Abstract
The development of cellular reprogramming methods to generate human induced pluripotent stem cells (iPSC) has led to the establishment of lines from hundreds of patients with a variety of neurologic and psychiatric diseases. One of the fundamental powers of iPSC technology lies in the competency of these cells to be directed to become any cell type in the body, thus allowing researchers to examine disease mechanisms and identify and test novel therapeutics in relevant cell types. The field has now exited the phase of “proof-of-principle” studies showing the potential of the model systems, and it has now entered an exciting new era where iPSC studies are contributing to the field’s understanding of mechanisms of disease. Here, we describe the challenges of iPSC modeling of neuropsychiatric disorders, and highlight studies where some of these challenges have been addressed to provide novel insights into disease mechanisms.
Introduction
Neurobiologists are increasingly conceptualizing many neuropsychiatric disorders as disorders of abnormal brain development that result in altered circuitry. Disruption of multiple developmental processes could result in abnormal circuitry: determination of a complex array of neuronal and glial fates in the correct numbers, migration of cells to precise locations, elaboration of dendritic and axonal architecture appropriate for communicating with particular target cells, and establishment of appropriate number and strength of synapses with sufficient plasticity to respond to experience. While imaging has provided clues to the possible circuits disrupted, and genetics has provided important insight into the molecular players, the cellular mechanisms underlying the associated developmental defects are not well understood. The advent of induced pluripotent stem cell (iPSC) technology has allowed researchers a window into neural development in human cells derived from patients. This rapidly advancing and growing field already has offered new insights into human neurodevelopmental pathways linked to neuropsychiatric disorders, and promises to expand our understanding of normal and pathological human circuit development. Here, we review the recent progress in modeling neuropsychiatric disorders, and provide our perspective on the greatest hurdles and challenges facing the field today.
Making and analyzing the relevant cell types
Theoretical considerations of cell fate
One challenge in modeling neuropsychiatric diseases is in choosing and generating the cell types most relevant to explore. For autism spectrum disorder (ASD) and schizophrenia (SCZ), studies to date have in most cases directed iPSCs to cerebral cortical fates of the forebrain. Cortex is likely to play an important role in these disorders, and while other brain regions are also likely involved, cell types of these other brain regions remain largely under studied to date. The cerebral cortex is made up of a diverse array of cell types including upper and lower layer glutamatergic projection neurons, inhibitory GABAergic interneurons of various fates, as well as different subtypes of astrocytes, oligodendrocytes and microglia. Layered upon this is the added complexity of the different functional regions of the cortex. In most cases, at the outset it is unclear which of these cell types are likely to be most affected in iPSC models of neuropsychiatric disease. With broad diagnostic criteria for the heterogeneous circuit disorders such as schizophrenia or autism, it is likely that initiating insults in different subsets of cells in the brain could result in convergent symptoms in patients. Due to this potential heterogeneity, a focus upon strong mutations and copy number variants (CNVs) may increase the probability of identifying statistically significant developmental changes in particular cell fates in vitro. In this manner, the study of rare but strong mutations could be helpful for ultimately identifying the points of convergence of multiple genetic alterations which in turn may inform the field of the pathways that may be best targeted for therapeutic intervention.
Specific examples of studies demonstrating cell fate differences
One impressive example of where the field has gained insights into the relevant cell types and disease mechanisms is with iPSC studies of Timothy syndrome (TS). TS is a very rare disorder (less than 20 cases reported), and many individuals with TS have neurodevelopmental abnormalities resulting in ASD-like symptoms, intellectual disability (ID) and/or seizures along with heart and digit malformations. All of the known cases are caused by mutations in the gene CACNA1C, which encodes the calcium channel CaV1.2. Mutant iPSC-derived cortical neurons and neuronal precursor cells (NPCs) showed phenotypes linked to known functions of this channel (defects in calcium signaling and activity-dependent gene expression)1. In addition, neurons with mutant channels exhibited activity-dependent dendrite retraction, and this phenotype was shown to be independent of altered calcium flow through the mutant channel2. Further, using single cell analyses of gene expression in neurally-differentiated iPSCs, an effect of the mutation was observed on the number of Satb2+, lower layer neurons present and on expression of tyrosine hydroxylase (TH)1. TH-expressing individual cells co-expressed forebrain and not midbrain markers, arguing against an alteration of cell fate with this mutation1. Further studies are warranted to interrogate whether this finding is an artifact of the in vitro culture system, or if TH upregulation and resultant catecholamine dysregulation, are features of the pathogenesis of the disease.
The same study described above went on to confirm the Satb2 phenotype in a mouse model of the disease1. The combination of studies in human (patient-derived) neurons using iPSCs with studies in mouse in vivo may be particularly powerful. While the iPSC studies may allow investigation of in vitro mechanism in a human cellular system, in vivo studies should not be abandoned. Another strength of the studies on Timothy syndrome described is that the genetic construct validity of the mouse mutation is carefully considered. They knocked in the G406R dominant, gain-of-function mutation and they study the mouse as a heterozygote, which has high genetic construct validity with the human condition. Thus, studies in iPSCs (corroborated by animal studies in vivo where possible) from this ultrarare but strong mutation revealed developmental phenotypes that implicate certain cell types in potentially contributing to the syndrome.
Novel approaches relevant to issues of cell fate
One option in searching for disease-relevant phenotypes is to generate a heterogeneous mixture of neural cell types, and to interrogate functional changes on a cell-by-cell level. Numerous classical techniques such as immunostaining and patch clamp electrophysiology allow for analysis on a cell-by-cell level. In addition, new technologies are allowing researchers to interrogate single cells using higher throughput methodologies. Recent studies by the Walsh lab revealed the heterogeneity in neural progenitor cells present in the developing human fetal cerebral cortex3. This study defined transcriptional profiles of radial progenitor subtypes, and compared these to profiles of mouse and ferret cortical progenitor cells using single cell RNA sequencing. This required sequencing of hundreds of cells. In the past, the large numbers of single cell profiles necessary to establish the structure of the population would have been cost-prohibitive. However, using a new technique called DropSeq, a recent study sequenced over 40,000 single murine retinal cells4. In DropSeq, single cells are separated from one another into nanoliter-sized droplets, where the RNA from each cell is associated with a bar code. By assigning a unique barcode to each cell, mRNA transcripts from thousands of cells can be pooled and sequenced simultaneously, and the data deconvoluted based upon the barcode. Using this strategy, the authors estimated that 10,000 single cell libraries can be prepared and analyzed in 12 hours for ~6.5 cents per sample4. One can use this or other single cell methods to identify populations of cells within heterogeneous iPSC-derived neural cultures, and then compare this population structure to cells from cultures with an engineered mutation and/or derived from patients with disease. The application of these single cell analysis platforms have the potential to transform how we think about identifying relevant cell types and phenotypes in iPSC models of complex diseases.
Directing iPSCs to the relevant cell fate
Upon choosing which subset(s) of cells to analyze for a particular study, the next consideration is the method to be used to direct the differentiation from the iPSC state. Multiple protocols exist for generating over 40 different cell fates found in the nervous system. Many decades of research of mammalian brain development have guided the rationale behind which factors to add and when in these protocols, and the protocol chosen will influence the types of phenotypes that can be detected. For example, direct conversion protocols may bypass critical developmental events that are necessary for the generation of certain cell types, and the precise concentration and timing of treatment with patterning factors will determine the subset of neurons produced.
Direct conversion from iPSCs with Neurogenin-2 (Ngn2) rapidly and efficiently generates neurons that express markers of telencephalic layer 2/3 excitatory projection neurons5. In the initial report of this protocol, NGN2-induced neurons were characterized by RNA profiles and electrophysiological measures. These cells are reported to express AMPA-type but not NMDA-type glutamate receptors at the RNA level, and when co-cultured with glia they exhibit spontaneous and evoked EPSCs that are efficiently blocked with AMPA receptor antagonists. While this raises the question of the fidelity of NGN2-induced neurons relative to their in vivo counterparts, there are three major advantages to this protocol relative to existing protocols. The first is that the gene expression profiles of the resultant neurons are quite homogenous between individual cells within a differentiation. Second, profiles are reproducible between independent lines. Finally, the protocol is quite rapid, with cells acquiring neuronal gene expression patterns and morphology within two weeks. Thus, this protocol may be optimal for screening purposes. Developmental phenotypes and phenotypes expressed only in specific subsets of cells may be missed if direct conversion protocol are employed. For example, our lab (TYP) has recently analyzed the consequences of genomic DISC1 disruption on early aspects of neurodevelopment using isogenic iPSC-derived cells6. DISC1 interruption has been implicated in the pathophysiology of major mental illness based on rare mutations that predispose patients to disease7. The most powerful of these mutations, a balanced translocation identified in a Scottish pedigree, interrupts the coding sequence of DISC18. We modeled the effects of DISC1 disruption either near the site of this translocation or at another site in DISC1, using genome editing techniques to generate mutations in hiPSCs6. In telencephalic neural progenitor cells derived from these lines, we observed increased canonical Wnt signaling, and alterations in gene expression profiles consistent with a decrease in the number of intermediate progenitor (IP) cells6. In agreement with altered gene expression profiles, we observed a decrease in the percentage of TBR2 positive NPCs, as well as an elevation in EdU incorporation with DISC1 disruption6. The effects observed are relatively subtle, but our work highlights that it is possible to identify modest but significant alterations in neurodevelopment that are attributable to disruption of a single gene, using thorough analyses of multiple isogenic hiPSC lines over many differentiations with high numbers of wells. However, these phenotypes may be missed if alternative differentiation protocols (for ex., those that do not generate IPs) are employed.
Summary
The examples cited above highlight some of the challenges in identifying cell-type specific phenotypes. One further consideration is that while approaches for analysis of cellular and cell-autonomous phenotypes are advancing, studies of connectivity pose additional challenges that need to be addressed. Since the pathology of many neuropsychiatric disorders resides at the level of connectivity, circuit development and plasticity, studies of connectivity are likely to be essential. Unfortunately, these studies are challenging in vitro and particularly challenging in human neurons wherein the differentiation period is very long and circuit development occurs in response to experience. Initial attempts to model connectivity in a dish using electrophysiological measures (MEAs, calcium imaging, fluorescence-based voltage sensors), anterograde and/or retrograde-labeling with retroviruses9, and 3-dimensional organoids10,11 are potential methods for improving the field’s ability to address issues surrounding connectivity. Current protocols generally produce cells with gene expression profiles most similar to human fetal neurons12. Thus, there is an inherent limitation in the field for study of some later onset events in brain development that may be relevant to neuropsychiatric disease. Xenografting of human cells into developing animals and studies of these cells in the maturing brain in vivo may offer substantial insight, although these approaches also pose technical and practical limitations.
Overcoming variability and increasing reproducibility
Theoretical issues
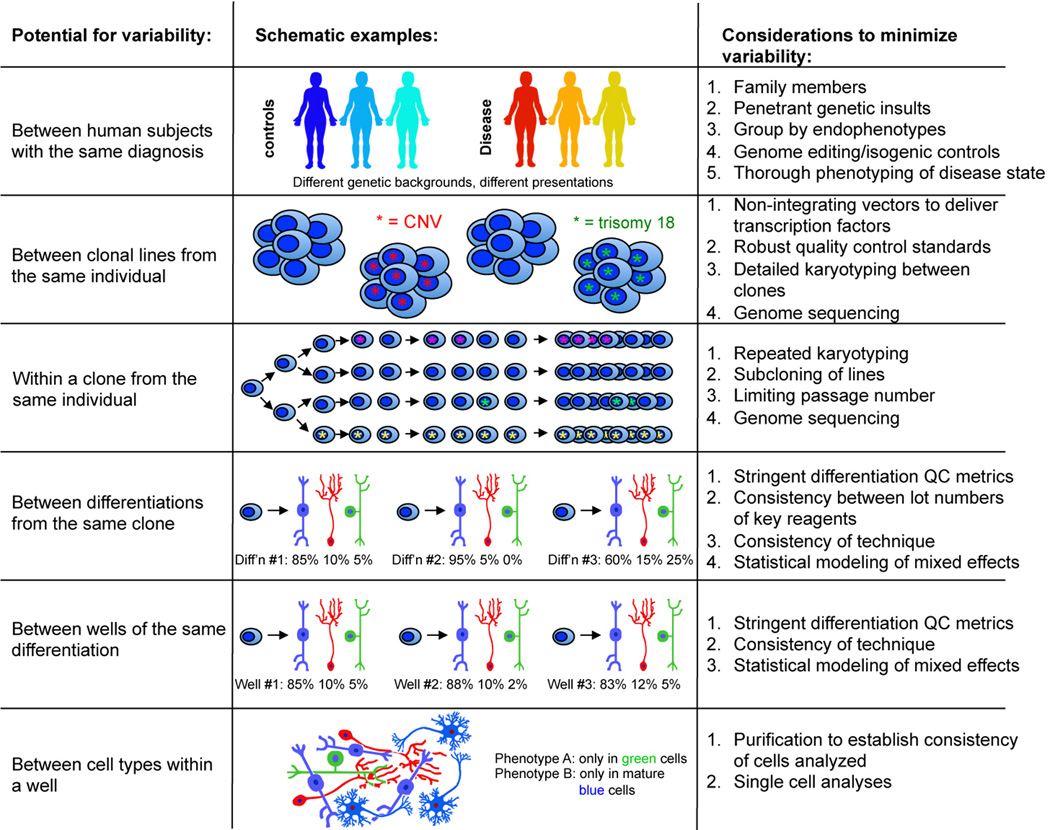
Experiments in iPSCs are subject to a number of factors that introduce substantial variability (Figure 1). Some of these factors include: heterogeneous genetic background of cases and controls; stochastic events in reprogramming; and heterogeneity in differentiation experiments, within wells and across wells. The variability introduced by each of these may be minimized in part with strategies outlined in Figure 1. To accurately assign cellular phenotypes to the genetic background or disease of study, it may be necessary to analyze these phenotypes over multiple clones from the same line over several differentiations and many wells. Transparency in reporting of technical and statistical methods is always important in scientific publication, but is critical for interpretation of reported results regarding iPSC modeling of disease.
Figure 1. Sources of variability in iPSC modeling of neuropsychiatric disease, and considerations for minimizing this variability.
Numerous biological and technical variables in iPSC modeling have the potential to contribute to inconsistent results within a study and difficulties in replication between laboratories. There are multiple points in the process where this variability can be introduced, and details of experimental design can minimize variation at each level from the selection of subjects included in the study to the analysis of particular cell types within a well. Outlined here are the hierarchical levels at which variability has been observed, as well as strategies used to minimize or bypass this variability.
Specific studies
One example where measures were taken to control for the inherent variability of the system is in an iPSC study of Phelan-McDermid syndrome (PMDS)13. PMDS is characterized by ID, developmental delay, impaired speech and increased risk of ASD-like symptoms, and is caused by heterozygous deletion of chromosome 22q13. In this study, the expression profiles and electrophysiological properties of individual neurons derived from iPSCs of two patients with PMDS and ASD where analyzed13. In order to minimize the effects of heterogeneity in differentiations and maturation between lines and experiments, CAMKIIalpha-GFP was used to label only mature forebrain neurons13. Further, to control for density and other variable environmental effects, wild type and PMDS cells were differentially labeled and co-cultured in the same dishes for analyses of electrophysiological properties13. Under these carefully controlled conditions, the authors were able to show that PMDS neurons show defects in excitatory but not inhibitory synaptic transmission13.
Modeling neuropsychiatric disease of known strong genetic influence versus idiopathic disease
Theoretical considerations
As outlined above, multiple sources of variability in iPSC-derived neural cells heighten the challenge in observing statistically significant cell and molecular phenotypes. It is likely that observing disease phenotypes in vitro is much more amenable to those disorders or syndromes caused by a known strong genetic lesion. The strength of iPSCs is that the genome of these cells is identical to the patient from whom the cells were derived. Therefore, the intrinsic abnormalities governing neurodevelopmental processes may be present in the DNA and mitochondria of these cells. The exception to this is the de novo, somatic mutations that occurred within the patient’s body (unless of course the iPSCs were derived from those cells where the somatic mutation occurred). In contrast, environmental insults are not encoded within the cells, unless the insults are such that they leave an indelible epigenetic mark on the genome of cells used for derivation. For example, a head trauma experienced in a toddler that contributes to pathology would not be encoded in skin or blood cells used for derivation of iPSC lines. For these reasons, the majority of studies to date revealing significant developmental phenotypic effects have studied strong genetic lesions.
Gene editing has greatly improved the field’s sensitivity to detect significant phenotypes, and has facilitated our ability to attribute these phenotypes to the genetic lesion being studied. While incredibly powerful, it is important to be mindful of the variables that genome editing cannot control for between clonal lines. The first of these is the de novo mutations that will invariably accumulate with passage number. Some studies have suggested by whole genome sequencing of CRISPR- and TALEN-targeted iPSC lines that off target mutations from these genome editing technologies may be minimal. These studies have argued that the more serious obstacle to generation of truly isogenic lines was the clonal heterogeneity that results from the accumulation of de novo mutations with passage number14–16. Of course, off-target mutations may remain a concern in some situations despite ongoing improvements in methods to enhance target specificity. These findings suggest that even in studies of putatively isogenic iPSC lines, it will likely be necessary to examine multiple clones of each genotype.
Another variable to consider that may differ between lines is allele-specific gene expression due to X-inactivation, genomic imprinting, or stochastic monoallelic expression. X-inactivation occurs stochastically during the development of an organism to one or the other X-chromosome in females. Some studies have suggested that human pluripotent stem cells (hPSCs) maintain a clonal X-chromosome active state17 while others suggest that X-inactivation patterns are heterogenous in hPSCs18, 19. Genomic imprinting is a process by which certain genes are expressed in a parent-of-origin specific manner. Imprinting plays a central role in the manifestation of certain neuropsychiatric disorders such as Prader-Willi syndrome (PWS) and Angelman syndrome (AS). Studies of iPSCs from patients with these disorders suggest that imprinting status is maintained following reprogramming20,21. In addition to the 100 known imprinted genes22, stochastic monoallelic expression is now appreciated to be more widespread. In a recent study using RNAseq of human iPSC-derived neurons, 801 genes were identified that were expressed in an allele-biased manner23. Importantly, different culturing conditions affected both the X-inactivation status and stochastic monoallelic versus biallelic expression23. Thus, while the ability to generate isogenic lines is a valuable tool for determining cause-effect relationships between gene mutation and phenotype, it is still important to interrogate multiple clonal lines to ensure reproducibility between experiments.
Examples of studies modeling idiopathic disorders
A recent paper modeling bipolar disorder (BD) provides an illustrative example of how well-informed subject selection, coupled with careful characterization of cells, can identify novel cell and molecular phenotypes24. In this study, iPSC lines were made from a quartet containing two BD brothers and two unaffected parents24. Selection of a pedigree with a high incidence of mental illness may have increased the likelihood of the accumulation of deleterious alleles, and indeed, in depth genetic analysis of these subjects revealed the elevated “genetic load” for each subject24. The incorporation of close family members as controls in this study simplifies the confounding issues relating to genetic diversity. To study NPCs, neural differentiation was induced by FGF withdrawal followed by culture in neural induction media. Neural rosettes were then selected and expanded, and CNS NPCs purified via a FACs protocol. The authors reported difficulties in establishing immunopurified NPC lines specifically from the two BD patients, and not from the two parents24. A single NPC line was developed from each of the BD subjects, and these NPCs showed reduced BrdU incorporation, reduced viability following differentiation to neuronal fates, and alterations of expression of genes involved in neuroplasticity relative to lines derived from the cells of the parents24.
In another recent study, Mariani et al. (2015) utilized iPSCs derived from patients with autism with increased head circumference (HC)25. In this study, unaffected, first-degree family members were used as controls. Using an organoid culture system the investigators conducted transcriptome analysis,, and showed upregulation of genes in several pathways involved in cell proliferation, neuronal differentiation and synaptogenesis25. It may be tempting to speculate that these pathways are related to the underlying cause of macrocephaly and/or autism in the proband. Perhaps more surprising was the discovery of the overproduction of GABAergic neurons25. The authors also present data that the GABAergic phenotype could be ameliorated by reduction of expression of the transcription factor FOXG1, which was upregulated in the autism organoids25. An additional intriguing finding in this study (albeit with small sample size) was a significant correlation between FOXG1 levels and autism symptom severity25. This last observation reflects the valuable approach of drawing correlates between iPSC phenotypes and patient symptoms. This interesting new study has potentially revealed novel cellular and molecular mechanisms in autism pathogenesis. This and related studies can guide hypotheses which can be further interrogated in other systems, such as in postmortem studies.
Managing expectations in identifying cellular phenotypes in vitro
Theoretical considerations
In vitro modeling of strong genetic influences coupled with measures to limit technical variability increase likelihood of identifying neural phenotypes relevant to disease. While certain neurodevelopmental abnormalities cause dramatic malformations of cortical development (microcephaly, lissencephaly, periventricular heterotopias), the majority of neuropsychiatric diseases show only subtle morphological and pathological findings in imaging and postmortem studies26,27. With these relatively subtle morphological findings, what might we expect to observe when modeling these disorders in a dish? iPSCs may offer an opportunity to discover the most early cellular defects in human cells which may not be readily apparent in other systems. These early defects may be closer to the primary disease mechanism wherein the postmortem studies may reflect late stages of disease.
Specific examples of studies demonstrating strong phenotypes
Given the discussed variability observed between wells, lines, and differentiations, coupled to the often microscopic and subtle pathology observed in postmortem brain, one might consider it surprising to acquire new information about the mechanisms of neuropsychiatric disease with iPSC modeling. However, multiple studies to date have presented clear cellular phenotypes in iPSC-derived neurons for both genetic and idiopathic disorders. In the first published study using iPSCs to model schizophrenia, the authors showed robust defects in neuronal connectivity, neurite number and synaptic protein levels in iPSC-derived neurons9. In this pioneer study, iPSC lines were derived in three cases from patients with a parent with psychiatric disease, and in one case from a patient with childhood-onset schizophrenia9. Thus, patient selection may have assisted with discovery of profound neurodevelopment defects. In another study, neurons derived from subjects with a 4bp deletion in the C-terminus of DISC1 showed dramatic decreases in expression of presynaptic proteins, and a defect in depolarization-induced vesicle release, along with a number of morphological abnormalities28. For both studies, cells were directed to telencephalic fates, and the phenotypes identified by analyzing heterogeneous cultures of multiple fates. The widespread phenotypes observed suggest that the defects are at least somewhat global in nature within these neuronal subtypes.
Resolving subtle in vivo phenotypes with robust in vitro phenotypes
How might we resolve the relatively subtle pathology found in the postmortem studies with the strong phenotypes observed in the dish in some published studies? One possibility is that our understanding of the true magnitude of the pathology present in the developing human brain is masked by compensatory mechanisms such that by the time a patient with neuropsychiatric disease comes to autopsy, the pathological “scar” that we observe in postmortem analyses is minimal due to as-of-yet undetermined mechanisms involving repair, remodeling, and/or removal of maldeveloped cells. The developing human brain initially produces excessive numbers of cells and synapses that are pruned in later stages of development. Thus, mechanisms exist as part of normal brain developmental to cull those cells and synapses that are not necessary. There is evidence for the hypothesis that pathology may be obscured by compensatory mechanisms in postmortem studies of patients with ASD who died as children. In a relatively recent study29, a detailed postmortem analysis of cerebral cortical tissue was performed for subjects between the ages of 2 and 15. Interestingly, focal patches of abnormal laminar cytoarchitecture were observed in 10/11 children with ASD and in only 1/11 unaffected controls29. As there was no preselection for endophenotypes or specific genetic insults, these results suggest that defects such as these are much more prominent during development of the ASD brain than previously appreciated.
Another possible explanation for the strong phenotypes observed in some iPSC models is that the in vitro environment is highly sensitized such that the developmental effects of gene mutations are amplified. Selective pressures of the substrates upon which the cells are plated and abnormal environmental stressors of the culturing conditions may play a role in phenotype presentation. One salient feature of some iPSC-derived neuronal cultures is the lack of astrocytes, oligodendrocytes, and microglia, which in vivo may ameliorate some of the effects observed in vitro. For example, astrocytes play an important role in neuronal development, with roles in metabolic support, neurotransmitter recycling, synapse development and elimination and neuron survival. Since neurons and astrocytes share a common progenitor cell, most differentiation protocols result in the production of astrocytes at late differentiation time points30–32, while some do not5. However, culturing conditions are designed to promote neuronal health and survival, and endogenous astrocyte production can be variable between lines and differentiation conditions. The precise numbers and health of astrocytes in iPSC-derived neural cultures may have an important impact upon developmental phenotypes and should not be overlooked. In fact, recent studies have shown that disrupted astrocyte development may underlie certain neurodevelopmental disorders (reviewed in33). In a recent iPSC-based study of Costello syndrome (CS), the importance of proper astrocyte development on neural development was highlighted34. CS is a RASopathy characterized by macrocephaly, cognitive impairment, ASD-like traits and tumor formation. CS results from mutation of HRAS (Harvey rat sarcoma viral oncogene homolog), which induces hyperactive Ras signaling. Using iPSCs from four patients with CS, the study showed that astrocytes with HRAS mutation differentiated more rapidly, exhibit altered morphology, and released excessive extracellular matrix remodeling factors and proteoglycans34. Complemented by in vivo studies in a mouse model of the disease, the authors showed that this altered astrocyte development had an impact on synapse development and function, resulting in enhanced or premature inhibitory synaptic strength34.
Further still, it appears that iPSCs are allowing the identification of cellular phenotypes that might otherwise not be feasible in human systems. For example, in NPCs derived from iPSCs from patients with 15q11.2 microdeletion, a candidate susceptibility variant in schizophrenia and other neuropsychiatric conditions, there are defects in adherens junctions and apical polarity35. These observations led to specific follow-up hypotheses in mice that then were corroborated in vivo, again demonstrating the synergy of the in vitro iPSC and in vivo mouse experiments35.
Conclusions
In summary, the advancing state of the iPSC field appears to be presenting unprecedented opportunities to identify cellular and developmental phenotypes with relevance to neuropsychiatric disorders. The clear strengths in this approach involve the study of human neurons, those derived directly from patients or genetically-engineered. Studies in cells with both strong mutations as well as studies in cells from patients with idiopathic disease hold promise. Researchers in the field have been rapidly developing methods that begin to address some of the challenges. These include methods for addressing sources of variability as well as methods for development of robust controls. Novel approaches to study connectivity and 3D aspects of brain development also are rapidly evolving. Despite the clear progress and important role that iPSC methods now occupy in the field, there are natural limitations. Studies that corroborate the in vitro human iPSC studies with in vivo animal studies where possible will be a particularly powerful approach.
Highlights.
Considerations for choosing and making the appropriate cell types to model neuropsychiatric disease.
Understanding the sources of variability in iPSC models of neurodevelopmental disorders, and considerations for minimizing or bypassing this variability.
Challenges to modeling neuropsychiatric disease of known strong genetic influence versus idiopathic disease.
Managing expectations in identifying cellular phenotypes in vitro
Acknowledgments
This work was supported by NIMH R01MH101148 (TLY-P), NIA R33AG049864 (TLY-P), the Brain and Behavior Research Foundation (TLY-P), NIMH R01MH102418 (EMM) and NIMH R01MH105442 (EMM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. Pasca SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, Pasca AM, Cord B, Palmer TD, Chikahisa S, Nishino S, et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. * This study, in concert with Krey et al, demonstrates how detailed analyses of iPSC-derived neurons harboring a powerful mutation can yield novel insights into neurodevelopmental processes.
- 2.Krey JF, Pasca SP, Shcheglovitov A, Yazawa M, Schwemberger R, Rasmusson R, Dolmetsch RE. Timothy syndrome is associated with activity-dependent dendritic retraction in rodent and human neurons. Nat Neurosci. 2013;16:201–209. doi: 10.1038/nn.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson MB, Wang PP, Atabay KD, Murphy EA, Doan RN, Hecht JL, Walsh CA. Single-cell analysis reveals transcriptional heterogeneity of neural progenitors in human cortex. Nat Neurosci. 2015;18:637–646. doi: 10.1038/nn.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. ** This study describes the development of a new methodology for rapidly and inexpensively sequencing RNA from single cells. This technology could have a major impact on studies of neuropsychiatric disorders, aiding in the discovery of the cell types most affected by genetic insults.
- 5.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Srikanth P, Han K, Callahan DG, Makovkina E, Muratore CR, Lalli MA, Zhou H, Boyd JD, Kosik KS, Selkoe DJ, Young-Pearse TL. Genomic DISC1 Disruption in hiPSCs Alters Wnt Signaling and Neural Cell Fate. Cell Reports. 2015;15:851–857. doi: 10.1016/j.celrep.2015.07.061. ** This study highlights that it is possible to identify modest but significant alterations in neurodevelopment that are attributable to disruption of a single gene, using thorough analyses of multiple isogenic hiPSC lines over many differentiations with high numbers of wells.
- 7.Porteous DJ, Millar JK, Brandon NJ, Sawa A. DISC1 at 10:connecting psychiatric genetics and neuroscience. Trends Mol Med. 2011;17(12):699–706. doi: 10.1016/j.molmed.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9(9):1415–1423. doi: 10.1093/hmg/9.9.1415. 22. [DOI] [PubMed] [Google Scholar]
- 9.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014;9:2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. ** This study, along with the detailed protocol in Lancaster et al 2014, describe a powerful technique that allows one to follow human neural development from iPSCs in a three dimensional structure.
- 12.Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, Beaumont KG, Kim HJ, Topol A, Ladran I, Abdelrahim M, Matikainen-Ankney B, Chao SH, Mrksich M, Rakic P, Fang G, Zhang B, Yates JR, 3rd, Gage FH. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. 2015;20(3):361–368. doi: 10.1038/mp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shcheglovitov A, Shcheglovitova O, Yazawa M, Portmann T, Shu R, Sebastiano V, Krawisz A, Froehlich W, Bernstein JA, Hallmayer JF, et al. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature. 2013;503:267–271. doi: 10.1038/nature12618. ** This study used creative and well-designed experiments to circumvent the heterogeneity observed in neural differentiations of iPSCs, to show defects of excitatory, but not inhibitory, synaptic transmission.
- 14.Smith C, Gore A, Yan W, Abalde-Atristain L, Li Z, He C, Wang Y, Brodsky RA, Zhang K, Cheng L, et al. Whole-genome sequencing analysis reveals high specificity of CRISPR/Cas9 and TALEN-based genome editing in human iPSCs. Cell Stem Cell. 2014;15:12–13. doi: 10.1016/j.stem.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veres A, Gosis BS, Ding Q, Collins R, Ragavendran A, Brand H, Erdin S, Cowan CA, Talkowski ME, Musunuru K. Low incidence of off-target mutations in individual CRISPR-Cas9 and TALEN targeted human stem cell clones detected by whole-genome sequencing. Cell Stem Cell. 2014;15:27–30. doi: 10.1016/j.stem.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K, Yu C, Qu J, Li M, Yao X, Yuan T, Goebl A, Tang S, Ren R, Aizawa E, et al. Targeted gene correction minimally impacts whole-genome mutational load in human-disease-specific induced pluripotent stem cell clones. Cell Stem Cell. 2014;15:31–36. doi: 10.1016/j.stem.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tchieu J, Kuoy E, Chin MH, Trinh H, Patterson M, Sherman SP, Aimiuwu O, Lindgren A, Hakimian S, Zack JA, et al. Female human iPSCs retain an inactive X chromosome. Cell Stem Cell. 2010;7:329–342. doi: 10.1016/j.stem.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruck T, Benvenisty N. Meta-analysis of the heterogeneity of X chromosome inactivation in human pluripotent stem cells. Stem Cell Res. 2011;6:187–193. doi: 10.1016/j.scr.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Kim DH, Jeon Y, Anguera MC, Lee JT. X-chromosome epigenetic reprogramming in pluripotent stem cells via noncoding genes. Semin Cell Dev Biol. 2011;22:336–342. doi: 10.1016/j.semcdb.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Germain ND, Chen PF, Plocik AM, Glatt-Deeley H, Brown J, Fink JJ, Bolduc KA, Robinson TM, Levine ES, Reiter LT, et al. Gene expression analysis of human induced pluripotent stem cell-derived neurons carrying copy number variants of chromosome 15q11-q13.1. Mol Autism. 2014;5:44. doi: 10.1186/2040-2392-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamberlain SJ, Chen PF, Ng KY, Bourgois-Rocha F, Lemtiri-Chlieh F, Levine ES, Lalande M. Induced pluripotent stem cell models of the genomic imprinting disorders Angelman and Prader-Willi syndromes. Proc Natl Acad Sci U S A. 2010;107:17668–17673. doi: 10.1073/pnas.1004487107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radford EJ, Ferron SR, Ferguson-Smith AC. Genomic imprinting as an adaptative model of developmental plasticity. FEBS Lett. 2011;585:2059–2066. doi: 10.1016/j.febslet.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 23. Lin M, Hrabovsky A, Pedrosa E, Wang T, Zheng D, Lachman HM. Allele-biased expression in differentiating human neurons: implications for neuropsychiatric disorders. PLoS ONE. 2012;7:e44017. doi: 10.1371/journal.pone.0044017. * This study used iPSC technology and RNA sequencing to identify 801 genes expressed in an allele-biased manner in human neurons, highlighting an often overlooked and possibly important aspect of the biology of these cells.
- 24. Madison JM, Zhou F, Nigam A, Hussain A, Barker DD, Nehme R, van der Ven K, Hsu J, Wolf P, Fleishman M, et al. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol Psychiatry. 2015;20:703–717. doi: 10.1038/mp.2015.7. * This study generated and analyzed iPSCs from a pedigree enriched for bipolar disorder, and with careful characterization of differentiated cells identified neurodevelopmental phenotypes associated with BD.
- 25. Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, Gerstein M, Grigorenko EL, Chawarska K, Pelphrey KA, Howe JR, Vaccarino FM. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell. 2015;162(2):375–390. doi: 10.1016/j.cell.2015.06.034. * This pioneering study utilizes organoid cultures in idiopathic autism with macrocephaly to model the cellular and molecular processes potentially involved in disease. They discover a novel overproduction of GABAergic cells.
- 26.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2011;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. * This study is one of the recent large studies of postmortem brain in autism. They identify increased dendritic spine density in autism temporal lobe. The spine deficits correlate with hyperactivated mTOR and impaired autophagy. They then corroborate findings in relevant mouse mutants.
- 28.Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014;515:414–418. doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Wynshaw-Boris A, Colamarino SA, Lein ES, Courchesne E. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370:1209–1219. doi: 10.1056/NEJMoa1307491. ** The findings of this study bring to light the widespread nature of focal defects of cortical laminar architecture in young patients with autism that is not evident in adult subjects. This provides evidence that morphological phenotypes in neuropsychiatric disorders may be more pronounced when examined during developmental time windows.
- 30.Muratore CR, Srikanth P, Callahan DG, Young-Pearse TL. Comparison and optimization of hiPSC forebrain cortical differentiation protocols. PLoS ONE. 2014;9:e105807. doi: 10.1371/journal.pone.0105807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE. 2011;6:e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng H, Guo M, Martins-Taylor K, Wang X, Zhang Z, Park JW, Zhan S, Kronenberg MS, Lichtler A, Liu HX, et al. Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells. PLoS ONE. 2010;5:e11853. doi: 10.1371/journal.pone.0011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sloan SA, Barres BA. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr Opin Neurobiol. 2014;27:75–81. doi: 10.1016/j.conb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krencik R, Hokanson KC, Narayan AR, Dvornik J, Rooney GE, Rauen KA, Weiss LA, Rowitch DH, Ullian EM. Dysregulation of astrocyte extracellular signaling in Costello syndrome. Sci Transl Med. 2015;7:286ra266. doi: 10.1126/scitranslmed.aaa5645. ** This interesting study coupled hiPSC and rodent models to show a defect in astrocyte function with a mutation that causes Costello syndrome, a RASopathy.
- 35.Yoon KJ, Nguyen HN, Ursini G, Zhang F, Kim NS, Wen Z, Makri G, Nauen D, Shin JH, Park Y, Chung R, Pekle E, Zhang C, Towe M, Hussaini SM, Lee Y, Rujescu D, St Clair D, Kleinman JE, Hyde TM, Krauss G, Christian KM, Rapoport JL, Weinberger DR, Song H, Ming GL. Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell. 2014;15(1):79–91. doi: 10.1016/j.stem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]