Abstract
This clinical study assessed and compared the efficacy of tea tree oil (TTO), an alternative form of medicine, with clotrimazole (i.e., allopathy) and a conservative form of management in the treatment of oral fungal infection.
In this interventional, observational, and comparative study, we enrolled 36 medically fit individuals of both sexes who were aged 20–60 years old. The participants were randomly assigned to three groups. Group I was given TTO (0.25% rinse) as medicament, Group II was given clotrimazole, and Group III was managed with conservative treatment. The results were analyzed from the clinical evaluation of lesions, changes in four most common clinical parameters of lesions, and subjective symptoms on periodic follow-up. Based on the results, the percentage efficiency of the two groups were taken and compared through a bar graph on the scale of 1.
No toxicity to TTO was reported. Group I (TTO) was found to be more efficient than the other two groups, as changes in four parameter indices of lesions were noted, and results for all three groups were compared on a percentage basis.
The study concluded that TTO, being a natural product, is a better nontoxic modality compared to clotrimazole, in the treatment of oral fungal infection and has a promising future for its potential application in oral health products.
Keywords: candidiasis, clotrimazole, conservative management, fungal infection, tea tree oil
Graphical abstract

1. Introduction
Tea tree oil (TTO) is derived from the paper bark tea tree, which belongs to the family of Myrtaceae; it belongs to two genera, Leptospermum and Melaleuca.1 TTO medications have been used for thousands of years by Australian aborigines both internally and externally for alleviation of pain and promotion of wound healing, cure colds, and influenza. Although tree tea oil is used around the world in a small number of cosmetic and medicinal products, its use as an ingredient of oral health care products remains limited. 1.8-Cineole and terpinen-4-ol are the main active ingredients of TTO.2, 3, 4 TTO is a uniquely defined combination of monoterpenes, sesquiterpenes, and terpene alcohol with outstanding therapeutic properties. It is recognized as having broad-spectrum antibacterial,5 antiviral (Carson et al 2005)6, antimycoplasmal, and antiprotozoal activities (Furneri et al, 2006)7, as well as anti-inflammatory (Finlay-Jones et al 2001)8 and anticancer properties (Greay et al 2010).9, 10 It acts by damaging the microorganisms' cellular membrane and subsequently denaturing the cell contents.5 Anecdotally, TTO is known as an excellent treatment for fungal infections, in particular, vaginal candidiasis and dermatophytoses, but relative data showing its oral antifungal property is rather sparse.11, 12, 13
1.1. Aims and objectives
The potential usefulness of TTO in oral health care products has not been assessed, and hence, through this study, our aim was to assess and compare the efficacy of TTO as an alternative form of medicine with clotrimazole (i.e., allopathy) and a conservative form of management in the treatment of oral fungal infection. Our objective was to create directly comparable and quantitative antifungal data for TTO, for which little literature data exist and which can be a useful addition to the current range of oral antifungal drugs.
2. Materials and methods
The Maharaj Vinayak Global University (MVGU) Research Ethics Committee gave ethical approval for the study. Patients visiting the Oral Medicine Department of Jaipur Dental College, Kukas, India were screened for oral fungal infection. Fungal infection included: (1) leukoplakia superimposed candidiasis, (2) denture-induced candidiasis, and (3) pseudomembranous candidiasis. The most common presenting clinical features of the above-mentioned fungal infections included erythema (sign), burning sensation (symptom), inflammation (sign), and fungal hyphae (pathological) as suggested in the literature.8, 9, 10 The efficiency of the three groups was compared on the basis of four parameter indices that were selected based on the most common clinical features of fungal infection mentioned so far in the literature.
The inclusion criteria called for male and female participants who were: (1) willing to cooperate, (2) available for regular follow-up, and (3) within the age group of 20–60 years. The exclusion criteria included patients who were: (1) uncooperative, (2) on antifungal medication, (3) HIV-positive, (4) known to have critical diseases (e.g., leukemia and lymphoma), (5) undergoing radiation therapy, and those (6) who discontinued the medication and follow-up and (7) have reported side effects from TTO. Histopathological smear of the lesion and the prosthesis used were taken to confirm the diagnosis of fungal infection. Patients diagnosed with fungal infection were randomly divided into (1) TTO group, (2) Allopathy group (i.e., clotramizole), and (3) Conservative group, based on the treatment to be carried out.
Evaluation of lesion was done on the basis of four parameter indices: (1) erythema; (2) visual analog scale, burning sensation; (3) inflammation; and (4) fungal hyphae pretreatment. Complete medical, drug, and any allergy history were noted for selected patients, and any subjective and objective symptoms associated with the lesion were also recorded. Prestructured performa was filled with all the above details and with specific emphasis on the frequency of use of prosthesis and its maintenance.
After the confirmation of the diagnosis, treatment was started, and those patients who were willing to undergo treatment signed a consent form. During their first visit, participants selected for treatment with Group I were instructed to perform on-spot rinse with TTO and were placed on a 24-hour observation for any toxic effect from the essential oil. The participants were handed a pamphlet that outlined the use of TTO, in the form of a rinse (diluting 5 ml oil/50 ml water – concentration 0.10%) to be done thrice daily until the first follow-up (after a meal). Patients were instructed not to eat/drink anything until 30 minutes after rinsing. Patients selected for treatment under Group II (Allopathy) had to apply clotrimazole ointment thrice daily after meals until the first follow-up. Under Group III (conservative management), participants were asked to carry out regular cleaning and washing of the prosthesis on a daily basis and removal of prosthesis during the night in case fungal infection turned out to be denture-induced. The participants were asked to refrain from using proprietary mouthwashes during the study, and no auxiliary cleaning aids were allowed to be used during the course of the study. Medication was distributed to the participants in labeled (A, TTO; B, Allopathy) dispensing bottles. The participants were not aware of the contents of these bottles.
Follow-up was carried out after the 1st week, 2nd week, and 3rd week to evaluate the subsequent changes in the lesion on the basis of the four parameter indices (Fig. 1A–C). On the recall visit, any changes in clinical features associated with lesions were noted. Patient performa posttreatment was filled up again, and the medication was continued if any symptoms and signs of the lesion persisted. No patient reported any adverse effects in relation with the oil. The recorded data were preserved for analysis of results.
Fig. 1.
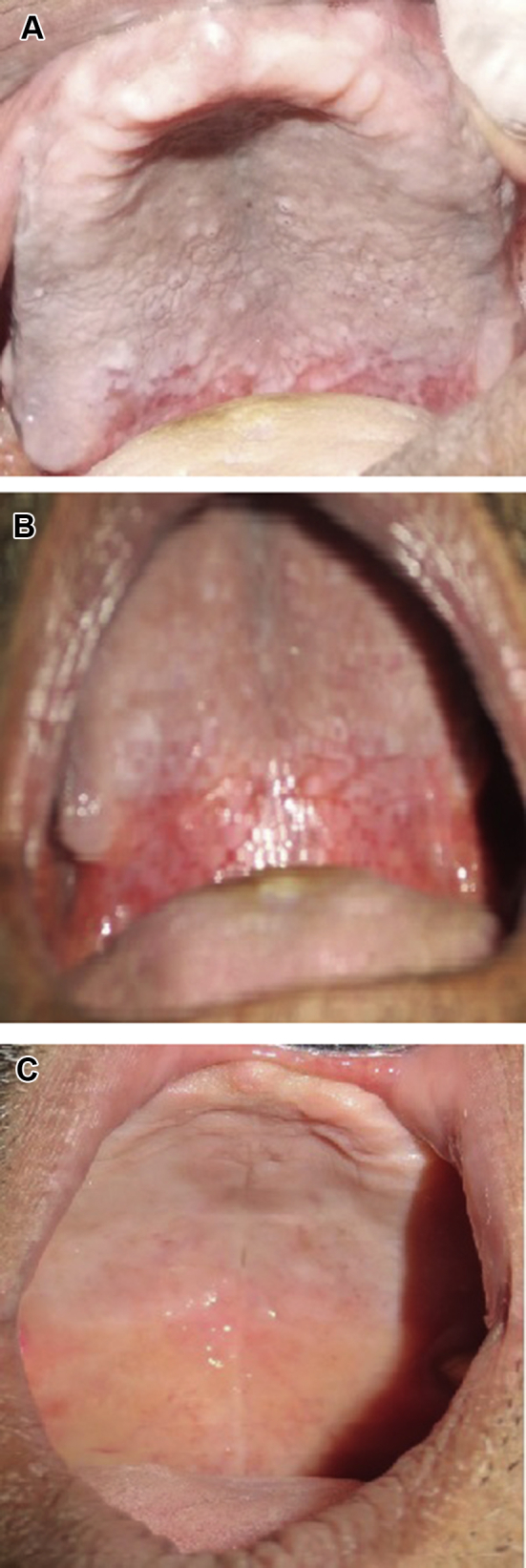
(A) Fungal infection in the palatal region – pre treatment. (B) First visit – After 1 week erythema & burning sensation reduced – Tea tree oil treated. (C) Second visit – After 2 weeks complete relief.
2.1. Data analysis
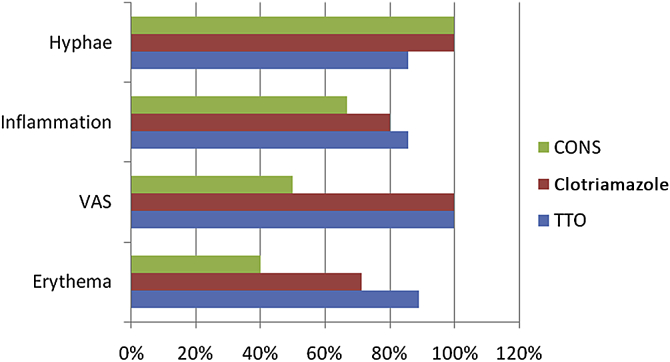
The data for each participant were added, averaged, and weighted depending on the positive (improvement) or negative (no improvement) changes in the lesion in subsequent recalls. The efficiency, compliance, and safety index of the two drugs were evaluated and compared using a relative percentage chart for both groups. Results were analyzed using bargraph for all clinical indices. The efficiency of all three treatment methods was compared on a bar graph on the scale of 1.
3. Results and discussion
A total of 36 participants, consisting of 11 females and 25 males (Table 1), were recruited for the study. Those not included in the study were either dropouts or were omitted based on the exclusion criteria. Statistical analysis showed that intraexaminer error was within acceptable limits. Overall, there were 13 patients in Group I, 13 patients in Group II, and 10 patients in Group III.
Table 1.
Distribution in treatment groups of subjects by age and gender.
| Group | Female | Male | n | Mean age (y) |
|---|---|---|---|---|
| TTO | 5 | 8 | 13 | 64.92 |
| Clotriamazole | 4 | 9 | 13 | 63.24 |
| Cons | 2 | 8 | 10 | 64.51 |
TTO = tea tree oil.
A percentage analysis for all three groups was done, and a comparative chart in terms of percentage for all four parameter indices (Table 2) was drawn up.
Table 2.
Percentage efficiency of each group in treatment of all four parameter indices.
| Indices | TTO | Clotriamazole | Cons |
|---|---|---|---|
| Erythema | 89% | 71% | 40% |
| VAS | 100% | 100% | 50% |
| Inflammation | 85.70% | 80.00% | 66.67% |
| Hyphae | 85.70% | 100% | 100% |
Data are presented as %.
TTO = tea tree oil; VAS = visual analog scale.
The following results were deduced (Table 2). (1) The TTO-treated group showed an 89% reduction in erythema and inflammation compared to the other two groups. Thus, TTO proved to be a better alternative to clotrimazole in reducing the two main clinical features associated with oral fungal infection. (2) In the TTO group, there was a 100% reduction in visual analog scale burning sensation score. A complete elimination of fungal hyphae was achieved only by clotrimazole. Clotrimazole proved to be 100% successful in the reduction of clinical and histopathological features in this study. 100% reduction in burning sensation was achieved in subjects treated under group I & group II, but only 50% reduction was achieved in group III.
From the above findings, it was preliminarily concluded that overall both Group I and Group II are at par in giving symptomatic relief to patients suffering from oral fungal infection.
As the study was directed toward comparing allopathy and alternative medicine in the treatment of oral fungal infection, we further filtered the results and compared all three modalities through a bar graph on the scale of 1 (Fig. 2).
Fig. 2.
Bar graph analysis of indices for every group. Results: 1. TTO is effective in reducing inflammation and erythema better than other two modalities. 2. Clotriamazole is equally at a par with TTO in reducing irritation and VAS score. 3. Conservative Management is equally at a par with the other two modalities in reducing irritation and hyphae and less effective in reducing inflammation, VAS, and erythema. TTO = tea tree oil; VAS = visual analog scale.
A bar graph analysis was performed to determine the most efficient modality. It showed the following results. (1) TTO (Group I) was the most efficient of all three modalities. (2) Although clotrimazole (Group II) scored 100% on two indices, it came only a close second in overall efficiency. (3) Conservative management (Group III) was the least efficient of all three treatments.
The allopathic antifungal agents are effective in the treatment of oral fungal infection, but present several major negative effects that cannot be overlooked. Polyene antifungals have a broad spectrum of antifungal activities, but because of their poor absorption through the gut their use in the treatment of oral candidiasis is limited. Fewer cases of polyene toxicity to animal cells have been reported; however, at therapeutic doses, some (e.g., amphotericin B) may bind to animal membrane cholesterol, thus increasing the risk of human toxicity. Amphotericin B is also nephrotoxic when given intravenously. Some recent case reports also suggest the toxicity of nystatin if it is used in the long run. As a polyene's hydrophobic chain is shortened, its sterol binding activity is increased. Therefore, further reduction of the hydrophobic chain may result in it binding to cholesterol, making it toxic to humans. Among azole antifungals, fluconazole is the agent of first choice for all forms of oral candidosis. However, several mechanisms of azole resistance have been reported including: (1) an alteration in the chemical structure of the demethylase enzyme, (2) removal of the azole from the cell by multidrug transporter pumps, and (3) compensation by other sterol synthesis enzymes in membrane biosynthesis.14 These resistance mechanisms of fungal microorganisms can increase the chances of failure of these antifungal drugs in the future. The clinical effectiveness of agents that can only be delivered topically, such as amphotericin or nystatin, is limited owing to problems in maintaining sufficient levels of these drugs at the site of infection. The taste of topical agents stimulates salivary secretion, which rapidly dilutes and removes the antifungal agent from the mouth. In view of this, their clinical use is limited (K. Hammer et al 2002).15 Fluconazole has a good safety profile when given systemically, with few contraindications or side effects. Important interactions occur with coumarin anticoagulants and sulfonylurea antidiabetic agents though. Apart from certain side effects such as liver damage or effect on estrogen levels, many antifungal medicines can cause allergic reactions in some people. For example, the azole group of drugs is known to have caused anaphylaxis.16
Hence, allopathic antifungals is beneficial but also has several major side effects. In vitro studies regarding TTO have been performed on filamentous fungi, dermatophytes, and Candida albicans. The literature suggests that TTO in vitro substantially alters the membrane permeability of C. albicans and Candida glabrata. TTO is also less misicible in water compared to clotramizole. Therefore, compared to clotramizole TTO will be less misicible in saliva and will remain at the effected site for a longer length of time, thereby producing a better pharmacological effect. As discussed in detail, allopathic antifungals have numerous toxic effects if used in the long run, whereas TTO, being purely a herbal plant, has no toxic effects if used for a long duration.17, 18
3.1. Limitations
Although the authors have tried their best to include all possible parameters in the study, certain limitations/drawbacks remain (as detailed below).
Research can be elaborated with an increase in the sample size with special emphasis on a specific candidal pathogen that is mainly the causative factor for denture-induced candidiasis.
As candida is one of the predisposing factors for potentially premalignant and malignant lesions, specific Candida species can be investigated.
The efficacy of TTO can be compared on the different grades of denture-induced candidiasis.
The future of tea trees is interesting and challenging, and more aspects of its properties and potential therapeutic applications can be investigated.
4. Conclusion
Within the limitations of the study, it was apparent that Group I patients showed improvement in relation to symptoms associated with lesion(s). TTO, being a better alternative to clotrimazole, is justifiable because it has good membrane permeability and has no toxic effects, whereas allopathic antifungals have several side effects that cannot be overlooked. From the study results, we conclude that efficiency of both medications are at a par, but TTO, being nontoxic, economical, and compliable, remains a better alternative to allopathy in the treatment of oral fungal infections.
The components of TTO are known to have lipophilic properties that facilitate its diffusion through the epithelium. If TTO is readily absorbed after its topical application or rinse into gingival tissues and has antifungal properties once it has entered connective tissues, it would be a unique nontoxic agent that would be a useful addition to the current range of chemotherapeutic antifungal options.
Conflicts of interest
All authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Barr A., Chapman J., Smith N., Beveridge M. Greenhouse Publications; Victoria: 1988. Traditional Bush Medicines – An Aboriginal pharmacopoeia; pp. 23–46. [Google Scholar]
- 2.Juergens U.R., Stober M., Schmidt-Schilling L., Kleuver T., Vetter H. Anti-inflammatory effects of eucalyptol (1.8-cineole) in bronchial asthma: inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. Eur J Med Res. 1998;3:407–412. [PubMed] [Google Scholar]
- 3.Juergens U.R., Stober M., Vetter H. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. Eur J Med Res. 1998;3:508–510. [PubMed] [Google Scholar]
- 4.Santos F.A., Rao V.S. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res. 2000;14:240–244. doi: 10.1002/1099-1573(200006)14:4<240::aid-ptr573>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Cox S.D., Mann C.M., Markham J.L. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil) J Appl Microbiol. 2000;88:170–175. doi: 10.1046/j.1365-2672.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 6.Carson C.F., Hammer K.A., Riley T.V. Melaleuca alternifolia (tea tree oil): A review of antimicrobial and other medicinal properties. Clin Microbiol Rev. Jan 2006;19(1):50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furneri P.M., D’Arrigo M., Ginestra G., Giuseppina M., Bisignano M. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia against S. aureus and E. coli. Phytomedicine. 2010;17:317–322. doi: 10.1016/j.phymed.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Finlay-Jones J., Hart P., Riley T.V., Carson C. Antiinflammatory. Activity of Tea Tree Oil, Rural Industries Research & Development Corporation Publication No 01/10, Chapter 2. 2002. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia suppresses inflammatory mediator production by activated human monocytes. [Google Scholar]
- 9.Greay SJ, Hammer KA. Recent developments in bioactivity of mono and di-terpenes anti-cancer and anti-microbial activity. Phytochemistry Reviews. http://dx.doi.org/1007/s11101-011-9212-6.
- 10.Hart P.H., Brand C., Carson C.F., Riley T.V., Prager R.H., Finlay-Jones J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm Res. 2000;49:619–626. doi: 10.1007/s000110050639. [DOI] [PubMed] [Google Scholar]
- 11.Veien N.K., Rosner K., Skovgaard G. Is tea tree oil an important contact allergen? Contact Dermatitis. 2004;50:378–379. doi: 10.1111/j.0105-1873.2004.0350f.x. [DOI] [PubMed] [Google Scholar]
- 12.Ellepola A.N.B., Samaranayake L.P. Oral candidal infections and antimycotics. Crit Rev Oral Biol Med. 2000;11:172–198. doi: 10.1177/10454411000110020301. [DOI] [PubMed] [Google Scholar]
- 13.Renstrup G. Occurrence of Candida in oral leukoplakias. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78:421–424. doi: 10.1111/j.1699-0463.1970.tb04322.x. [DOI] [PubMed] [Google Scholar]
- 14.Baginski M., Czub J. Amphotericin B and its new derivatives – Mode of action. Current Drug Metabolism. June 2009;10(5):459–469. doi: 10.2174/138920009788898019. [DOI] [PubMed] [Google Scholar]
- 15.Hammer K.A., Carson C.F., Riley T.V. In vitro activity of Melaleuca alternifolia oil against dermatophytes and other filamentous fungi. J Antimicrob Chemother. 2002;53:1081–1085. doi: 10.1093/jac/dkf112. [DOI] [PubMed] [Google Scholar]
- 16.Hammer K.A., Carson C.F., Riley T.V. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 2004;53:1081–1085. doi: 10.1093/jac/dkh243. [DOI] [PubMed] [Google Scholar]
- 17.Jandourek A., Vaishampayan J.K., Vazquez J.A. Efficacy of melaleuca oral solution for the treatment of fluconazole refractory oral candidiasis in AIDS patients. AIDS. 1998;12:1033–1037. [PubMed] [Google Scholar]
- 18.Vazquez J.A., Zawawi A.A. Efficacy of alcohol-based and alcohol-free melaleuca oral solution for the treatment of fluconazole-refractory oropharyngeal candidiasis in patients with AIDS. HIV Clin Trials. 2002;3:379–385. doi: 10.1310/99dy-8q52-306a-v0aj. [DOI] [PubMed] [Google Scholar]