Abstract
Background and Objective
The Ghana Randomized Air Pollution and Health Study (GRAPHS) is a community-level randomized controlled trial of cookstove interventions for pregnant women and their newborns in rural Ghana. Given that household air pollution from biomass burning may be implicated in adverse cardiovascular outcomes, we sought to determine whether exposure to carbon monoxide (CO) from woodsmoke was associated with blood pressure (BP) among 817 adult women.
Methods
Multivariate linear regression models were used to evaluate the association between CO exposure, determined with 72-hour personal monitoring at study enrollment, and BP, also measured at study enrollment. At the time of these assessments, women were in the first or second trimester of pregnancy.
Results
A significant positive association was found between CO exposure and diastolic blood pressure (DBP): on average, each 1ppm increase in exposure to CO was associated with 0.43 mmHg higher DBP [0.01, 0.86]. A non-significant positive trend was also observed for systolic blood pressure (SBP).
Conclusion
This study is one of very few to have examined the relationship between household air pollution and blood pressure among pregnant women, who are at particular risk for hypertensive complications. The results of this cross-sectional study suggest that household air pollution from wood-burning fires is associated with higher blood pressure, particularly DBP, in pregnant women at early to mid-gestation. The clinical implications of the observed association toward the eventual development of chronic hypertension and/or hypertensive complications of pregnancy remain uncertain, as few of the women were overtly hypertensive at this point in their pregnancies.
Keywords: Household air pollution, cookstoves, biomass, blood pressure, pregnancy, cardiovascular disease
Introduction
Worldwide, close to 3 billion households rely on biomass fuels for cooking and heating (Bonjour et al. 2013). Household Air Pollution (HAP) is created by the combustion of these fuels in open fires, and has been associated with many adverse health outcomes. In 2010 HAP was ranked the fourth contributing risk factor to the burden of disease worldwide (Lim et al. 2012). For the country of Ghana specifically, HAP is the number one risk factor, contributing to the highest national burden of disease including cardiovascular disease (CVD) (Institute for Health Metrics and Evaluation 2013). Despite the huge estimated CVD burden associated with HAP, only one study to date has directly investigated the association between HAP and cardiovascular morbidity (Lee et al. 2012a). Given the long latencies in CVD, such studies are very challenging. Most research has consequently focused on risk factors for CVD.
Blood Pressure (BP) is a known risk factor for CVD (Lewington et al. 2002), and has been shown to respond to changes in air pollution, particularly with respect to particulate matter less than 2.5 microns in aerodynamic diameter (PM2.5) (Brook et al. 2010). Mechanisms that may underlie the response of BP to exposure to particulate matter include autonomic imbalance and oxidative stress (Brook et al. 2009). Several controlled experiments in humans have demonstrated almost immediate increases in BP in response to short-term increases in PM2.5 (Cosselman et al. 2012; Langrish et al. 2009; Urch et al. 2005), although other studies have not found this association (Brook et al. 2002). A review of epidemiologic studies, largely conducted in economically advanced countries, indicates that a 10 µg/m3 increase in ambient PM2.5 is associated with BP elevations of approximately 1–8 mmHg (Brook and Rajagopalan 2009). Meanwhile, exposures to PM2.5 can be much higher in the developing world (Clark et al. 2013); in the geographic region of this study, personal air sampling has previously recorded 24-hour exposure levels averaging 128.5 µg/m3 among women who are the primary cooks for their households (Van Vliet et al. 2013).
HAP from biomass burning contains a complex mixture of volatile and particulate pollution (McCracken et al. 2012), including PM2.5 and carbon monoxide (CO). Due to its relative ease of monitoring, CO monitoring has been used as an indicator of woodsmoke exposure in previous cookstove intervention studies, including the RESPIRE trial in Guatemala (Smith et al. 2011); however, the use of CO as a proxy for PM2.5 exposure has been brought into question by several studies that have found poor correlations between the two pollutants in biomass settings (Dionisio et al. 2012; Klasen et al. 2015). Other studies have found stronger correlations between PM and CO, both in cooking areas and using personal measures (Dionisio et al. 2008; McCracken et al. 2013; Pollard et al. 2014). CO may itself be related to cardiovascular disease, with studies demonstrating associations between short-term increases in ambient CO and CVD hospital admissions in Taiwan (Yang et al. 2004) and the United States (Bell et al. 2009), and with CVD emergency department visits in Canada (Stieb et al. 2009), in statistical analyses that controlled for levels of co-pollutants such as PM and NO2. The adverse health effects of chronic, low-level exposure to CO appear to reflect mechanisms other than the hypoxia that is implicated in acute CO poisoning – including endothelial inflammation and immune activation (World Health Organization (WHO) 2010). Few studies have so far been conducted to assess the relationship between CO and blood pressure, and those among pregnant women have yielded conflicting results. A large study of pregnant women in the United States found that 0 to 4-day-lagged ambient exposures to CO, but not PM, were associated with higher blood pressure at delivery among women who were normotensive or diagnosed with chronic hypertension (Männistö et al. 2014). Conversely, a cohort study of healthy pregnant women in Pennsylvania found that chronic exposure to PM, but not CO, was associated with higher BP in late pregnancy (Lee et al. 2012b).
The epidemiologic basis for our understanding of the association between HAP and BP currently rests on eight studies conducted in five locations: Guatemala (McCracken et al. 2007), India (Dutta et al. 2011), Nicaragua (Clark et al. 2011), China (Baumgartner et al. 2011), and Nepal (Neupane et al. 2015). Notably, all eight analyses have reported a positive association between HAP and BP, although the studies differ in the methods used to categorize HAP exposure (McCracken et al. 2012). While personal exposure monitoring is widely considered to be preferable to static monitoring techniques in terms of capturing health-relevant exposures to air pollutants (Clark et al. 2013; Steinle et al. 2013), few of these studies have employed personal monitoring. Those that have done so have been limited by sample sizes that were fairly small. These studies, meanwhile, have largely taken place among populations of non-pregnant adults, but pregnancy presents a period of particular concern for elevations in blood pressure. Hypertension complicates an estimated 5–10% of pregnancies worldwide (Duley 2009; Lindheimer et al. 2010) and contributes substantially to global maternal mortality, accounting for an estimated 18% of all maternal deaths (Abalos et al. 2014). Hypertensive disorders during pregnancy also contribute to other adverse outcomes in both mother and child (Barker 1995; Männistö et al. 2013). To our knowledge, only two studies (Agrawal and Yamamoto 2014; Wylie et al. 2015) have previously investigated the relationship between biomass smoke exposure and hypertension in pregnancy, and neither has used personal monitoring to determine exposure to HAP.
The fact that HAP is a potentially modifiable environmental risk factor for both BP and CVD (Brook et al. 2011b) makes the case for the public health importance of scientific studies to clarify the relationship between HAP and cardiovascular risk factors, and to evaluate the potential impact of interventions to reduce HAP. Here we report the results of a cross-sectional analysis of the association between CO exposure and blood pressure among 807 pregnant women in a predominantly rural setting of Ghana.
Methods
Data Sources
Participants were studied upon initial enrollment into the study, “Ghana Randomized Air Pollution and Health Study (GRAPHS)”. In brief, GRAPHS is a community-level clustered randomized controlled trial of a cookstove intervention in a predominantly rural area of Ghana (Jack et al. 2015) (NCT01335490). Inclusion criteria for enrollment include being in the first or second trimester of pregnancy, living in one of 35 communities in the Kintampo Health Research Center (KHRC) catchment area, being the primary cook of the household, and being a non-smoker. Exclusion criteria include carrying multiple fetuses or a gestational age greater than 28 weeks (determined via ultrasound) (Boamah et al. 2014). The predominant type of cookstove used by households in this area of Ghana is a three-stone fire, fed by firewood. GRAPHS is a collaboration between Columbia University and KHRC, and the location of the study is the Kintampo North Municipality and Kintampo South District of the Brong Ahafo Region, located in the middle belt of Ghana. In the study area, hypertension is one of the leading causes of adult visits to health facilities (Ghana Health Service 2013).
Ethical Approvals
Ethical approvals for GRAPHS were obtained from the Institutional Review Boards of Columbia University Medical Center, Massachusetts General Hospital/Partners Healthcare, the Ghana Health Service Ethical Review Committee, and the Kintampo Health Research Centre Institutional Ethics Committee.
Study Participants
Enrollment and data collection for GRAPHS began in September 2013. Potential participants in this analysis included women who had been enrolled in GRAPHS and for whom both a baseline BP and an initial CO measurement session had been conducted by September 2014 (n = 1183).
Exposure Monitoring
Personal CO measurements were collected for 72-hour periods immediately after the GRAPHS women were enrolled into the study, prior to delivery of improved cookstoves. Each woman was outfitted with a Lascar (Erie, PA) EL-CO-USB Carbon Monoxide (CO) data logger set to record CO measurements every 10 seconds. The Lascar EL-CO-USB device reports concentrations between 0–1000 parts per million (ppm) CO and has a manufacturer-reported precision of ±6%. The monitor was worn in the woman’s breathing zone (attached to clothing) during the day, and women were instructed to keep the monitors near them while they slept. During the CO monitoring period, trained fieldworkers visited the women every 24 hours to record the past day’s cooking events and to check on any issues with monitoring compliance. CO monitors were periodically checked at the KHRC laboratory against a known concentration of National Institute of Standards and Technology (NIST) traceable CO gas (50ppm) mixed in air, and all CO data was post-processed to account for a monitor- and time-specific correction factor.
Baseline Blood Pressure Monitoring
Systolic and diastolic blood pressure measurements (SBP; DBP) were recorded at the Kintampo Municipal and Jema District Hospitals during the study women’s initial visit to the hospital to determine study eligibility, using a Watch BP Home-Microlife (Microlife Corporation, Widnau, Switzerland) digital automatic blood pressure monitor operated by trained GRAPHS study staff. The Watch BP Home-Microlife instrument has been previously validated against sphygmomanometer readings for blood pressure measurement in adults (Stergiou et al. 2007) and in pregnancy (Chung et al. 2009). Blood pressure was measured with the cuff around the upper left arm of the seated, resting study participant following five minutes of rest. A single blood pressure measurement (consisting of one SBP and one DBP measurement) was recorded for each participant.
Covariates
Gestational age was determined via ultrasound at study enrollment. Maternal weight and height measurements and a verbal medical history were also recorded during this initial hospital visit. Other potential covariates, including marital status, level of education, indicators of socioeconomic status (e.g. occupation, asset ownership, housing construction, and the household’s toilet and water facilities) were recorded by questionnaire during a home visit shortly after study enrollment. Activities that could have influenced CO exposure during the monitoring session (e.g. use of mosquito coils, smoking in the household) were recorded by questionnaire after each 24 hours of the 72-hour CO monitoring session.
Data management and analysis
The final population for analysis was 817 women. Of the initial 1183 potential participants, we excluded 328 whose CO data did not meet validity criteria. The CO validity criteria comprised three components: 1) “high” confidence in the unit-specific Lascar CO monitor correction factor (i.e., a correction factor between 0.6–1.2 as calculated from the CO span gas procedure: 215 records did not meet this criterion); 2) a CO monitoring session duration of ≥44 hours (71 records did not meet this criterion); and 3) a coding of “1” or “high” confidence of validity from a visual evaluation of the tracing of the minutewise CO data (174 records were coded otherwise). This resulted in a sample size of 855 for further analysis. We further excluded two participants whose DBP measurements were extreme outliers (defined as more than 3.5 times the interquartile range beyond the 3rd quartile of the data; no SBP measurements were outlying to this degree). Lastly, we excluded 36 women with missing covariate data. All data analysis was conducted using R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Outcome and Exposure measures
SBP and DBP were the dependent variables in separate linear regression analyses. We estimated the strength of the association between these variables and CO exposure using multivariate linear regression models with one-way cluster-robust standard errors to account for the clustering of individuals within communities.
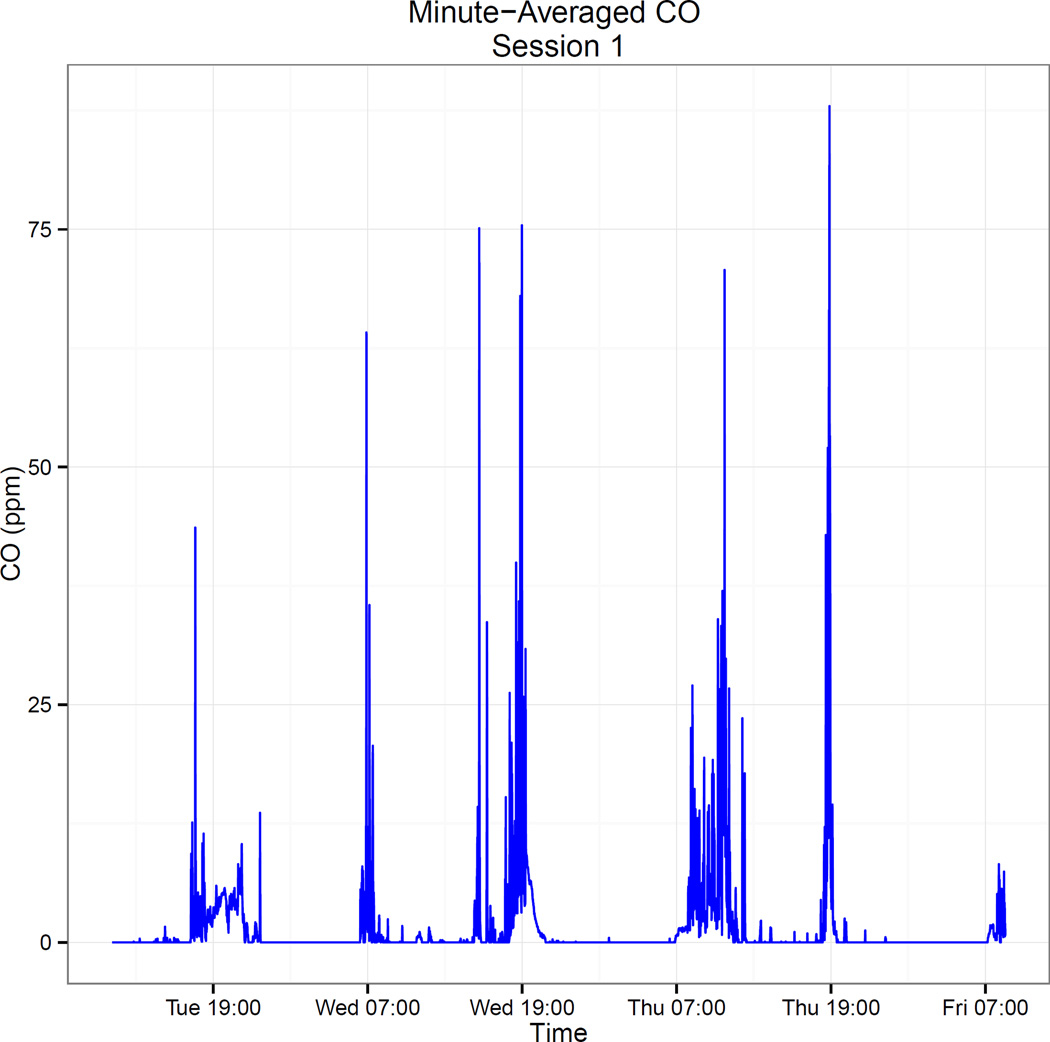
In calculating CO exposure, we first averaged each woman’s CO exposure over every minute of her monitoring session (which ranged in length from 44 to 90 hours, mean = 71.5 hours). The resulting time series generally was characterized by numerous recordings near 0 punctuated by spikes of much higher CO exposure, typically corresponding to cooking periods (see Figure 1 for an example). We selected the mean of the minute-averaged CO as the metric representing exposure to cooking smoke.
Figure 1.
Sample minute-averaged CO plot from a 72-hour sampling session
Statistical Modeling
We employed a multivariate linear regression model with cluster-robust standard errors to account for the grouping of individuals within communities. The regression equation was constructed separately for SBP and DBP as outcomes, and can generally be represented as follows:
BP = β̂0 + β̂1CO + γX + ε,
where β̂0 is a constant and β̂1 is the effect estimate for carbon monoxide exposure on BP, holding other covariates constant. X represents a matrix of potential covariates that vary at the individual or community level (e.g. age, gestational age, BMI, location of community in relation to the main road). The variance of the slope estimates β and γ is calculated using a sandwich estimator to obtain a variance-covariance matrix – and hence standard errors – that take into account the clustering of individual observations within discrete communities (Arai 2015).
Covariate Selection
Our variable selection process was motivated by the desire to include additional factors that may influence BP levels and to account for other possible sources of CO exposure beyond cooking smoke. We retained covariates in the final model if they were associated with SBP or DBP in univariate a nalyses with p < 0.1 and/or changed the parameter estimate for BP by more than 10%.
At the individual level, potential covariates included age, body mass index (BMI), occupation, day of week of BP measurement, and gestational age (in weeks). Although individual smoking and history of hypertension are also known to predict BP, in our cohort very few women had ever smoked (0.4% of the participants, Table 1) or had previously been diagnosed with hypertension (1.4%). These two variables were therefore not assessed as potential covariates.
Table 1.
Baseline characteristics of 1183 women in the GRAPHS cohort
| Characteristic | Included in analysis (n = 817) |
Excluded from analysis (n = 366) |
|||
|---|---|---|---|---|---|
| Mean ± SD | p-value from two-sample t-test |
||||
| Age (years) | 27.3 ±7.1 | 27.8 ± 7.6 | 0.29 | ||
| BMI (kg/m2) | 23.3 ± 3.3 | 23.4 ± 3.5 | 0.64 | ||
| Gestational age (weeks) | 16.0 ± 4.3 | 15.8 ± 4.4 | 0.42 | ||
| Asset Index (centered at 0)a | −0.02 ± 2.2 | 0.07 ± 2.3 | 0.53 | ||
| Percent |
p-value from Fisher’s exact test |
||||
| Known history of hypertension (%) | 1.4 | 1.9 | 0.45 | ||
| Known history of diabetes (%) | 0 | 0.001 | 0.10 | ||
| Ever smoker (%) | 0.4 | 0.5 | 0.65 | ||
| Smoker in household or compound (%) | 21 | 20 | 0.82 | ||
| Education (%): |
None | 38 | 40 | 0.59 | |
| Primary School | 27 | 23 | |||
| Middle School or above | 35 | 30 | |||
| NA | 0 | 6 | |||
| Marital status (%): |
Married | 53 | 54 | 0.37 | |
| Living with partner | 32 | 31 | |||
| Single | 14 | 10 | |||
| Widowed/Divorced/Separated | 0.4 | 0 | |||
| NA | 0 | 5 | |||
| Employment status (%): |
None | 31 | 28 | 0.67 | |
| Farmer/laborer/domestic worker | 40 | 40 | |||
| Trader/food seller/businesswoman | 21 | 17 | |||
| Other | 8 | 9 | |||
| NA | 0 | 5 | |||
| Cooking location (%): |
Primary: | Totally open | 60 | 55 | 0.90 |
| Partially enclosed | 21 | 21 | |||
| Fully enclosed | 18 | 18 | |||
| NA | 0 | 6 | |||
| Secondary: | Totally open | 14 | 12 | 0.65 | |
| Partially enclosed | 30 | 27 | |||
| Fully enclosed | 16 | 13 | |||
| None | 41 | 42 | |||
| NA | 0 | 6 | |||
| Fuel type (%): |
Primary | Wood | 96 | 88 | 0.07 |
| Crop Residue | 0.1 | 0 | |||
| Charcoal | 4 | 5 | |||
| LPG | 0.2 | 1 | |||
| NA | 0 | 6 | |||
| Secondary: | Wood | 6 | 7 | 0.19 | |
| Crop Residue | 2 | 2 | |||
| Charcoal | 49 | 40 | |||
| None | 42 | 46 | |||
| NA | 0 | 6 | |||
The Asset Index was constructed using a Principal Component Analysis of variables including: type of housing materials, type of toilet facility, primary water source, type of home ownership, household ownership of livestock animals, and household ownership of consumer durables (e.g. table, mattress, radio, television, bicycle, motorcycle, car, phone, radio, fan, computer).
As exposure to secondhand smoke may affect CO exposure and influence BP, we included a binary indicator variable for tobacco smoking by anyone in the household during the CO monitoring session. We constructed a household asset index as a proxy for socioeconomic status, as household-level socioeconomic status may predict BP, possibly through diet and other means. The asset index was constructed using a principal components analysis of the study participants’ housing characteristics and ownership of household durables (including the material of the home’s walls, roof, and floor, the household’s primary source type of drinking water and type of toilet facility, and ownership of items such as tables, mattresses, radios, phones, and TVs). The asset data was centered and scaled prior to analysis, such that the mean of the asset index was 0, and a higher score indicated higher household socioeconomic status (Filmer and Pritchett 2001; Wagstaff et al. 2007; Vyas and Kumaranayake 2006).
Additional variables were considered for inclusion, including self-reported exposure to other sources of smoke during the monitoring session (e.g. trash burning, mosquito coils), rainy season vs. dry season, and presence/absence of a major road passing through the community. These factors were not associated with BP and did not significantly affect model parameters, and so were not included in the final models.
The final adjusted model contained covariates for: age, BMI, gestational age, occupation, secondhand smoke exposure, and day of week of BP measurement.
Results
Table 1 summarizes the descriptive characteristics of the GRAPHS women in this study. The baseline characteristics of the women included in the final analysis (n = 817) did not differ significantly from those excluded due to invalid CO data or other reasons (n = 366). The included women were largely in the early second trimester of pregnancy at the time of enrollment into the study. Very few of them were previously known to be hypertensive (1.4%) or diabetic (0%). The majority of the women were either working as farmers/laborers (40%) or not employed outside the home (31%). Although the women were non-smokers, about one-fifth (21%) were exposed to secondhand tobacco smoke in their household or compound. The majority of women (60%) primarily cook in a totally open location, although about half the women have a partially or fully enclosed location in which they do some of their cooking (e.g. when it is raining). The predominant cooking fuel in this setting is wood (96%), with charcoal being used as a secondary fuel for about half the women.
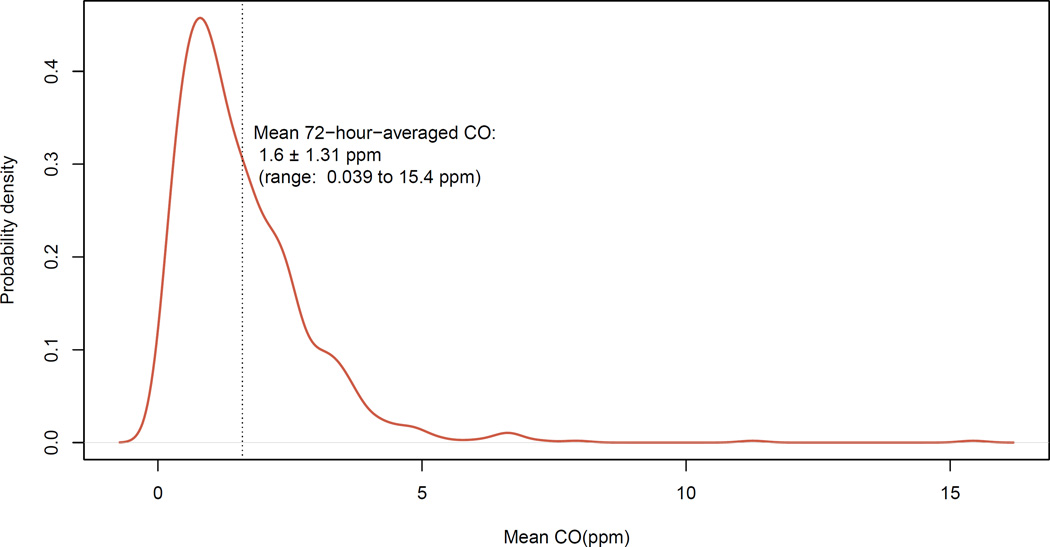
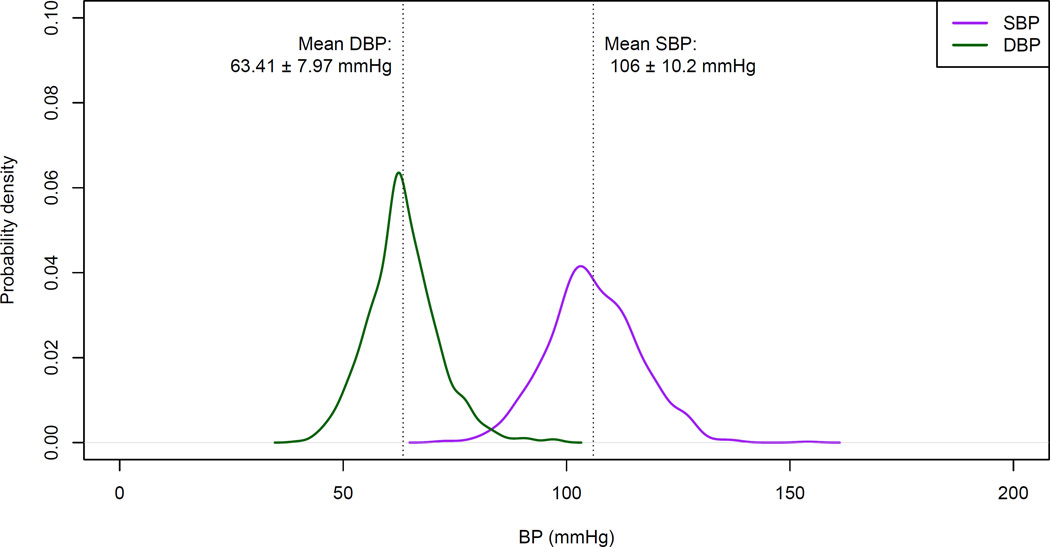
Compliance with the CO monitoring protocol was quite good, with research workers reporting that women were wearing the devices 93.7% of the time at the start of their daily check-up visits. The mean duration of CO monitoring sessions was 71.5 hours (range: 44.2–90.3, with 97.5% of the sessions reaching at least 90% of the target sampling interval of 72 hours). The mean CO measured was 1.6 ppm, with a distribution with a long right tail (Figure 2). The pregnant women were largely normotensive, with a mean SBP of 106 mmHg and a mean DBP of 63 mmHg (Figure 3). Only six individuals (<1% of the participants) were overtly hypertensive, defined as SBP > 140 mmHg and/or DBP > 90 mmHg.
Figure 2.
Distribution of mean 72-hour-averaged CO
Figure 3.
Distribution of baseline BP
The crude and adjusted estimates for the effect of CO exposure on BP are shown in Table 2. We found that higher CO exposure was significantly associated with higher DBP in our adjusted models. We observed a non-significant trend in the same direction for SBP. A 1ppm increase in mean CO exposure was associated, on average, with 0.39 mmHg higher SBP (95% confidence interval: [−0.12, 0.90]), and with 0.43 mmHg higher DBP [0.01, 0.86].
Table 2.
Crude and adjusted effects of personal CO exposure on BP. The associations are the estimated change in SBP or DBP (mmHg) associated with a 1 ppm increase in mean 72-hour-averaged personal CO exposure.
| Crude Associationa | Adjusted Associationa,b | |||
|---|---|---|---|---|
| Estimate (mmHg) |
95% CI | Estimate (mmHg) |
95% CI | |
| SBP | 0.31 | [−0.28, 0.91] | 0.39 | [−0.12, 0.90] |
| DBP | 0.59 | [0.14, 1.03] | 0.43 | [0.01, 0.86] |
Standard Errors are cluster-robust. In each model, results are reported after removal highly influential variables with Cook’s Distance > 0.04; maximum number removed observations = 2.
Adjusted for age, BMI, gestational age, occupation, secondhand smoke exposure, and day of week of BP measurement.
The full output of the adjusted model can be found in the Supplemental Digital Content, Table 1. The coefficients for the included confounders (age, BMI, gestational age, and secondhand smoke exposure) were in the expected direction, with the exception of age in the SBP model only. While increased age is generally associated with higher SBP, in our cohort age was associated with lower SBP, perhaps because the age range in our cohort was quite narrow (range 15–48 years).
Our results reflect the fact that regression diagnostics indicated that a small number of outlying and highly influential observations were swaying our model effect estimates, particularly for DBP. We used the criterion of Cook’s Distance (using a universal cutoff value of Cook’s Distance > 0.04) to remove a maximum of two influential observations from the data prior to reporting the final results. We found that one influential observation led to an overestimation the association between CO and DBP in the adjusted model, while the behavior of the SBP models was less influenced by outlying observations (see Supplemental Digital Content, Figure 1 for an example of the change in beta coefficient in response to piecewise removal of influential observations).
We conducted an additional sensitivity analysis in which we excluded ten women from the analysis who reported never cooking during the exposure monitoring period. The results from this sensitivity analysis can be found in the Supplemental Digital Content, Table 2. The results for SBP and DBP were quite similar to the main result, suggesting that community-level exposures to CO may contribute to the observed effect on BP.
Discussion
SBP and DBP were both higher in pregnant women exposed to higher levels of CO at mid-gestation, although only the results for DBP reached statistical significance at alpha = 0.05. This finding supports evidence from prior studies that exposure to HAP from cooking smoke is related to increases in blood pressure (Baumgartner et al. 2011; Clark et al. 2011; McCracken et al. 2007). Furthermore, this study suggests that these relationships hold during early- and mid-pregnancy. Pregnancy has a known effect on BP due to changes in cardiac output and intravascular volume, with BP falling during early pregnancy, reaching its nadir near mid-pregnancy, and rising thereafter (Lindheimer et al. 2010). The analysis here controlled for gestational age in weeks, and found that CO exposure is associated with higher BP even at early to mid-gestational stages. This work also suggests that the association between HAP and blood pressure may be more robust for DBP than for SBP. Several previous studies have similarly shown a stronger effect of particulate air pollution on DBP than SBP (Brook et al. 2009; Neupane et al. 2015; Urch et al. 2005), although others have found significant associations for SBP only (Baumgartner et al. 2011; Brook et al. 2011a), or for both parameters(McCracken et al. 2007). In this study, we observed positive trends for both SBP and DBP. It is possible that the greater variability of SBP measurements, as compared to DBP, led to the greater statistical instability observed here for SBP.
The magnitude of the association between HAP exposure and BP observed in this cohort (~0.4 mmHg higher SBP and DBP for each 1ppm elevation in CO exposure) is comparable in scale to the associations between HAP and BP that have been observed in Nicaragua, China, and Guatemala, where BP was between 0.5–3.7 mmHg higher per unit of HAP exposure (McCracken et al. 2012). For this cohort of pregnant women in particular, it is worth noting that the size of the association observed here is the same order of magnitude as the differences in BP that have been observed in studies of maternal smoking. Smoking, which results in higher levels of CO exposure than those observed here, is associated with numerous adverse pregnancy outcomes including low birth weight and preterm birth. In a study of 7106 pregnant women in the Netherlands, statistically significant differences in BP were observed between women who never smoked during pregnancy versus those who smoked throughout the pregnancy or smoked only during the first trimester, and the differences in SBP and DBP between these groups at 16 weeks pregnancy were <1 mmHg (Bakker et al. 2010). Interestingly, the study found that DBP at 16 weeks was actually higher among the nonsmokers than the smokers, and that this pattern reversed by the time of delivery. SBP followed a more predictable pattern, with smokers having higher SBP throughout gestation and the differences between groups increasing as the pregnancy progressed. In the current analysis, we found that both SBP and DBP were higher in GRAPHS women exposed to more CO at early to mid-gestation.
While the clinical relevance of such small elevations in BP may be hard to discern at the individual level, especially in the young and largely healthy GRAPHS population, this study adds to the growing body of evidence that HAP exposure raises both SBP and DBP among normotensive adults. This finding, in turn, lends support to hypotheses that changes in long-term basal BP levels may be one pathway through which long-term exposure to air pollution adversely affects cardiovascular risk (Brook et al. 2009, 2010).
Limitations
This cross-sectional study assessed CO exposure and BP among women at early to mid-pregnancy. BP was not reliably assessed later in gestation across in GRAPHS, so we could not examine differences in BP closer to the time of delivery, nor assess the effects of CO exposure on hypertensive disorders of pregnancy that manifest later in gestation. Further, BP readings can vary within an individual, and accurate BP assessment for clinical purposes often requires more than the single measurement that was conducted here. A certain amount of measurement error may therefore exist in the BP data analyzed here. Due to its nondifferential nature, any bias resulting from this error would be expected to influence the results toward the null.
It is also possible that other sources of CO, besides cookstove smoke, contributed to the women’s CO data. However, the fact that inclusion of other known sources of CO (e.g. exposure to trash burning, involvement in charcoal production) did not affect the main parameter of interest makes it more likely that the observed association is attributable to CO from cookstove smoke. The length of the monitoring period (approximately three days) and the fact that personal CO monitoring was conducted lend strength to the assertion that these values may represent the individual women’s “typical” exposures to CO due to cooking with their traditional three-stone fires. Since CO was the only pollutant measured in this baseline air pollution monitoring, it is difficult to know whether the effects found are due to CO itself or to another pollutant that co-varies with CO in the context of biomass burning. Monitoring of more than one pollutant would allow for better analysis of the source components of HAP that may be responsible for the higher observed BP; as it is, we do not know whether the effects seen, if attributable to HAP, are due to CO, PM, or to other pollutants produced during biomass burning.
Finally, this is an observational study, and although we controlled for many known predictors of BP, it is possible that confounding by an unmeasured covariate influenced our results. For example, ambient temperature may be associated both with energy use patterns and with blood pressure, and has often been included as a covariate in blood pressure models. A limitation of our study is that this data was not available in our study area. The climate of the study region is tropical, however, with mean temperatures staying within a fairly narrow range all year (Kintampo South Assembly. 2007) and with seasonal differences in precipitation (two “rainy” seasons per year versus drier periods). The lack of wide temperature variability would likely attenuate any observable effect of temperature on the relationship observed here. Information on certain other potential predictors of BP, such as diet and time of day of the BP measurement, was likewise not available and therefore could not be included in our analyses. Future work based on the randomized provision of cleaner stoves, in this cohort and others, will help to determine whether HAP is a cause of increased blood pressure in pregnant women and other adults.
Conclusions
This study of over 800 pregnant women in rural Ghana provides evidence of a positive association between CO exposure and mid-pregnancy blood pressure, with results stronger for DBP than SBP.
Supplementary Material
Acknowledgments
Sources of Funding Support:
Funding for AQ provided by the Interdisciplinary Training Grant in Climate and Health (T32 ES023770). Funding for GRAPHS is provided through the United States National Institute of Environmental Health Sciences (NIH 1R01ES019547; P30 ES009089), The Global Alliance for Clean Cookstoves, the Thrasher Research Fund, and the Ghana Ministry of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests:
All authors declare they have no actual or potential competing financial interest.
References
- Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel J, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG Int. J. Obstet. Gynaecol. 2014;121(Suppl 1):14–24. doi: 10.1111/1471-0528.12629. [DOI] [PubMed] [Google Scholar]
- Agrawal S, Yamamoto S. Effect of Indoor air pollution from biomass and solid fuel combustion on symptoms of preeclampsia/eclampsia in Indian women. Indoor Air n/a–n/a. 2014 doi: 10.1111/ina.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M. Cluster-robust standard errors using R. [accessed 1 October 2015];2015 Available: http://www.ne.su.se/polopoly_fs/1.216115.1426234213!/menu/standard/file/clustering1.pdf. [Google Scholar]
- Bakker R, Steegers EA, Mackenbach JP, Hofman A, Jaddoe VW. Maternal smoking and blood pressure in different trimesters of pregnancy: the Generation R study. J. Hypertens. 2010;28:2210–2218. doi: 10.1097/HJH.0b013e32833e2a3d. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, et al. Indoor air pollution and blood pressure in adult women living in rural China. Environ. Health Perspect. 2011;119:1390–1395. doi: 10.1289/ehp.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Peng RD, Dominici F, Samet JM. Emergency hospital admissions for cardiovascular diseases and ambient levels of carbon monoxide: results for 126 United States urban counties, 1999–2005. Circulation. 2009;120:949–955. doi: 10.1161/CIRCULATIONAHA.109.851113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boamah EA, Asante K, Ae-Ngibise K, Kinney PL, Jack DW, Manu G, et al. Gestational Age Assessment in the Ghana Randomized Air Pollution and Health Study (GRAPHS): Ultrasound Capacity Building, Fetal Biometry Protocol Development, and Ongoing Quality Control. JMIR Res. Protoc. 2014;3:e77. doi: 10.2196/resprot.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Prüss-Ustün A, et al. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environ. Health Perspect. 2013;121:784–790. doi: 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Bard RL, Burnett RT, Shin HH, Vette A, Croghan C, et al. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup. Environ. Med. 2011a;68:224–230. doi: 10.1136/oem.2009.053991. [DOI] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J. Am. Soc. Hypertens. JASH. 2009;3:332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate Matter Air Pollution and Cardiovascular Disease An Update to the Scientific Statement From the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Weder AB, Rajagopalan S. “Environmental Hypertensionology” The Effects of Environmental Factors on Blood Pressure in Clinical Practice and Research. J. Clin. Hypertens. 2011b;13:836–842. doi: 10.1111/j.1751-7176.2011.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, de Greeff A, Shennan A. Validation and compliance of a home monitoring device in pregnancy: microlife WatchBP home. Hypertens. Pregnancy. 2009;28:348–359. doi: 10.1080/10641950802601286. [DOI] [PubMed] [Google Scholar]
- Clark ML, Bazemore H, Reynolds SJ, Heiderscheidt JM, Conway S, Bachand AM, et al. A baseline evaluation of traditional cook stove smoke exposures and indicators of cardiovascular and respiratory health among Nicaraguan women. Int. J. Occup. Environ. Health. 2011;17:113–121. doi: 10.1179/107735211799030942. [DOI] [PubMed] [Google Scholar]
- Clark ML, Peel JL, Balakrishnan K, Breysse PN, Chillrud SN, Naeher LP, et al. Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environ. Health Perspect. 2013;121:1120–1128. doi: 10.1289/ehp.1206429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosselman KE, Krishnan RM, Oron AP, Jansen K, Peretz A, Sullivan JH, et al. Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension. 2012;59:943–948. doi: 10.1161/HYPERTENSIONAHA.111.186593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio KL, Howie S, Fornace KM, Chimah O, Adegbola RA, Ezzati M. Measuring the exposure of infants and children to indoor air pollution from biomass fuels in The Gambia. Indoor Air. 2008;18:317–327. doi: 10.1111/j.1600-0668.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- Dionisio KL, Howie SRC, Dominici F, Fornace KM, Spengler JD, Adegbola RA, et al. Household Concentrations and Exposure of Children to Particulate Matter from Biomass Fuels in The Gambia. Environ. Sci. Technol. 2012;46:3519–3527. doi: 10.1021/es203047e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Dutta A, Mukherjee B, Das D, Banerjee A, Ray MR. Hypertension with elevated levels of oxidized low-density lipoprotein and anticardiolipin antibody in the circulation of premenopausal Indian women chronically exposed to biomass smoke during cooking. Indoor Air. 2011;21:165–176. doi: 10.1111/j.1600-0668.2010.00694.x. [DOI] [PubMed] [Google Scholar]
- Filmer D, Pritchett LH. Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- Ghana Health Service. Brong Ahafo Regional Annual Health Report. 2013 [Google Scholar]
- Institute for Health Metrics and Evaluation. Global Burden of Disease Country Profiles | Ghana. [accessed 14 February 2014];2013 Available: http://www.healthmetricsandevaluation.org/gbd/country-profiles#g. [Google Scholar]
- Jack DW, Asante KP, Wylie BJ, Chillrud SN, Whyatt RM, Ae-Ngibise KA, et al. Ghana randomized air pollution and health study (GRAPHS): study protocol for a randomized controlled trial. Trials. 2015;16:420. doi: 10.1186/s13063-015-0930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintampo South Assembly. Ghana » Brong Ahafo Region » Kintampo South District Climate and Vegetation. [accessed 29 September 2015];2007 Available: http://kintamposouth.ghanadistricts.gov.gh/?arrow=dnf&_=45&r=10&rlv=climate.
- Klasen EM, Wills B, Naithani N, Gilman RH, Tielsch JM, Chiang M, et al. Low correlation between household carbon monoxide and particulate matter concentrations from biomass-related pollution in three resource- poor settings. Environ. Res. 2015;142:424–431. doi: 10.1016/j.envres.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish JP, Mills NL, Chan JK, Leseman DL, Aitken RJ, Fokkens PH, et al. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part. Fibre Toxicol. 2009;6:8. doi: 10.1186/1743-8977-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-S, Hang J, Zhang F, Dai H, Su L, Christiani DC. In-home solid fuel use and cardiovascular disease: a cross-sectional analysis of the Shanghai Putuo study. Environ. Health. 2012a;11:18. doi: 10.1186/1476-069X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P-C, Talbott EO, Roberts JM, Catov JM, Bilonick RA, Stone RA, et al. Ambient air pollution exposure and blood pressure changes during pregnancy. Environ. Res. 2012b;117:46–53. doi: 10.1016/j.envres.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2010;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindheimer MD, Taler SJ, Cunningham FG. Hypertension in pregnancy. J. Am. Soc. Hypertens. JASH. 2010;4:68–78. doi: 10.1016/j.jash.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Männistö T, Mendola P, Liu D, Leishear K, Sherman S, Laughon SK. Acute Air Pollution Exposure and Blood Pressure at Delivery Among Women With and Without Hypertension. Am. J. Hypertens. 2014 doi: 10.1093/ajh/hpu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männistö T, Mendola P, Vääräsmäki M, Järvelin M-R, Hartikainen A-L, Pouta A, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681–690. doi: 10.1161/CIRCULATIONAHA.112.128751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JP, Schwartz J, Diaz A, Bruce N, Smith KR. Longitudinal Relationship between Personal CO and Personal PM2.5 among Women Cooking with Woodfired Cookstoves in Guatemala. PLoS ONE. 2013;8:e55670. doi: 10.1371/journal.pone.0055670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JP, Smith KR, Díaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ. Health Perspect. 2007;115:996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JP, Wellenius GA, Bloomfield GS, Brook RD, Tolunay HE, Dockery DW, et al. Household Air Pollution from Solid Fuel Use: Evidence for Links to CVD. Glob. Heart. 2012;7:223–234. doi: 10.1016/j.gheart.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Neupane M, Basnyat B, Fischer R, Froeschl G, Wolbers M, Rehfuess EA. Sustained use of biogas fuel and blood pressure among women in rural Nepal. Environ. Res. 2015;136:343–351. doi: 10.1016/j.envres.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard SL, Williams DL, Breysse PN, Baron PA, Grajeda LM, Gilman RH, et al. A cross-sectional study of determinants of indoor environmental exposures in households with and without chronic exposure to biomass fuel smoke. Environ. Health Glob. Access Sci. Source. 2014;13:21. doi: 10.1186/1476-069X-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. The Lancet. 2011;378:1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- Steinle S, Reis S, Sabel CE. Quantifying human exposure to air pollution—Moving from static monitoring to spatio-temporally resolved personal exposure assessment. Sci. Total Environ. 2013;443:184–193. doi: 10.1016/j.scitotenv.2012.10.098. [DOI] [PubMed] [Google Scholar]
- Stergiou GS, Giovas PP, Gkinos CP, Patouras JD. Validation of the Microlife WatchBP Home device for self home blood pressure measurement according to the International Protocol. Blood Press. Monit. 2007;12:185–188. doi: 10.1097/MBP.0b013e3280b083ce. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Szyszkowicz M, Rowe BH, Leech JA. Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis. Environ. Health Glob. Access Sci. Source. 2009;8:25. doi: 10.1186/1476-069X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, et al. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ. Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet EDS, Asante K, Jack DW, Kinney PL, Whyatt RM, Chillrud SN, et al. Personal exposures to fine particulate matter and black carbon in households cooking with biomass fuels in rural Ghana. Environ. Res. 2013;127:40–48. doi: 10.1016/j.envres.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- Wagstaff A, O’Donnell O, Doorslaer EV, Lindelow M, editors. Analyzing Health Equity Using Household Survey Data: A Guide to Techniques and their Implementation. 1 edition. Washington, D.C.: World Bank Publications; 2007. [Google Scholar]
- World Health Organization (WHO) WHO guidelines for indoor air quality: selected pollutants. 2010 [PubMed]
- Wylie BJ, Singh MP, Coull BA, Quinn A, Yeboah-Antwi K, Sabin L, et al. Association between wood cooking fuel and maternal hypertension at delivery in central East India. Hypertens. Pregnancy. 2015:1–14. doi: 10.3109/10641955.2015.1046604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-Y, Chen Y-S, Yang C-H, Ho S-C. Relationship between ambient air pollution and hospital admissions for cardiovascular diseases in kaohsiung, taiwan. J. Toxicol. Environ. Health A. 2004;67:483–493. doi: 10.1080/15287390490276502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.