Abstract
Accumulating evidence indicates that the efficacy of tumor targeted therapies relies on the host immune response, including targeted small molecule and antibody approaches that were not previously thought to have an immune component. Here we review the current understanding of how targeted therapies on tumor cells could have a major impact on the immune response, and how this relates to the therapeutic efficacy of these approaches. In this context we evaluate different strategies that combine targeted therapies with immunotherapy approaches, and discuss past and ongoing clinical trials. We highlight gaps in knowledge, and argue that significant progress for combined therapies will require a better understanding of the complex interactions between immune cells, the tumor and the tumor microenvironment in different cancer settings.
Introduction
More comprehensive understanding of cancer biology has given impetus to molecularly targeted cancer therapies in the past decade. Furthermore, large scale mutational analysis of human cancer has increased our understanding of cancer etiology, targeting, and drug resistance [1, 2]. Many targeted anticancer agents available for clinical use have been generated and have shown remarkable clinical responses in most patients [3, 4, 5]. Nevertheless, these targeted therapies have limited use due to a high frequency of tumor drug resistance [6, 7]. The cell-intrinsic factors by which cancers adapt to grow and evade targeted therapy have been considered to be major mechanisms controlling the overall response [8, 9, 10, 11]. Therefore, until recently, research and drug development has primarily focused on blocking second mutations or compensatory signals [12, 13, 14]. For decades, mechanistic studies of targeted therapy in vitro or in xenograft models have resulted in inherent long-term ignorance of the immune system, especially the adaptive immune system, in response to these therapies. Clinical observations and even trials under such influence in the past has largely ignore the role of therapy-induced adaptive immunity for tumor control. Through the utilization of syngeneic mouse tumor models, a pivotal set of studies made conceptual progress demonstrating that the mechanisms of tumor progression by anti-HER2 monoclonal antibody (mAb) and anti-EGFR mAb require an adaptive immune response [15, 16, 17]. Since then, accumulating evidence showed that in addition to cell-intrinsic factors, the immune-mediated cytotoxic effects elicited by targeted therapies are the determined factor for tumor regression [18, 19, 20, 21, 22]. These recent studies have driven tremendous interest in understanding the role of adaptive immunity; approaches that combine targeted therapy with immunotherapy are now being developed with a goal to achieve better immunity and prolonged duration of tumor control, even, in some cases, eradication [22, 23]. Since the immune regulatory mechanism of each targeted therapy could be different for different tumors, identifying and understanding how each targeted therapy affects the immune system in a specific tumor and host setting are important for optimizing combinatorial strategies and for personalized medicine. Here we review the previously underappreciated crucial immunomodulatory aspects of targeted therapies, focusing on tumor targeting mAbs and small molecule inhibitors, and provide an overview of the recent progresses in the integration of immunotherapy and targeted therapy.
Immunomodulatory effects of Ab-based targeted therapies
Antagonists of the oncogenic receptor tyrosine kinases, such as anti-EGFR and anti-HER2, block tumor cell growth signaling and trigger apoptosis pathways. mAbs can bind to Fc receptors (FcRs) on macrophages, neutrophils, and natural killer cells (NK), and induce cell death by activating the complement-dependent cytotoxicity (CDC) or antibody-dependent cellular cytotoxicity (ADCC). Targeted antibodies such as trastuzumab and cetuximab are clinically efficacious oncogene-targeted treatments with proven survival benefits in some patients with HER2 and EGFR tumor mutations, respectively [24, 25]. In addition to the established direct cell death mechanisms. We have demonstrated the ability of these mAbs to activate the host’s immune system that control tumor growth (Figure 1). Patients who were previously treated with trastuzumab exhibited a substantial increase in CD8 T cells and NK cells, which was correlated with improved clinical outcomes [26]. In a tumor vaccination clinical trial, patients receiving trastuzumab therapy had a significant HER2/neu E75 peptide-specific CD8 T cell response compared to the control arm, highlighting a positive correlation of the adaptive immune response and clinical benefits [27]. While many studies have revealed such a correlation, the mechanism by which the adaptive immune responses mediate antibody-mediated tumor control is still unknown.
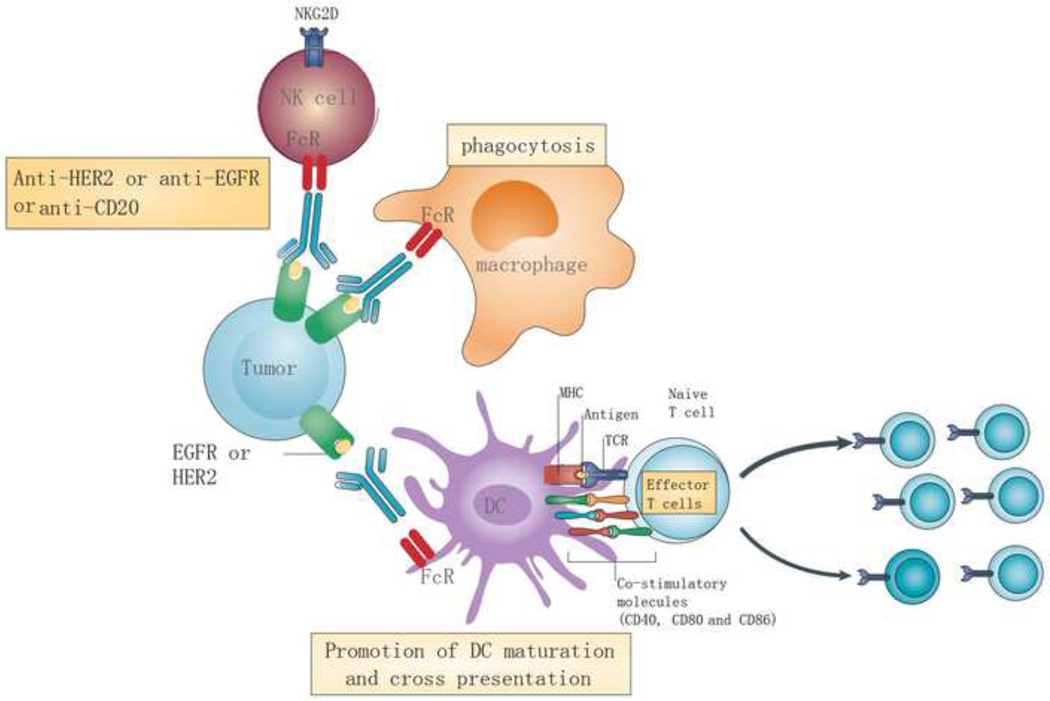
Figure 1. The effect of Ab-based targeted therapy on immune responses.
The immune modulatory effects of antibody-based targeted therapies include enhancing macrophage phagocytosis, NK cytotoxicity, promoting DC priming, and stimulating tumor-specific T cells. For example, monoclonal antibodies against EGFR and HER2 promote macrophage phagocytosis and DC cross-presentation of tumor antigens through FcRs. FcR-dependent opsonization also elevates the expression of co-stimulatory molecules such as CD40, CD80, and CD86 on the DC surface, increasing TCR signaling and promoting T cell activation.
Anti-HER2/neu antibody therapies have been reported to mediate tumor regression by interrupting oncogenic signals and inducing FcR-mediated cytotoxicity for decades. For mechanistic studies studying tumor burden after different therapies, xenograft models were primarily used with prolonged high dose of antibody. However, using a mouse mammary tumor line derived from Her2/neu Tg mice, our team demonstrated that the therapeutic effect of anti-HER2/neu antibody depends mainly on both innate and adaptive immunity [15]. We observed that both MyD88 and stress protein signaling are required for T cell responses and tumor control. Intriguingly, the addition of intensive chemotherapeutic drugs after antibody treatment further reduces tumor burden. Intriguingly, if chemotherapeutic drugs were given prior to antibody treatment, the antibody-mediated immunity was significantly diminished and tumor burdened was uncontrolled and subjected to relapses. This observation was later confirmed by another study [16]. Together, both groups showed that an increased influx of innate and adaptive immune cells into the tumor microenvironment which can be further enhanced by selected immunotherapy, leading to increased tumor eradication and resistance to re-challenge [15, 16].
Notably, the key cell crucial for developing a tumor-specific T cell responses could be DC, which expresses an array of FcRs for internalization of antigen-antibody complexes and antigen presentation for cross priming [28]. Antigen uptake through opsonization of apoptotic tumor cells is also associated with enhanced antigen presentation by DCs [29]. mAbs can facilitate the uptake of tumor antigens by DCs, which helps to stimulate the activation and expansion of CD4+ and CD8+ tumor-specific T cells. In addition, targeted therapy mediated cell death can release large amounts of tumor antigens and damage-associated molecular pattern molecules (DAMPs), which can augment antigen presentation by dendritic cells (DCs) to enhance the priming of cytotoxic T lymphocytes (CTLs) [15, 16] (Box 1). In accordance with these findings, the effect of anti-HER2 was demonstrated to depend on not only FcR-mediated phagocytosis, but also DC cross-priming through a MyD88-dependent mode in two distinct immunocompetent murine HER2 breast cancer models [15, 16]. Those two studies show that novel immune-mediated effects elicited by oncogenic receptor blockade by antibodies is essential for overall tumor control.
Box 1. Danger associated molecular patterns (DAMPs) signaling in targeted therapy.
Targeted agents interrupting oncogenic ignals may lead to cellular stress or cell apoptosis. This in turn results in the release of DAMPs and activates their corresponding pattern recognition receptors (PRRs). Multiple DAMP signal activation seems to be necessary to trigger an adaptive immune response. For example, active ATP secretion recognized by purinergic receptors P2Y2 and P2X7 have wide-ranging immunostimulatory functions across DCs, natural killer cells, macrophages, and T cells. High-mobility-group box 1 (HMGB1) release activates Toll-like receptor 2 (TLR2) and TLR4, which in turn triggers downstream MYD88 signaling in DCs and increased production of inflammatory cytokines. Nucleotide acids including DNA and RNA could also facilitate innate sensing of stressed tumor cells, which promote the production of type I interferon and increase cross-priming.
Epidermal growth factor receptor (EGFR), another oncogenic receptor over-expressed on tumor cells, can lead to more aggressive malignancies. The antitumor effect of cetuximab, an FDA approved anti-EGFR antibody, has been also considered to depend on its intrinsic oncogenic-signal blockade and antibody dependent cell-mediated cytotoxic effect (ADCC). Using a newly developed xenograft model with reconstituted immune cells in Rag mice, it was clearly demonstrated that the antitumor effect of cetuximab became more pronounced in immune reconstituted mice, and the EGFR(+) human tumor burden was reduced in the presence of an adaptive immune system [17, 30]. The EGFR antibody (cetuximab) can enhance the capacity of cross-priming by DC and augment the tumor specific T cell response [17, 30]. Consistently, utilizing a human colon cancer cell line, cetuximab was shown to promote opsonization and phagocytosis of colon cancer cells by human dendritic cells (DCs) that are subsequently engaged in antigen-cross presentation and CTL activation through both FcR and MyD88 pathways [30]. Notably, the cross-talk between NK mediated ADCC effect and DCs mediated antigen presentation can together contribute to activation of tumor antigen specific T cells [31, 32]. Cetuximab-activated NK cells promote DC maturation and CD8+ T-cell priming, leading to the spreading of tumor antigen and the activation of tumor-specific T cells in cetuximab-treated head and neck cancer patients [33]. It raises the possibility that the immune-cell cross-talk elicited by these tumor targeted mAbs could be essential for rejuvenating the tumor microenvironment and overall anti-tumor efficacy.
Bevacizumab, a distinct type of antibody, binds and sequesters vascular endothelial growth factor-A (VEGFA), a dominant molecule that is required for blood vessel growth. VEGF, produced by most tumors, not only plays an important role in tumor angiogenesis, but also, in a previously underappreciated role, inhibit DCs maturation, and promote the expansion of MDSCs and expression of PD-1 or other inhibitory checkpoints involved in CD8+ T cell exhaustion [34, 35]. Bevacizumab was shown to facilitate DCs maturation and increase DC population in peripheral blood from patients with lung, breast, and colorectal carcinoma [36]. In an experimental mouse model, anti-VEGF antibody significantly improved the number and function of DCs in draining lymph nodes and spleens of tumor-bearing mice. Consistent with these findings, a study carried out in a mouse model of breast cancer found that the blockade of VEGF led to increased maturation of DCs and inhibited infiltration of immune-suppressive cells like Tregs and MDSCs [37]. In addition, low doses of anti-VEGF can inhibit the transition of tumor-associated macrophages from an immune inhibitory M2-like phenotype toward an immune stimulatory M1-like phenotype to facilitate CD4+ and CD8+ T-cell tumor infiltration [38]. In total, these studies have demonstrated how targeted anticancer mAbs agents modulate the composition and function of the tumor microenvironment (TME) for control of the tumor.
Immunomodulatory effects of small-molecule inhibitors
Besides positive Ab-based targeted therapies, the discovery of mutated kinases as drug targets has motivated an intensive effort to develop small molecule kinase inhibitors for tumor treatment. Since the first kinase inhibitors were developed in the early 1980s, there are now more than 20 small molecule inhibitors approved by FDA and actively pursued as promising targeted therapeutics for killing cancer cells [39].
The first small molecule targeted agent, imatinib, was approved for the treatment of a chronic myeloid leukemia (CML) patient with the “Philadelphia chromosome (Ph)” mutation, which encodes the oncogenic fusion protein BCR-ABL1 [40]. As a tyrosine kinase inhibitor (TKI), imatinib was shown as having broader activity that inhibits the platelet-derived growth factor receptor-alpha (PDGFRα) and beta (PDGFRβ), and the stem cell receptor (c-Kit) [41, 42, 43]. For a long time, the therapeutic effects of imatinib had been predominantly attributed to the inhibition of signals for tumor-cell survival and proliferation. In a mouse model of spontaneous gastrointestinal stromal tumor (GIST), imatinib can relieve c-Kit dependent immunosuppression by Tregs within the tumor via a reduction of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO), which in turn can expand CD8+ T cells responses[19]. The antitumor effect of imatinib was abrogated by deleting CD8+ T cells and was enhanced by cytotoxic T lymphocyte-associated antigen (CTLA-4) blockade [19]. In accordance with these results, an imatinib-combined DC-based vaccine in an imatinib-resistant BCR-ABL negative lymphoma resulted in fewer metastases, with a decreased number of Tregs and increased T cell-derived IFNγ production, compared with monotherapy [39]. The complete mechanism for how imatinib for activates the immune response to inhibit tumor growth remains to be determined.
Second generation compounds dasatinib and nilotinib are used to treat imatinib-resistant CML, and are highly effective in patients who fail to response to imatinib, as well as in newly diagnosed patients [44]. Short-term treatment with dasatinib, another wide-spectrum TKI drug, can increase levels of tumor antigen-specific T cells which are crucial for its antitumor effect [41]. However, prolonged dasatinib treatment did not provide any enhanced antitumor effects or survival benefit compared with short-term dasatinib treatment in lung squamous cell cancer. Further studies revealed that antigen- specific T-cell responses can be impaired with the continuous presence of dasatinib [41], These studies suggest that the optimal dose and timing of dasatinib should be considered in order to avoid the development of immunosuppressive side effects.
Ibrutinib, is a confirmed irreversible inhibitor of Bruton’s tyrosine kinase (BTK) with outstanding clinical activity in relapsed/refractory CLL and mantle cell lymphoma [45, 46]. This drug also inhibits other tyrosine kinases, including interleukin-2-inducible T-cell kinase (ITK), an enzyme important for the survival of Th2 T cells [47], suggesting that it might have immunomodulatory effects in addition to direct anti-lymphoma effects. Indeed, in murine models of B cell lymphoma, intratumoral injection of ibrutinib combined with TLR9 agonist CpG induce complete tumor regression not only in the injected site, but also at distant sites [20]. Transplanting CD4 and CD8 T cells from animals treated with intratumoral CpG and ibrutinib to treatment-naive mice can prevent the outgrowth of tumors, indicating an essential role of the immune system involved in the therapeutic efficacy of ibrutinib [20]. Notably, ibrutinib can sculpt the antitumor T cell response mediated by PD1/PD-L1 blockade and lead to impressive therapeutic effects when combined with anti-PD-L1 in mouse models of lymphoma [48], This suggests that the combination of PD1 blockade and ibrutinib might also be effective even for patients with BTK negative tumors.
Analogous to monoclonal antibodies that target HER2, EGFR and VEGF, small molecule kinase inhibitors targeting these oncogenes also have important effects on anticancer immunity. For example, the dual ERBB1/ERBB2 kinase tyrosine inhibitor lapatinib, either alone or combined with doxorubicin, was shown to inhibit the growth of HER2-positive breast cancer with intensified tumor infiltration of IFNγ-producing CTLs. Further depletion experiments revealed that CD8+ but not CD4+ T cells are required for the antitumor effect when both drugs were administered [49]. It remains unclear which one actually induces the immune response for tumor regression. FDA-approved EGFR kinase inhibitors gefitinib and erlotinib have demonstrated a ~75% response rate for patients whose tumors harbor somatic mutations in exons encoding the tyrosine kinase domain of EGFR such as L858R [50, 51, 52]. In lung cancer cell lines, both gefitinib and erlotinib can strengthen immunosurveillance by up-regulating the expression of NKG2D ligands on tumor cells, which in turn enhance the susceptibility of malignant cells to NK cell mediated cytotoxicity [53, 54]. Whether the innate and adaptive antitumor responses are essential in gefitinib and erlotinib mediated tumor control remains to be determined.
Unlike bevacizumab, which targets extracellular VEGF, tyrosine kinase inhibitors sorafenib and sunitinib target the intracellular signaling pathways of VEGF receptors. Recently, it has been demonstrated that these inhibitors also alter the immune system [55, 56]. In both mouse models and RCC patients, sunitinib was shown to inhibit Stat3 in MDSCs, down-regulate angiogenic gene expression, and reduce tumor infiltrating Treg cells [57, 58]. Similarly, in vitro experiments proved that sunitinib can increase the apoptosis of MDSCs and reverse its immune suppression function [34]. Intriguingly, sunitinib treatment also resulted in reduced expression of interleukin (IL)-10, transforming growth factor-beta (TGFβ), and enhanced expression of Th1 cytokine IFNγ, and increased CTL responses in isolated tumor-infiltrating leukocytes [34]; therefore, sunitinib may favor an immune stimulatory environment. Although sorafenib shares many targets with sunitinib, it displays an almost opposite role in immune effects. Sorafenib, but not sunitinib, induced DC apoptosis and attenuated primary T cell responses in vivo. Consistently, sorafenib caused an irrecoverable inhibition of primary human T cell proliferation by targeting Lck phosphorylation, even after drug withdrawal [59]. Interestingly, axitinib, the most specific VEGFR inhibitor[60], does not cause the same attenuation of the host immune response as in sorafenib-treated hosts [61]. Therefore, axitinib might be the preferred potential candidate for exploring combinatorial treatment with immunotherapy.
The activating mutation BRAFV600E was originally reported in melanoma, but has subsequently been identified as a driver mutation in several other cancers, such as colon cancer and thyroid cancer [62, 63]. Selected targeting inhibitors including vemurafenib and dabrafenib have been tested in clinical trials for melanoma since 2008, and demonstrated high response rates and a significant prolongation in survival [63, 64]. There is accumulating evidence indicating that the therapeutic efficacy of BRAF inhibitors depends on their ability to generate a tumor specific immune response. Treatment with a BRAF inhibitor in patients with metastatic melanoma has a profound effect on the tumor microenvironment, with increased melanoma antigen expression, CD8+ T cell infiltration, and a decrease in immunosuppressive cytokines [21, 64, 65]. In murine melanoma tumor models, treatment with BRAF inhibitor correlated with reduced numbers of melanoma infiltrating Tregs and MDSCs, with a relative increase in CD40L and IFNγ expression on intratumoral TILs [66]. The depletion of CTLs and NK cells, or inhibition of IFNγ signaling, resulted in lose of tumor control and increased metastatic dissemination.
Supporting these data, line BRAF inhibitor PLX4720, a research analogue of vemurafenib, lost antitumor efficacy at controlling a metastatic BRAFV600E mutant melanoma tumor growth and metastasis dissemination while depleting CTLs or NK cells and neutralizing IFNγ [67]. Although the tumor infiltrating T cells in patients treated with BRAF inhibitors demonstrated an activated phenotype, the exhaustion markers Tim-3 and PD-1 were also up-regulated by treatment. Two weeks of the initiation of BRAF inhibitor treatment, such increase in PD-1 expression was coupled with a significant increase in the expression of its ligand PD-L1 within the tumor stroma cells [21]. Additional evidence indicated that the up-regulation of PD-L1 on melanoma may be due to IFNγ production by T cells, which represent one of immune events for acquired resistance to BRAF inhibition [68]. These data suggest that immune checkpoint blockade (particularly blockade of the PD-1 pathway) coupled with BRAF inhibitors may augment antitumor responses (Table 1).
| Drugs | Targets | First approval |
Indications | Impact on immune system |
Ref. |
|---|---|---|---|---|---|
| Antibody-based drugs | |||||
| Bevacizumab | VEGF | 2009 | Metastatic colorectal cancer, NSCLC, glioblastoma, metastatic kidney cancer |
Depletes TREG cells; Enhance DCs maturation and numbers in peripheral blood; Increase in T-cells and B- cells; |
[18, 19,20,21] |
| Cetuximab | EGFR | 2009 | Metastatic colorectal cancer, head and neck cancer |
Cetuximab-activated NK cells; Promote DC maturation and CD8(+) T-cell priming; |
[28, 29,30] |
| Trastuzumab | HER2 | 1998 | Breast cancer GIST |
Reduce circulating T regulatory cells (Tregs); Alter the balance between Tregs and Th2; Increasing the number of specific antitumor circulating CTLs; |
[6, 10,13] |
| Small-molecular drugs | |||||
| Gefitinib | EGFR | 2003 | NSCLC | Enhances NK cell cytotoxicity; Results in PD-L1 downregulation on tumor cells; |
[39,40.41] |
| Ibrutinib | BTK | 2013 | Transplantable murine lymphoma; Immortalized human T lymphocytes |
Promotes ICD; Robustly inhibits TH2 Polarization; |
[33,34,35,36] |
| Erlotinib | EGFR | 2004 | Metastatic NSCLC, locally advanced, unresectable or metastatic pancreatic cancer |
Results in increased susceptibility to NK-cell cytotoxicity; May favor the accumulation of MDSCs in cancer patients; |
[39,40.41,42,43] |
| Lapatinib | EGFR,ErbB2 | 2007 | Metastatic breast cancer |
Enhances T-cell and IFN- γ-based immunity; |
[38] |
| Sorafenib | Multiple tyrosine kinases |
2005 | Advanced RCC, unresectable HCC |
Favors the accumulation of peripheral CD4+NKG2D+T cells; Depletes tumorinfiltrating TREG cells; Depletes circulating TREG cells and MDSCs; |
[44,45,50] |
| Sunitinib | Multiple tyrosine kinases |
2006 | Advanced RCC, imatinib- resistant GIST |
Promotes an increase in the CTL/TREG-cell ratio Depletes TREG cells; Decreases levels of exhaustion markers amongst TILs; Favors the recruitment of CD3+ T cells to the tumor bed; Increase the apoptosis of MDSCs; |
[44,45,46,47] |
| Axitinib | VEGFRs, PDGFR, c-KIT |
2012 | RCC | Increases proportion of CD8+ T cells and reduced the proportion of myeloid-derived suppressor cells; Induces differentiation of monocytic MDSC toward an antigen-presenting phenotype; |
[25, 26, 27] |
| Vemurafenib Dabrafenib |
BRAF | 2011 | Unresectable or metastatic melanoma |
Favors tumor infiltration by CD4+ cells and CTLs; |
[51,52] |
| Trametinib | MEK | 2013 | Melanoma Transplantable murine CRC |
Increases tumor infiltration by CTLs; Inhibitory effects on T cell; Proliferation, pS6 proteins and cytokine expression; |
[58] |
| Dasatinib Nilotinib |
Multiple tyrosine kinases |
2006 | Resistant CML | Short-term treatment increase levels of tumor antigen-specific T cells; Prolonged treatment impaire antigen-specific T-cell responses; Differentially affect NK cell reactivity; |
[32,41] |
| Imatinib | KIT,PDGFRα | 2001 | CML GIST |
Promotes the expansion of circulating NK cells; Promotes the accumulation of BCR-ABLl-specific CTLs; Activates peripheral NK cells to produce IFNγ; Promotes tumor infiltration by CTLs and NK cells; Promotes an activatory crosstalk between DCs and NK cells; |
[24,25,26,27] |
Fine-tuning the immune system: Combination immunotherapies
The breakthrough of checkpoint blockers such as ipilimumab, the mAb blocking cytotoxic T lymphocyte-associated protein 4 (CTLA4), and pembrolizumab, the mAb blocking programmed cell death 1 (PD1) initiated a new era in cancer research and treatment. Clinical success of immune checkpoint blockers regulating T cell response showed long-term survival and durable antitumor responses in patients with metastatic melanoma as well as in patients with several other tumor types [69, 70, 71, 72]. Parallel to these advances in checkpoint blockade, the recent successes of chimeric antigen receptor (CAR) T cell therapy in lymphoma have reinstated the principle that immunotherapy can induce long-lasting responses and extend cancer patient survival [73]. This raises the possibility that proper combination of these immunotherapies with current cancer treatments could give a rise to long-term tumor control while eliciting active immune effectors that sometimes lead to curative responses.
The strengths and weaknesses of targeted agents raise the hypothesis that combining immunotherapy with targeted agents might have synergistic effects in cancer treatment. There is increasing evidence showing that host immunity contributes to the clinical therapeutic outcome of targeted therapies, suggesting the possibility that targeted therapies may help to optimize the antitumor immune response (Figure 2). The premise is that the rapid release of antigenic debris on tumor cell apoptosis induced by target agents may contribute to the enhancement of tumor antigen presentation. The targeted therapies can also promote favorable changes within the TME by directly modulating immune responses to improve immune-cell function. A number of these combination therapies are currently being investigated (Table 2).
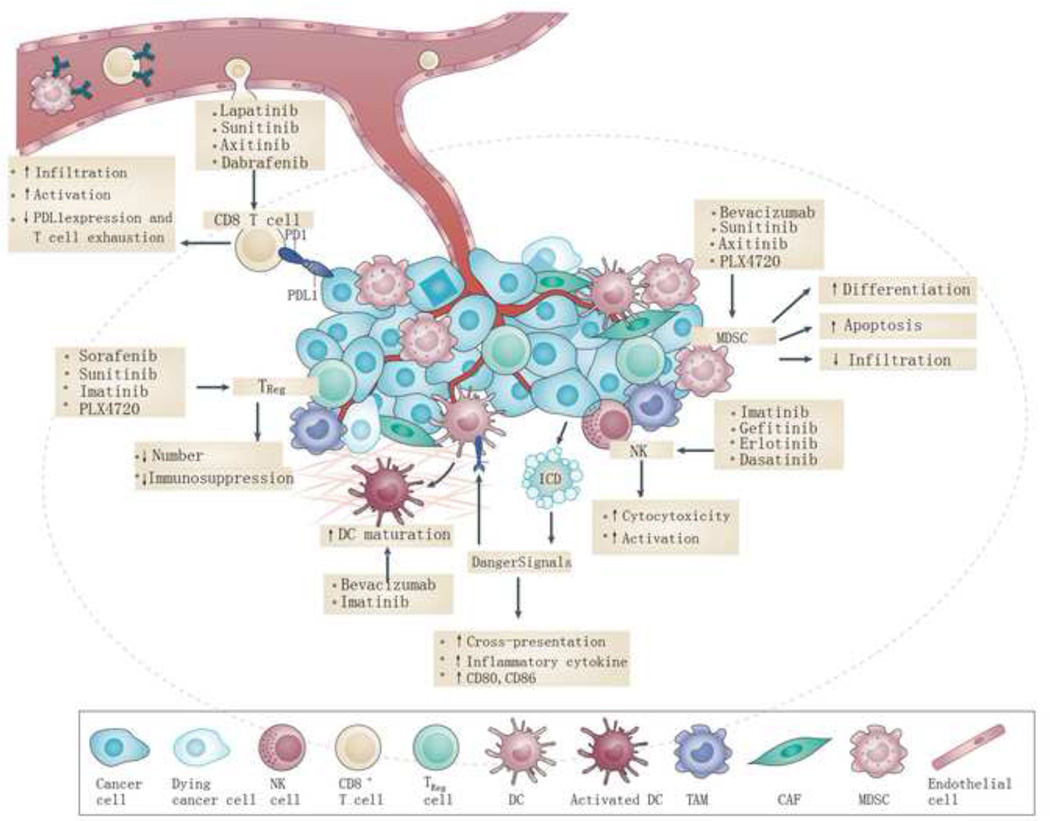
Figure 2. The effect of small molecule inhibitors on tumor microenvironment.
Targeted therapies have been shown to alter the TME in multiple ways, including direct and indirect effects. Some agents could reverse the immunosuppressive environment by inhibiting the function and infiltration of MDSCs and Tregs. Numerous therapies increase the expression of tumor antigens on the tumor cell surface, increasing cross priming of DCs for enhanced T cell activation. Therapies also can increase expression of NK cells expressing member D (NKG2D) ligands, which serve as co-stimulatory molecules for CTLs, as well as activators of NK cells, which also increase the cytotoxicity of NK cells.
| Combination | ClinicalTrials.gov Identifier | Combination | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Target therapy and checkpoint blockade | Targeted Therapy and Cytokine Therapy | ||
| Dabrafenib+/Trametinib+lpilimumab | (NCT01767454;NCT01940809; NCT01673854;NCT02200562) |
Vemurafenib+High-dose IL-2 | (NCT01754376; NCT01683188) |
| Nivolumab + Wunitinib, Pazopanib, or Ipilimumab |
(NCT01472081) | Vemurafenib+IL-2 (infusional 96 hour)+IFN-α |
(NCT01603212) |
| Dabrafenib+Trametinib followed by Ipilimumab+Nivolumab or vice versa |
(NCT02224781) | Vemurafenib+Pegylated IFN | (NCT01959633) |
| Vemurafenib+Anti−PD-Ll | (NCT01656642) | Nilotinib+ Peginterferon α2b | (NCT01657604; NCT01866553; NCT02001818; NCT01220648) |
| Dabrafenib+Trametinib+Anti−PD-1 | (NCT02130466) | Vemurafenib+High-dose IFN-α2b | (NCT01943422) |
| Trametinib+/− Dabrafenib+Anti−PD- L1 |
(NCT02027961) | Pegylated IFN-alpha 2B+ Dasatinib | (NCT01725204; NCT01872442) |
| Imatinib mesylate+lpilimumab | (NCT01738139) | ||
| lpilimumab+Cetuximab+/− Intensity- Modulated Radiation Therapy |
(NCT01935921) | ||
| Sunitinib +Nivolumab | (NCT02400385) | ||
| Pembrolizumab +Axitinib in advanced RCC |
(NCT02133742) | ||
| Dasatinib+lpilimumab | (NCT01643278) | ||
| Targeted Therapy and Cytokine Therapy | |||
| Vemurafenib+Tumor-infiltrating Lymphocytes |
(NCT00338377; NCT01585415; CT01659151) | ||
| Dasatinib+DC vaccine | (NCT01876212) | ||
| Vaccine-peptide derived from the protein IDO (IDO Long)+lpilimumab+ vemurafenib) |
(NCT02077114) | ||
| Cetuximab+ GVAX+ Cyclophosphamide | (NCT00305760) | ||
In a transgenic mouse model of breast cancer, mice that received a combination of neu-specific mAbs along with neu-targeted GM-CSF-secreting tumor vaccine had longer survival rates than those receiving either therapy alone. Such combined therapy increased the uptake of cancer cells by DCs, and enhanced the expression of co-stimulatory molecules such as CD40, CD80, and CD86, which contributed to the improvement of HER2-specific T cell function [74]. Since many EGFR+ tumors fail to respond to cetuximab, we wonder whether the lack of strong oncogenic signaling might limit cetuximab-trigger innate sensing. Therefore, extra TLR agonistic might provoke innate responses that link to T cell response. Indeed, cetuximab conjugated with CpG for increasing sensing, amplifying cross-priming for CTL response inside tumor tissues showed more potent antitumor effect than cetuximab alone. Armed cetuximab could lead to complete tumor regression and resistance to tumor re-challenge [17]. Notably, a recent study revealed that tumor-infiltrating activated CD8+ T cells could express VEGFR. Addition of VEGF-A after TCR engagement on CD8+ T cells increases expression of inhibitory checkpoints (PD-1, Tim-3, CTLA-4, and Lag-3). In the CT26 colon cancer model, a VEGFA antibody combined with anti-PD-1 intensified the infiltration of CD8+ T cells and down-regulated the expression of inhibitory receptors on CD8+ T cells [35]. Given the immunomodulatory properties of these targeted mAbs, incorporating immunotherapeutic strategies into treatment should provide many clinical benefits for synergies.
Thus, the development of antibody-based combination therapy or fusion proteins has attracted great interest. For example, HER2 positive tumor cells treated with trastuzumab up-regulated an inducible costimulatory molecule CD137 expression on human NK cells. An agonistic antibody targeting CD137 can synergize with trastuzumab to boost NK mediated cytotoxicity and trigger a potent tumor specific immune memory response as shown by protection from tumor re-challenge [16, 75]. Notably, the activity of anti-HER2 and anti-EGFR treatment relies on type I and type II interferon, which in turn promote MHC class I and PD-L1 expression on tumor cells [76]. Blockade of PD1/PD-L1 interaction synergizes with anti-HER2 mAb. Partly on the basis of these results, mAbs against HER2 and EGFR are currently being evaluated in clinical trials in combination with blockade of checkpoints CTLA-4 and PD-1, and with immunostimulatory cytokines such as IL-12 (NCT01860430, NCT02129556, NCT01468896). Although a combinatorial therapeutic approach remains promising, systemic administration of cytokines or immunomodulatory mAbs usually has severe dose-limiting toxicities, thereby preventing the further use in its therapeutically active dose range.
Antibody-based tumor targeting fusion protein with a preferential accumulation inside tumor tissue could provide a means to reduce the administered dose and consequently systemic side effects [77]. There have been developed several anti-HER2 based fusion proteins. For example, anti-HER2/neu–IL-2 caused significant tumor growth inhibition of murine intestinal tumor cells expressing human HER2/neu, while antibodies alone had no effect [78]. In a syngeneic tumor model, anti-EGFR mAb with IFNβ was more potent than the first generation of Ab for controlling Ab-resistant tumors [76]. The underlying mechanism of this strategy is that limited type I IFN in growing tumors might hinder antigen presentation inside tumor tissues. Ab-IFNβ therapy directly targets intratumoral dendritic cells, which reactivates CTL by increasing antigen cross-presentation within the tumor microenvironment. Although anti-EGFR-IFNβ achieved a more effective antitumor effect than anti-EGFR Ab alone, the host eventually relapsed even after complete regression in first 20–30 days, due to increased PD-L1 on tumor cells induced by IFNs. Therefore, blocking PD-L1 after Ab-IFN treatment can overcome treatment-acquired resistance and completely eradicates established tumors.
Combining immunotherapy to enhance the anti-tumor effect of targeted small-molecule inhibitors
Immunotherapy with two agents, interferon (IFN) α and interleukin-2 (IL-2), has shown promising activity in cancer therapies, especially in metastatic melanoma treatment [79]. Thus, another approach to extend or enhance the efficacy of target therapy for patients with BRAFV600 mutant melanoma is to combine immunotherapy such as IL-2 or IFNα with BRAF inhibitors such as vemurafenib or nilotinib to maintain the high frequency of tumor responses. These responses can be sustained during the entire therapy. Given the potential synergy, some phase I/II clinical trials of concurrent administration of vemurafenib or nilotinib with IL2 and/or IFNα are being conducted in melanoma patients (NCT01603212; NCT01959633; NCT01657604; NCT01866553; NCT02001818; NCT01220648).
Since there is strong preclinical evidence showing that combined therapy with the BRAFV600-specific inhibitor vemurafenib and Adaptive T cell transfer (ACT) resulted in superior antitumor effects against a fully syngeneic BRAFV600E mutant melanoma, several initial phase I/II trials of a combination of vemurafenib with ACT are being conducted (NCT02354690; NCT01585415; NCT01659151). Given preclinical reports suggesting that dasatinib serves as an effective adjuvant to peptide-based DC vaccination in an M05 (B16.OVA) melanoma mouse model, a phase I clinical study of dasatinib in combination with a DC-cell based vaccine loaded with tumor blood vessel Ag (TBVA) in metastatic melanoma patients is currently ongoing (NCT01876212). A phase I clinical study for targeting IDO by a synthetic peptide vaccine in combination with ipilimumab or vemurafenib in metastatic melanoma patients was recently completed (NCT02077114). In addition, GVAX, a granulocyte-macrophage colony- stimulating factor (GM-CSF) gene-transfected tumor cell vaccine has been given together with cyclophosphamide and cetuximab in treating patients with metastatic or locally advanced pancreatic cancer in a phase II trial (NCT00305760). A more complete understanding of the impact of these small-molecule inhibitors on immune cells regarding optimizing dose, sequence, and timing will be helpful when designing and integrating these agents with immunotherapy in clinical trials (Box 2).
Box 2. Timing parameters of specific targeted therapies when combining with immunotherapies.
The optimal timing and sequence of combination therapy is essential for better clinical outcome. Targeted agents that can boost antigen presentation and initiate T cell priming should be administered before vaccines or checkpoint blockade. Transient drug delivery should be considered if the drug has a deleterious effect on effector T cell functions. Agents promoting T cell memory or enhancing T cell function should be given after T cell priming, and continued throughout treatment to amplify the antitumor immune response. Agents mitigating the tumor suppressive microenvironment should be administrated before immunotherapy and continued throughout treatment.
Concluding remarks
The outcome of the targeted therapy is dictated by not only cell-intrinsic effects, but also by cell-extrinsic immune-mediated cytotoxic effects. The complex interplay between cell intrinsic and extrinsic effects will ultimately dictate the magnitude of the tumor response to targeted therapy. First, therapy-mediated tumor cell death releases DAMPs and tumor antigens that could be presented by APC to drive tumor-specific adaptive immune responses. Interestingly, recent evidence established an unexpected and essential role of type I IFN signaling in multiple therapies. One mechanistic study revealed that DNA or RNA derived from tumor tissue could be the source driving type I IFNs production for bridging innate and T cell responses. However, the regulation, function, and importance of the type I IFNs pathway in targeted therapy is largely unknown and need to be dissected.
Targeted therapy can also alter the immunosuppressive cytokines milieu of tumor cells and enhance infiltration of immune effector cells. One example is that BRAF inhibition reduced the immunomodulatory cytokines IL-10, VEGF, and IL-6. In this case, CTLs could be actively recruited to tumor tissue. However, oncogenic programs, such as BRAF, can still evade immunesurveillance through PDL1 expression. Tumor samples from patients treated with BRAF inhibitors with increased T-cells infiltration also showed increase expression of T cell exhaustion markers like TIM3, PD-1 and PD-L1. It is possible that BRAF inhibitors activate the c-JUN and STAT3 signaling pathway for PD-L1 expression. These immunosuppressive pathways or molecules like PD-L1 can allow tumor cells to escape immune detection. It is worth noting that high dosing of target therapy could exert deleterious effect on T-cell function. Therefore, it is important to consider the optimal dose, order of treatments and timing of targeted therapy when designing clinical trials for combinational therapy in order to maximize the overall antitumor efficacy and minimize the toxicity profiles. Among these factors, the administration schedule or timing may be the major determinant for overall antitumor effect and more mechanistic studies in animal models and clinical trials are needed to fully generate effective antitumor responses. It is worth noting that high dosing of target therapy could exert a deleterious effect on T-cell function. Therefore, the important parameters regarding optimizing dose, sequence, and timing of targeted therapy should be considered when designing clinical trials for combinational therapy, in order to maximize the overall antitumor efficacy and minimize the toxicity profiles. Among these factors, the administration schedule or timing may be the major determinant for overall antitumor effect and more mechanistic studies in animal models
Trend Box.
The adaptive immune system can play an essential role in the efficacy of targeted therapies.
Promotion of DCs/NKs maturation and activation by targeted therapies that inhibit oncogenic pathways can positively impact the generation of effective tumor-reactive CTLs
Current targeted oncology therapies can synergize with immunotherapy approaches aimed at activating or re-activating tumor-reactive T cells.
Different targeted therapies have varied effects on immune cells in the tumor, and in some cases have systemic impact on the patient’s immune system.
The treatment course for cancer patients should be considered to prevent adversely promoting immunosuppression and/or dampening antitumor immune responses.
The immune system could play a role in the emergence of resistance to targeted therapies
Outstanding Questions.
What is the contribution of DAMPs released by cancer cells after targeted therapy to the overall anti-tumor immune response? What PRRs detect these DAMPs, and what are the cell types involved?
How can one avoid severe toxicity upon treating patients with combinations of targeted therapies and immunotherapy? Can administration of low-dose targeted therapy prior to immunotherapy reduce or avoid toxic side effects? The optimal timing and sequence of combination therapies may be essential for improved clinical outcome.
What roles IFNs play in the response to targeted therapies?
Does the dose of specific targeted therapies impact the function of T cells, both systemically and at the tumor site? Can high doses of specific targeted therapies result in deleterious effects on T cells, and if so, what are these doses?
How can the anti-tumor immune response to combination therapies be monitored in real-time in individual patients, to inform treatment decisions?
What mechanisms underlie resistance to immunotherapies? Are these cell-intrinsic mechanisms or do they rely on changes to the tumor microenvironment?
Do immunosuppressive cells such as MDSC and Treg cells influence the outgrowth of tumors resistant to targeted therapies?
Acknowledgements
We thank Daryl Harmon and Jian Qiao for helpful editing and discussions. This work was in part supported by the U.S. National Institutes of Health through National Cancer Institute grants CA141975
Glossary
- HER2/neu
A tyrosine kinase related to the epidermal growth factor receptor, t has a role in the pathogenesis of breast cancer and is a target of treatment (with the monoclonal antibody trastuzumab and the small molecule inhibitor lapatinib) in 25 percent of patients with breast cancer in which HER2/neu is overexpressed. Overexpression of HER2/neu is associated with disease recurrence and worsen prognosis. HER2 is so named because it has a similar structure to human epidermal growth factor receptor (HER1). neu is so named because it was derived from a neuroglioblastoma cell line. ErbB belongs to the EGFR receptor family. erbB1 (EGFR/HER-1), erbB2 (HER-2), erbB3 (HER-3), and erbB4 (HER-4) are the four members that comprise this receptor family
- EGFR
Also known as HER-1, EGFR (epidermal growth factor receptor) belongs to a family of receptors – HER-2 HER-3, HER-4 are other members of the family – and binds to the EGF TGF-α, and other related proteins, leading to the generation of proliferative and survival signals within the cell. It also belongs to the larger family of tyrosine kinase receptors and is generally overexpressed in several solid tumors of epithelial origin
- TKI
Molecules that inhibit the activity of tyrosine kinase receptors. They are small molecules developed to inhibit the binding of ATP to the cytoplasmic region of the receptor (eg, gefitinib), thus further blocking the cascade of reactions that is activated by the pathway
- Somatic mutation
A change in the genotype of a cancer cell. This is distinguished from a germline mutation, which is a change in the genotype of all the normal cells in a patient's body. Germline mutations may be passed to offspring, but somatic mutations may not
- BRAF V600E
V600E is the most common oncogenic mutation of BRAF in cancer. A V600E amino acid change results in constitutive activation of the BRAF kinase and promotes cell transformation
- PI3K-PTEN-AKT pathway
Signal transduction pathways involving the signaling molecules phosphatidylinositol-3 kinase (PI3K), PTEN, and Akt. PI3K generates phosphorylated inositides at the cell membrane, which are required for the recruitment and activation of the serine kinase Akt. PTEN is a lipid phosphatase which counteracts the effect of PI3K. Accordingly, mutated PI3K and AKT act as dominant oncogenes, while PTEN is a tumor
- BRAF
BRAF is an isoform of RAF. RAF proteins (Raf-1, A-Raf, B-Raf) are intermediate to Ras and MAPK in the cellular proliferative pathway. Raf proteins are typically activated by Ras via phosphorylation, and activated Raf proteins in turn activate MAPK via phosphorylation. However, Raf proteins may also be independently activated by other kinases
- VEGF
VEGF (vascular endothelial growth factor) is a cytokine that mediates numerous functions of endothelial cells including proliferation, migration, invasion, survival, and permeability. VEGF naturally occurs as a glycoprotein and is critical for angiogenesis. Many tumors overexpress VEGF, which correlates to poor prognosis. VEGF-A, -B, -C, -D, and -E are members of the larger family of VEGF-related proteins
- KIT
A member of the PDGFR family, c-kit is a tyrosine kinase receptor that dimerizes following ligand binding and is autophosphorylated on intracellular tyrosine residues
- PDGFRA and PDGFRB
The receptor for PDGF exists distinctly as the dimeric αα or ββ form. All dimer combinations of PDGF A and B signal through PDGFR-αα. PDGF BB signals through PDGFR-β. PDGF CC signals through the αα and αβ receptors. PDGF DD signals through the ββ and αβ receptors.
- BCR-ABL
BCR-ABL is a constitutively activated tyrosine kinase that arises from the formation of the Philadelphia chromosome. This enzyme is a characteristic molecular abnormality present in almost all cases of chronic myeloid leukemia
- CML
Chronic melanoma leukemia CML is a cancer of the white blood cells. It is a form of leukemia characterized by the increased and unregulated growth of predominantly myeloid cells in the bone marrow and the accumulation of these cells in the blood. CML is a clonal bone marrow stem cell disorder in which a proliferation of mature granulocytes (neutrophils, eosinophils, and basophils) and their precursors are found. It is a type of myeloproliferative disease associated with a characteristic chromosomal translocation called the Philadelphia chromosome
- GIST
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract. GISTs arise in the smooth muscle pacemaker interstitial cell of Cajal or similar cells. They are defined as tumors whose behavior is driven by mutations in the KIT gene (85%), PDGFRA gene (10%), or BRAF kinase (rare). 95% of GISTs stain positively for KIT (CD117).
- BTK
Bruton's tyrosine kinase (abbreviated Btk or BTK), also known as tyrosine-protein kinase, BTK is an enzyme in humans that is encoded by the BTK gene. BTK is a kinase that plays a crucial role in B-cell development. BTK contains a PH domain that binds phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 binding induces Btk to phosphorylate phospholipase C, which in turn hydrolyzes PIP2, a phosphatidylinositol, into two second messengers, inositol triphosphate (IP3) and diacylglycerol (DAG), which then go on to modulate the activity of downstream proteins during B-cell signaling
- ITK
Tyrosine-protein kinase ITK/TSK, also known as interleukin-2-inducible T-cell kinase, or simply ITK, is a protein that in humans is encoded by the ITK gene. ITK is a member of the TEC family of kinases and is highly expressed in T cells
- GVAX
GVAX is a cancer vaccine composed of whole tumor cells genetically modified to secrete the immune stimulatory cytokine, granulocyte-macrophage colony-stimulating factor (GM-CSF), and then irradiated to prevent further cell division. The product exists as both autologous (patient specific) and allogeneic (non-patient specific) therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Baselga J. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Larson RA. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 5.Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Joensuu H. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 6.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, Bardelli A. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, Pao W. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 8.Diaz LA, Jr, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, Vogelstein B. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–540. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loibl S, von Minckwitz G, Schneeweiss A, Paepke S, Lehmann A, Rezai M, Denkert C. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (her2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol. 2014;32:3212–3220. doi: 10.1200/JCO.2014.55.7876. [DOI] [PubMed] [Google Scholar]
- 10.Misale S, Arena S, Lamba S, Siravegna G, Lallo A, Hobor S, Bardelli A. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med. 2014;6:224ra226. doi: 10.1126/scitranslmed.3007947. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, Fletcher JA. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764–4774. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 12.Boussen H, Cristofanilli M, Zaks T, DeSilvio M, Salazar V, Spector N. Phase II study to evaluate the efficacy and safety of neoadjuvant lapatinib plus paclitaxel in patients with inflammatory breast cancer. J Clin Oncol. 2010;28:3248–3255. doi: 10.1200/JCO.2009.21.8594. [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Schadendorf D. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 14.Corcoran RB, Atreya CE, Falchook GS, Kwak EL, Ryan DP, Bendell JC, Kopetz S. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol. 2015;33:4023–4031. doi: 10.1200/JCO.2015.63.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Fu YX. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Smyth MJ. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108:7142–7147. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Zhang X, Mortenson ED, Radkevich-Brown O, Wang Y, Fu YX. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol Ther. 2013;21:91–100. doi: 10.1038/mt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A, Smyth MJ. Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest. 2015 doi: 10.1172/JCI66236. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, DeMatteo RP. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagiv-Barfi I, Kohrt HE, Burckhardt L, Czerwinski DK, Levy R. Ibrutinib enhances the antitumor immune response induced by intratumoral injection of a TLR9 ligand in mouse lymphoma. Blood. 2015;125:2079–2086. doi: 10.1182/blood-2014-08-593137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Wargo JA. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albiges L, Fay AP, Xie W, Krajewski K, McDermott DF, Heng DY, Choueiri TK. Efficacy of targeted therapies after PD-1/PD-L1 blockade in metastatic renal cell carcinoma. Eur J Cancer. 2015 doi: 10.1016/j.ejca.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, Ribas A. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015;7:279ra241. doi: 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baselga J, Albanell J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann Oncol. 2001;12(Suppl 1):S35–S41. doi: 10.1093/annonc/12.suppl_1.s35. [DOI] [PubMed] [Google Scholar]
- 25.Baselga J. The EGFR as a target for anticancer therapy--focus on cetuximab. Eur J Cancer. 2001;37(Suppl 4):S16–S22. doi: 10.1016/s0959-8049(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 26.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Coudert B. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benavides LC, Gates JD, Carmichael MG, Patil R, Holmes JP, Hueman MT, Peoples GE. The impact of HER2/neu expression level on response to the E75 vaccine: from U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2009;15:2895–2904. doi: 10.1158/1078-0432.CCR-08-1126. [DOI] [PubMed] [Google Scholar]
- 28.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 30.Correale P, Botta C, Cusi MG, Del Vecchio MT, De Santi MM, Gori Savellini G, Tagliaferri P. Cetuximab +/− chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer. 2012;130:1577–1589. doi: 10.1002/ijc.26181. [DOI] [PubMed] [Google Scholar]
- 31.Moretta L, Ferlazzo G, Bottino C, Vitale M, Pende D, Mingari MC, Moretta A. Effector and regulatory events during natural killer-dendritic cell interactions. Immunol Rev. 2006;214:219–228. doi: 10.1111/j.1600-065X.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Lou Y, Lizee G, Qin H, Liu S, Rabinovich B, Hwu P. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, Ferris RL. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alfaro C, Suarez N, Gonzalez A, Solano S, Erro L, Dubrot J, Perez-Gracia JL. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer. 2009;100:1111–1119. doi: 10.1038/sj.bjc.6604965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Terme M. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Morse MA. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roland CL, Lynn KD, Toombs JE, Dineen SP, Udugamasooriya DG, Brekken RA. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One. 2009;4:e7669. doi: 10.1371/journal.pone.0007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Poznansky MC. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu P, Nielsen TE, Clausen MH. Small-molecule kinase inhibitors: an analysis of FDA-approved drugs. Drug Discov Today. 2015 doi: 10.1016/j.drudis.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Koretzky GA. The legacy of the Philadelphia chromosome. J Clin Invest. 2007;117:2030–2032. doi: 10.1172/JCI33032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carroll M, Ohno-Jones S, Tamura S, Buchdunger E, Zimmermann J, Lydon NB, Druker BJ. CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood. 1997;90:4947–4952. [PubMed] [Google Scholar]
- 42.Dewar AL, Cambareri AC, Zannettino AC, Miller BL, Doherty KV, Hughes TP, Lyons AB. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005;105:3127–3132. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]
- 43.Melnick JS, Janes J, Kim S, Chang JY, Sipes DG, Gunderson D, Caldwell JS. An efficient rapid system for profiling the cellular activities of molecular libraries. Proc Natl Acad Sci U S A. 2006;103:3153–3158. doi: 10.1073/pnas.0511292103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marin D, Rotolo A, Milojkovic D, Goldman J. The next questions in chronic myeloid leukaemia and their answers. Curr Opin Hematol. 2013;20:163–168. doi: 10.1097/MOH.0b013e32835dd922. [DOI] [PubMed] [Google Scholar]
- 45.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, O'Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Blum KA. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, Byrd JC. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A. 2015;112:E966–E972. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannesdottir L, Tymoszuk P, Parajuli N, Wasmer MH, Philipp S, Daschil N, Doppler W. Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur J Immunol. 2013;43:2718–2729. doi: 10.1002/eji.201242505. [DOI] [PubMed] [Google Scholar]
- 50.Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol. 2005;23:2556–2568. doi: 10.1200/JCO.2005.07.799. [DOI] [PubMed] [Google Scholar]
- 51.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 52.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 53.He S, Yin T, Li D, Gao X, Wan Y, Ma X, Wang Y. Enhanced interaction between natural killer cells and lung cancer cells: involvement in gefitinib-mediated immunoregulation. J Transl Med. 2013;11:186. doi: 10.1186/1479-5876-11-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim H, Kim SH, Kim MJ, Kim SJ, Park SJ, Chung JS, Kang CD. EGFR inhibitors enhanced the susceptibility to NK cell-mediated lysis of lung cancer cells. J Immunother. 2011;34:372–381. doi: 10.1097/CJI.0b013e31821b724a. [DOI] [PubMed] [Google Scholar]
- 55.Seliger B, Massa C, Rini B, Ko J, Finke J. Antitumour and immune-adjuvant activities of protein-tyrosine kinase inhibitors. Trends Mol Med. 2010;16:184–192. doi: 10.1016/j.molmed.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Porta C, Paglino C, Imarisio I, Ganini C, Pedrazzoli P. Immunological effects of multikinase inhibitors for kidney cancer: a clue for integration with cellular therapies? J Cancer. 2011;2:333–338. doi: 10.7150/jca.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, Bukowski R. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 59.Zhao W, Gu YH, Song R, Qu BQ, Xu Q. Sorafenib inhibits activation of human peripheral blood T cells by targeting LCK phosphorylation. Leukemia. 2008;22:1226–1233. doi: 10.1038/leu.2008.58. [DOI] [PubMed] [Google Scholar]
- 60.Zakharia Y, Zakharia K, Rixe O. Axitinib: from preclinical development to future clinical perspectives in renal cell carcinoma. Expert Opin Drug Discov. 2015;10:925–935. doi: 10.1517/17460441.2015.1045411. [DOI] [PubMed] [Google Scholar]
- 61.Stehle F, Schulz K, Fahldieck C, Kalich J, Lichtenfels R, Riemann D, Seliger B. Reduced immunosuppressive properties of axitinib in comparison with other tyrosine kinase inhibitors. J Biol Chem. 2013;288:16334–16347. doi: 10.1074/jbc.M112.437962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 63.Rahman MA, Salajegheh A, Smith RA, Lam AK. Multiple proliferation-survival signalling pathways are simultaneously active in BRAF V600E mutated thyroid carcinomas. Exp Mol Pathol. 2015;99:492–497. doi: 10.1016/j.yexmp.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 65.Khalili JS, Liu S, Rodriguez-Cruz TG, Whittington M, Wardell S, Liu C, Lizee G. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18:5329–5340. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steinberg SM, Zhang P, Malik BT, Boni A, Shabaneh TB, Byrne KT, Turk MJ. BRAF inhibition alleviates immune suppression in murine autochthonous melanoma. Cancer Immunol Res. 2014;2:1044–1050. doi: 10.1158/2326-6066.CIR-14-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrari de Andrade L, Ngiow SF, Stannard K, Rusakiewicz S, Kalimutho M, Khanna KK, Smyth MJ. Natural killer cells are essential for the ability of BRAF inhibitors to control BRAFV600E–mutant metastatic melanoma. Cancer Res. 2014;74:7298–7308. doi: 10.1158/0008-5472.CAN-14-1339. [DOI] [PubMed] [Google Scholar]
- 68.Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 69.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Rosenberg SA. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 72.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim PS, Armstrong TD, Song H, Wolpoe ME, Weiss V, Manning EA, Jaffee EM. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;118:1700–1711. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Levy R. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, Fu YX. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu EF, Gai SA, Opel CF, Kwan BH, Surana R, Mihm MC, Wittrup KD. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell. 2015;27:489–501. doi: 10.1016/j.ccell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Penichet ML, Dela Cruz JS, Shin SU, Morrison SL. A recombinant IgG3-(IL-2) fusion protein for the treatment of human HER2/neu expressing tumors. Hum Antibodies. 2001;10:43–49. [PubMed] [Google Scholar]
- 79.Payne MJ, Argyropoulou K, Lorigan P, McAleer JJ, Farrugia D, Davidson N, Middleton MR. Phase II pilot study of intravenous high-dose interferon with or without maintenance treatment in melanoma at high risk of recurrence. J Clin Oncol. 2014;32:185–190. doi: 10.1200/JCO.2013.49.8717. [DOI] [PubMed] [Google Scholar]