Abstract
The serotonin (5-hydroxytryptamine, 5-HT) system plays an important role in stress-related psychiatric disorders and substance abuse. Stressors and stress hormones can inhibit the dorsal raphe nucleus (DRN)-5-HT system, which composes the majority of forebrain-projecting 5-HT. This inhibition is mediated via stimulation of GABA synaptic activity at DRN-5-HT neurons. Using swim stress-induced reinstatement of morphine conditioned place-preference, recent data from our laboratory indicate that morphine history sensitizes DRN-5-HT neurons to GABAergic inhibitory effects of stress. Moreover, GABAA receptor-mediated inhibition of the serotonergic DRN is required for this reinstatement. In our current experiment, we tested the hypothesis that GABAergic sensitization of DRN-5-HT neurons is a neuroadaptation elicited by multiple classes of abused drugs across multiple models of stress-induced relapse by applying a chemical stressor (yohimbine) to induce reinstatement of previously extinguished cocaine self-administration in Sprague-Dawley rats. Whole-cell patch-clamp recordings of GABA synaptic activity in DRN-5-HT neurons were conducted after the reinstatement. Behavioral data indicate that yohimbine triggered reinstatement of cocaine self-administration. Electrophysiology data indicate that 5-HT neurons in the cocaine group exposed to yohimbine had increased amplitude of inhibitory postsynaptic currents compared to yoked-saline controls exposed to yohimbine or unstressed animals in both drug groups. These data, together with previous findings, indicate that interaction between psychostimulant or opioid history and chemical or physical stressors may increase postsynaptic GABA receptor density and/or sensitivity in DRN-5-HT neurons. Such mechanisms may result in serotonergic hypofunction and consequent dysphoric mood states which confer vulnerability to stress-induced drug reinstatement.
Keywords: Addiction, Cocaine, Electrophysiology, GABA, Stress-induced reinstatement, The dorsal raphe nucleus (DRN)-5-HT system
1. Introduction
Drug addiction is characterized by repeated relapse to drug use even after a prolonged period of abstinence. Stress, in addition to re-exposure to drug and exposure to drug-associated environmental cues, is a particularly potent trigger of relapse (Sinha, 2008). Stress can also reinstate drug-seeking behavior in animal models of drug abuse (Shaham et al., 2003), which are applied to understand the neurobiological mechanisms underlying stress-induced drug relapse.
The dorsal raphe nucleus (DRN) serotonin (5-hydroxytryptamine, 5-HT) system has been long known to play a critical role in stress and stress-related disorders (Charney et al., 1990), and recently its role in reward-seeking behavior and drug abuse captured a great deal of attention (Kirby et al., 2011). For example, optogenetic activation of DRN neurons produces reward-related behaviors (Liu et al., 2014; McDevitt et al., 2014) and modifies motivation to acquire rewards (Miyazaki et al., 2014). Moreover, major classes of drugs of abuse all modulate the 5-HT system (Kirby et al., 2011), and dysfunction of the 5-HT system is believed to mediate some of the psychological symptoms of withdrawal, e.g. anxiety and depression (Parsons et al., 1995; Tao et al., 1998; Weiss et al., 1996), as well as high levels of impulsivity (Cunningham and Anastasio, 2014), which may increase the vulnerability to relapse.
Exposure to stress can elicit the release of the stress neurohormone, corticotropin-releasing factor (CRF) into the DRN (Price et al., 2002). CRF regulates DRN-5-HT neurons in a bidirectional and CRF receptor subtype-specific manner (Kirby et al., 2000; Valentino et al., 2010). Although there is evidence that 24 h after exposure to swim stress, DRN-5-HT neurons exhibited increased excitability (Lamy and Beck, 2010), acute stress as well as intra-DRN injection of low doses of CRF primarily inhibit DRN-5-HT activity (Kirby et al., 2000; Price et al., 2002). Additional support for these findings come from the observation that the neuronal excitability of DRN-5-HT neurons is decreased in Wistar-Kyoto rats, a stress hyperresponsive strain (Lemos et al., 2011). Moreover, anatomical and electrophysiological studies show that this inhibitory effect is indirectly mediated by GABAergic interneurons in the DRN (Kirby et al., 2008; Roche et al., 2003).
Recent emerging evidence shows that GABA transmission in DRN mediates drug-related stress and stress-induced reinstatement. For example, infusion of GABA agonists into the DRN reduces cocaine-induced anxiety (Ettenberg et al., 2011) and escalates alcohol-heightened aggression (Takahashi et al., 2010). Using a swim stress-induced reinstatement of conditioned place preference (CPP) model, data from our laboratory indicate that morphine history sensitizes DRN-5-HT neurons to the GABAergic inhibitory effects of swim stress (Staub et al., 2012). Moreover, we provided direct evidence that stimulation of GABA signaling in the DRN is both necessary and sufficient for swim stress-induced reinstatement of morphine CPP (Li et al., 2013). Collectively, our data suggest that subjects with morphine history may be more vulnerable to the effects of stress on DRN circuits. However, it is not known whether GABAergic sensitization of DRN-5-HT neurons is a neuroadaptation elicited only by an interaction between morphine exposure and swim stress or whether it is more broadly related to stress-induced drug reinstatement. Moreover, although CPP model is widely used for inferring the hedonic value of addictive drugs, questions remain whether a non-contingent paradigm is an adequate procedure to represent active drug-seeking behaviors that characterize drug addiction in humans (Sanchis-Segura and Spanagel, 2006). Therefore, in our current study we tested GABA synaptic activity in DRN-5-HT neurons by whole-cell patchclamp electrophysiology in brain slices from rats exposed to the chemical stressor yohimbine to induce reinstatement of previously extinguished cocaine self-administration (Feltenstein and See, 2006). Self-administration provides contingent drug exposure and can measure voluntary drug intake in animals, thus better representing addiction-like behavior and resolving the limitation of CPP (Sanchis-Segura and Spanagel, 2006).
2. Experimental procedures
2.1. Subjects
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing 180–200 g upon arrival in the laboratory were housed 2 per cage under standard temperature (20 °C) and humidity (40%) on a 12/12 h light/dark reverse cycle (lights off at 9:00 AM). After 7–8 days of acclimation, rats received i.v. catheter surgeries, and were singly housed throughout the rest of experiment. Food and water were provided ad libitum. All subjects were observed and/or weighed daily to assess general health and responsiveness to drug exposure. Animal protocols were approved by the Temple University Institutional Animal Care and Use Committee and were conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
2.2. Behavior
2.2.1. Surgery
Under isoflurane anesthesia (2–3% in O2; Piramal Healthcare limited, Andhra Pradesh, India), rats were implanted with a silastic i.v. catheter (CamCaths, Cambridgeshire, UK; tubing length 3 cm) into the right jugular vein as previously described (Li and Frantz, 2009). During recovery, catheters were flushed with 0.1 ml saline containing Baytril (Bayer, Shawnee Mission, KS, 0.2 mg/ml) and heparin (Sagent Pharmaceuticals, Schaumburg, IL; 100 USP units/1 ml) twice daily for the first two days post-surgery, then once daily throughout the rest of the experiment (after daily self-administration sessions). Catheter patency was confirmed in all subjects by full loss of muscle tone within 5 s of i.v. infusion of the short acting anesthetic agent, 1% Methohexital sodium Pharmaceuticals, Parsippany, NJ) one day before the first and after the last self-administration session. Subjects that failed either patency test were eliminated from the study.
2.2.2. Drugs
Cocaine HCl (generously supplied by the National Institute of Drug Abuse drug supply program) was dissolved in saline (2 mg/ml) and infused in a volume of 0.25 ml/kg/infusion (i.v.). Yohimbine (Sigma-Aldrich, St. Louis, MO) was dissolved in distilled water (2.5 mg/ml) and injected in a volume of 1 ml/kg (i.p.). Doses were chosen based on their efficacy in this model as described previously (Feltenstein and See, 2006).
2.2.3. Equipment
Behavioral tests were conducted in operant conditioning chambers enclosed in sound-attenuating, ventilated environmental cubicles (Med Associates, Inc., St. Albans, VT). To start each session, a house light turned on and two levers extended into the chamber. Lever presses on the inactive lever were recorded but had no scheduled consequences. Presses on the active lever triggered a syringe pump (Med Associates, Inc., St. Albans, VT) to deliver drug solution via a stainless steel swivel (Instech Laboratories, Inc., Plymouth Meeting, PA) and polyethylene tubing attached to a catheter portal on each animal’s back. Each reinforced response lit a cue light above the lever for 2 s. Drug delivery, the cue light and house light were not present during a 20 s time-out (TO20) after each infusion. Drug delivery and data collection were controlled by Med Associates software (Med PC IV).
2.2.4. Cocaine self-administration, extinction and stress-induced reinstatement
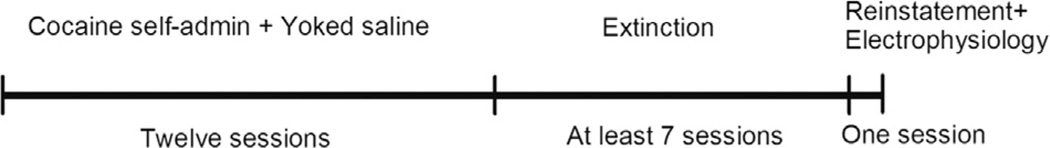
Timeline of the experimental design is shown in Figure 1. Following a 6–7-day post-surgical recovery, rats were trained to self-administer cocaine in daily 2 h sessions over 12 days conducted during the dark phase of the light–dark cycle. Lever-pressing on the active lever was reinforced by i.v. infusion of cocaine (0.5 mg/kg/ infusion) under a Fixed-Ratio 1 (FR1) TO20 schedule of reinforcement. The volume of cocaine solution for each rat was titrated according to body weight (0.044 ml/kg/s)
Figure 1.
Experimental design time-line for cocaine self-administration, extinction and yohimbine stress-induced reinstatement. Electrophysiology recordings were conducted immediately following stress-induced reinstatement test.
Following cocaine self-administration rats underwent daily 2 h extinction. During extinction, rats were connected to the metal coil tether but not the infusion tubing. Only the house light remained on throughout the session. No consequences were presented after presses on either lever.
Once extinction criterion was reached (a minimum of seven extinction sessions and maintained ≤ 15 active lever responses over two consecutive days), chemical stress-induced reinstatement of cocaine seeking was conducted on the following day. Rats received an injection (i.p.) of either yohimbine (2.5 mg/kg), widely used as an α2-adrenergic receptor antagonist that increases noradrenaline release but also has moderate affinity for other types of receptors (see details in discussion) (Millan et al., 2000) and produces anxiety in both humans (Lader and Bruce, 1986) and animals (Johnston and File, 1989), or saline 30 min prior to a 2 h reinstatement session. Parameters for stress-induced reinstatement sessions were identical to extinction sessions, i.e. no drug-paired cues were presented.
2.2.5. Yoked saline controls
A subset of controls received yoked saline infusions in this experiment. Lever presses by these subjects did not produce any scheduled consequences in any session. However, during self-administration, they received an infusion of saline accompanied by cues and time-out sequences whenever their self-administering counterparts received a cocaine infusion. They also received an injection (i.p.) of either yohimbine or saline 30 min prior to the reinstatement session.
2.2.6. Electrophysiology
Immediately following the reinstatement test, animals were euthanized and brain slices prepared for electrophysiology as described previously (Kirby et al., 2008). Subjects were euthanized by rapid decapitation and brains were placed into ice-cold artificial cerebrospinal fluid (ACSF) in which sucrose (248 mM) was substituted for NaCl. Slices (250 µm thick) were cut through the DRN using a Vibratome 3000 Plus (Vibratome, Bannockburn, IL) and placed in ACSF ((in mM): 124 NaCl, 2.5 KCl, 2 NaH2PO4, 2.5 CaCl2, 2 MgSO4, 10 dextrose, and 26 NaHCO3) with l-tryptophan (50 mM) at 35 °C bubbled with 95% O2/5% CO2 for 1 h. Slices were then maintained in room temperature ACSF bubbled with 95% O2/5% CO2.
Slices were transferred to a recording chamber (Warner Instruments, Hamden, CT) and continuously perfused with ACSF at 1.5–2.0 ml/min. Only one cell was recorded per brain slice. DRN neurons were visualized using a Nikon E600 upright microscope (Optical Apparatus, Ardmore, PA). The resistance of the electrode was 4–6 MΩ when filled with an intracellular solution ((in mM): 70 Kgluconate, 70 KCl, 2 NaCl, 4 EGTA, 10 HEPES, 4 MgATP, 0.3 Na2GTP, 0.1% biocytin, pH 7.3).
Spontaneous inhibitory postsynaptic current (IPSC) recordings were made in cells located in the ventromedial subdivision of the DRN at mid-caudal levels that correspond to 7.32–8.16 mm caudal to bregma in Paxinos and Watson (2007) where 5-HT neurons are most densely populated. Whole-cell recordings were conducted in voltage-clamp mode (Vm= −70 mV) with a HEKA patch-clamp EPC-10 amplifier (HEKA Elecktronik Lambrecht, Pfalz, Germany). Series resistance was monitored throughout the experiment. If the series resistance was unstable or exceeded four times the electrode resistance, the cell was discarded. Series resistance ranges were also noted for each experimental group to ensure consistent overlap across groups. Signals were filtered at 1 kHz and digitized at 10 kHz. The liquid junction potential was −9 mV between the pipette solution and the ACSF and was not subtracted from the data obtained.
The non-NMDA receptor antagonist 6,7-dinitroquinoxaline-2,3 (1H,4H)-dione (DNQX; 20 µM; Sigma-Aldrich, St. Louis, MO) was bath applied to isolate GABAergic IPSCs. Total spontaneous IPSCs (sIPSCs) were recorded for 200 s in the absence and miniature spontaneous IPSCs (mIPSCs) were recorded for 200 s in the presence of tetrodotoxin (TTX; 1 µM; Enzo Life Science, Farmingdale, NY) to block action potential-dependent events. At the end of some experiments the GABAA receptor antagonist bicuculline (20 µM) was used to confirm the GABAergic nature of IPSC events.
2.2.7. Immunohistochemistry
Following electrophysiology experiments, brain slices were post fixed in 4.0% paraformaldehyde for 20–24 h and kept in 20% sucrose solution until standard fluorescent immunohistochemical methods were performed to visualize the recorded biocytin-filled cell and tryptophan hydroxylase 2 (TPH2) as a marker of 5-HT neurons. A rabbit-anti mouse TPH2 antibody (1:1000; Millipore, Billerica, MA) was visualized using an Alexa 594-conjugated donkey anti-rabbit secondary antiserum (1:200; Life Technologies, Carlsbad, CA). Biocytin was visualized using Alexa 488-conjugated streptavidin (1:500; Life Technologies, Carlsbad, CA). Sections were viewed and images captured by Leica Sp5 Confocal Microscope (Leica Microsystems, Exton, PA). Electrophysiological data were excluded from the study if recorded cells could not be conclusively identified as either TPH2-positive or -negative by immunohistochemistry.
2.2.8. Data analysis
For behavioral tests, the primary dependent measures were total active lever presses and total inactive lever press. For self-administration and extinction phases, measures were analyzed with a three-way repeated measures analysis of variance (ANOVA) with self-administration drug (saline vs. cocaine) and reinstatement drug (saline vs. yohimbine) as between-subject factors and session as a within-subject factor. For the reinstatement test, measures in last extinction session vs. reinstatement were analyzed with a three-way repeated measures ANOVA with self-administration drug (saline vs. cocaine) and reinstatement drug (saline vs. yohimbine) as between-subject factors and session (last extinction session vs. reinstatement) as a within-subject factor.
For electrophysiological results, MiniAnalysis software (Synapto-soft, Decatur, GA) was used to analyze IPSC events from last 60 s of either sIPSC or mIPSC recording on the basis of amplitude, rate of rise, duration, and area. Initially, noise analysis was conducted for each cell and amplitude detection thresholds set to exceed noise values. Events were automatically selected, analyzed for double peaks, and then visually inspected and confirmed. Event amplitude histograms were generated and compared to noise histograms to ensure that they did not overlap. Holding current was recorded and GABA synaptic activity analyzed for frequency, amplitude, rise time (calculated from 10% to 90% of peak amplitude) and decay time (calculated by averaging all events and fitting a double-exponential function from 10% to 90% of the decay phase to generate an initial fast component and a subsequent slow component of the decay phase). All data are represented as mean±SEM. Results were then analyzed using two-way ANOVAs with self-administration drug and reinstatement drug as independent variables. Paired t-test was used to compare frequency of sIPSC and mIPSC within each experimental group.
The correlation of behavioral and electrophysiological data was analyzed via Pearson's Product–Moment Correlation. Post hoc Tukey test or paired t-test was used where applicable. A probability of p < 0.05 was considered significant.
3. Results
3.1. Behavior: yohimbine reinstated cocaine-seeking behavior in rats with a history of cocaine self-administration (Figure 2)
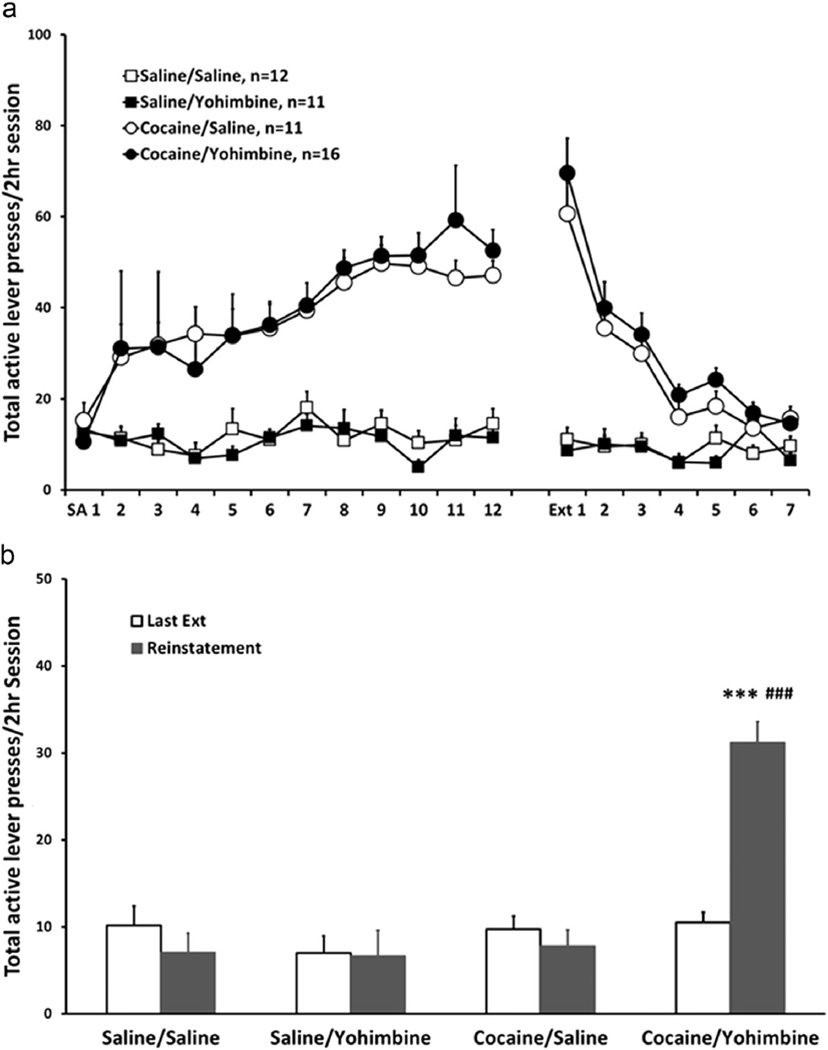
Figure 2.
Cocaine self-administration, extinction of cocaine-seeking behavior and reinstatement test. (a) Points represent mean±SEM of total active lever presses/2 h session of self-administration and extinction in cocaine groups and yoked saline controls. (b) Last extinction session and reinstatement test. Bars represent mean±SEM of total active lever presses/2 h session. Yohimbine reinstated cocaine-seeking behavior in animals with a history of cocaine self-administration (***p < 0.001 vs. saline/saline, saline/yohimbine and cocaine/yohimbine groups; p < 0.001 vs. last extinction session in cocaine/yohimbine group).
During self-administration, both cocaine/saline and cocaine/yohimbine groups established a stable active lever pressing behavior after 12 sessions of training. However, both saline groups did not acquire self-administration, only pressing the active lever for a few times within each session (p < 0.001) (Figure 2a). None of the groups pressed the inactive lever at high numbers (data not shown). During extinction training, the lever pressing behavior in cocaine groups gradually extinguished (p < 0.001) and reached the numbers similar to saline controls by days 6–7.
During the reinstatement test, only the cocaine/yohimbine group reinstated lever pressing behavior (Figure 2b). A three-way repeated measures ANOVA with self-administration drug (saline vs. cocaine) and reinstatement drug (saline vs. yohimbine) as between-subject factors and session (last extinction session vs. reinstatement) as a within-subject factor revealed a self-administration drug × reinstatement drug × session interaction [F(1, 46)=12.711, p=0.001] and main effects of self-administration drug [F(1,46)=20.75, p < 0.001], reinstatement drug [F(1,46)=10.90, p < 0.01] and session [F(1,46)=7.58, p < 0.01]. Post hoc paired t-test showed that the cocaine/yohimbine group exhibited a higher level of active lever presses in the reinstatement test than the last extinction session (t15=−9.185, p < 0.001), and post hoc Tukey tests showed that in the reinstatement test the cocaine/yohimbine group exhibited a higher level of active lever presses than other three groups (p < 0.001 for all three comparisons).
3.2. Electrophysiology: GABA synaptic activity is selectively sensitized in DRN-5-HT neurons following yohimbine stress-induced reinstatement of cocaine seeking (Figure 3)
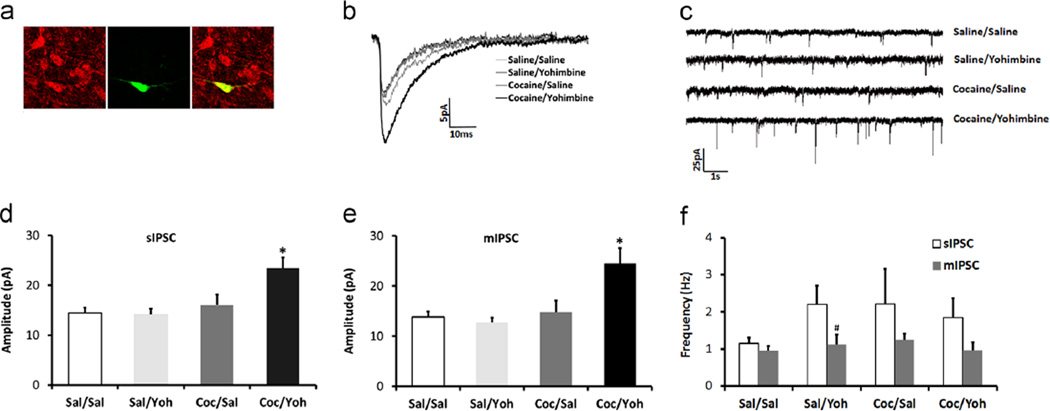
Figure 3.
GABA synaptic activity is selectively sensitized in DRN-5-HT neurons following yohimbine stress-induced reinstatement of cocaine-seeking. (a) Immunohistochemical identification of the biocytin-filled recorded neuron (green) with TPH2-IR (red) is shown in a 5-HT cell that is double-labeled (yellow) in the merged panel. (b) Representative traces of averaged mIPSCs from saline/saline, saline/yohimbine, cocaine/yohimbine, and cocaine/yohimbine groups. (c) Representative raw traces of mIPSCs. (d) and (e) Mean amplitudes of sIPSC and mIPSC respectively in cocaine/yohimbine group were greater than three control groups (*p < 0.05 cocaine/yohimbine vs. three control groups). (f) Mean frequency of sIPSC was higher than that of mIPSC in saline/yohimbine group (p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
A total of 72 DRN cells were recorded from 50 animals after behavior tests, and their cell types (5-HT vs. non-5-HT) were identified via immunohistochemistry (Figures 3a and 4a). Eleven cells were excluded from the study due to inconclusive immunohistochemical identification of their cell types, leaving a total of 61 cells in the electrophysiology data set. IPSC characteristics and holding current from 5-HT vs. non-5-HT cells in each treatment group are shown in Table 1.
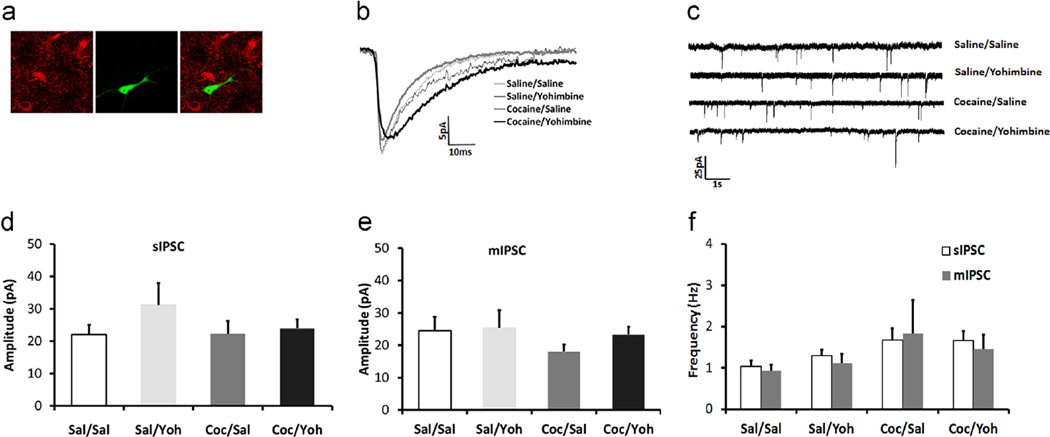
Figure 4.
GABA synaptic activity is unchanged in DRN- non-5-HT neurons following yohimbine stress-induced reinstatement of cocaine-seeking. (a) Immunohistochemical identification of the biocytin-filled recorded neuron (green) in a non-5-HT neuron without TPH-IR (red) is shown. (b) Representative traces of averaged mIPSCs from saline/saline, saline/yohimbine, cocaine/yohimbine, and cocaine/yohimbine groups. (c) Representative raw traces of mIPSCs. (d) Mean sIPSC and (e) mIPSC amplitudes (±SEM) of non-5-HT neurons from the four groups. IPSC amplitudes are not different across treatment groups. (f) Mean frequency of sIPSC did not differ from that of mIPSC in the four groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Electrophysiological characteristics in DRN-5-HT and non-5-HT neurons.
| 5-HT | Saline/saline n=7 (7 animals) |
Saline/yohimbine n=8 (6) |
Cocaine/saline n=8 (6) |
Cocaine/yohimbine n=9 (7) |
|---|---|---|---|---|
| Amplitude (pA) | ||||
| sIPSC | 14.51±1.00 | 14.16±1.17 | 16.05±2.11 | 23.43±2.12 * |
| mIPSC | 13.80±1.15 | 12.76±0.92 | 14.89±2.21 | 24.48±3.04 * |
| Frequency (Hz) | ||||
| sIPSC | 1.16±0.15 | 2.20±0.51 | 2.22±0.94 | 1.86±0.51 |
| mIPSC | 0.96±0.12 | 1.12±0.27 | 1.24±0.17 | 0.97±0.20 |
| mIPSC kinetics | ||||
| Rise time (ms) | 2.87±0.39 | 2.84±0.41 | 2.41±1.56 | 2.17±0.18 |
| Fast decay (tau) | 9.42±0.84 | 10.33±3.41 | 6.91±0.89 | 9.37±0.91 |
| Slow decay (tau) | 43.38±11.87 | 49.26±23.48 | 23.74±2.60 | 21.72±1.76 |
| Holding current (pA) | −20.86±8.63 | −23.59±8.95 | −47.56±19.24 | −36.36±10.57 |
| Non-5-HT | n=9 (7 animals) | n=6 (5) | n=8 (6) | n=6 (6) |
| Amplitude (pA) | ||||
| sIPSC | 22.02±3.04 | 31.20±6.71 | 22.27±3.96 | 23.96±2.67 |
| mIPSC | 24.56±4.30 | 25.36±5.48 | 18.04±2.21 | 23.30±2.42 |
| Frequency (Hz) | ||||
| sIPSC | 1.03±0.14 | 1.30±0.28 | 1.68±0.42 | 1.67±0.46 |
| mIPSC | 0.94±0.13 | 1.12±0.22 | 1.83±0.82 | 1.45±0.36 |
| mIPSC kinetics | ||||
| Rise time (ms) | 2.24±0.33 | 2.49±0.36 | 2.49±0.34 | 2.11±0.42 |
| Fast decay (tau) | 8.82±1.07 | 7.83±0.85 | 10.75±2.50 | 5.94±1.10 |
| Slow decay (tau) | 32.08±6.96 | 26.02±6.91 | 48.77±16.96 | 19.41±4.54 |
| Holding current (pA) | 0.21±12.20 | −18.54±13.02 | −12.38±7.94 | −9.13±17.69 |
Note: Amplitude of sIPSCs and mIPSCs was significantly elevated selectively in DRN-5-HT neurons following stress-induced reinstatement (*p<0.05, unpaired Student’s t-test). All other electrophysiological characteristics in DRN neurons were not different between treatment groups. Frequency of sIPSCs was significantly higher than that of mIPSC in DRN-5-HT neurons from saline/yohimbine group (p<0.05 paired Student’s t-test), and there was a trend of higher frequency of sIPSCs than that of mIPSC in cocaine/yohimbine group (p=0.065).
Serotonin neurons from the cocaine/yohimbine group had a greater amplitude of sIPSCs and mIPSCs than all other three groups (Figure 3b: averaged representative mIPSC event; Figure 3c: representative raw trace of mIPSCs; Figure 3d: mean sIPSCs; Figure 3e: mean mIPSCs). Two-way ANOVAs for sIPSC or mIPSC amplitude both revealed an interaction between self-administration drug and reinstatement drug [sIPSCs: F(1,28)=5.008, p < 0.05; mIPSCs: F(1,28)=6.139, p < 0.05], and a main effect of self-administration drug [sIPSCs: F(1,28)=9.80, p < 0.01; mIPSCs: F(1,28)=8.92, p < 0.01]. Post hoc Tukey tests showed that the amplitude of sIPSCs and mIPSCs in the cocaine/yohimbine group was greater than the other three groups (sIPSCs: p < 0.01 vs. saline/saline; p < 0.01 vs. saline/yohimbine; p < 0.05 vs. cocaine/saline; mIPSCs: p < 0.01 vs. saline/saline; p < 0.01 vs. saline/yohimbine; p < 0.05 vs. cocaine/saline). No correlation between the level of active lever presses in stress-induced reinstatement and sIPSC [r(7)=0.043, p=0.912] or mIPSC [r(7)= −0.18, p=0.642] amplitude was observed. Frequency of sIPSCs was higher than that of mIPSCs in DRN-5-HT cells in saline/yohimbine group [t(7)=2.465, p < 0.05], and cocaine/yohimbine group showed a trend of higher sIPSC frequency than mIPSC frequency [t(8)=2.137, p=0.065], suggesting that yohimbine may increase the action potential-dependent release of GABA onto DRN-5-HT cells (Figure 3f).
For non-5-HT neurons the IPSC amplitude did not differ across the four groups (Figure 4b: averaged representative mIPSC event; Figure 4c: representative raw trace of mIPSCs; Figure 4d: mean sIPSCs; Figure 4e: mean mIPSCs), and sIPSC frequency also did not differ from mIPSC frequency in the four groups (Figure 4f). Moreover, for both DRN-5-HT and -non-5-HT cells, sIPSC or mIPSC frequency, mIPSC kinetics (rise time, fast or slow decay) or holding current did not differ across the four groups (Table 1).
4. Discussion
In our current study, behavioral data indicate that the chemical stressor yohimbine triggered reinstatement of cocaine self-administration. Electrophysiology data indicate that 5-HT neurons in the cocaine group exposed to stress had increased amplitude of postsynaptic GABAergic currents compared to yoked-saline controls exposed to stress or unstressed animals in both drug groups.
Technical considerations
One thing to note is that the frequencies of IPSCs for both DRN-5-HT and -non-5-HT neurons in our current study were low compared with our previous studies (Kirby et al., 2008; Staub et al., 2012). This is possibly the result of using older animals (~11–14 week old) in the current study, necessitated by the lengthy behavioral training, compared to our previous studies with CPP-trained (11–12 weeks; Staub et al., 2012) and naïve rats (~4–5 week old; Kirby et al., 2008). For example, using vehicle treated or naïve Sprague-Dawley rats in our laboratory under the same electrophysiological conditions, we have recorded the following mIPSC frequencies in 5-HT DRN neurons: 4–5-week-old: mean=6.21 ± 0.59, range=0.80–14.82 (N=39); 11–12-week-old: mean=3.98 ± 0.54, range=1.09–6.55 (N=12); 11–14-week-old: mean=1.16 ± 0.15, range=0.67–1.71 (N=7). Synaptic overproduction and pruning in the brain is a known general consequence of maturation of young animals into adults (Brenhouse and Andersen, 2011). GABA synapses in particular are known to be affected by development and aging (Brenhouse and Andersen, 2011) including age-related alterations in GABAA receptor subunit expression (Yu et al., 2006) and decline in synaptic density during maturation (Cruz et al., 2003). Moreover, electrophysiology studies demonstrate age-related reductions in presynaptic GABA release, as indicated by reduced mIPSC frequency (Wong et al., 2006). It is also well-established that older animals yield brain slices with reduced viability as compared to juveniles (Moyer and Brown, 1998), potentially resulting in fewer functional presynaptic terminals releasing GABA in the slice. Another possible explanation for the low frequency are the behavioral paradigms in the current study. For example, some procedures, i.e. catheter surgery, daily catheter flush and attached drug delivery line, can cause extra stress, which is well known to change the electrophysiological characteristics of DRN neurons (Lemos et al., 2011; Valentino et al., 2010). For example in Lemos et al. 2011, the authors showed a trend for a reduction in basal mIPSC frequency in stress hyperresponsive WKY rats compared to age-matched Sprague-Dawley rats. However, consistent with our previous findings, the sIPSC frequency for DRN-5-HT neurons in Saline/Saline and Cocaine/Saline groups and DRN-non-5-HT neurons was not statistically different from mIPSC frequency indicating that the majority of spontaneous IPSCs in the DRN are non-action potential-dependent, representing spontaneous fusion of GABA vesicles with the presynaptic membrane. In contrast, the sIPSC:mIPSC frequency ratio was generally higher in yohimbine-treated subjects, indicating that yohimbine may increase action potential-dependent release of GABA onto DRN-5-HT neurons.
Yohimbine is generally used as high-affinity α2-adrenergic receptor antagonist in stress-induced reinstatement studies (Shalev et al., 2010; Shepard et al., 2004); however, it also has moderate affinity at other receptors such as α1-adrenergic receptors, 5-HT(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors (Doxey et al., 1984; Millan et al., 2000). There is also evidence that yohimbine causes the release of neuropeptides, which may also contribute to its anxiogenic effects (Rasmusson et al., 1998). Moreover, yohimbine decreases firing of serotonergic neurons in the DRN (Millan et al., 2000). This is consistent with our current finding that yohimbine increased the frequency of sIPSCs in DRN-5-HT neurons, which would inhibit excitability of DRN-5-HT neurons.
These data expand upon our previous findings showing a similar GABAergic sensitization selectively in DRN-5-HT neurons in a model of swim stress-induced reinstatement of morphine CPP (Staub et al., 2012), and suggest that the effect of interaction between addictive drugs and stress on DRN-5-HT neurons may be a general neuroadaptation linked to reinstatement that is not specific to drug classes, drug exposure methods (contingent vs. non-contingent), relapse models or stressor types. Moreover, together with the direct behavioral evidence that GABA signaling in the DRN is necessary for stress-induced reinstatement of morphine CPP (Li et al., 2013), we hypothesize that this GABAergic neuroadaptation in DRN-5-HT neurons may serve as a common neurochemical mechanism enhancing vulnerability to stress-induced reinstatement of drug-seeking. However, it is worth noting that the level of reinstatement does not correlate with the degree of GABAergic sensitization DRN-5-HT neurons in both CPP and self-administration models, suggesting that the DRN 5-HT system may have a general role in reinstatement rather than determining the precise level of motivation for drug-seeking in stress-induced reinstatement.
Many addictive drugs, though distinct in their primary mechanism of action, regulate the 5-HT system in a similar manner. Psychostimulants, opioids and ethanol all acutely increase extracellular 5-HT levels in many brain regions but reduce 5-HT basal levels and suppress 5-HT output after chronic exposure and during withdrawal (for review Kirby et al., 2011). These changes in the 5-HT system may contribute to both the acute euphoric effects of addictive drugs and the long-term transition to addiction. For example, genetic deletion studies have indicated a role of 5-HT as well as dopamine in psychostimulant reward (Sora et al., 2001, 1998). Recently, optogenetic activation of DRN neurons provides direct evidence that 5-HT neurons mediate reward and reward-related behavior (Liu et al., 2014; Miyazaki et al., 2014). During withdrawal, hypofunction of 5-HT system may contribute to mood disorders such as dysphoria, depression and anxiety (Koob, 2000; Parsons et al., 1995; Tao et al., 1998; Weiss et al., 1996), which then lead to vulnerability to relapse as a means of ‘self-medication’ and restoration of homeostasis within the 5-HT system (Markou et al., 1998).
Indeed, a growing literature implicates the 5-HT system in the neurobiological mechanisms underlying reinstatement of drug-seeking behavior. For example, systemic stimulation of the 5-HT system with 5-HT-selective reuptake inhibitors or 5-HT-releasing agents suppresses both cue-elicited cocaine reinstatement (Burmeister et al., 2003) and also stress-induced reinstatement of alcohol-seeking behavior in rats (Le et al., 1999). Studies focusing on serotonin receptor types suggest that different types of serotonin receptors may play opposite roles in reinstatement (Filip et al., 2010). For example, blockade of 5-HT2A receptors attenuates both drug- and cue-induced reinstatement (Fletcher et al., 2002; Nic Dhonnchadha et al., 2009), whereas stimulation of 5-HT2C receptors attenuates cocaine-induced, cue-induced (Fletcher et al., 2002, 2008; Neisewander and Acosta, 2007), and stress-induced cocaine reinstatement (Fletcher et al., 2008). Although only a few studies have investigated the role of the sources of the serotonergic projections in drug reinstatement, ours and other results all suggest that inhibition of 5-HT cell bodies reinstates drug-seeking behavior, whereas stimulation of 5-HT cell bodies blocks stress-induced reinstatement (Land et al., 2009; Le et al., 2008; Li et al., 2013). This moreover implies that hypofunction of DRN-5-HT neurons induced by stress may be a critical neuronal mechanism creating vulnerability to drug relapse.
Furthermore, our current study showed that the GABAergic sensitization induced by the interaction between cocaine history and stress was specific for 5-HT vs. non DRN-5-HT neurons. This cell-type specificity was consistent with our previous findings that only DRN-5-HT neurons showed the postsynaptic GABAergic sensitization following stress-induced reinstatement of morphine CPP (Staub et al., 2012). The DRN contains a neurochemically heterogeneous cell population which includes 5-HT, dopaminergic, GABAergic, and glutamatergic neurons (Vasudeva et al., 2011). Recent optogenetic studies demonstrated that precisely activating DRN-5-HT neurons in mice can reinforce a variety of instrumental tasks (Liu et al., 2014) and enhances patience for delayed reward (though no reinforcing effects of 5-HT neuronal activation were observed in this study) (Miyazaki et al., 2014). However, another recent study reported that non-5-HT glutamatergic DRN neurons mediate drug reward (McDevitt et al., 2014). These studies indicate that different types of DRN neurons may have different functions in drug addiction. To more precisely understand the functional significance of the GABAergic sensitization in DRN-5-HT neurons in stress-induced reinstatement of drug-seeking, we plan to use a conditional gene deletion approach in future studies to silence GABAA receptors selectively in DRN-5-HT neurons to test our hypothesis that GABAergic sensitization of DRN-5-HT neurons is necessary for stress-induced drug reinstatement. This approach will give us more insight to the 5-HT circuits that contribute to stress-induced vulnerability to reinstatement.
Another possible mechanism for the specificity of GABAergic sensitization in DRN-5-HT neurons is that the interaction of drug history and stress may be able to change selective GABAA receptor subtypes in the DRN. GABAA receptors are composed of five subunit proteins forming an associated chloride channel, and 19 subunits have been cloned. Different subunit combinations determine biophysical and pharmacological properties of the receptor subtypes and also subunit composition can be impacted by a variety of pharmacological agents including cocaine (Backes and Hemby, 2003; Uusi-Oukari and Korpi, 2010). There is evidence that the DRN-5-HT and –non-5-HT neurons express distinct subunits of GABAA receptors (Corteen et al., 2014; Gao et al., 1993), and behavioral history can change a selective subunit expression but leave others unaffected (Corteen et al., 2014). Moreover, a stress-hyperresponsive rat strain show altered expression of certain GABAA receptor subunits in the DRN-5-HT neurons (Lemos et al., 2011), suggesting that either stress exposure may alter expression of GABAA receptor subunits or that the composition of GABAA receptor subtypes in the DRN-5-HT neurons may affect the response to stress. Therefore, if drug history changes the expression of GABAA receptor subunits in the DRN-5-HT neurons it could in turn impact stress vulnerability.
In summary, our current data, together with previous findings in stress-induced reinstatement of morphine CPP, indicate that interaction between drug history and stress may increase postsynaptic GABA receptor density and/or sensitize DRN-5-HT neurons to GABAergic inhibition. Such mechanisms may result in serotonergic hypofunction and consequent dysphoric mood states which confer vulnerability to stress-induced drug reinstatement. Serotonin hypofunction has been observed in other stress models such as the Wistar-Kyoto rat which is thought to model chronic stress/depression-like behaviors as well as vulnerability to drugs of abuse (Lemos et al., 2011).
Acknowledgments
This research was supported by NIH Grants DA037523 and DA013429.
Role of funding source
Funding for the study was provided by NIH Grants DA037523 and DA013429; the NIH had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors
Authors Chen Li and Lynn G. Kirby contributed to the collection and analysis of data. Both authors have contributed to and have approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Backes E, Hemby SE. Discrete cell gene profiling of ventral tegmental dopamine neurons after acute and chronic cocaine self-administration. J. Pharmacol. Exp. Ther. 2003;307:450–459. doi: 10.1124/jpet.103.054965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci. Biobehav. Rev. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology (Berl.) 2003;168:146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Krystal JH, Heninger GR. Serotonin function and human anxiety disorders. Ann. NY Acad. Sci. 1990;600:558–572. doi: 10.1111/j.1749-6632.1990.tb16910.x. (discussion 572-553). [DOI] [PubMed] [Google Scholar]
- Corteen NL, Carter JA, Rudolph U, Belelli D, Lambert JJ, Swinny JD. Localisation and stress-induced plasticity of GABA receptor subunits within the cellular networks of the mouse dorsal raphe nucleus. Brain Struct. Funct. 2014 doi: 10.1007/s00429-014-0824-7. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Lewis DA. Postnatal development of pre- and postsynaptic GABA markers at chandelier cell connections with pyramidal neurons in monkey prefrontal cortex. J. Comp. Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76(Pt B):460–478. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey JC, Lane AC, Roach AG, Virdee NK. Comparison of the alpha-adrenoceptor antagonist profiles of idazoxan (RX 781094), yohimbine, rauwolscine and corynanthine. Naunyn Schmiedebergs Arch. Pharmacol. 1984;325:136–144. doi: 10.1007/BF00506193. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Ofer OA, Mueller CL, Waldroup S, Cohen A, Ben-Shahar O. Inactivation of the dorsal raphe nucleus reduces the anxiogenic response of rats running an alley for intravenous cocaine. Pharmacol. Biochem. Behav. 2011;97:632–639. doi: 10.1016/j.pbb.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav. Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Filip M, Alenina N, Bader M, Przegalinski E. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict. Biol. 2010;15:227–249. doi: 10.1111/j.1369-1600.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Gao B, Fritschy JM, Benke D, Mohler H. Neuronspecific expression of GABAA-receptor subtypes: differential association of the alpha 1- and alpha 3-subunits with serotonergic and GABAergic neurons. Neuroscience. 1993;54:881–892. doi: 10.1016/0306-4522(93)90582-z. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Yohimbine's anxiogenic action: evidence for noradrenergic and dopaminergic sites. Pharmacol. Biochem. Behav. 1989;32:151–156. doi: 10.1016/0091-3057(89)90225-6. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J. Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann. NY Acad. Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Lader M, Bruce M. States of anxiety and their induction by drugs. Br. J. Clin. Pharmacol. 1986;22:251–261. doi: 10.1111/j.1365-2125.1986.tb02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy CM, Beck SG. Swim stress differentially blocks CRF receptor mediated responses in dorsal raphe nucleus. Psychoneuroendocrinology. 2010;35:1321–1332. doi: 10.1016/j.psyneuen.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc. Natl. Acad. Sci. US A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Li Z, Fletcher PJ. Intra-median raphe nucleus (MRN) infusions of muscimol, a GABA-A receptor agonist, reinstate alcohol seeking in rats: role of impulsivity and reward. Psychopharmacology (Berl.) 2008;195:605–615. doi: 10.1007/s00213-007-0943-4. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Zhang G, Walsh T, Kirby LG, Akanwa A, Brooks-Kayal A, Beck SG. Stress-hyperresponsive WKY rats demonstrate depressed dorsal raphe neuronal excitability and dysregulated CRF-mediated responses. Neuropsychopharmacology. 2011;36:721–734. doi: 10.1038/npp.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology. 2009;204:725–733. doi: 10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- Li C, Staub DR, Kirby LG. Role of GABA receptors in dorsal raphe nucleus in stress-induced reinstatement of morphine-conditioned place preference in rats. Psychopharmacology (Berl.) 2013;230:537–545. doi: 10.1007/s00213-013-3182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Feng Q, Zhang JE, Wang D, Zeng J, Bao J, Kim JY, Chen ZF, El Mestikawy S, Luo M. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. 2014;81:1360–1374. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Tiran-Cappello A, Shen H, Balderas I, Britt JP, Marino RA, Chung SL, Richie CT, Harvey BK, Bonci A. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell. Rep. 2014;8:1857–1869. doi: 10.1016/j.celrep.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Coge F, Galizzi JP, Boutin JA, Rivet JM, Dekeyne A, Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Tanaka KF, Yamanaka A, Takahashi A, Tabuchi S, Doya K. Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr. Biol. 2014;24:2033–2040. doi: 10.1016/j.cub.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Brown TH. Methods for whole-cell recording from visually preselected neurons of perirhinal cortex in brain slices from young and aging rats. J. Neurosci. Methods. 1998;86:35–54. doi: 10.1016/s0165-0270(98)00143-5. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav. Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav. Neurosci. 2009;123:382–396. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. J. Pharmacol. Exp. Ther. 1995;274:1182–1191. [PubMed] [Google Scholar]
- Paxinos G, Watson CR. The Rat Brain in Stereotaxic Coordinates. sixth edition. Academic Press; 2007. [Google Scholar]
- Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology (Berl.) 2002;162:406–414. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Southwick SM, Hauger RL, Charney DS. Plasma neuropeptide Y (NPY) increases in humans in response to the alpha 2 antagonist yohimbine. Neuropsychopharmacology. 1998;19:95–98. doi: 10.1016/S0893-133X(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J. Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, vulnerability to addiction. Ann. NY Acad. Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc. Natl. Acad. Sci. USA. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc. Natl. Acad. Sci. USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub DR, Lunden JW, Cathel AM, Dolben EL, Kirby LG. Morphine history sensitizes postsynaptic GABA receptors on dorsal raphe serotonin neurons in a stress-induced relapse model in rats. Psychoneuroendocrinology. 2012;37:859–870. doi: 10.1016/j.psyneuen.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Kwa C, Debold JF, Miczek KA. GABA (A) receptors in the dorsal raphe nucleus of mice: escalation of aggression after alcohol consumption. Psychopharmacology (Berl.) 2010;211:467–477. doi: 10.1007/s00213-010-1920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Alteration in regulation of serotonin release in rat dorsal raphe nucleus after prolonged exposure to morphine. J. Pharmacol. Exp. Ther. 1998;286:481–488. [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol. Rev. 2010;62:97–135. doi: 10.1124/pr.109.002063. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudeva RK, Lin RC, Simpson KL, Waterhouse BD. Functional organization of the dorsal raphe efferent system with special consideration of nitrergic cell groups. J. Chem. Neuroanat. 2011;41:281–293. doi: 10.1016/j.jchemneu.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J. Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TP, Marchese G, Casu MA, Ribeiro-da-Silva A, Cuello AC, De Koninck Y. Imbalance towards inhibition as a substrate of aging-associated cognitive impairment. Neurosci. Lett. 2006;397:64–68. doi: 10.1016/j.neulet.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099:73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]