Abstract
Scope
We studied the impact of dietary supplementation with licorice root components on diet-induced obesity, fat accumulation and hepatic steatosis in ovariectomized C57BL/6 mice as a menopause model.
Materials and Methods
We evaluated the molecular and physiological effects of dietary licorice root administered to ovariectomized C57BL/6 mice as root powder (LRP), extracts (LRE) or isolated isoliquiritegenin (ILQ) on reproductive (uterus and mammary gland) and non-reproductive tissues important in regulating metabolism (liver, perigonadal, perirenal, mesenteric and subcutaneous fat). Quantitative outcome measures including body weight, fat distribution (MRI), food consumption, bone density and weight (DXA) and gene expression were assessed by the degree of restoration to the premenopausal health state. We characterized histological (H&E and oil red O staining) and molecular properties (expression of certain disease markers) of these tissues, and correlated these with metabolic phenotype as well as blood levels of bioactives.
Conclusions
Although LRE and ILQ provided some benefit, LRP was the most effective in reducing body weight gain, overall fat deposition, liver steatosis, and expression of hepatic lipid synthesis genes following ovariectomy. Our data demonstrate that licorice root provided improvement of multiple metabolic parameters under conditions of menopausal low estrogen and high-fat diets without stimulating reproductive tissues.
Keywords: Botanical estrogens, Dietary Supplements, Estrogen Receptor, Licorice Root, Metabolism, Menopause
1 Introduction
Increased cardiovascular disease (CVD)-related deaths associated with obesity and diabetes renders this triad one of the biggest public health problems and the number one cause of morbidity among women in the US [1]. For premenopausal women, the risk of diabetes and CVD is half that of men, but after menopause, the incidence of metabolic disease and CVD doubles for women, eventually reaching that for men [1]. With declining estrogen levels at menopause, there is an increase in abdominal fat, insulin resistance and CVD risk factors [2–4]. Sedentary life style, restricted mobility, and western-style, fat- and sugar-rich diets, combined with low estrogens in postmenopausal women, aggravate this problem, making postmenopausal women more susceptible to metabolic syndrome and associated diabetes and CVD [2, 5, 6].
Estrogen-containing hormone replacement therapies (HRT) improve insulin sensitivity, decrease type 2 diabetes, and overall fat deposition. They also maintain bone health, and suppress hot flush [2, 6, 7]. If initiated at the time of menopause, HRT can also maintain cardiovascular health, but if the start of HRT is delayed, other cardiovascular risks are increased [8–10]. Certain regimens of HRT, such as PremPro® (Estrogen+Progestin), also increase the risk of BC [8–10], and overall, estrogens are proproliferative for uterus and breast, raising the risk of cancer in these tissues. Considering the health concerns associated with traditional HRT, it is not surprising that older women seek alternatives to support their health and for relief from menopausal symptoms. In fact, over the past decade the use of HRT has steadily declined, whereas the use of botanical estrogen dietary supplements (BEDS) as alternatives has risen, so that now almost 50% of postmenopausal women are taking estrogenic dietary supplements [9, 11, 12]. The number of botanical products offered has also increased dramatically, including mixtures and chimeras of supplements used for multiple purposes. All these products are targeted to older women and are being consumed with the perceptions that they provide safe, “natural” alternatives to HRT.
Soy and soy isoflavones are still the most widely used botanical estrogens (BEs), and although they have been subject to considerable research, there is still some controversy surrounding their actions [9, 11, 13–16]. Beyond soy, botanical dietary supplements containing licorice root are taken by women who wish to maintain their health and seek relief from menopausal symptoms. All of these botanicals are known or reported to contain compounds with estrogenic activity, but, like soy, these compounds are structurally very different from the steroidal estrogens in HRT and thus likely to have mechanistically different actions. Licorice root, which we showed previously to contain isoliquiritigenin, acts as a low affinity estrogen through ERα [17, 18]. Licorice contains the flavone liquiritigenin (LIQ), which is in dynamic equilibrium with the isomeric chalcone isoliquiritigenin (ILQ) [19]. From our work [18] and that of others [19], the major targets of the prenylflavonoids are most likely the ERs, although their ERβ preference is less than that of the soy isoflavones [18], suggesting the involvement of other mechanisms.
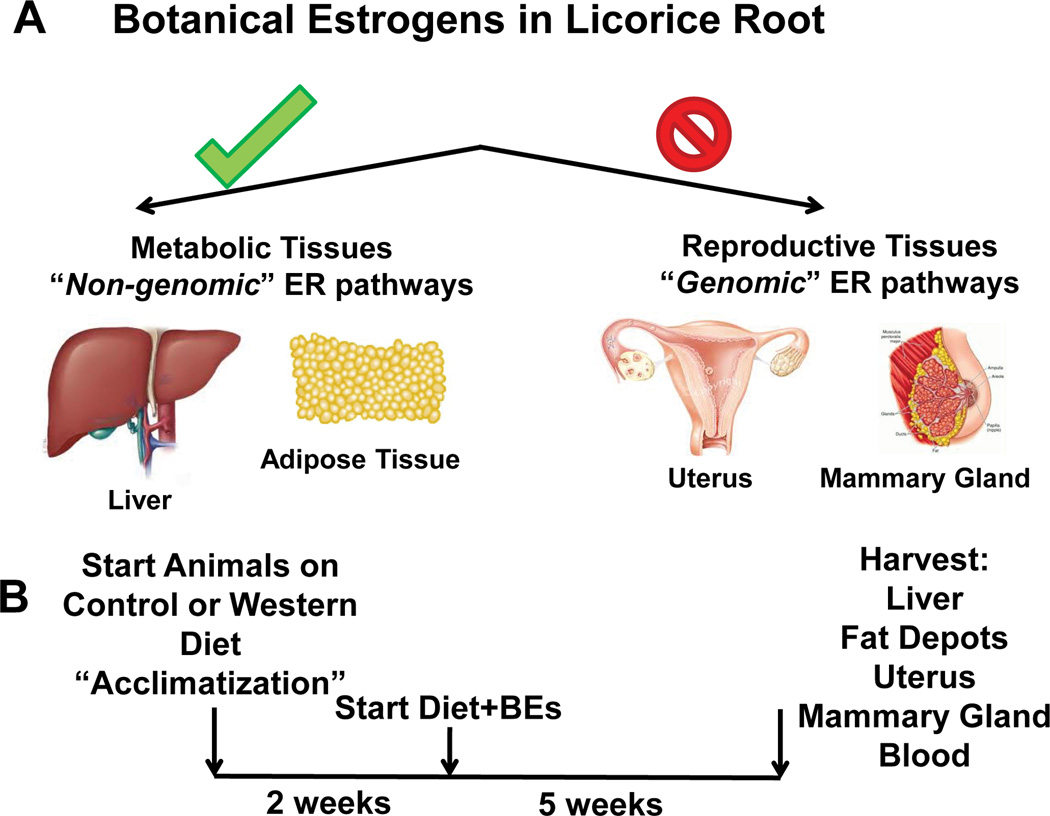
Women consuming BEDS might be facing unknown risks or might be reaping unexpected benefits as a result of the selective and mechanistically distinct actions that the structurally diverse BEs have on the full set of estrogen target tissues. In this study, we evaluated the effectiveness of BE from licorice root in maintaining metabolic health as well as providing resiliency to the detrimental effects of high fat diets, using preclinical mouse models. Estrogenic compounds decrease weight gain and improve glucose and insulin sensitivity in various animal models including high-fat diet fed, ovariectomized-mice [20]. Our central hypothesis is that Botanical Estrogens (BEs) from licorice root have preferential estrogenic activities in metabolic tissues, which would provide selective desired metabolic effects without stimulating reproductive tissues and would provide resiliency to menopause and high fat diet-induced metabolic syndrome and obesity (Figure 1A). In order to test our hypothesis, we investigated the impact of dietary supplementation of licorice root, as root powder, extract or pure ILQ in reproductive and non-reproductive metabolic tissues after ovariectomy and high-fat diets in C57BL/6 mice. We fed licorice root powder (LRP), licorice root extract (LRE) and ILQ as part of the diet to mimic daily intake as dietary supplement and monitored the molecular effects of licorice on various fat depots as well as the liver which is the metabolic control center of the body. Our findings suggest that licorice root powder (but not LRE or ILE) prevents obesity associated with loss of estrogens and high-fat diet without producing any stimulatory effects on reproductive tissues.
Figure 1.
(A) Hypothesis for preferential BE action in non-reproductive tissues vs. reproductive tissues. (B) Design of the study. C57BL6 Mice (8/group) were fed either a control AIN76A diet (normal, low fat diet, 16% Kcal from fat) or a High Fat Diet (HFD, 45% Kcal from fat) for 2 weeks and then switched to diets containing no licorice root botanical (control), licorice root powder (LRP), Licorice root extract (LRE) or isoliquiritigenin (ILQ) for 5 weeks prior to tissue and blood analyses
2 Materials and Methods
2.1 Animals and Diets
To test the impact of the BEs on resiliency to obesity and metabolic health after menopause, we used ovariectomized C57BL/6 mice that were fed a high fat diet and compared parameters also in animals on a control diet. This is a commonly used method for inducing metabolic disease associated with menopause in mice [21–23]. Because normal chow usually contains isoflavones, which can have estrogenic activities that could abrogate the metabolic effects of ovariectomy, we used a phytoestrogen-free semi-purified AIN76A (Harlan, CA.170481) diet having casein as the protein source. The high fat diet for these studies (Harlan TD.88137) is termed “western diet” and is based on an AIN76A diet (45% Kcal from fat). It is high in butterfat with sugar and some cholesterol (0.2%), and it was developed for metabolic studies [21, 22]. The very high fat diets (60% Kcal from fat) were not used to induce obesity, because we wanted to study a comparable level of fat consumed by humans in the US (~34% Kcal from fat; NHANES). For these studies, we used 8–10 week-old ovariectomized female animals that were randomized to normal and high fat diets plus various BEs supplemented in the food and monitored over several weeks.
The doses were selected for relevance to predicted human consumption in dietary supplements. Relevance to human exposure was determined using web-based supplementation estimates that were obtained for whole licorice root powder consumption by humans at up to 15 g/d and tablets made from a 10-fold concentrated extract of licorice root at 7.6 g/d of whole root equivalents [26]. The total flavanone + chalcone (liquiritigenin (LIQ) + ILQ) content in whole licorice root preparations from several Glycyrrhiza species are approximately around 10 mg LIQ + ILQ per g of root (1%) [27]. Therefore, when one considers the two representative supplementation scenarios discussed above for a 70 kg human, these consumption estimates correspond to approximately 1–2 mg of LIQ + ILQ per kg bw/d. Given the allometric scaling relationship based solely on bw3/4, a 25-g mouse would require approximately a 7-fold higher dose on a mg/kg bw basis (and a 250-g rat would require a 4-fold higher dose), to achieve an equivalent AUC [28]. The human equivalent dose in a mouse would be approximately 10–15 mg/kg bw/d. The dietary level chosen to evaluate biological effects was 5% LRP, which is equivalent to approximately 50 mg/kg bw/d based on consumption of diet equivalent to 10% of mouse bw/d. The LRE diet (0.5% of a 10-fold concentrated extract) was prepared to deliver the same dose of whole root equivalents (50 mg/kg bw/d). The ILQ diet (0.05%) was chosen to deliver a similar dose of purified ILQ (50 mg/kg bw/d). All of the experimental procedures were approved by the Illinois Institutional Animal Care and Use Committee (Protocol ID 13315) and were conducted in accordance with the regulations described in the Committee's Manual.
2.2 Administration of BEs and Dosages
All of the BEs used in these studies were provided by the Botanical Center at the University of Mississippi. The identity and purity of all botanical components were verified by chromatography and spectroscopy by the same group, as done in prior studies [18]. The BEs were administered to laboratory animals through pelleted AIN76 - based rodent diet into which the licorice root components have been incorporated either as powders, dried methanol extracts, or major active components. Because BEDS are consumed by humans as powders, extracts, and fractionated products, we believe that this approach should provide a realistic view of what effects might be expected in humans. BE administration in food is also very practical, and with pelleted feed, we could quantify food consumption. In our experience, daily dosing of animals by gavage frequently lead to stress and reduced food consumption that seriously confounds experimental results and was therefore not used.
2.3 Identification of licorice root components in the blood
The internal dosimetry of LIQ and ILQ was evaluated in serum from adult female C57Bl/6 mice collected at the time of sacrifice after 5 weeks on the respective diets A validated LC/MS/MS method with internal standard quantification (d3-daidzein and d4-genistein for LIQ and ILQ, respectively) was used to quantify total conjugated and aglycone forms of LIQ and ILQ (see Table 1, limits of detection 0.0002–0.006 µM depending on volume analyzed), without and with enzymatic hydrolysis, respectively (n = 8–10 mice). The timing of sacrifice with respect to the last consumption of dosed diets was not known, so the single point estimates contain considerable uncertainty about maximum serum concentrations and inter-animal variability.
Table 1.
Blood levels of LIQ and ILQ, as total and aglycones, in animals on control diets (C) or high fat diets (HF) containing licorice root powder (LRP), licorice root extract (LRE), or pure isoliquiritigenin (ILQ).
| Dose Group |
LIQ-Total (µM) |
LIQ-Aglycone (µM) |
% Aglycone |
LIQ/ILQ Ratio |
ILQ-Total (µM) |
ILQ-Aglycone (µM) |
% Aglycone |
|---|---|---|---|---|---|---|---|
| C | <LODa | <LOD | -- | -- | <LOD | <LOD | -- |
| C-LRP | 3.8 | 0.0034 | 0.1 | 8 | 0.46 | 0.0041 | 0.9 |
| C-LRE | 1.9 | 0.0175 | 0.9 | 11 | 0.17 | 0.0014 | 0.8 |
| C-ILQ | 0.037 | <LOD | -- | 0.3 | 0.13 | 0.0083 | 6.4 |
| HF | 0.014 | <LOD | -- | -- | <LOD | <LOD | -- |
| HF-LRP | 2.9 | 0.0054 | 0.2 | 11 | 0.27 | 0.0023 | 0.9 |
| HF-LRE | 1.4 | 0.0036 | 0.3 | 16 | 0.09 | 0.0008 | 0.9 |
| HF-ILQ | 0.055 | 0.0004 | 0.7 | 0.3 | 0.18 | 0.013 | 7.1 |
<LOD, below level of detection
2.4 Histological characterization of tissues
Hematoxylin and eosin (H&E) staining, Oil Red O (ORO) staining and whole mount staining were performed on paraffin-embedded mouse tissue sections [29].
2.5 Gene expression analysis
Tissues were immediately snap frozen upon removal from mice. HepG2-ERα expressing cells were maintained in William’s E medium. Cells were treated with 1 nM E2, 10 nM ILQ or LIQ in the presence or absence of 1 µM of the antiestrogen Fulvestrant for 24 hours. Gene expression was determined using quantitative real time PCR and primers designed for the indicated genes. Primer sequences were obtained from Primer Data Bank (http://pga.mgh.harvard.edu/primerbank/). Total RNA was isolated, reverse-transcribed, and analyzed by real-time PCR, as described previously [30, 31].
2.6 Dual-energy X-ray Absorptiometry (DXA) Analysis of bone
Femur bone mineral content (BMC, mg) and area (cm2) were measured using DXA (Piximus, Lunar Corp., Madison, WI, USA). Bone mineral density (BMD) was calculated as BMC/area (mg/cm2).
2.7 Statistics
Data from metabolism studies were analyzed using either one-way ANOVA to compare different BE effects or two-way ANOVA to compare the effects of different diets and BEs followed by Bonferroni post hoc test using GraphPad Prism 6. Data from gene expression studies were analyzed using t-test. Differences were considered statistically significant if * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. Data are shown as means ±SEM.
3 Results
3.1 Establishing the effects of BEs on weight gain and fat distribution after ovariectomy and high fat diet
It is now widely accepted that estrogens are protective in premenopausal women by preventing metabolic syndrome and associated diabetes and CVD risk [32]. Women who used estrogen or estrogen plus progesterone hormone replacement therapy had improved insulin sensitivity, decreased fat depots, and decreased CVD risk, suggesting a tissue-selective mode of action of estrogens on the health and maintenance of metabolic tissues [6, 33, 34]. Based on this data we proposed that licorice root, which contains ER-binding molecules, should improve the impaired metabolic phenotype due to loss of estrogens by normalizing body weight, decreasing overall adiposity, decreasing lipid accumulation in liver, and inducing gene expression changes in liver and fat depots in a manner similar to E2, while having minimal effects on uterus and mammary tissues (Figure 1A).
To test our hypothesis, we divided 10 week old ovariectomized wild type C57BL6 mice into two diet groups. Diets were formulated in both normal and high fat form, the latter to provide a dietary stress frequently encountered in humans. One group continued on normal diet (AIN76A) whereas the second group was placed on high fat diet for 2 weeks. Animals in the vehicle-control and E2-treated (5 µg/day; pellets containing 0.125 mg E2) groups received pellets subcutaneously, respectively, whereas animals treated with various licorice root components received the BEs supplemented in their food. Dietary feeding of BEs is a reasonable approximation of daily use of BEs as dietary supplements taken by women in the form of pills once or a few times daily.
We have found that by matching the concentrations in the plasma of the laboratory animals with that found in humans consuming BEDS (when known), we are best able to determine appropriate doses for our studies. In general, the BEs are administered in the diet at a dose of 5% w/w of the root powder, 0.5% of a dried methanol extract, or a lesser amount of principal components of the extract (Figure 1B). In this form, LRP provided the highest blood concentration of LIQ and ILQ both in total and aglycone form (Table 1). Table 1 shows that the LRP and LRE diets produced similar serum levels of LIQ and ILQ aglycones in the nM range, with LIQ levels consistently higher than ILQ, which presumably reflects the flavanone/chalcone ratio in G. uralensis [27] as well as the known interconversion between these two isomeric substances [35]. The diet containing purified ILQ produced serum levels of ILQ in the same range as the LRP and LRE diets. Consumption of the purified ILQ diet also produced measurable levels of LIQ in serum, but these levels were approximately 10-fold lower than those for ILQ. The aglycones were consistently below 1% of the total LIQ and ILQ present in serum for LRP and LRE diets, which is consistent with extensive Phase II metabolism after ingestion.
In our study on licorice root (LR), as powder (LRP), extract (LRE) and pure isoliquiritigenin (ILQ) we found that both E2 and LRP were effective in normalizing weight in control and western (high fat) diet-fed animals (Fig 2A), mainly due to changes in overall adiposity (Figure 2B) but not lean mass (Figure 2C) as verified by whole body magnetic resonance imaging (MRI). This result is in line with the blood levels of bioactive aglycone forms shown in Table 1 and suggests that these differences might likely become pronounced at higher dosages of LRE or ILQ in the diet and possibly with longer treatment times.
Figure 2. BEs are very effective in normalizing weight gain associated with ovariectomy under normal and high fat diets.
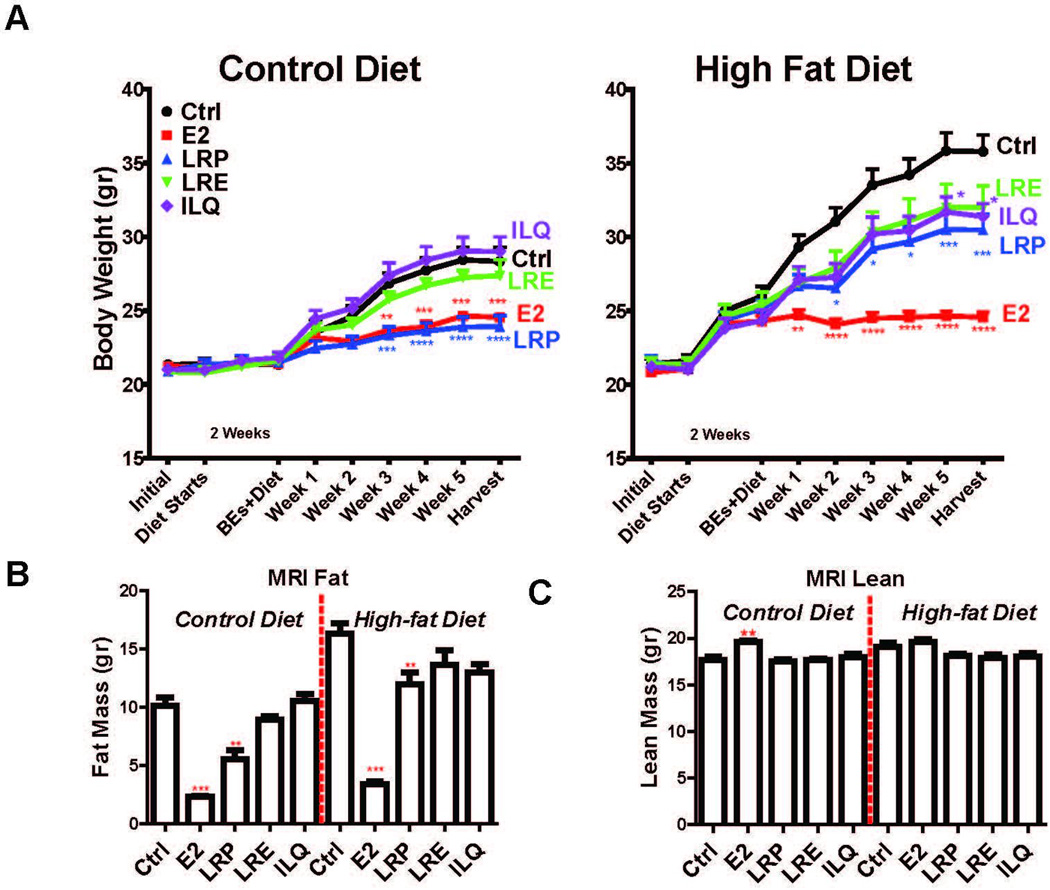
(A) Total Weight of animals on various diets supplemented with BEs. Two-way ANOVA, Bonferroni posttest, * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001. (B) BEs decrease adiposity of mice on normal and high fat diet without affecting lean mass. Fat and lean mass of animals on various diets supplemented with BEs as identified with whole body MRI. Two-way ANOVA, Bonferroni posttest, * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
To understand whether or not the changes in total weight were due to food intake, we monitored food consumption over the duration of the study (Figure 3). Animals that were on the high-fat diet consumed less food (Figure 3A), however energy intake was similar (Figure 3B). As expected, E2 decreased food intake in both normal and high-fat diet groups (Figure 3B). However, the licorice root component-containing diets did not alter the food intake (Figure 3B), indicating that this was not a determinant of differing body weights and changes in the fat depots. Thus, our studies suggest that BEs from licorice root are very effective in normalizing weight gain associated with ovariectomy under normal and high fat diets and BEs decrease adiposity of mice on normal and high fat diet without affecting lean mass.
Figure 3. BEs do not affect food intake.
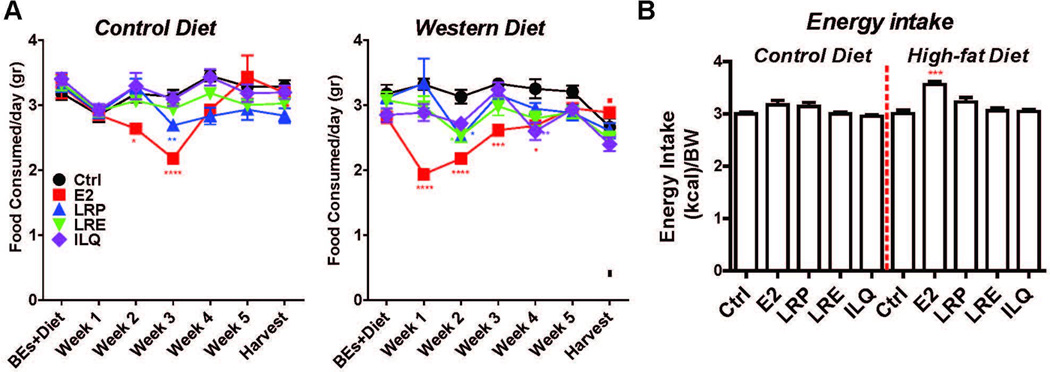
(A) Total weight of food consumed and (B) Energy intake on various diets supplemented with BEs. Two way ANOVA, Bonferroni posttest, * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001
3.2 BEs reduce weight of various fat depots without increasing weight of uterus or causing gene expression changes in reproductive tissues
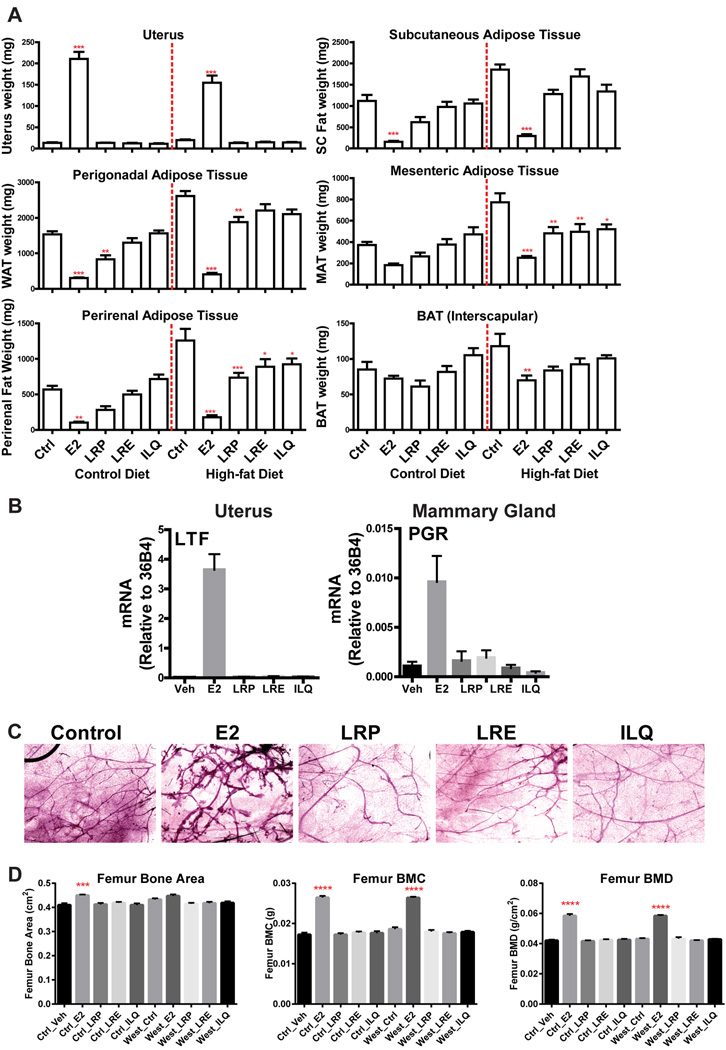
By monitoring changes in reproductive and non-reproductive tissues, we found E2 greatly increased growth of the uterus, as expected, whereas none of the BEs increased uterine weight. On the other hand, both E2 and LRP were very effective in reducing the weight of perigonadal, subcutaneous, mesenteric and perirenal adipose tissue in high fat diet-fed groups (Figure 4). Regardless of the regional differences [36], each of these white adipose depots was affected in a similar manner by the treatments, suggesting a general trend toward decreasing adiposity by BEs and E2. On the control AIN76A diet, E2 reduced adipose tissue depots but this was seen to be significant for LRP only in perigonadal adipose tissue, although trends toward a decrease with LRP were observed in other depots. By contrast, even though brown adipose tissue (BAT) is an important contributor to metabolism and energy homeostasis [37], BEs did not change the weight of this tissue (Figure 4A). Moreover, when we monitored gene expression changes in mammary gland and uterus none of the BEs induced expression of E2 responsive genes (Figure 4B). E2 was very effective in increasing mammary gland ductal formation, whereas LRE, LRP or ILQ did not induce such changes in mice (Figure 4C). In addition, neither of the BEs from licorice root affected femur bone area or bone weight (Figure 4D) further supporting tissue selective activities of licorice root components in liver and adipose tissue. Licorice root BEs do not activate the same molecular mechanisms as E2 does in uterus, mammary gland and bone. However, both the licorice root powder, extract and ILQ behaved like E2 in adipose depots, suggesting that they work through similar molecular pathways in these tissues. Thus, our findings show that licorice root bioactives are very effective in decreasing lipid deposition of various fat depots without stimulating mammary gland and uterus or affecting bone.
Figure 4. BEs reduce weight of various fat depots without increasing weight of the uterus.
(A) Weight of uterus and several fat depots from animals on various diets supplemented with BEs. (B) BEs don’t induce expression of E2 responsive genes in uterus or mammary gland. (C) BEs don’t induce mammary gland ductal branching. (D) BEs do not affect bone mass. Two-way ANOVA, Bonferroni posttest, * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
3.3 E2 and Licorice Root decrease lipid deposition in liver and fat depots and modulate the same molecular circuitries in metabolic tissues
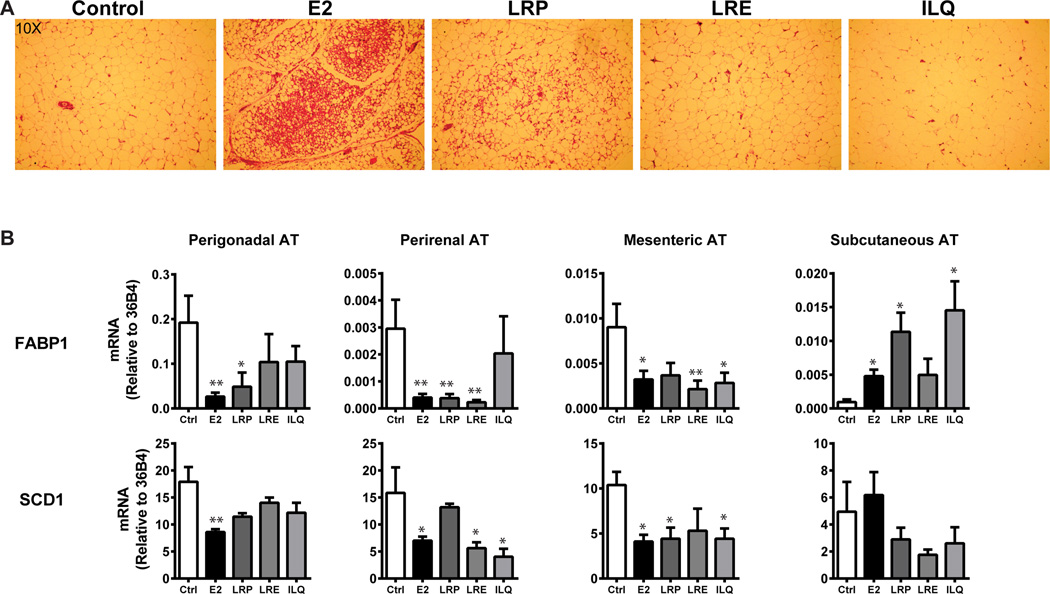
Since we saw differences in lipid deposition with BE supplementation in the diet, we monitored the histological and molecular changes in various tissues. We observed dramatic liver and adipose tissue phenotypes in E2 and LRP-treated animals, especially in those on the high fat diet (Figures 5, 6). Removal of estrogens by ovariectomy combined with high fat diet increased total adiposity in perigonadal adipose tissue, and treatment with E2 or LRP greatly decreased size of the adipocytes, as observed in H&E stained tissue sections (Figure 5A). In addition to these markers, we also monitored SCD1 [38] and FABP1 in adipose tissue [39]. These changes were consistent with the H&E staining and suggested a preventive role for LRP and LRE in high-fat induced lipid deposition in adipocytes. Both E2 and Licorice root components (root powder, extract or ILQ) modulated the genes that are critical for lipid deposition.
Figure 5. E2 and LRP decrease adipocyte area in perigonadal fat tissue of mice on high fat diet.
(A) Histology of perigonadal adipose tissue from animals on high fat diet supplemented with control, E2, LRE, LRP or ILQ. (B) Gene expression analysis of various fat depots from animals on high-fat diet supplemented with various BEs. t-test, * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001
Figure 6. E2 and LRP decrease hepatic steatosis through ERα.
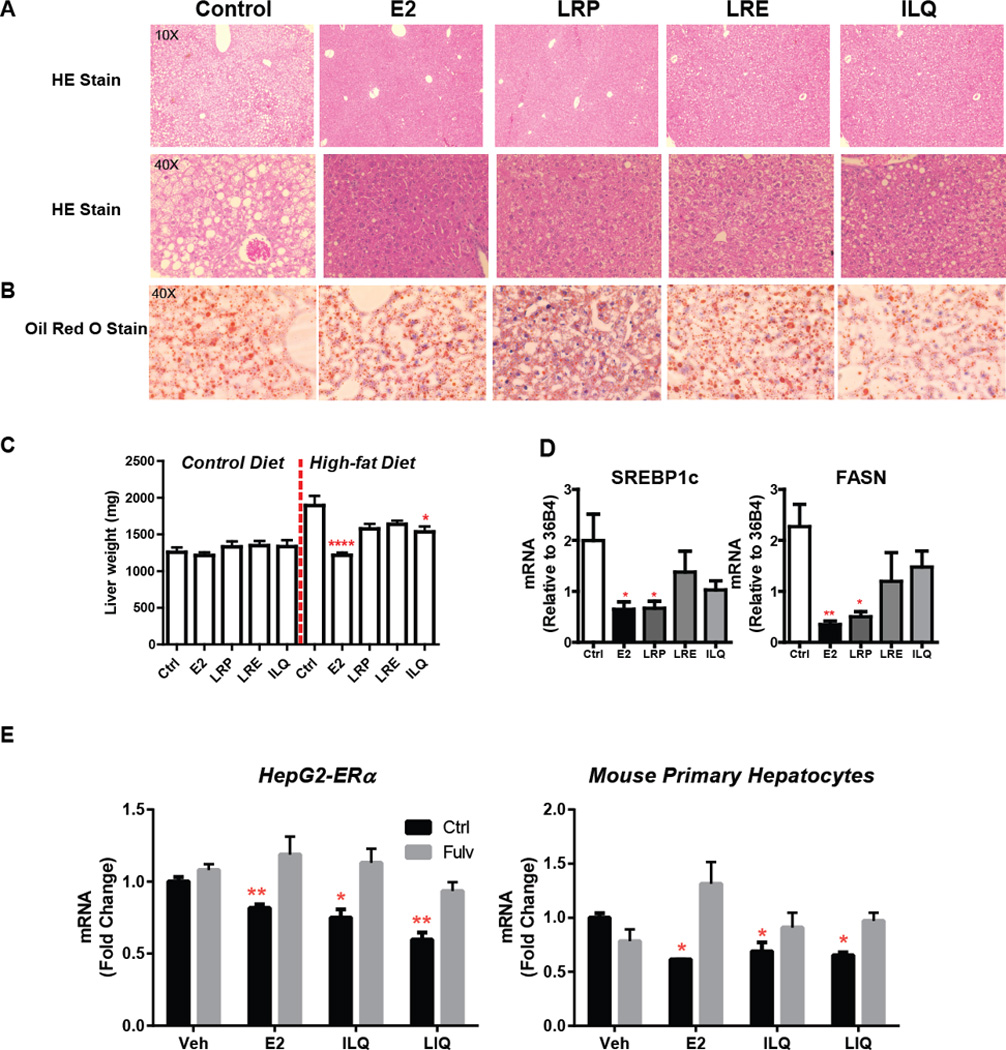
(A) Histology of liver (B) Oil Red O staining of liver (red dots in Veh sample are lipid droplets) (C) Weight of liver (D) Hepatic gene expression from animals on high fat diet with control, E2, LRP, LRE or ILQ (E) Gene expression of SREBP-1c in HepG2-ERα cells (left panel) or mouse primary hepatocytes (right panel). t-test, * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001
Similarly, increased lipid accumulation (lipidosis) in hepatocytes was observed in control animals on high fat diet, and E2 or LRP decreased hepatocyte lipid content, as observed in H and E staining, as decrease in the size of lipid droplets (Figure 6A) and Oil Red O staining (Figure 6B) of liver sections from treated animals. Moreover, weight of the liver was increased by high-fat diet and this was attenuated by E2 and BEs treatments as well (Figure 6C). This ectopic lipid accumulation is reminiscent of Non-Alcoholic Fatty Liver Disease (NAFLD) or hepatic steatosis, induced by obesity and high fat diet in humans and rodents [40–42], which predisposes the individual to type-2 diabetes and cardiovascular disease [43–45].
We verified molecular changes in fatty acid synthesis pathways by monitoring two biomarkers of hepatic steatosis, FASN [46, 47] and SREBP-1c [48, 49], and found that expression levels of these genes were reduced with E2 and LRP treatments (Figure 6D) suggesting an inhibitory role for ERα for these pathways. To further prove that BEs from Licorice root work through ERα, we treated HepG2 cells that stably express ERα or mouse primary hepatocytes with E2, ILQ or LIQ. Overall, the effect of ILQ and LIQ was more pronounced in HepG2-ERα cells compared to primary hepatocytes. Despite the differences in level of response to ligands ERα antagonist fulvestrant was very effective in blocking the downregulation of SREBP1c by E2 and BEs, suggesting that they act through hepatic ERα (Figure 6E). Thus, our studies provide a mechanistic basis for selective BE actions through ERα.
4 Discussion
Our studies suggest that dietary LRP supplementation provided resiliency to high-fat diet induced obesity in a menopause preclinical mouse model. Beneficial effects observed in metabolic tissues correlated with the blood level of liquiritigenin and isoliquiritigenin and mimicked those by E2, suggesting a protective role for licorice root supplementation through modulation of ERα pathways in metabolic tissues. At the observed blood levels of ILQ, we did not see any stimulation of reproductive tissues. In the study presented here, we provided an in vivo assessment of the selective actions of licorice root components LRP, LRE and ILQ in preclinical models relevant to the health of menopausal women. It is focused on molecular mechanisms of modulation of metabolism by BEs, as complex mixtures or isolates, from licorice root and also considers interaction of these end points with high fat diets. We took a comprehensive approach, monitoring several end points of BEs action in various metabolic tissues in addition to assessing risks in reproductive tissues. In this comprehensive approach, we monitored effects on total weight, lipid distribution (MRI), and food consumption and correlated these physiological end points with the histological and molecular changes in expression of critical genes for each tissue. Even though perigonadal fat is the main focus of other studies, we also monitored weight, histology and gene expression changes in perirenal, mesenteric, subcutaneous and brown adipose tissue and showed that licorice root supplementation mainly affected the visceral fat depots. Our findings are consistent with the sexually dimorphic roles of visceral vs. subcutaneous fat depots and their associations with diabetes and CVD risks [29, 50] and suggest that licorice root BEs reduce diabetes and CVD risks mainly through their actions in visceral fat depots and liver by preventing lipid deposition through ERα mediated pathways.
We studied the tissue-selective actions of licorice root components quantitatively, at morphological, physiological, and gene regulatory levels. These results showed that, LRP feeding in the diet, which provided highest concentration of ILQ as total and aglycones, mirrored E2 action in metabolic tissues by normalizing weight gain after ovariectomy, decreased overall lipid deposition in the body and decreased hepatic steatosis without stimulating mammary gland or uterus. These effects were because of the impact of licorice root BEs on the fat component of the body and were independent of changes in food intake. Even though BEs supplementation with BEs in the diet did not change food intake, at the end of the study these animals gained less weight compared to the control animals.
Previous studies have focused on licorice root [19, 51] and also reported that there were no detrimental effects on reproductive tissues, however in these studies licorice root extracts were provided as gavage to animals. This approach provides a one-time increase in the blood levels of bioactives. However, in our studies, we incorporated LRP, LRE and ILQ into the daily diet which mimicked dietary supplement uptake and provides a more constant blood level through-out the day. Feeding BEs in the diet also avoided stress associated with the gavage procedure which might impact metabolic parameters measured, such as overall food intake. Blood level measurements of active components, enabled direct comparisons with women consuming BEDS and showed that metabolic effects observed reflected the blood levels of ILQ and LIQ after dietary supplementation of various licorice root forms. ILQ and LIQ are in equilibrium in the blood. Interestingly, we achieved highest ILQ and LIQ concentrations in the blood when we fed the animals LRP. This might be due to matrix effects, or changes in the metabolism of ILQ when supplemented to animals in pure vs. root powder form. In our previous studies, we showed that both of these compounds had estrogenic activities through both ERα and ERβ [17, 18]. Mainly because of their lower binding affinity for the receptor compared to E2, these compounds required higher blood levels to properly function in the metabolic tissues. We believe, again because of this lower binding affinity, that they fail to form stable ER-coregulator complexes that are required for reproductive tissue stimulation, yet they still activate other ER modulated pathways that are required for metabolic tissue health maintenance.
Food consumption was not affected by ligand treatments showing that even though estrogens impact brain regions that control appetite, BEs did not work through regulation of food intake in our studies [4]. Even though the animals that were fed BEs consumed more food than did the E2 group, these animals gained less weight compared to the control group, which suggests that the main effect of BEs is on lipid deposition. Moreover, we extended our studies to liver, mesenteric, inguinal and perirenal adipose tissue and performed an in depth analysis of disease biomarker genes in these tissues. These approaches enabled us to identify and further characterize BEs from licorice root that might improve in menopausal women their metabolic health maintenance, which is aggravated by high-fat diet and sedentary life style. In addition, in the studies with primary hepatocytes and HepG2 cells overexpressing ERα, we showed that BEs treatment, down-regulated genes that were associated with lipid deposition. We believe that this effect was through ERα since ERα antagonist, fulvestrant, treatment reversed the BEs effect. We observed stronger responses to estrogens in HepG2-ERα cells, possibly, due to higher and more stable ERα content of these cells. However, our observations were similar in both cell models and consistent with our in vivo findings. These cell line models are well established, frequently used in similar studies to understand molecular mechanisms regulating hepatocyte physiology and provided initial data supporting the involvement of ERα in responses to BEs. Further studies need to be done in ERKO animals to further prove that in vivo effects of BEs are through ERα.
From these BE feeding experiments and the ensuing analyses, we obtained a large amount of morphological, physiological, biochemical, and gene expression data from which we can make an assessment of the tissue-selective effects of different BEs on metabolic parameters. We noted in particular the potential of BEs for maintaining a healthy metabolic state under conditions of low estrogen (as a model of menopause), and at the same time we observed little potential risks in stimulation of reproductive tissues. The results from these BE feeding studies done in animals, the gene expression profiles and pathway analyses in different target tissues and cell culture studies have revealed mechanistic aspects of the underlying molecular mechanisms that might account for the tissue-selective ERα mediated-effects that we observed with the BEs.
Acknowledgments
This work was supported by the NIH [P50AT006268] (WGH, BSK, JAK, DRD, IAK) from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS) and the National Cancer Institute (NCI) and National Institute of Food and Agriculture, U.S. Department of Agriculture, award ILLU-698-909 (ZME). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, U.S. Food and Drug Administration and U.S. Department of Agriculture.
Abbreviations and definitions
- BAT
brown adipose tissue
- BE
botanical estrogen
- BEDS
botanical estrogenic dietary supplements
- BRC
Botanical Research Center
- CVD
cardiovascular disease
- DXA
Dual-energy X-ray Absorptiometry
- ER
estrogen receptor
- H&E
hematoxylin and eosin
- HRT
hormone replacement therapy
- ILQ
isoliquiritigenin
- LIQ
liquiritigenin
- LRE
licorice root extract
- LRP
licorice root powder
- LXR
Liver X Receptor
- MFP
mammary fat pad
- MRI
magnetic resonance imaging
- ORO
oil red O
- SERM
selective estrogen receptor modulator
Footnotes
ZME, WGH, BSK and JAK conceived, designed he study, and wrote the manuscript. ZME, PG, YCH, LX, AC, JH, NCT and MW performed the animal experiments, harvested tissues and performed the ensuing molecular analysis. KW performed cell line experiments. UI and RT performed the DXA analysis. ZME, WGH, BSK, UI, RT, DRD, IAK and JAK gave conceptual advice and edited the manuscript.
The authors have declared no conflict of interest.
References
- 1.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard BV, Hsia J, Ouyang P, Van Voorhees L, et al. Postmenopausal hormone therapy is associated with atherosclerosis progression in women with abnormal glucose tolerance. Circulation. 2004;110:201–206. doi: 10.1161/01.CIR.0000134955.93951.D5. [DOI] [PubMed] [Google Scholar]
- 3.Rachon D, Teede H. Ovarian function and obesity--interrelationship, impact on women's reproductive lifespan and treatment options. Mol Cell Endocrinol. 2010;316:172–179. doi: 10.1016/j.mce.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozbey N, Sencer E, Molvalilar S, Orhan Y. Body fat distribution and cardiovascular disease risk factors in pre- and postmenopausal obese women with similar BMI. Endocrine journal. 2002;49:503–509. doi: 10.1507/endocrj.49.503. [DOI] [PubMed] [Google Scholar]
- 6.Trikudanathan S, Pedley A, Massaro JM, Hoffmann U, et al. Association of female reproductive factors with body composition: the Framingham Heart Study. J Clin Endocrinol Metab. 2013;98:236–244. doi: 10.1210/jc.2012-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuomikoski P, Mikkola TS. Postmenopausal hormone therapy and coronary heart disease in early postmenopausal women. Ann Med. 2014;46:1–7. doi: 10.3109/07853890.2013.854982. [DOI] [PubMed] [Google Scholar]
- 8.Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360:573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLennan AH, Gill TK, Broadbent JL, Taylor AW. Continuing decline in hormone therapy use: population trends over 17 years. Climacteric. 2009;12:122–130. doi: 10.1080/13697130802666251. [DOI] [PubMed] [Google Scholar]
- 10.Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy: physiological complexity belies therapeutic simplicity. Science. 2004;304:1269–1273. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- 11.Mahady GB, Parrot J, Lee C, Yun GS, Dan A. Botanical dietary supplement use in peri- and postmenopausal women. Menopause. 2003;10:65–72. doi: 10.1097/00042192-200310010-00011. [DOI] [PubMed] [Google Scholar]
- 12.Pinkerton JV, Constantine GD. Hyperandrogenic Disorders and Menopause. Endocrine Society. 2015 pp. FRI-124-FRI-124. [Google Scholar]
- 13.Carlson S, Peng N, Prasain JK, Wyss JM. Effects of botanical dietary supplements on cardiovascular, cognitive, and metabolic function in males and females. Gend Med. 2008;5(Suppl A):S76–S90. doi: 10.1016/j.genm.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geller SE, Studee L. Botanical and dietary supplements for mood and anxiety in menopausal women. Menopause. 2007;14:541–549. doi: 10.1097/01.gme.0000236934.43701.c5. [DOI] [PubMed] [Google Scholar]
- 15.Ye YB, Chen AL, Lu W, Zhuo SY, et al. Daidzein and genistein fail to improve glycemic control and insulin sensitivity in Chinese women with impaired glucose regulation: A double-blind, randomized, placebo-controlled trial. Mol Nutr Food Res. 2015;59:240–249. doi: 10.1002/mnfr.201400390. [DOI] [PubMed] [Google Scholar]
- 16.Andrade JE, Ju YH, Baker C, Doerge DR, Helferich WG. Long-term exposure to dietary sources of genistein induces estrogen-independence in the human breast cancer (MCF-7) xenograft model. Mol Nutr Food Res. 2015;59:413–423. doi: 10.1002/mnfr.201300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong P, Madak-Erdogan Z, Li J, Cheng J, et al. Transcriptomic analysis identifies gene networks regulated by estrogen receptor alpha (ERalpha) and ERbeta that control distinct effects of different botanical estrogens. Nucl Recept Signal. 2014;12:e001. doi: 10.1621/nrs.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Y, Gong P, Madak-Erdogan Z, Martin T, et al. Mechanisms enforcing the estrogen receptor beta selectivity of botanical estrogens. Faseb J. 2013;27:4406–4418. doi: 10.1096/fj.13-234617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunier EF, Vivar OI, Rubenstein A, Zhao X, et al. Estrogenic plant extracts reverse weight gain and fat accumulation without causing mammary gland or uterine proliferation. PLoS One. 2011;6:e28333. doi: 10.1371/journal.pone.0028333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riant E, Waget A, Cogo H, Arnal JF, et al. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 21.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 22.Leiter EH. Selecting the "right" mouse model for metabolic syndrome and type 2 diabetes research. Methods Mol Biol. 2009;560:1–17. doi: 10.1007/978-1-59745-448-3_1. [DOI] [PubMed] [Google Scholar]
- 23.Barrera J, Chambliss KL, Ahmed M, Tanigaki K, et al. Bazedoxifene and conjugated estrogen prevent diet-induced obesity, hepatic steatosis, and type 2 diabetes in mice without impacting the reproductive tract. Am J Physiol Endocrinol Metab. 2014;307:E345–E354. doi: 10.1152/ajpendo.00653.2013. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Department of Agriculture; Agricultural Research Service; Beltsville Human Nutrition Research Center; Food Surveys Research Group (Beltsville MD) What we eat in America, NHANES [Google Scholar]
- 25. Drugs.com. Licorice. http://www.drugs.com/npp/licorice.html. [Google Scholar]
- 26.Sahelian R. Licorice root supplement health benefit Glycyrrhizae radix [Google Scholar]
- 27.Hajirahimkhan A, Simmler C, Yuan Y, Anderson JR, et al. Evaluation of estrogenic activity of licorice species in comparison with hops used in botanicals for menopausal symptoms. PLoS One. 2013;8:e67947. doi: 10.1371/journal.pone.0067947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Environmental Protection Agency. Recommended use of body weight3/4 as a default method in derivation of the oral reference dose. 2011 [Google Scholar]
- 29.Davis KEMDN, Sun KWMS, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2:227–242. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frasor J, Barnett DH, Danes JM, Hess R, et al. Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) alpha activity by ERbeta in the uterus. Endocrinology. 2003;144:3159–3166. doi: 10.1210/en.2002-0143. [DOI] [PubMed] [Google Scholar]
- 31.Jia Y, Kim S, Kim J, Kim B, et al. Ursolic acid improves lipid and glucose metabolism in high-fat-fed C57BL/6J mice by activating peroxisome proliferator-activated receptor alpha and hepatic autophagy. Mol Nutr Food Res. 2015;59:344–354. doi: 10.1002/mnfr.201400399. [DOI] [PubMed] [Google Scholar]
- 32.Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60:10–18. doi: 10.1016/j.maturitas.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Hevener AL, Drew BG. In: Integrative Biology of Women’s Health. Spangen EE, editor. New York: Springer; 2013. pp. 87–122. [Google Scholar]
- 34.Korljan B, Bagatin J, Kokic S, Berovic Matulic N, et al. The impact of hormone replacement therapy on metabolic syndrome components in perimenopausal women. Med Hypotheses. 2010;74:162–163. doi: 10.1016/j.mehy.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Simmler C, Hajirahimkhan A, Lankin DC, Bolton JL, et al. Dynamic residual complexity of the isoliquiritigenin-liquiritigenin interconversion during bioassay. J Agric Food Chem. 2013;61:2146–2157. doi: 10.1021/jf304445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17:644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chechi K, Carpentier AC, Richard D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol Metab. 2013;24:408–420. doi: 10.1016/j.tem.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Cao H, Gerhold K, Mayers JR, Wiest MM, et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurencikiene J, Ryden M. Liver X receptors and fat cell metabolism. Int J Obes (Lond) 2012;36:1494–1502. doi: 10.1038/ijo.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2300–2308. doi: 10.3748/wjg.v18.i19.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 42.Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:195–203. doi: 10.1038/nrgastro.2010.21. [DOI] [PubMed] [Google Scholar]
- 43.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perlemuter G, Bigorgne A, Cassard-Doulcier AM, Naveau S. Nonalcoholic fatty liver disease: from pathogenesis to patient care. Nat Clin Pract Endocrinol Metab. 2007;3:458–469. doi: 10.1038/ncpendmet0505. [DOI] [PubMed] [Google Scholar]
- 45.Sun Z, Lazar MA. Dissociating fatty liver and diabetes. Trends Endocrinol Metab. 2013;24:4–12. doi: 10.1016/j.tem.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yecies JL, Zhang HH, Menon S, Liu S, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yecies JL, Manning BD. Transcriptional control of cellular metabolism by mTOR signaling. Cancer Res. 2011;71:2815–2820. doi: 10.1158/0008-5472.CAN-10-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higuchi N, Kato M, Shundo Y, Tajiri H, et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res. 2008;38:1122–1129. doi: 10.1111/j.1872-034X.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- 49.Beaven SW, Matveyenko A, Wroblewski K, Chao L, et al. Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metab. 2013;18:106–117. doi: 10.1016/j.cmet.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amato P, Christophe S, Mellon PL. Estrogenic activity of herbs commonly used as remedies for menopausal symptoms. Menopause. 2002;9:145–150. doi: 10.1097/00042192-200203000-00010. [DOI] [PubMed] [Google Scholar]