implicated in regulation of fear and anxiety behavior.
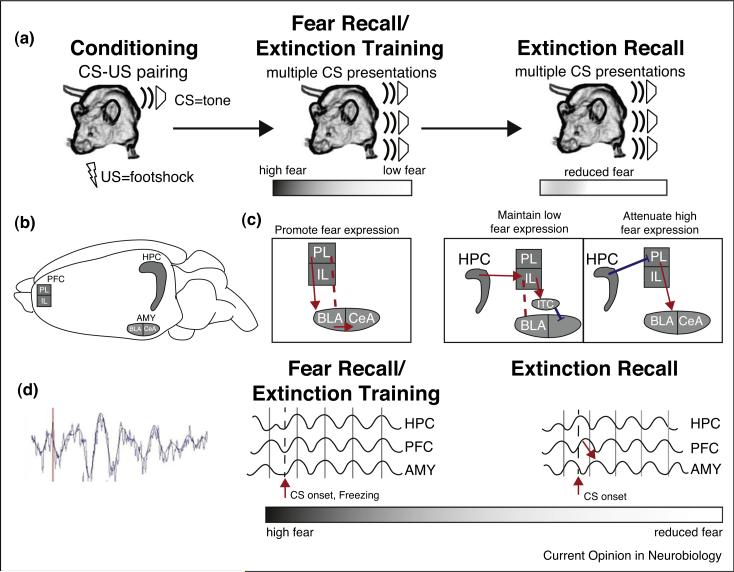
Figure 1.
Behavioral fear paradigms and their anatomical and physiological correlates. (a) Behavioral paradigms for fear learning and memory. Rodents learn to associate a neutral tone (conditioned stimulus, CS) with an aversive outcome, a footshock (unconditioned stimulus, US). Learning for this association is measured by cessation of movement (freezing). Memory for the association is measured at a later time point during which the CS is presented in the absence of the US. During the fear recall trial, the animal expresses high freezing/fear, displaying its memory for the CS–US pairing. As extinction trials (CS exposure without the US) progress, the animal learns that the CS no longer predicts the US, and freezing decreases. Memory for extinction can be tested in a subsequent session by assessing freezing to the CS during an extinction recall session. (b) Structural representation of the areas important in fear learning. The hippocampus (HPC), prefrontal cortex (PFC), and amygdala (AMY) are the main interconnected regions of the fear circuit. The AMY regions depicted include basolateral (BLA), central nucleus (CeA) and the intercalcated cells (ITCs). PFC is divided into prelimbic (PL) and infralimbic (IL) subdivisions. (c) Circuits active during extinction learning and extinction recall. During states of high fear during the fear recall/extinction training session PL activates BLA neurons, leading to excitatory output from CeA and fear expression. Activation of 2 pathways inhibits fear expression during extinction recall. To inhibit fear expression, HPC activates IL, which projects to the GABAergic ITC neurons and inhibits fear output from CeA. To decrease activity of the extinction fear expression circuit, the HPC inhibits PL, leading to an indirect decrease of CeA output. (d) The physiological activity correlated with fear memory and learning. Left, an example of a raw LFP trace with data filtered between 1 and 12 Hz to display the increase in theta activity following freezing behavior. During states of high fear theta frequency activity is synchronized across the fear circuit. During extinction recall, there is less theta synchrony in response to the CS. In addition, theta phase activity in PFC leads the AMY, which is hypothesized to be a signal of learned safety (see text).
Circuit and network activity in limbic regions influences fear and anxiety behavior
Studies in animals and humans suggest that changes in plasticity underlie function in amygdala (AMY)–prefrontal cortex (PFC)–hippocampus (HPC) fear circuits [3•• ,6] (Figure 1b). AMY is the fear acquisition and expression hub, while PFC critically controls fear inhibition and extinction. Within PFC, regional differences in fear regulation between infralimbic (IL) and prelimbic (PL) subdivisions are noted. Specifically, IL activation enhances extinction, while PL promotes fear expression [7•,8,9]. HPC modulates AMY and PFC activity, and provides contextual information about the fear memory. Regulation of fear behavior depends on coordinated activity and communication between these regions. As PFC plasticity in IL and PL is critical for fear regulation, communication between these regions and AMY has been extensively investigated (Figure 1c). Research suggests that the opposing effects of IL and PL are mediated by differences in their respective connections with AMY [7•]. Specifically, IL projects to the intercalated cell masses (ITCs) and lateral division of the central nucleus, which contain GABAergic neurons that inhibit output neurons of central amygdala (CeA). Alternatively, PL promotes fear by activating basolateral amygdala (BLA) neurons. The BLA stores the CS–US association, and BLA neurons project to and excite CeA. IL and PL also have reciprocal connections with AMY and HPC that modulate fear expression. Recent studies combining retrograde tracer techniques with immediate early gene activation in discrete projections from BLA to HPC and PFC provided new insight about circuit connectivity during fear recall and extinction [10•,11]. Specifically, these findings showed that a subpopulation of BLA to PL projection neurons become active during states of high fear, while BLA to IL projections are selectively recruited during extinction. Supporting studies demonstrated that BLA cells projecting to PL exhibit firing patterns induced by plasticity in conditioned mice, while BLA-IL cells show these changes only following extinction [10•]. These findings support the idea that cellular plasticity is required for interregional communication that regulates both fear acquisition and its extinction.
New technologies, including optogenetics, now allow researchers to directly activate or inhibit cell type specific populations, and such studies manipulating AMY and PFC cells have increased our understanding of mechanisms controlling fear regulation [10•,12,13,14••,15]. Investigations into the role of interneurons highlighted the importance of inhibitory control in temporal coordination of neural activity patterns [13,14••]. Consistent with the importance of AMY in fear acquisition, manipulating parvalbumin (PV) inhibitory interneurons in BLA during CS–US pairings correlated with freezing during CS re-exposure. Activating PV cells during conditioning caused decreased freezing during re-exposure, whereas PV cell inhibition caused increased freezing [13]. These changes were attributed to PV interneuron silencing causing disinhibition of target principal neurons; that is PV cell inhibition resulted in increased excitatory BLA activity in response to CS–US pairing. Inhibition of PV interneurons in PFC following conditioning also caused increased freezing behavior during CS re-exposure [14••]. Interestingly, inhibiting PV cells in PFC led to phase resetting of theta-frequency oscillatory activity as measured by local field potential recording (LFPs). This resulted in increased synchronized spiking of PFC output excitatory neurons targeting the BLA [14••], uncovering a possible mechanism that facilitates synchronous communication between these regions. As optogenetics and related techniques now allow for investigating how cellular changes impact physiology and behavior, an important area of future research will be utilizing these methods in combination with genetic strategies to manipulate cells reporting changes in activity-dependent plasticity [16,17].
Mechanisms by which electrophysiological activity in AMY–HPC–PFC circuits influences fear behavior are emerging. Coordinated oscillations and neuronal synchrony facilitate communication across brain regions. This is studied in behaving animals by recording LFPs or electroencephalogram (EEG), which reflect summations of oscillatory activity from surrounding neurons. In animal models, single cell recordings are also often incorporated in order to examine changes in neuronal firing in relation to the regional oscillatory activity. The power (magnitude) and synchronization of oscillations in delta (0–4Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz) and gamma (30–100 Hz) frequencies influence coordination of large-scale networks. Low-frequency theta oscillations were first shown to be important in HPC, with firing of CA1 place fields correlating with an animal's location [18]. It has since been demonstrated that both theta magnitude as well as the temporal synchronization of theta phase are important for interregional communication during spatial memory acquisition and recall [19•,20]. Theta phase timing is important for controlling synaptic plasticity [21], and synchronous, or coherent, theta activity is implicated in emotional learning and behavior. Increases in theta power and synchrony in HPC–PFC– AMY circuits are observed during states of high fear and anxiety, while decreases in phase synchrony are observed after extinction [22–25] (Figure 1d). Following conditioning and initial CS re-exposure animals concurrently demonstrate high levels of freezing and synchronous theta activity in HPC–PFC–AMY. This synchronous theta activity during early extinction is important for consolidation, as manipulating HPC–AMY theta synchrony during extinction by electrical stimulation alters fear expression following subsequent CS re-exposure [23]. Consistent with a role for PFC in extinction, once an animal successfully learns that the CS no longer predicts the US, theta synchrony between PFC–AMY shifts. Specifically, after successful extinction, LFP theta oscillations in PFC begin to ‘lead’ AMY theta oscillations [26]. The AMY firing rate is synchronized to PFC theta phase, suggesting that AMY is receiving information from PFC that inhibits freezing [24,26]. Lending further support to the idea that PFC–AMY directionality is behaviorally relevant, mice demonstrating increased generalized fear and poor extinction do not demonstrate this theta shift [24].
It has been demonstrated that theta activity plays a role in human learning and memory. The importance of theta synchrony was illustrated in a study conducting LFP and single cell recordings from AMY and HPC during memory recall in epilepsy patients implanted with microwire electrodes [27•]. Individuals were shown a set of images, and then later a second set in which 50% of the images were repeated. Similar to animal studies described previously [24,26], time-locked firing of single neurons in coordination with the region's theta activity was significantly higher when a subject correctly recognized a repeated image. Consistent with the animal literature, human EEG studies revealed changes in PFC theta and gamma oscillations during discrete stages of fear [28•]. Further paralleling animal findings, individuals with impaired extinction learning lacked the observed increase in gamma activity associated with CS extinction in the ventromedial prefrontal cortex (vmPFC), an area corresponding to IL in rodents. Another recent study utilizing a fear conditioning and extinction paradigm found that individuals reporting higher anxiety in response to uncertainty had a generalized increase in AMY activation during CS+ and CS− presentations in early extinction, as well as higher vmPFC activity during late extinction [29]. A limited amount of work has thus far investigated EEG biomarkers in PTSD patients [30], including studies investigating whether there are abnormalities in EEG activity in different frequency bands. While many results have been inconsistent, some have found altered theta/alpha activity in PTSD patients, which correlated with symptom severity [30,31]. Together, the available findings suggest that consistent markers may be associated with fear regulation, which is highly relevant for translating PTSD research.
Activity-dependent plasticity as a mediator of the circuit dynamics that control fear and anxiety behavior
As discussed above, animal models suggest that changes in oscillatory activity may represent biomarkers that could be used for improving diagnostics and treatment outcome. Both targeted genetic deletions and environment manipulations impacting activity-dependent gene expression in rodents alter electrophysiological activity in vivo as well as behavioral performance in fear-related tasks [32–36]. Specifically, disruption of molecules has been associated with activity-dependent plasticity and inhibitory control alter fear expression during CS re-exposure and theta frequency oscillatory activity [33,35]. For example, expression of the 65 kDa isoform of the GABA synthesizing enzyme, glutamic acid decarboxylase (GAD65) is transiently regulated following fear conditioning, and its deletion in mice leads to increased generalized fear expression and decreased AMY-HPC theta phase synchrony during fear memory recall [35]. Manipulation of GAD65 is of particular relevance given that this isoform is activity-dependent and its synaptic localization renders it responsible for providing GABA for phasic inhibition, which is important for network synchronization. There is also evidence that environmental factors influence the physiological aspects of fear learning, at least in part via their impact on expression of plasticity molecules. For example, chronic alcohol exposure leads to impaired extinction, which is attributed to downregulation of N-methyl-d-aspartate receptor (NMDA) receptors and decreased IL firing [32]. These findings contribute to an increasing body of literature highlighting the importance of plasticity molecules in regulating the fear circuitry at the behavioral and physiological levels.
Brain-derived neurotrophic factor (BDNF) is an activity-dependent molecule that has been extensively implicated in fear regulation and anxiety [37•, 38–41]. BDNF regulates synaptic plasticity in the developing and adult brain, and is enriched in regions associated with fear behavior including AMY, HPC, and PFC. BDNF signaling is critical at all levels of the fear circuitry-the behavior deficits that occur depend upon the brain regions in which BDNF signaling is affected. Given the important role of AMY in fear acquisition, decreasing BDNF signaling in this region significantly impacts fear learning and consolidation [42,43]. Specifically, mice with less AMY expression of BDNF display decreased fear expression to the CS following conditioning. Alternatively, BDNF disruption in HPC or PFC is associated with impairments in fear extinction [38–40,44]. Specifically, mice with virally induced HPC-specific BDNF deletions exhibit persistent fear compared to controls even after multiple CS re-exposures [44].
BDNF is a prototypical activity-dependent molecule with both its transcription and secretion controlled by neural activity. Many levels of regulation, including multiple transcript production, control BDNF signaling. At least 9 upstream promoters drive BDNF expression [45], with 2 being highly dependent on induction of neural activity [46]. Epigenetic regulation at specific BDNF promoters has been correlated with impaired fear regulation and anxiety [47,48]. Early exposure to adverse events results in chromatin remodeling that influences BDNF expression in regions important for fear regulation and anxiety during adulthood [49,50]. At the genetic level, a single-nucleotide polymorphism (SNP) at BDNF codon 66 is implicated in fear regulation and anxiety [51,52]. This valine-to-methionine substitution (Val66Met) causes abnormal BDNF trafficking and reduced activity-dependent release [52]. A role for BDNF in emotional learning was translated from animal models to humans with the finding that both mice and people carrying the Met allele display impaired fear extinction [40,53]. Met allele carriers demonstrating impaired extinction also show reduced vmPFC activation during extinction compared to Val-allele counterparts [40]. Finally, harboring the Met allele is predictive of poorer response to exposure therapy [54]. Following 8 weeks of cognitive behavioral therapy PTSD patients carrying the Met allele showed a smaller reduction in behavioral symptoms compared to Val carriers. These findings provide evidence that tight control of activity-dependent BDNF expression is essential for regulating fear and anxiety, and provide translational support for the idea that extinction deficits observed in animal models may be recapitulated in humans with similar genetic variants.
BDNF influences learning and extinction in fear circuits through its role in neural activation and memory formation. Abnormal NMDAR-mediated transmission in AMY, HPC and PFC contributes to altered synaptic plasticity in mice modeling the BDNF Val66Met polymorphism [55–57]. Decreased late phase long-term potentiation (LLTP) hippocampal plasticity is also observed in animals where activity-dependent BDNF signaling is selectively attenuated [38]. Moreover, exogenous BDNF application influences neuronal excitability in key brain regions during fear regulation. Specifically, ventral HPC (vHPC) BDNF infusion increases IL firing rate [9], and decreases fear expression when treatment occurs before extinction [39]. Signaling downstream of BDNF activates pathways important for protein translation that are critical for LTP induction, including mammalian target of rapamycin (mTOR) and extracellular signal-related kinases (ERK). Activation of these pathways is implicated in fear memory, typically in the context that decreased activation results in less protein synthesis and impaired memory formation or consolidation [58–60].
Activity-dependent BDNF signaling significantly impacts excitatory/inhibitory (E/I) balance via its regulation of both glutamatergic and GABAergic neurotransmission. As demonstrated in a number of genetic models, proper E/I balance is critical in regulating fear and anxiety [35,38,61]. While BDNF is primarily expressed in glutamatergic cells, tropomysin receptor kinase B (TrkB), BDNF's cognate receptor, is expressed in both excitatory and inhibitory neurons [62,63]. BDNF-TrkB signaling is implicated in inhibitory synapse function and controls the maturation of cortical inhibition [64]. Since BDNF potently regulates GABAergic synapses, BDNF signaling is theorized to be a key mechanism in the homeostatic plasticity that maintains E/I balance [65]. This idea is supported by the fact that neural activity induces Bdnf expression, and the subsequently produced BDNF promotes inhibition to dampen excitability. Evidence that disrupting activity-dependent Bdnf expression and secretion impairs inhibitory synapses and GABAergic transmission provides additional empirical support for this hypothesis [56,66]. Genetically altered mice in which activity-dependent BDNF signaling is attenuated have fewer fast-spiking PV interneurons and reduced inhibitory post-synaptic currents (IPSC) in PFC, contributing to impaired GABAergic transmission [38,67]. Several inter-neuron subtypes express TrkB, providing a mechanistic basis for controlling inhibitory synaptic potentiation [63]. This possibility is strengthened by evidence that TrkB deletion in PV-interneurons decreases their action potential generation [68]. Evidence that heterozygous TrkB deletion in PV-interneurons causes fear extinction deficits suggests that TrkB signaling may contribute to the ability of PV-interneurons to regulate fear [61]. Thus, BDNF's ability to properly regulate fear learning and extinction may be mediated at least in part by its critical role in inhibitory plasticity and E/I balance.
Conclusions
Recent work has increased our understanding of how network communication within fear circuits controls fear and anxiety. The molecular and cellular mechanisms regulating oscillatory activity during fear recall and extinction are not yet clearly understood. However, sophisticated systems neuroscience techniques are providing researchers with tools to answer these questions. The available evidence suggests that it is important to investigate the role of activity-dependent molecules, including BDNF, as these molecules are in a powerful position to coordinate the cellular, physiological, and behavioral events that dictate expression of fear and anxiety. In humans, EEG changes associated with abnormal fear learning and extinction could serve as non-invasive, translational biomarkers to improve diagnosis and treatment response. A growing body of work suggests that consistent physiological changes across animal and human studies are identifiable in individuals showing heightened generalized fear, and that these alterations are correlated with genetic variation in molecules that regulate activity-dependent processes. By understanding the genetic and environmental factors that influence plasticity and associating them with biomarkers that report physiological responses to these events, the ability to manipulate plasticity to improve fear and anxiety outcome may be realized.
Acknowledgements
Research in the Martinowich group related to this manuscript is supported by the National Institute of Mental Health (PI: KM, MH105592), the Brain and Behavior Research Foundation and the Lieber Institute for Brain Development.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
of special interest
of outstanding interest
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Morrison FG, Ressler KJ. From the neurobiology of extinction to improved clinical treatments. Depress Anxiety. 2013;31:279–290. doi: 10.1002/da.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36:23–31. doi: 10.1016/j.tins.2012.11.003. [A discussion of the factors which can contribute to PTSD — such as stress, alcohol, and epigenetic changes — and the development of potential PTSD therapies. Work from both rodent and human literature is considered.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Likhtik E, Paz R. Amygdala–prefrontal interactions in (mal) adaptive learning. Trends Neurosci. 2015;38:158–166. doi: 10.1016/j.tins.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lissek S, van Meurs B. Learning models of PTSD: theoretical accounts and psychobiological evidence. Int J Psychophysiol. 2014 doi: 10.1016/j.ijpsycho.2014.11.006. http://dx.doi.org/10.1016/j.ijpsycho.2014.11.006. [DOI] [PMC free article] [PubMed]
- 6.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [A review of the differing roles of infralimbic cortex and prelimbic cortex in fear extinction, with a discussion of the potential benefits of complex mPFC–amygdala interacitons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenton GE, Pollard AK, Halliday DM, Mason R, Bredy TW, Stevenson CW. Persistent prelimbic cortex activity contributes to enhanced learned fear expression in females. Learn Mem. 2014;21:55–60. doi: 10.1101/lm.033514.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ. Hippocampal–prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology. 2014;39:2161–2169. doi: 10.1038/npp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Senn V, Wolff SBE, Herry C, Grenier F, Ehrlich I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, Lüthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [The complex interactions between the amygdala and its target neurons in the hippocampus and prefrontal cortex are investigated in this work through retrograde labeling, optogenetics, and in vitro/in vivo recordings. The results suggest that changes in plasticity between amygdala and prefrontal cortex impact fear learning and extinction.] [DOI] [PubMed] [Google Scholar]
- 11.Hübner C, Bosch D, Gall A, Lüthi A, Ehrlich I. Ex vivo dissection of optogenetically activated mPFC and hippocampal inputs to neurons in the basolateral amygdala: implications for fear and emotional memory. Front Behav Neurosci. 2014;8:64. doi: 10.3389/fnbeh.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho J-H, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC–amygdala circuits. Neuron. 2013;80:997–1003. doi: 10.1016/j.neuron.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff SBE, Gründemann J, Tovote P, Krabbe S, Jacobson GA, Müller C, Herry C, Ehrlich I, Friedrich RW, Letzkus JJ, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- 14••.Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TCM, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [Although several studies have found increased theta frequency phase synchrony between the amygdala and prefrontal cortex during fear recall, this study offers a potential mechanism by which synchrony is impacted through inhibition of parvalbumin interneurons.] [DOI] [PubMed] [Google Scholar]
- 15.Do-Monte FH, Manzano-Nieves G, Quiñones-Laracuente XK, Ramos-Medina L, Quirk GJ. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci. 2015;35:3607–3615. doi: 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denny C, Kheirbek A, Alba MA, Tanaka EL, Brachman KF, Laughman RA, Tomm KB, Turi NK, Losonczy GF, Hen AR. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth L, Kentros C, Mayford M. Generation of a synthetic memory trace. Science. 2014;335:1513–1516. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 19•.Colgin LL. Mechanisms and functions of theta rhythms. Annu Rev Neurosci. 2013;36:295–312. doi: 10.1146/annurev-neuro-062012-170330. [This review provides a broad overview of the brain's generation of theta oscillations, as well as an in-depth history of research implicating theta oscillations in learning and interregional communication.] [DOI] [PubMed] [Google Scholar]
- 20.Lisman JE, Jensen O. The θu–γ neural code. Neuron. 2013;77:1002–1016. doi: 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyman JM, Wyble BP, Goyal V, Rossi C, Hasselmo AME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci. 2003;23:11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adhikari A, Topiwala M, Gordon J. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape H-C. Patterns of coupled theta activity in amygdala– hippocampal–prefrontal cortical circuits during fear extinction. PLoS ONE. 2011;6:e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Likhtik E, Stujenske JM, Topiwala M, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidenbecher T, Laxmi TR, Stork O, Pape H-C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- 26.Lesting J, Daldrup T, Narayanan V, Himpe C, Seidenbecher T, Pape HC. Directional theta coherence in prefrontal cortical to amygdalo-hippocampal pathways signals fear extinction. PLOS ONE. 2013;8:e77707. doi: 10.1371/journal.pone.0077707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [A demonstration that the timing of oscillatory activity is important to human memory retention, and that this timing is intrinsically linked to mechanisms of synaptic plasticity.] [DOI] [PubMed] [Google Scholar]
- 28•.Mueller EM, Panitz C, Hermann C, Pizzagalli DA. Prefrontal oscillations during recall of conditioned and extinguished fear in humans. J Neurosci. 2014;34:7059–7066. doi: 10.1523/JNEUROSCI.3427-13.2014. [This study is one of the first to conduct time-frequency analysis on EEG data collected from human subjects during extinction recall. Its findings demonstrate that there are potential correlates in human EEG that are comparable to previous results in rodent models.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morriss J, Christakou A, van Reekum CM. Intolerance of uncertainty predicts fear extinction in amygdala–ventromedial prefrontal cortical circuitry. Biol Mood Anxiety Disord. 2015;5:4. doi: 10.1186/s13587-015-0019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobo I, Portugal LC, Figueira I, Volchan E, David I, Garcia Pereira M, de Oliveira L. EEG correlates of the severity of posttraumatic stress symptoms: a systematic review of the dimensional PTSD literature. J Affect Disord. 2015;183:210–220. doi: 10.1016/j.jad.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Wahbeh H, Oken BS. Peak high-frequency HRV and peak alpha frequency higher in PTSD. Appl Psychophysiol Biofeedback. 2013;38:57–69. doi: 10.1007/s10484-012-9208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes A, Fitzgerald PJ, Macpherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, et al. Chronic alcohol remodels prefrontal neurons and disrupts NMDA receptor-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald PJ, Pinard CR, Camp MC, Feyder M, Sah A, Bergstrom HC, Graybeal C, Liu Y, Schlüter OM, Grant SG, et al. Durable fear memories require PSD-95. Mol Psychiatry. 2014 doi: 10.1038/mp.2015.44. http://dx.doi.org/10.1038/mp.2014.161. [DOI] [PubMed]
- 34.Fitzgerald PJ, Whittle N, Flynn SM, Graybeal C, Pinard CR, Gunduz-Cinar O, Kravitz AV, Singewald N, Holmes A. Prefrontal single-unit firing associated with deficient extinction in mice. Neurobiol Learn Mem. 2014;113:69–81. doi: 10.1016/j.nlm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape HC, Stork O. Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory. Learn Mem. 2008;15:163–171. doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayanan V, Heiming RS, Jansen F, Lesting J, Sachser N, Pape HC, Seidenbecher T. Social defeat: impact on fear extinction and amygdala–prefrontal cortical theta synchrony in 5-HTT deficient mice. PLoS ONE. 2011;6:e22600. doi: 10.1371/journal.pone.0022600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity: memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci. 2014;122:169–192. doi: 10.1016/B978-0-12-420170-5.00006-4. [This review offers an extensive overview of the role of BDNF in the amygdala, hippocampus and prefrontal cortex in different learning paradigms. Also discussed is the potential benefit to targeting BDNF in the treatment of psychiatry disorders.] [DOI] [PubMed] [Google Scholar]
- 38.Sakata K, Martinowich K, Woo NH, Schloesser RJ, Jimenez DV, Ji Y, Shen L, Lu B. Role of activity-dependent BDNF expression in hippocampal–prefrontal cortical regulation of behavioral perseverance. Proc Natl Acad Sci U S A. 2013;110:15103–15108. doi: 10.1073/pnas.1222872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal–infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heldt S, Zimmermann K, Parker K, Gaval M, Ressler KJ. Bdnf deletion or TrkB impairment in amygdala inhibits both appetitive and aversive learning. J Neurosci. 2014;34:2444–2450. doi: 10.1523/JNEUROSCI.4085-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou D, Huang CC, Hsu KS. Brain-derived neurotrophic factor in the amygdala mediates susceptibility to fear conditioning. Exp Neurol. 2014;255:19–29. doi: 10.1016/j.expneurol.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Heldt SA, Stanek L, Chhatwal JP, Ressler K. Hippocampusspecific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2008;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 46.Aid T, Kazantseva A, Piirsoo M, Palm K. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;535:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandey SC, Sakharkar AJ, Tang L, Zhang H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis. 2015 doi: 10.1016/j.nbd.2015.03.019. http://dx.doi.org/10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed]
- 50.Roth TL, Matt S, Chen K, Blaze J. Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Dev Psychobiol. 2014 doi: 10.1002/dev.21218. http://dx.doi.org/ 10.1002/dev.21218. [DOI] [PMC free article] [PubMed]
- 51.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 52.Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, Siao C-J, Herrera DG, Toth M, Yang C, McEwen BS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lonsdorf TB, Golkar A, Lindström KM, Haaker J, Ohman A, Schalling M, Ingvar M. BDNFval66met affects neural activation pattern during fear conditioning and 24 h delayed fear recall. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu102. http://dx.doi.org/10.1093/scan/nsu102. [DOI] [PMC free article] [PubMed]
- 54.Felmingham KL, Dobson-Stone C, Schofield PR, Quirk GJ, Bryant RA. The brain-derived neurotrophic factor Val66Met polymorphism predictrs response to exposure therapy in posttraumatic stress disorder. Biol Psychiatry. 2009;73:1059–1063. doi: 10.1016/j.biopsych.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galvin C, Lee FS, Ninan I. Alteration of centromedial amygdala glutamatergic synapses by the BDNF Val66Met polymorphism. Neuropsychopharmacology. 201540:2269–2277. doi: 10.1038/npp.2015.76. http://dx.doi.org/10.1038/npp.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pattwell SS, Bath KG, Perez-Castro R, Lee FS, Chao MV, Ninan I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J Neurosci. 2012;32:2410–2421. doi: 10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, Chao MV. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci. 2010;30:8866–8870. doi: 10.1523/JNEUROSCI.1405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cestari V, Rossi-Arnaud C, Saraulli D, Costanzi M. The MAP(K) of fear: from memory consolidation to memory extinction. Brain Res Bull. 2014;105:8–16. doi: 10.1016/j.brainresbull.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Huynh TN, Santini E, Klann E. Requirement of mammalian target of rapamycin complex 1 downstream effectors in cued fear memory reconsolidation and its persistence. J Neurosci. 2014;34:9034–9039. doi: 10.1523/JNEUROSCI.0878-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mac Callum PE, Hebert M, Adamec RE, Blundell J. Systemic inhibition of mTOR kinase via rapamycin disrupts consolidation and reconsolidation of auditory fear memory. Neurobiol Learn Mem. 2014;112:176–185. doi: 10.1016/j.nlm.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Lucas EK, Jegarl A, Clem RL. Mice lacking TrkB in parvalbumin-positive cells exhibit sexually dimorphic behavioral phenotypes. Behav Brain Res. 2014;274:219–225. doi: 10.1016/j.bbr.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cellerino A, Maffei L, Domenici L. The distribution of brain-derived neurotrophic factor and its receptor trkB in parvalbumin-containing neurons of the rat visual cortex. Eur J Neurosci. 1996;8:1190–1197. doi: 10.1111/j.1460-9568.1996.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 63.Gorba T, Wahle P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur J Neurosci. 1999;11:1179–1190. doi: 10.1046/j.1460-9568.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 64.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 65.Rutherford LC, Dewan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, Lu B. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:5942–5947. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng K, An JJ, Yang F, Xu W, Xu Z-QD, Wu J, Hökfelt TGM, Fisahn A, Xu B, Lu B. TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc Natl Acad Sci U S A. 2011;108:17201–17206. doi: 10.1073/pnas.1114241108. [DOI] [PMC free article] [PubMed] [Google Scholar]