Abstract
Nitroglycerin (glycerol trinitrate, GTN) induces headache in migraineurs, an effect that has been used both diagnostically and in the study of the pathophysiology of this neurovascular pain syndrome. An important feature of this headache is a delay from the administration of GTN to headache onset that, because of GTN’s very rapid metabolism, cannot be due to its pharmacokinetic profile. It has recently been suggested that activation of perivascular mast cells, which has been implicated in the pathophysiology of migraine, may contribute to this delay. We reported that hyperalgesia induced by intradermal GTN has a delay to onset of ~30 min in male and ~45 min in female rats. This hyperalgesia was greater in females, was prevented by pretreatment with the anti-migraine drug, sumatriptan, as well as by chronic pretreatment with the mast cell degranulator, compound 48/80. The acute administration of GTN and compound 48/80 both induced hyperalgesia that was prevented by pretreatment with octoxynol-9, which attenuates endothelial function, suggesting that GTN and mast cell-mediated hyperalgesia are endothelial cell-dependent. Furthermore, A-317491, a P2X3 antagonist, which inhibits endothelial cell-dependent hyperalgesia, also prevents GTN and mast cell-mediated hyperalgesia. We conclude that delayed onset mechanical hyperalgesia induced by GTN is mediated by activation of mast cells, which in turn release mediators that stimulate endothelial cells to release ATP, to act on P2X3, a ligand-gated ion channel, in perivascular nociceptors. A role of the mast and endothelial cell in GTN-induced hyperalgesia suggest potential novel risk factors and targets for the treatment of migraine.
Keywords: Vascular pain, nitroglycerin, endothelium, mast cells, migraine, P2X3
INTRODUCTION
Migraine, a chronic pain disorder whose pathophysiology is still incompletely understood, affects approximately 15% of the adult population, with women about twice as likely to suffer from this condition (Blackwell et al., 2014). Neurovascular mechanisms are considered important in the pathophysiology of migraine (Blackwell et al., 2014; Gupta et al., 2011), and a role for nitric oxide (NO), a potent vasodilator, has been implicated by finding that intravenous infusion of the NO donor, glyceryl trinitrate (GTN), reliably induces an attack in migraineurs (Olesen, 2008). However, while release of NO following GTN infusion is very rapid (Persson et al., 1994) and NO can act directly on nociceptors (Holthusen and Arndt, 1995), onset of GTN-induced headache does not occur until at least 45 – 60 min after its infusion (Sances et al., 2004; Thomaides et al., 2003), and persists for hours (Olesen et al., 1995). This markedly delayed onset of GTN-induced migraine-like headache indicates that NO is acting indirectly to induce a migraine attack.
While perivascular nociceptor mechanisms play a role in migraine (Asghar et al., 2011), there are challenges to directly studying the role of dural nociceptors in behavioral models of migraine-like pain. Therefore, in this study we explored whether we could produce delayed onset mechanical hyperalgesia using local administration of the classical inducer of experimental migraine, GTN, at the site of nociceptive testing, as well as by intravenous administration. We also tested the contribution of perivascular targets (mast cells, endothelium and leukocytes) in this GTN-induced delayed onset hyperalgesia.
EXPERIMENTAL PROCEDURES
Animals
Experiments were performed on adult male and female Sprague Dawley rats, (200–250 g; Charles River, Hollister, CA). Experimental animals were housed three per cage, under a 12 h light/dark cycle, in a temperature- and humidity-controlled environment. Food and water were available ad libitum. All behavioral nociceptive testing was performed between 10:00 AM and 4:00 PM. Experimental animals were acclimatized to the testing environment by bringing them to the room in which the experiments were to be performed in their home cages, where they were left for 15 – 30 min. After this acclimatization period, rats were placed in cylindrical acrylic restrainers that have side openings that allow extension of the hind limbs for nociceptive testing.
Nociceptive threshold was defined as the mean of three readings of mechanical threshold for hind leg withdrawal, taken at 5-min intervals. All experimental protocols were approved by the University of California, San Francisco Committee on Animal Research and conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Testing cutaneous nociception
The nociceptive flexion reflex was quantified with an Ugo Basile Analgesymeter® (Stoelting, Wood Dale, IL), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw. Nociceptive threshold was defined as the force, in grams, at which the rat withdrew its paw from this stimulus. Hyperalgesia was defined as a decrease in mechanical nociceptive threshold, here presented as percentage change from baseline nociceptive threshold. Both paws of the same rat received the same treatment, and baseline paw withdrawal threshold was defined as the mean of the three readings taken just before administration of a test agent. Each experiment was performed on separate groups of rats. Animals acted as their own controls, with a test agent injected either intradermally, on the dorsal surface of the hind paw at the site of nociceptive testing, or intravenously, before the intradermal administration of GTN. In one group of rats, GTN was administered intravenously (4 µg/kg/min, for 20 min) via a 25 ga butterfly infusion set in a lateral tail vein; mechanical nociceptive testing in this group began 30 min after start of infusion. Paw-withdrawal thresholds before and after drug treatment were compared.
Mast cell degranulation
Repeated administration of compound 48/80 causes long-lasting local mast cell depletion in the skin (Jaffery et al., 1994). We employed a protocol as previously described (Piovezan et al., 2004), wherein 3 daily escalating doses (1, 3 and 10 mg) of compound 48/80 were administered intradermally to the dorsal surface of the hind paw, at the site of nociceptor testing. On the fourth day, a 10 mg test dose of compound 48/80 was administered and nociceptive threshold was evaluated 30 min after administration. In animals receiving this protocol, compound 48/80 on the fourth day did not produce hyperalgesia, indicating mast cell degranulation, as acute administration of compound 48/80 produces robust mechanical hyperalgesia (Chatterjea et al., 2012) (and see Fig. 5).
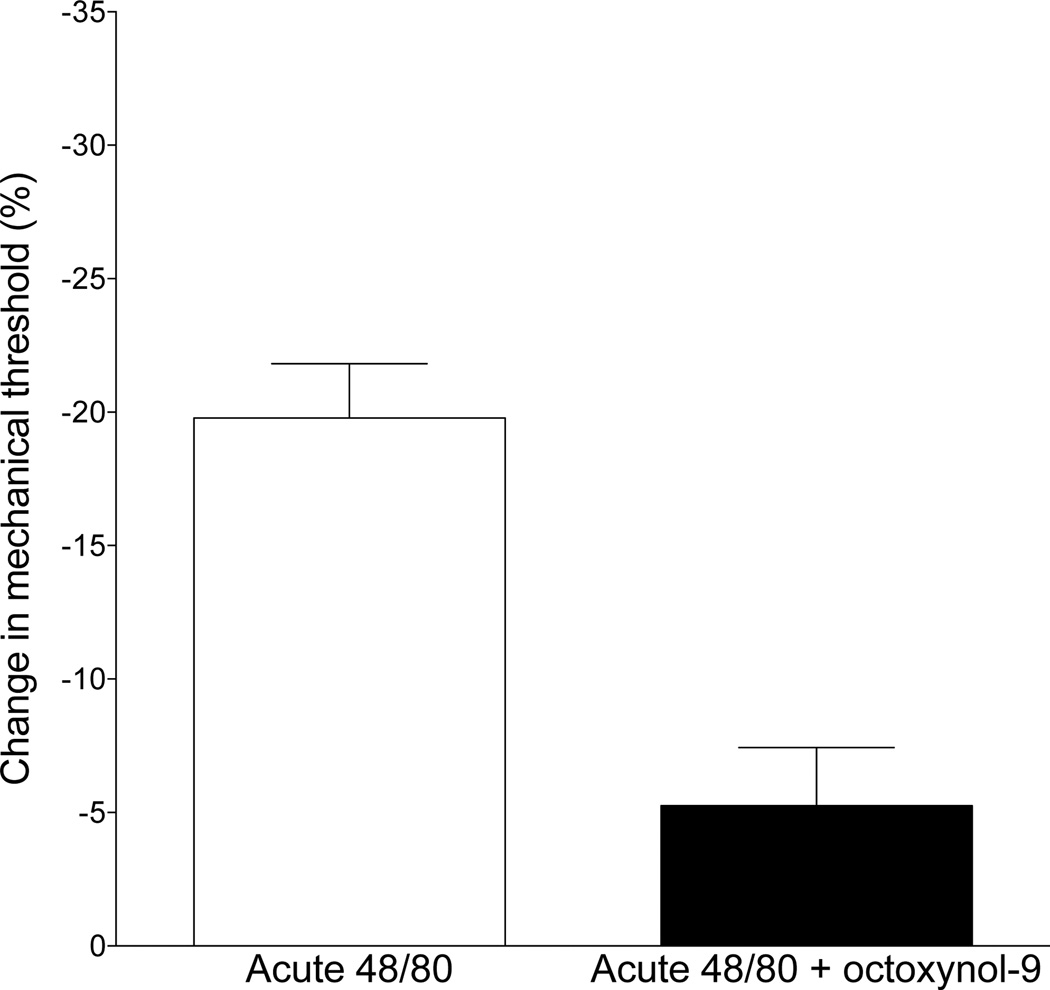
Fig. 5. Effect of octoxynol-9 on compound 48/80-induced hyperalgesia.
The repeated, escalating doses of compound 48/80, which depletes mast cells (see Methods and Fig. 3), attenuated compound 48/80 induced hyperalgesia. A single injection of compound 48/80 (10 µg intradermal in hind paw) causes mast cell degranulation, releasing pronociceptive mediators (Xanthos et al., 2011), that in turn produces mechanical hyperalgesia. Disrupting endothelial cell function by pretreatment with octoxynol-9 markedly attenuated the hyperalgesia induced by a single injection of compound 48/80 (one-tailed Student’s t-test, P<0.0004).
Attenuating endothelial function
Intravenous administration of octoxynol-9 impairs the function of the endothelial cell lining of blood vessels (Bourreau et al., 1993; McLeod and Piper, 1992; Sun et al., 1997), disrupting its contribution to nociceptor sensitization (Chen et al., 2014). To evaluate the role of the endothelial cell in GTN-induced by hyperalgesia, rats received an intravenous injection, through a tail vein, of a 0.5% solution of octoxynol-9, at a volume of 1 ml/kg body weight (Joseph and Levine, 2012a), 15 minutes before evaluation of GTN hyperalgesia.
Drugs
Glyceryl trinitrate (GTN), compound 48/80, fucoidin, sumatriptan, octoxynol-9 and A-317491 (Sigma Chemical Co., St. Louis, MO) were dissolved in saline. Drugs administered by intradermal injection were given in a volume of 2.5 µl/paw; octoxynol-9 was administered by intravenous injections in a volume of 1 ml/kg body weight. Doses of the drugs employed in this study were based on the result of dose response studies performed during preliminary experiments for this study, or during prior studies (Joseph and Levine, 2012a; Piovezan et al., 2004).
Statistical analyses
Group data are represented as mean ± SEM. Statistical significance was determined by unpaired Student’s t-test or by two-way repeated-measures ANOVA, followed by Sidek’s multiple comparison post hoc test. P<0.05 was considered statistically significant.
RESULTS
Effect of GTN on nociceptive threshold
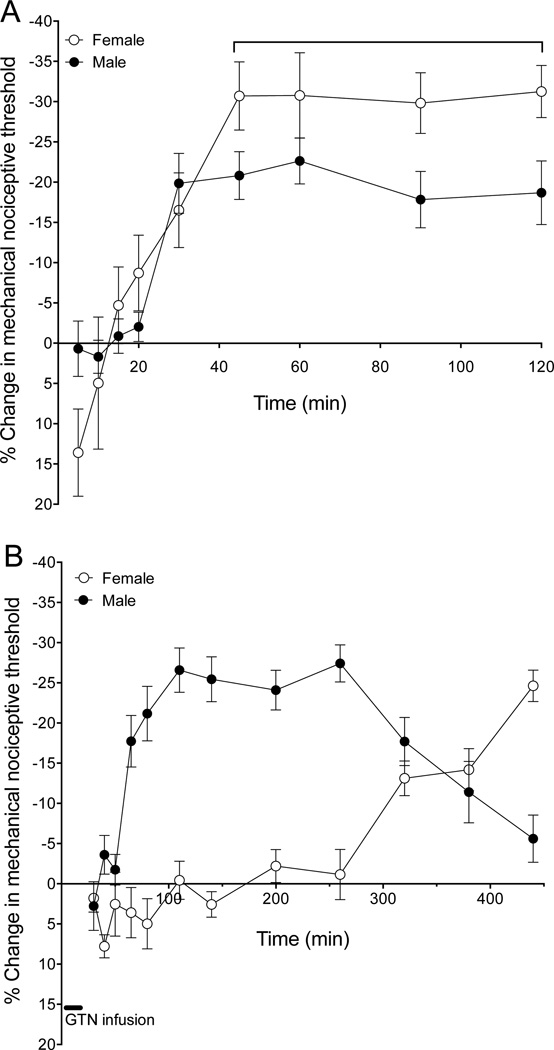
Intradermal injection of GTN (0.1 ng) produced delayed onset mechanical hyperalgesia, peaking 30 min after administration in male and 45 min in female rats, and remaining unattenuated over the remainder of the 120-minute observation period (Fig. 1A). The magnitude of GTN hyperalgesia during its plateau phase (45 – 120 min) is markedly greater in female rats (2-way repeated measures ANOVA, male vs. female P=0.039).
Fig. 1. GTN-induced delayed onset mechanical hyperalgesia.
(A). Nitroglycerin (GTN) was administered intradermally (0.1 ng, i.d.) on the dorsum of the hind paw. Mechanical nociceptive threshold was measured using the Randall-Selitto analgesymeter every 5 min for 120 min post GTN administration. GTN produced a decrease in nociceptive threshold, beginning ~30 min post-injection in males and ~45 min in females that remained undiminished for the duration of the testing period. During the plateau phase (30–120 min post administration), GTN-induced hyperalgesia was significantly greater in females (unpaired Student’s t-test, P<0.039)
(B). GTN was administered intravenously (4 µg/kg/min for 20 min) and mechanical nociceptive threshold evaluated at multiple time points between 30 and 440 min after starting GTN infusion. GTN produced a decrease in nociceptive threshold, beginning ~65 min after starting infusion in males but not until ~320 min in females. There is a significant difference in the effect of GTN in males and females (two-way repeated ANOVA, P<0.0001).
Intravenous injection of GTN (4 µg/kg/min, for 20 min) in males produced a marked decrease in nociceptive threshold beginning ~60 min after starting the perfusion, plateauing from 100 – 260 min, with threshold returning to baseline by ~440 min. In females hyperalgesia began much later, ~320 min after the start of intravenous GTN and reached a plateau ~440 min (Fig. 1B); there is a significant difference between males and females (two-way repeated measures ANOVA, P<0.0001).
Analysis of the neurovascular mechanism mediating this GTN-induced delayed onset mechanical hyperalgesia was performed using the intradermal route of administration in male rats.
Effect of triptan on GTN hyperalgesia
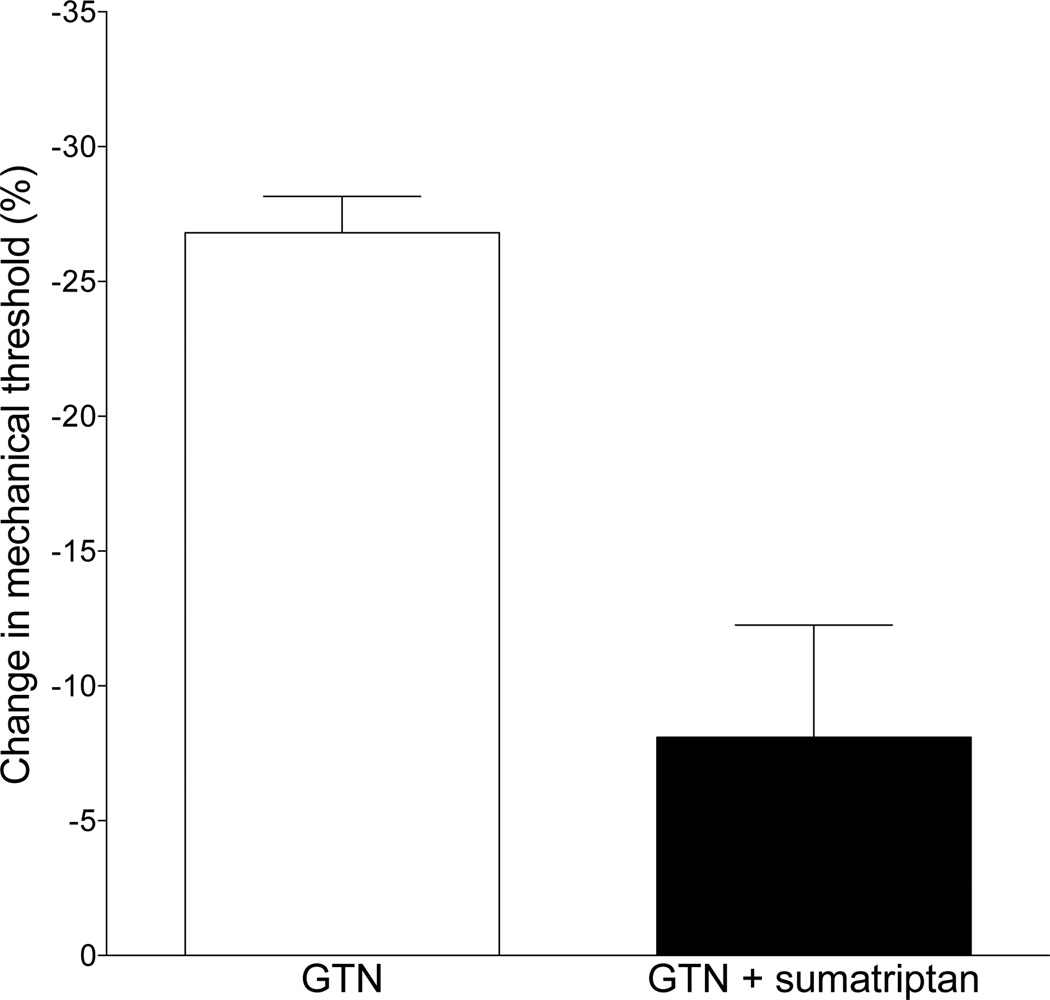
To test whether GTN hyperalgesia can be prevented by a standard therapy used to prevent an attack of migraine, sumatriptan (1 µg 10 min prior to GTN administration) was administered at the site of GTN administration and nociceptive testing. Sumatriptan markedly attenuated mechanical hyperalgesia induced by a subsequent injection of GTN (control vs. sumatriptan, P<0.001, unpaired Student’s t-test, Fig. 2).
Fig. 2. Effect of sumatriptan on GTN-induced delayed onset hyperalgesia.
The intradermal administration of sumatriptan (1 µg i.d.), 10 min before intradermal GTN administration, markedly attenuated GTN-induced delayed onset mechanical hyperalgesia (one-tailed Student’s t-test, P<0.0005).
Role of mast cell
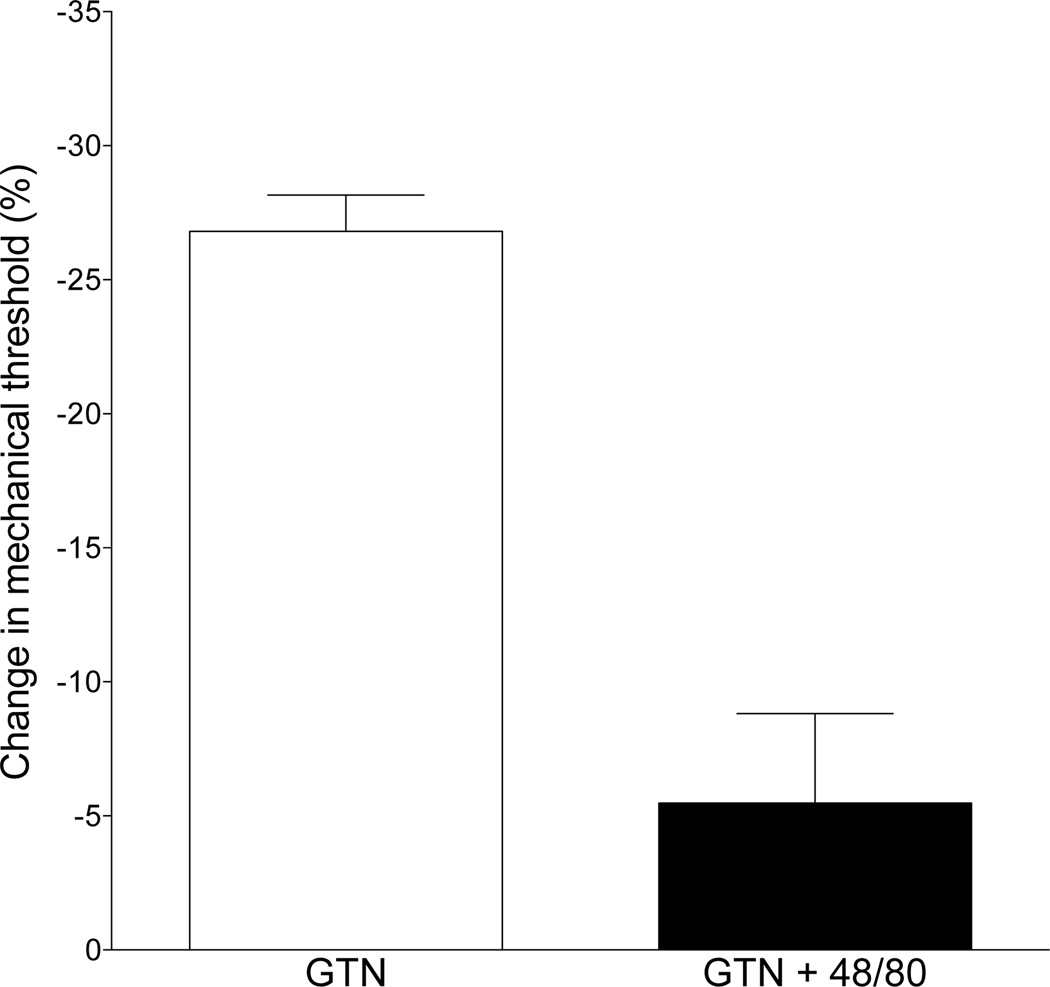
To test whether GTN-induced delayed onset mechanical hyperalgesia is mast cell dependent, as hypothesized by Olesen and colleagues (Olesen, 1992; Pedersen et al., 2015), we pretreated rats with compound 48/80 (see Methods for depletion protocol) at the site of nociceptive testing. Compound 48/80 markedly attenuated the delayed onset mechanical hyperalgesia induced by the subsequent injection of GTN (control vs. 48/80-treatment, P<0.0001, unpaired Student’s t-test, Fig. 3).
Fig. 3. Effect of the mast cell degranulator, compound 48/80, on GTN-induced delayed onset hyperalgesia.
Three daily injection of compound 48/80 (see Methods section for details) to deplete mast cells of their pronociceptive mediators markedly attenuated GTN-induced hyperalgesia (one-tailed Student’s t-test, P<0.0001).
Role of endothelial cell
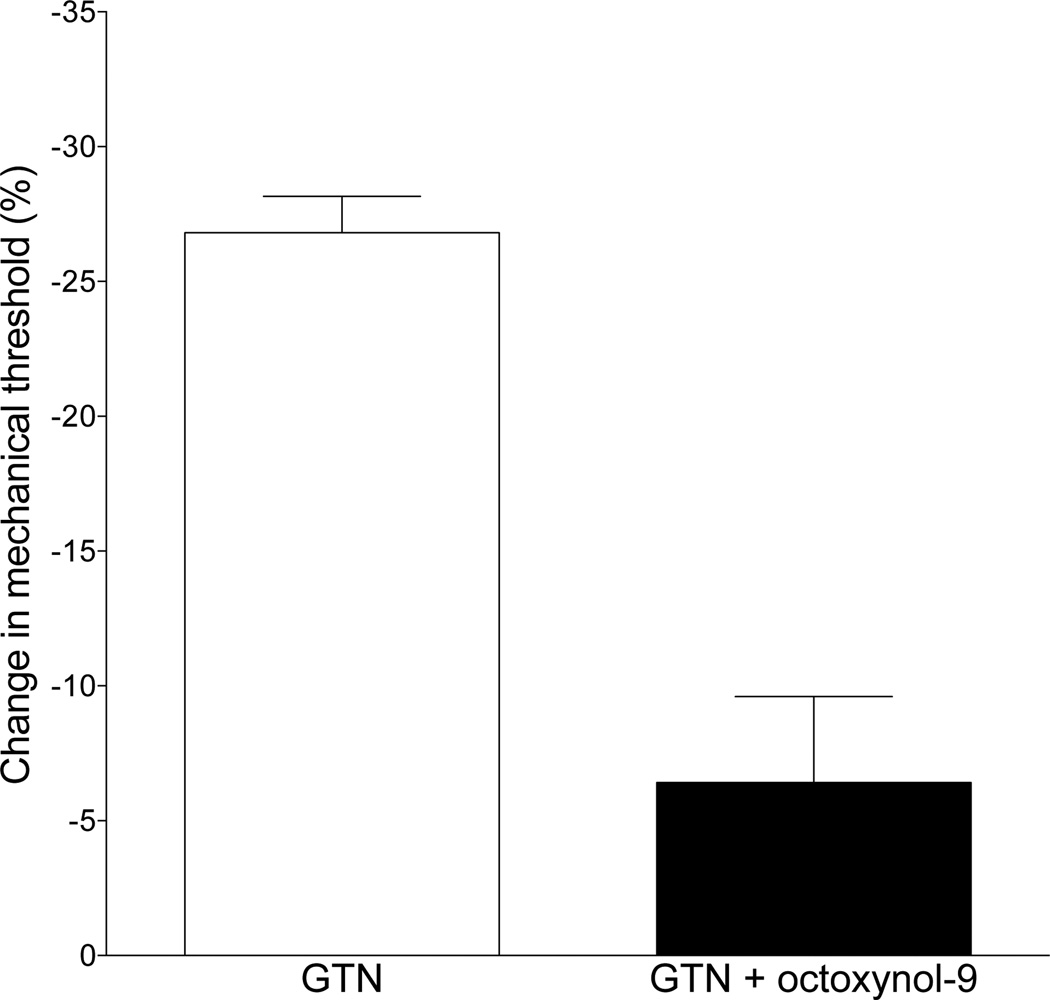
We have previously demonstrated a role for the vascular endothelial cell in mechanical hyperalgesia associated with vascular pain (Joseph et al., 2013; Joseph et al., 2015). Since mast cells are found in relatively high density adjacent to blood vessels in the microvasculature (Metcalfe et al., 1997), we next tested the hypothesis that attenuating vascular endothelial cell function, using intravenous octoxynol-9 administration, as previously described (Joseph and Levine, 2012a) would attenuate GTN-induced delayed onset hyperalgesia. GTN-induced hyperalgesia was markedly attenuated following impairment of endothelial cell function with octoxynol-9 pre-treatment (control vs. octoxynol-9 treatment, P<0.0005 unpaired Student’s ttest, Fig. 4).
Fig. 4. Effect of octoxynol-9 on GTN-induced delayed onset hyperalgesia.
Administration of the endothelial cell disruptor, octoxynol-9 (0.5% 1 ml/kg body weight, 30 min prior to GTN), markedly attenuated GTN-induced delayed onset mechanical hyperalgesia (one-tailed Student’s t-test, P<0.0001).
Relationship between mast and endothelial cell
We observed that GTN-induced hyperalgesia is dependent on both mast (Fig. 3) and endothelial (Fig. 4) cells. We next tested the hypothesis that GTN acts on perivascular mast cells to induce the release of a mediator(s) that acts on neighboring endothelial cells to release a purinergic pronociceptive mediator(s) that, in turn, acts on the peripheral terminal of nociceptors to sensitize them to mechanical stimulation. Therefore, we evaluated whether compound 48/80, which acutely induces mast cell-dependent hyperalgesia (Chatterjea et al., 2012), is affected by attenuating endothelial cell function. Octoxynol-9 pretreatment markedly attenuated hyperalgesia induced by acute administration of compound 48/80 (10 µg) (control vs. octoxynol-9 treatment, P<0.001 unpaired Student’s t-test, Fig. 5).
Role of P2X3
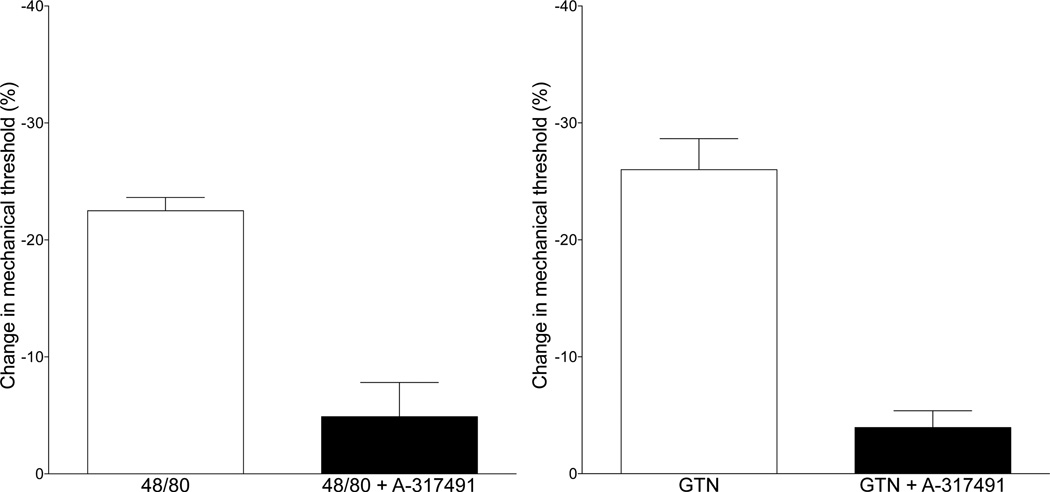
We have previously shown that endothelial cell-dependent mechanical hyperalgesia is mediated by release of a purinergic mediator, probably ATP, from the endothelial cell, which, in turn, acts at P2X3 receptors on the primary afferent nociceptor (Joseph et al., 2013; Joseph and Levine, 2012a). Therefore, we tested whether acute hyperalgesia produced by compound 48/80, as well as that induced by GTN, is P2X3 receptor-dependent, using A-317491, a selective P2X3 receptor antagonist. A-317491 markedly attenuated hyperalgesia induced by compound 48/80 (control vs. A-317491, P<0.0005 unpaired Student’s t-test, Fig. 6A). Similarly, antagonizing P2X3 receptors significantly attenuated hyperalgesia produced by GTN (Fig. 6B).
Fig. 6. Effect of the P2X3 receptor antagonist A-317491 on hyperalgesia induced by compound 48/80 and GTN.
We hypothesized that mediator(s) released from mast cells act on endothelial cells (see Fig. 5) to release ATP, which, in turn, can act at P2X3 receptors on primary afferent nociceptors, to produce mechanical hyperalgesia. Pretreatment with the selective P2X3 receptor antagonist, A-317491 markedly attenuated hyperalgesia produced by acute administration of compound 48/80 (10 µg intradermal in the hind paw; one-tailed Student’s t-test, P<0.0001, Panel A), as well as the delayed onset hyperalgesia induced by the administration of GTN (one-tailed Student’s t-test, P<0.0001, Panel B).
Role of neutrophils
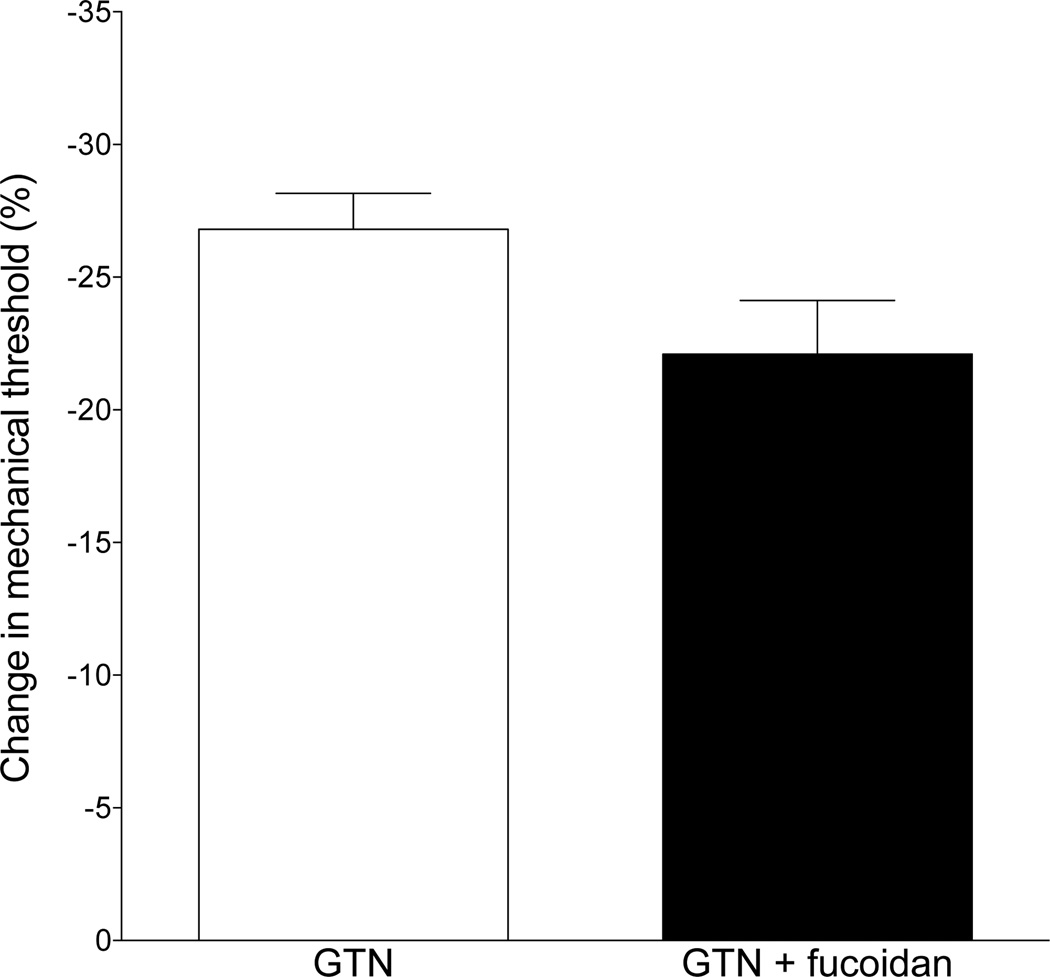
Since NO enhances the migration of neutrophils out of the vasculature (VanUffelen et al., 1996), and neutrophils can contribute to mechanical hyperalgesia (Cunha et al., 2008; Levine et al., 1984), we tested whether the nonspecific selectin inhibitor fucoidan (Cumashi et al., 2007), which prevents adherence of the neutrophil to the luminal side of the vascular endothelium, attenuates GTN-induced hyperalgesia. When fucoidan was given at a dose that has been shown to prevent the contribution of leukocytes to mechanical hyperalgesia in rats (25 mg/kg, i.v., 20 min before GTN administration, (Schiavuzzo et al., 2015)), it failed to attenuate GTN-induced delayed onset mechanical hyperalgesia (control vs. fucoidan treatment, P=N.S., Fig. 7).
Fig. 7. Effect of an inhibitor of neutrophil recruitment, fucoidan, on GTN-induced hyperalgesia.
Pretreatment of rats with fucoidan (25 mg/kg i.v.), to attenuate neutrophil recruited at the site of GTN administration and nociceptive threshold testing, did not significantly affect GTN-induced hyperalgesia (P=N.S.).
DISCUSSION
While the treatment of migraine was significantly advanced by the introduction of triptans in the 1990s (Tfelt-Hansen et al., 2000), because they are only partially effective (Diener et al., 2015; Tfelt-Hansen and Olesen, 2012), adequate management of pain for many migraineurs remains a major health care issue. Developing novel animal models that mimic characteristic features of migraine may provide a way forward in our understanding of migraine pathophysiology, and could also facilitate more rapid screening of potential therapeutic agents, as illustrated by the rational development of the triptans as effective treatments for migraine (Marmura et al., 2015).
In the present study, we developed a model of the delayed onset hyperalgesia induced by GTN, the research agent used to provoke an attack in migraineurs (Olesen et al., 1993; Olesen, 2008; Tfelt-Hansen and Tfelt-Hansen, 2009). When we injected GTN intradermally to a neurovascular unit of function in a peripheral tissue, in the rat, we observed a delayed onset long-lasting mechanical hyperalgesia, presumably via its ability to act as a nitric oxide donor. This mechanical hyperalgesia was greater in magnitude in female rats. Furthermore, it was mast and endothelial cell dependent, and was prevented by a P2X3 purinergic receptor antagonist. We have previously shown that P2X3 receptors on the peripheral terminal of the primary afferent nociceptor mediate vascular endothelial cell-dependent hyperalgesia (Joseph and Levine, 2012a). The clinical relevance of our model of delayed-onset mechanical hyperalgesia is supported by the observation that pretreatment with sumatriptan, a standard migraine therapeutic agent, markedly prevented the delayed onset mechanical hyperalgesia induced by GTN, as it does migraine attacks. Although we have shown that 5-HT1B and 5-HT1D receptors, on which sumatriptan acts, are located on nociceptors (Pierce et al., 1996b), sumatriptan is considered to be selective only for migraine pain (Bartsch et al., 2004; Cumberbatch et al., 1998). While systemically administered sumatriptan did not affect thermal or mechanical nociceptive threshold, or inflammatory hyperalgesia (Nikai et al., 2008), this may require a neurovascular component to the peripheral mechanisms generating allodynia/hyperalgesia. Thus, we have previously demonstrated (Joseph and Levine, 2012a) that sumatriptan administered at peripheral sites inhibits stimulus-dependent mechanical hyperalgesia by acting on 5-HT receptors located on endothelial cells (Machida et al., 2013); the data from the current study are consistent with this explanation. This model of GTN-induced hyperalgesia has advantages over other pre-clinical models in that it allows for the rapid screening of candidate therapeutic agents and has the potential to evaluate the effect of local modulatory interventions on nociception in an awake behaving animal.
In the present experiments we observed that depleting mast cells abolished GTN-induced delayed onset mechanical hyperalgesia. Clinically, it has been suggested that the mast cell is involved in both GTN-induced experimental and idiopathic migraine (Pedersen et al., 2015; Sicuteri, 1963). Mast cells are present in abundance around dural (Dimlich et al., 1991) as well as other blood vessels (Moon et al., 2010), and are well documented to be able to release pro-inflammatory and pro-nociceptive mediators (Dong et al., 2014; Xanthos et al., 2011). In our current experiments, we observed that hyperalgesia produced by both GTN and the mast degranulator, compound 48/80, is dependent on an intact functioning endothelial cell; endothelium-dependent hyperalgesia mediated by the release of a mediator implicated in endothelial cell dependent hyperalgesia that acts on P2X3 purinergic receptors on nociceptors to sensitize them to mechanical stimuli (Joseph and Levine, 2012a). Administration of GTN causes mast cell degranulation in vivo (Pedersen et al., 2015), and while it has been shown that products from mast cell degranulation excite meningeal nociceptors (Levy et al., 2007), known mast cell-derived mediators also act on the endothelium (Dileepan and Stechschulte, 2006; Kunder et al., 2011; Tharp, 1989). Migraine is thought to involve neurovascular mechanisms, and there is evidence that there is endothelial cell dysfunction in migraineurs (Tietjen et al., 2009; Tietjen and Khubchandani, 2015), with ATP synthesis and release from endothelial cells significantly increased during migraine attacks (Cieślak et al., 2015). Thus, we propose a neurovascular contribution to GTN-induced delayed onset mechanical hyperalgesia mediated by GTN-induced activation of mast cells, which in turn releases a variety of vasoactive mediators. Some of these mediators activate endothelial cells, to release ATP or another pronociceptive purine that acts at P2X3 receptors on the peripheral terminals of perivascular nociceptors, to lower their mechanical nociceptive threshold.
One difference between the present study and those using GTN to induce a migraine attack is that the present experiments were performed on spinal not trigeminal nociceptors. However, the basic neurovascular unit at both spinal and trigeminal levels contains mast cells, endothelial cells and innervation by nociceptors that contain 5HT1B/1D receptors (Classey et al., 2010; Kai-Kai and Keen, 1985; Pierce et al., 1996a); support for shared mechanisms in spinal and trigeminal distributions comes from both the presence of a migraine variant, abdominal migraine (Carson et al., 2011), which has been reported to be treatable with triptans in some patients (Kakisaka et al., 2010), as well as the observation that migraineurs do have lowered threshold in extra-cranial sites (Burstein et al., 2000).
There remains some controversy as to whether systemic administration of GTN to migraineurs produces extracranial symptoms. Based on our observation of robust GTN-induced hyperalgesia in our model, it might be expected that hyperalgesia or allodynia would be detectable in human subject receiving GTN infusion. However, while GTN has been administered to a large number of patients with, for example coronary artery disease with angina pectoris, GTNs ability to elicit or enhance pain syndromes is remarkably lacking in the medical literature. It should be noted that in the few studies in which the effect of GTN on extracranial pain has been evaluated, in non-migraineurs, GTN does not induce allodynia/hyperalgesia in either trigeminal-innervated or spinal-innervated tissues (Di Clemente et al., 2009; Perrotta et al., 2011; Sandrini et al., 2002). While in one study in migraineurs GTN administration did not produce allodynia or hyperalgesia as measured by quantitative sensory testing (Ladda et al., 2006), in contrast, in other studies GTN has been shown to have extracranial effects on nociception in migraineurs, but not in non-migraineurs (Di Clemente et al., 2009; Perrotta et al., 2011; Sandrini et al., 2002) and has been shown to reduce nociceptive thresholds relative to placebo (Thomsen et al., 1996). These latter studies used 0.9 – 1.2 mg GTN, oral or sublingual, which gives rise to plasma levels in the ng/ml range (Noonan and Benet, 1985), similar to that following µg/min intravenous administration (Bogaert, 1994; Imhof et al., 1982). This raises the intriguing possibility that migraine is a more generalized condition, impacting structures outside the head and neck. In support of this possibility, there is a marked co-morbidity of migraine with other pain syndromes, including irritable bowel syndrome and fibromyalgia syndrome and temporomandibular disorder (van Hemert et al., 2014; Vij et al., 2015). Furthermore, there are migrant variants that affect other body regions, such as abdominal migraine (Woodruff et al., 2013), which has been suggested to be responsive to triptans in some patients (Russell et al., 2002). It is possible that dural nociceptors have unique properties for migraine-related changes in the neurovascular unit in the dura; a systematic evaluation of whether administration of GTN enhances pain in other body domains in humans is required to address this possibility. However, in the current study in our rat model, we provide data demonstrating that intravenous GTN produces a robust decrease in mechanical nociceptive threshold, in the hind paw, consistent with the effects of systemic GTN administration in previous animal studies (Bates et al., 2010; Tassorelli et al., 2003; Tipton et al., 2015).
We observed that locally administered GTN produces a significantly greater hyperalgesia in females and that systemically administered GTN produces hyperalgesia. It has previously been reported that systemic GTN administration produces a greater mechanical hyperalgesia in female mice (Pradhan et al., 2014) and a greater increase expression of FOS protein (a marker of neuronal activity) in brain nuclei associated with pain processing of females rats (Greco et al., 2013). Although there is a markedly higher prevalence of migraine in women and a marked reduction in pain severity following depletion of female sex steroids (Brandes, 2006), to our knowledge sex differences in migraine severity induced by GTN infusion in human studies has not been evaluated. We also observed a sexual dimorphism in hyperalgesia following intravenous GTN with a ~60 min onset delay in males and ~320 min in females. The mechanism for this sexual dimorphism in delay to onset of GTN-induced is not known, but it has been observed that 4 h after intravenous GTN in female rats there is a significantly greater neuronal activation (measured by Fos protein expression) in several brain nuclei implicated in the modulation of migraine pain (Greco et al., 2013), and we have observed a marked delay to endothelial cell dependent pronociceptive effects in another model of vascular pain, stimulus-dependent hyperalgesia (Joseph and Levine, 2012b). However, how these findings relate to sexual dimorphism in migraine remains to be elucidated.
CONCLUSIONS
Our data support the suggestion that the action of GTN to induce a delayed onset migraine attack may be produced by a local neurovascular effect, dependent on mast and endothelial cell function. Compatible with a neurovascular contribution to the sexual dimorphism observed in patients with migraine, we also observed a greater GTN-induced hyperalgesia in females, which may be of relevance to the greater prevalence (Buse et al., 2013) of migraine in women. Migraine has a complex pathophysiology that likely involves multiple mediators acting on neurovascular targets. Interactions between the vascular endothelial system, perivascular mast cell and the peripheral nociceptor, can be explored in the model we have used in the current study, and may allow for a fuller understanding of interactions between the peripheral terminal of the primary afferent nociceptor and the vascular system it innervates, in the pathophysiology of pain syndromes.
Highlights.
Intradermal glycerol trinitrate (GTN) induces a delayed-onset mechanical hyperalgesia
GTN-induced hyperalgesia was greater in female rats
GTN-induced hyperalgesia appeared to be dependent on endothelial and mast cells
A-317491, a P2X3 antagonist, prevents GTN-induced hyperalgesia
Acknowledgments
This work was supported by NIH grant NS085831. We would like to thank Professors Jes and Ingrid Olesen, University of Copenhagen, for helpful comments and suggestions during the development and writing of this manuscript.
Abbreviations
- GTN
Glycerol trinitrate
- ANOVA
Analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
REFERENCES
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–645. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Knight YE, Goadsby PJ. Activation of 5-HT(1B/1D) receptor in the periaqueductal gray inhibits nociception. Ann Neurol. 2004;56:371–381. doi: 10.1002/ana.20193. [DOI] [PubMed] [Google Scholar]
- Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, Ptácek LJ, Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2010;30:170–178. doi: 10.1111/j.1468-2982.2009.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat. 2014;10:1–161. [PubMed] [Google Scholar]
- Bogaert MG. Clinical pharmacokinetics of nitrates. Cardiovasc Drugs Ther. 1994;8:693–699. doi: 10.1007/BF00877116. [DOI] [PubMed] [Google Scholar]
- Bourreau JP, Banijamali HS, Challice CE. Modification of excitation-contraction coupling in cat ventricular myocardium following endocardial damage. Can J Physiol Pharmacol. 1993;71:254–262. doi: 10.1139/y93-040. [DOI] [PubMed] [Google Scholar]
- Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006;295:1824–1830. doi: 10.1001/jama.295.15.1824. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–624. [PubMed] [Google Scholar]
- Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM, Serrano D, Lipton RB. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–1299. doi: 10.1111/head.12150. [DOI] [PubMed] [Google Scholar]
- Carson L, Lewis D, Tsou M, McGuire E, Surran B, Miller C, Vu TA. Abdominal migraine: an under-diagnosed cause of recurrent abdominal pain in children. Headache. 2011;51:707–712. doi: 10.1111/j.1526-4610.2011.01855.x. [DOI] [PubMed] [Google Scholar]
- Chatterjea D, Wetzel A, Mack M, Engblom C, Allen J, Mora-Solano C, Paredes L, Balsells E, Martinov T. Mast cell degranulation mediates compound 48/80-induced hyperalgesia in mice. Biochem Biophys Res Commun. 2012;425:237–243. doi: 10.1016/j.bbrc.2012.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Green PG, Levine JD. Does the antihyperalgesic disruptor of endothelial cells, octoxynol-9, alter nociceptor function? J Neurophysiol. 2014;112:463–466. doi: 10.1152/jn.00034.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieślak M, Czarnecka J, Roszek K, Komoszyński M. The role of purinergic signaling in the etiology of migraine and novel antimigraine treatment. Purinergic Signal. 2015;11:307–316. doi: 10.1007/s11302-015-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classey JD, Bartsch T, Goadsby PJ. Distribution of 5-HT(1B), 5-HT(1D) and 5-HT(1F) receptor expression in rat trigeminal and dorsal root ganglia neurons: relevance to the selective anti-migraine effect of triptans. Brain Res. 2010;1361:76–85. doi: 10.1016/j.brainres.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, Usov AI, Ustyuzhanina NE, Grachev AA, Sanderson CJ, Kelly M, Rabinovich GA, Iacobelli S, Nifantiev NE Consorzio Interuniversitario Nazionale per la Bio-Oncologia, I. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- Cumberbatch MJ, Hill RG, Hargreaves RJ. Differential effects of the 5HT1B/1D receptor agonist naratriptan on trigeminal versus spinal nociceptive responses. Cephalalgia. 1998;18:659–663. doi: 10.1046/j.1468-2982.1998.1810659.x. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008;83:824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- Di Clemente L, Coppola G, Magis D, Gérardy PY, Fumal A, De Pasqua V, Di Piero V, Schoenen J. Nitroglycerin sensitises in healthy subjects CNS structures involved in migraine pathophysiology: evidence from a study of nociceptive blink reflexes and visual evoked potentials. Pain. 2009;144:156–161. doi: 10.1016/j.pain.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Diener HC, Charles A, Goadsby PJ, Holle D. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010–1022. doi: 10.1016/S1474-4422(15)00198-2. [DOI] [PubMed] [Google Scholar]
- Dileepan KN, Stechschulte DJ. Endothelial cell activation by mast cell mediators. Methods Mol Biol. 2006;315:275–294. doi: 10.1385/1-59259-967-2:275. [DOI] [PubMed] [Google Scholar]
- Dimlich RV, Keller JT, Strauss TA, Fritts MJ. Linear arrays of homogeneous mast cells in the dura mater of the rat. J Neurocytol. 1991;20:485–503. doi: 10.1007/BF01252276. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang X, Qian Y. Mast cells and neuroinflammation. Med Sci Monit Basic Res. 2014;20:200–206. doi: 10.12659/MSMBR.893093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco R, Tassorelli C, Mangione AS, Smeraldi A, Allena M, Sandrini G, Nappi G, Nappi RE. Effect of sex and estrogens on neuronal activation in an animal model of migraine. Headache. 2013;53:288–296. doi: 10.1111/j.1526-4610.2012.02249.x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Nahas SJ, Peterlin BL. Chemical mediators of migraine: preclinical and clinical observations. Headache. 2011;51:1029–1045. doi: 10.1111/j.1526-4610.2011.01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthusen H, Arndt JO. Nitric oxide evokes pain at nociceptors of the paravascular tissue and veins in humans. J Physiol. 1995;487:253–258. doi: 10.1113/jphysiol.1995.sp020876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof PR, Sieber A, Hodler J, Müller P, Ott B, Fankhauser P, Chu LC, Gérardin A. Plasma concentrations and haemodynamic effects of nitroglycerin during and after intravenous infusion in healthy volunteers. Eur J Clin Pharmacol. 1982;23:99–106. doi: 10.1007/BF00545962. [DOI] [PubMed] [Google Scholar]
- Jaffery G, Coleman JW, Huntley J, Bell EB. Mast cell recovery following chronic treatment with compound 48/80. Int Arch Allergy Immunol. 1994;105:274–280. doi: 10.1159/000236769. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Green PG, Bogen O, Alvarez P, Levine JD. Vascular endothelial cells mediate mechanical stimulation-induced enhancement of endothelin hyperalgesia via activation of P2X2/3 receptors on nociceptors. J Neurosci. 2013;33:2849–2859. doi: 10.1523/JNEUROSCI.3229-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Green PG, Ferrari LF, Levine JD. Homocysteine-induced attenuation of vascular endothelium-dependent hyperalgesia in the rat. Neuroscience. 2015;284:678–684. doi: 10.1016/j.neuroscience.2014.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Role of endothelial cells in antihyperalgesia induced by a triptan and beta-blocker. Neuroscience. 2012a;232:83–89. doi: 10.1016/j.neuroscience.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Sexual dimorphism in endothelin-1 induced mechanical hyperalgesia in the rat. Exp Neurol. 2012b;233:505–512. doi: 10.1016/j.expneurol.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai-Kai MA, Keen P. Localization of 5-hydroxytryptamine to neurons and endoneurial mast cells in rat sensory ganglia. J Neurocytol. 1985;14:63–78. doi: 10.1007/BF01150263. [DOI] [PubMed] [Google Scholar]
- Kakisaka Y, Wakusawa K, Haginoya K, Saito A, Uematsu M, Yokoyama H, Sato T, Tsuchiya S. Efficacy of sumatriptan in two pediatric cases with abdominal painrelated functional gastrointestinal disorders: does the mechanism overlap that of migraine. J Child Neurol. 2010;25:234–237. doi: 10.1177/0883073809336875. [DOI] [PubMed] [Google Scholar]
- Kunder CA, St John AL, Abraham SN. Mast cell modulation of the vascular and lymphatic endothelium. Blood. 2011;118:5383–5393. doi: 10.1182/blood-2011-07-358432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladda J, Straube A, Förderreuther S, Krause P, Eggert T. Quantitative sensory testing in cluster headache: increased sensory thresholds. Cephalalgia. 2006;26:1043–1050. doi: 10.1111/j.1468-2982.2006.01134.x. [DOI] [PubMed] [Google Scholar]
- Levine JD, Lau W, Kwiat G, Goetzl EJ. Leukotriene B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes. Science. 1984;225:743–745. doi: 10.1126/science.6087456. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida T, Iizuka K, Hirafuji M. 5-hydroxytryptamine and its receptors in systemic vascular walls. Biol Pharm Bull. 2013;36:1416–1419. doi: 10.1248/bpb.b13-00344. [DOI] [PubMed] [Google Scholar]
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the american headache society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3–20. doi: 10.1111/head.12499. [DOI] [PubMed] [Google Scholar]
- McLeod JD, Piper PJ. Effect of removing the endothelium on the vascular responses induced by leukotrienes C4 and D4 in guinea-pig isolated heart. Eur J Pharmacol. 1992;212:67–72. doi: 10.1016/0014-2999(92)90073-d. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Moon TC, St Laurent CD, Morris KE, Marcet C, Yoshimura T, Sekar Y, Befus AD. Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol. 2010;3:111–128. doi: 10.1038/mi.2009.136. [DOI] [PubMed] [Google Scholar]
- Nikai T, Basbaum AI, Ahn AH. Profound reduction of somatic and visceral pain in mice by intrathecal administration of the anti-migraine drug, sumatriptan. Pain. 2008;139:533–540. doi: 10.1016/j.pain.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan PK, Benet LZ. Incomplete and delayed bioavailability of sublingual nitroglycerin. Am J Cardiol. 1985;55:184–187. doi: 10.1016/0002-9149(85)90325-x. [DOI] [PubMed] [Google Scholar]
- Olesen J. Cerebral blood flow in migraine with aura. Pathol Biol (Paris) 1992;40:318–324. [PubMed] [Google Scholar]
- Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther. 2008;120:157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Olesen J, Iversen HK, Thomsen LL. Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. Neuroreport. 1993;4:1027–1030. doi: 10.1097/00001756-199308000-00008. [DOI] [PubMed] [Google Scholar]
- Olesen J, Thomsen LL, Lassen LH, Olesen IJ. The nitric oxide hypothesis of migraine and other vascular headaches. Cephalalgia. 1995;15:94–100. doi: 10.1046/j.1468-2982.1995.015002094.x. [DOI] [PubMed] [Google Scholar]
- Pedersen SH, Ramachandran R, Amrutkar DV, Petersen S, Olesen J, Jansen-Olesen I. Mechanisms of glyceryl trinitrate provoked mast cell degranulation. Cephalalgia. 2015 doi: 10.1177/0333102415574846. [DOI] [PubMed] [Google Scholar]
- Perrotta A, Serrao M, Tassorelli C, Arce-Leal N, Guaschino E, Sances G, Rossi P, Bartolo M, Pierelli F, Sandrini G, Nappi G. Oral nitric-oxide donor glyceryl-trinitrate induces sensitization in spinal cord pain processing in migraineurs: a double-blind, placebo-controlled, cross-over study. Eur J Pain. 2011;15:482–490. doi: 10.1016/j.ejpain.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Persson MG, Agvald P, Gustafsson LE. Detection of nitric oxide in exhaled air during administration of nitroglycerin in vivo. Br J Pharmacol. 1994;111:825–828. doi: 10.1111/j.1476-5381.1994.tb14812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce PA, Xie GX, Peroutka SJ, Levine JD. Dual effect of the serotonin agonist, sumatriptan, on peripheral neurogenic inflammation. Reg Anesth. 1996a;21:219–225. [PubMed] [Google Scholar]
- Pierce PA, Xie GX, Levine JD, Peroutka SJ. 5-Hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: a polymerase chain reaction study. Neuroscience. 1996b;70:553–559. doi: 10.1016/0306-4522(95)00329-0. [DOI] [PubMed] [Google Scholar]
- Piovezan AP, D’Orléans-Juste P, Frighetto M, Souza GE, Henriques MG, Rae GA. Endothelins contribute towards nociception induced by antigen in ovalbumin-sensitised mice. Br J Pharmacol. 2004;141:755–763. doi: 10.1038/sj.bjp.0705663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155:269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G, Abu-Arafeh I, Symon DN. Abdominal migraine: evidence for existence and treatment options. Paediatr Drugs. 2002;4:1–8. doi: 10.2165/00128072-200204010-00001. [DOI] [PubMed] [Google Scholar]
- Sances G, Tassorelli C, Pucci E, Ghiotto N, Sandrini G, Nappi G. Reliability of the nitroglycerin provocative test in the diagnosis of neurovascular headaches. Cephalalgia. 2004;24:110–119. doi: 10.1111/j.1468-2982.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- Sandrini G, Tassorelli C, Cecchini AP, Alfonsi E, Nappi G. Effects of nimesulide on nitric oxide-induced hyperalgesia in humans--a neurophysiological study. Eur J Pharmacol. 2002;450:259–262. doi: 10.1016/s0014-2999(02)02188-x. [DOI] [PubMed] [Google Scholar]
- Schiavuzzo JG, Teixeira JM, Melo B, da Silva dos Santos DF, Jorge CO, Oliveira-Fusaro MC, Parada CA. Muscle hyperalgesia induced by peripheral P2X3 receptors is modulated by inflammatory mediators. Neuroscience. 2015;285:24–33. doi: 10.1016/j.neuroscience.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Sicuteri F. Mast cells and their active substances: their role in the pathogenesis of migraine. Headache. 1963;3:86–92. doi: 10.1111/j.1526-4610.1963.hed0303086.x. [DOI] [PubMed] [Google Scholar]
- Sun ZW, Wang XD, Deng XM, Wallen R, Gefors L, Hallberg E, Andersson R. The influence of circulatory and gut luminal challenges on bidirectional intestinal barrier permeability in rats. Scand J Gastroenterol. 1997;32:995–1004. doi: 10.3109/00365529709011216. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Greco R, Wang D, Sandrini M, Sandrini G, Nappi G. Nitroglycerin induces hyperalgesia in rats--a time-course study. Eur J Pharmacol. 2003;464:159–162. doi: 10.1016/s0014-2999(03)01421-3. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine. Drugs. 2000;60:1259–1287. doi: 10.2165/00003495-200060060-00003. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Olesen J. Taking the negative view of current migraine treatments: the unmet needs. CNS Drugs. 2012;26:375–382. doi: 10.2165/11630590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen PC, Tfelt-Hansen J. Nitroglycerin headache and nitroglycerin-induced primary headaches from 1846 and onwards: a historical overview and an update. Headache. 2009;49:445–456. doi: 10.1111/j.1526-4610.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- Tharp MD. The interaction between mast cells and endothelial cells. J Invest Dermatol. 1989;93:107S–112S. doi: 10.1111/1523-1747.ep12581221. [DOI] [PubMed] [Google Scholar]
- Thomaides T, Karagounakis D, Spantideas A, Katelanis S. Transcranial Doppler in migraine attacks before and after treatment with oral zolmitriptan or sumatriptan. Headache. 2003;43:54–58. doi: 10.1046/j.1526-4610.2003.03009.x. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Brennum J, Iversen HK, Olesen J. Effect of a nitric oxide donor (glyceryl trinitrate) on nociceptive thresholds in man. Cephalalgia. 1996;16:169–174. doi: 10.1046/j.1468-2982.1996.1603169.x. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, Herial NA, White L, Utley C, Kosmyna JM, Khuder SA. Migraine and biomarkers of endothelial activation in young women. Stroke. 2009;40:2977–2982. doi: 10.1161/STROKEAHA.109.547901. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, Khubchandani J. Vascular biomarkers in migraine. Cephalalgia. 2015;35:95–117. doi: 10.1177/0333102414544976. [DOI] [PubMed] [Google Scholar]
- Tipton AF, Tarash I, McGuire B, Charles A, Pradhan AA. The effects of acute and preventive migraine therapies in a mouse model of chronic migraine. Cephalalgia. 2015 doi: 10.1177/0333102415623070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert S, Breedveld AC, Rovers JM, Vermeiden JP, Witteman BJ, Smits MG, de Roos NM. Migraine associated with gastrointestinal disorders: review of the literature and clinical implications. Front Neurol. 2014;5:241. doi: 10.3389/fneur.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanUffelen BE, de Koster BM, Van den Broek PJ, VanSteveninck J, Elferink JG. Modulation of neutrophil migration by exogenous gaseous nitric oxide. J Leukoc Biol. 1996;60:94–100. doi: 10.1002/jlb.60.1.94. [DOI] [PubMed] [Google Scholar]
- Vij B, Whipple MO, Tepper SJ, Mohabbat AB, Stillman M, Vincent A. Frequency of Migraine Headaches in Patients With Fibromyalgia. Headache. 2015;55:860–865. doi: 10.1111/head.12590. [DOI] [PubMed] [Google Scholar]
- Woodruff AE, Cieri NE, Abeles J, Seyse SJ. Abdominal migraine in adults: a review of pharmacotherapeutic options. Ann Pharmacother. 2013;47:e27. doi: 10.1345/aph.1R620. [DOI] [PubMed] [Google Scholar]
- Xanthos DN, Gaderer S, Drdla R, Nuro E, Abramova A, Ellmeier W, Sandkühler J. Central nervous system mast cells in peripheral inflammatory nociception. Mol Pain. 2011;7:42. doi: 10.1186/1744-8069-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]