Abstract
Purpose
Two viral oncoproteins, E6 and E7, are expressed in all human papillomavirus (HPV)-infected cells, from initial infection in the genital tract to metastatic cervical cancer. Intramuscular vaccination of women with high grade cervical intraepithelial neoplasia (CIN2/3) twice with a naked DNA vaccine, pNGVL4a-sig/E7(detox)/HSP70, and a single boost with HPVE6/E7 recombinant vaccinia vaccine (TA-HPV) elicited systemic HPV-specific CD8 T cell responses that could traffic to the lesion and was associated with regression in some patients (NCT00788164).
Experimental Design
Here we examine whether alteration of this vaccination regimen by administration of TA-HPV vaccination in the cervicovaginal tract, rather than IM delivery, can more effectively recruit antigen-specific T cells in an orthotopic syngeneic mouse model of HPV16+ cervical cancer (TC-1 luc).
Results
We found that pNGVL4a-sig/E7(detox)/HSP70 vaccination followed by cervicovaginal vaccination with TA-HPV increased accumulation of total and E7-specific CD8+ T cells in the cervicovaginal tract and better controlled E7-expressing cervicovaginal TC-1 luc tumor than IM administration of TA-HPV. Furthermore, the E7-specific CD8+ T cells in the cervicovaginal tract generated through the cervicovaginal route of vaccination expressed the α4β7 integrin and CCR9, which are necessary for the homing of the E7-specific CD8+ T cells to the cervicovaginal tract. Finally, we show that cervicovaginal vaccination with TA-HPV can induce potent local HPV-16 E7 antigen-specific CD8+ T cell immune responses regardless of whether an HPV DNA vaccine priming vaccination was administered IM or within the cervicovaginal tract.
Conclusions
Our results support future clinical translation using cervicovaginal TA-HPV vaccination.
Introduction
Persistent infection with an oncogenic human papillomavirus (HPV) is a necessary, but insufficient cause of cervical cancer (1), the third most common cancer in women worldwide (2). Despite the availability of prophylactic vaccines, to date, uptake has been uneven, and so HPV disease remains common. Although most infections are cleared without intervention or clinical sequelae, mechanisms of immune-mediated clearance in humans are not well understood. Emerging data from human cohorts demonstrate that not all high grade dysplastic lesions in the cervix, cervical intraepithelial neoplasia 2/3 (CIN2/3), progress to invasive disease (3). Lesions associated with HPV16, the genotype most commonly associated with cancer, undergo complete regression in 20–25% of immune-competent women (3). Because expression of two viral proteins, E6 and E7, is functionally required for initiation and persistence of disease, they both represent rational, non-‘self’ antigenic targets for immune therapies (4).
Recent clinical data from a trial testing a heterologous DNA-prime, recombinant vaccinia vector-based boost (TA-HPV) demonstrate tissue localization of effector immune responses following peripheral, intramuscular vaccination in the deltoid muscles prior to standard therapeutic resection (5). Because memory T cells display pronounced tropism for the tissue in which they first encounter their cognate antigen, ongoing clinical trials are also testing the feasibility and immunogenicity of direct, intralesional vaccination in HPV16+ CIN2/3 (6). We developed a candidate therapeutic HPV vaccine, pNGVL4a-sig/E7(detox)/HSP70, based upon a naked DNA expressing a chimeric protein consisting of a signal peptide (sig) linked to HPV-16 E7 antigen and also heat shock protein 70 (HSP70), described previously (7). Intramuscular administration of pNGVL4a-sig/E7(detox)/HSP70 DNA vaccine has been well tolerated by patients with HPV16+ CIN2/3. However unlike the preclinical murine models, vaccination with this construct,in humans elicited weak systemic E7-specific CD8+ T cell responses that did not correlate with lesion regression (8). TA-HPV is a recombinant vaccinia virus vaccine that encodes HPV-16/18 E6 and E7 proteins. In humans, TA-HPV has elicited limited detectable systemic HPV-specific cellular immune responses (9–13). However, peripheral vaccination with this construct has elicited striking changes in the target lesions, suggesting that vaccine-induced immune responses are capable of trafficking to the site of antigen (5).
Many investigators have demonstrated that in preclinical murine models, heterologous vaccination regimens consisting of DNA vaccine priming, followed by boosting with viral vector constructs elicit effector responses that are far greater in magnitude than vaccination with either DNA alone or viral vectors alone (14–19). In our TC-1 model, vaccination with an E7-expressing DNA vaccine followed by boosting with E7 recombinant vaccinia virus also elicited greater immune responses compared to repeat immunization with either vaccine (14). In humans, Maldonado et al demonstrated that intramuscular vaccination with two doses of pNGVL4a-sig/E7(detox)/HSP70 DNA, followed by TA-HPV was well tolerated as well as immunogenic in patients with HPV16+ CIN2/3 (NCT00788164) (5, 20). This vaccination regimen also elicited effector memory CD8 T cell infiltrates in and around the CIN lesions, a phenotype associated with lesion clearance, suggesting that systemic immunization can elicit local immunity.
Tissue-resident memory T cells (Trm) have been shown to play a central role in the local control of infection, including in the genital tract (for reviews see (21, 22)). Site-specific vaccination in the setting of established disease may present a strategy for induction of therapeutic immunity for HPV+ mucosal tumors (for review see (23)). Indeed, in a preclinical murine model using an orthotopic HPV+ tumor, intranasal mucosal administration of a candidate head and neck cancer vaccine targeting E7 generated significantly enhanced therapeutic effects and mucosal-targeted antigen-specific CD8+ T cell responses compared to intramuscular administration of the vaccine (24).
Here, we examine whether intramuscular vaccination with E7-expressing DNA followed by intratumoral vaccination with TA-HPV would elicit enhanced clearance of vaginal HPV16+ tumors, as compared to peripheral boosting with intramuscular injection of TA-HPV, and dissect the signals attracting the HPV-specific CD8+ T cells to the genital tract in an orthotopic model of HPV associated cervical cancer.
Materials and Methods
Mice
Six- to eight- week old female C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD). β7 integrin deficient C57BL/6 mice (C57BL/6-Itgb7tm1cgn/J) were purchased from The Jackson Laboratory (Bar Harbor, Maine). Rag1 knockout OT-1 transgenic C57BL/6 mice were purchased from Taconic (Hudson, NY, USA). All animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Cells
TC-1 cells expressing the HPV16 E6–E7 proteins (25) and the TC–1 cells expressing the firefly luciferase gene (TC-1 luc) were developed in our laboratory and have been described previously (26).
Antibodies, tetramer and other reagents
FITC, PE and APC-conjugated anti-mouse CD8a (clone 53.6.7), FITC-conjugated anti-mouse IFN-γ (clone XMG1.2), PerCP-Cy5.5-conjugated anti-mouse CD103 (clone M290) antibodies were purchased from BD Pharmingen (San Diego, CA). APC-conjugated anti-mouse α4β7 (clone DATK32) was purchased from eBiosciences. PE-conjugated anti-mouse S1P1/EDG-1 (clone 713412) was purchased from R&D systems (Minneapolis, MN). Purified anti-mouse CD4 antibody (clone GK1.5) was purchased from Bio X Cell (West Lebanon, NH). FITC-conjugated anti-mouse CCR9 (clone CW-1.2) and purified rat anti-mouse MAdCAM-1 antibody (clone MECA-367) were purchased from BioLegend (San Diego, CA). Target retrieval solution, peroxidase block, HRP-conjugated rabbit anti-rat secondary antibody, anti-rabbit labelled polymer and diaminobenzidine (DAB) were all purchased from DAKO (Carpinteria, CA). HPV16 E7aa49-57 and HPV16 E7aa43-62 peptides were synthesized by GenScript (Piscataway, NJ) at purity over 80%. CpG-ODN 1826 was synthesized by Invitrogen (Grand Island, NY). PE or APC-conjugated, HPV16 E7aa49-57 peptide loaded H-2Db tetramer was provided by National Institute of Allergy and Infectious Diseases tetramer core facility. Retinoic acid receptor beta (RAR-β) antagonist, LE540 was purchased from STEMCELL Technologies (Vancouver, Canada). All trans retinoic acid (RA) was purchased from Sigma (St Louis, MO). CD11c positive selection kit was purchased from Miltenyi Biotech (San Diego, CA). Carboxyfluorescein succinimidyl ester (CFSE) was purchased from BD Pharmingen. Ammonium chloride solution (ACK) was purchased from Quality Biological INC.
Immunization procedures
The DNA vaccine, pNGVL4a- sig/E7(detox)/HSP70 used in this study has been described previously (7). A recombinant vaccinia virus expressing HPV16 and HPV18 E6 and E7, TA-HPV, has also been described previously (9). Mice were immunized by cervicovaginal vaccination (lateral wall of the cervicovaginal tract) or intramuscular (biceps femoris muscle of hind leg) injection on day 0 with 50 μg of pNGVL4a-sig/E7(detox)/HSP70 DNA vaccine, and boosted with 1x107 PFU of TA-HPV 7 days later. For peptide vaccination, 10 μg of HPV16 E7aa43-62 peptide was mixed with 1 μg of CpG1826 before the injection. For subcutaneous vaccination, the vaccines were injected subcutaneously at the base of tail. The total volume injected was 50 μL for both routes. Mice were anesthetized before immunization.
In vivo CD4+ T cell depletion
To deplete CD4+ T cells, mice were injected with 100 μg of anti-mouse CD4 antibody (Clone GK1.5) for 3 days via intraperitoneal injection. The depletion was maintained through the experiment by injecting the antibody once per week. The success of depletion was confirmed by staining peripheral blood cells with an anti-mouse CD4 antibody that recognizes a different epitope than the depleting antibody.
Lymphocyte preparation
PBMCs were obtained by bleeding from the tail vein of mice and after obtained red blood cells were lyzed with ACK lysis buffer. Vaginal (cervical and vaginal tissues) cell suspensions were obtained by enzymatic dispersion in RPMI 1640 digestion buffer for 1 hour at 37 °C while shaking. Cervicovaginal cells were passed through a 70-μM cell strainer (BD). Iliac lymph node and spleen cell suspensions were mechanically disrupted and filtered through a 70μM cell strainer. PBMC, cervicovaginal, spleen and lymph node cell suspensions were washed in RPMI/FBS 2% and freed from erythrocytes by treatment with ACK.
Flow cytometry analysis
For cell surface staining, single cells from spleen, lymph node or cervicovaginal tissue were resuspended in 50 μL of FACS buffer (PBS+0.5% BSA). To avoid nonspecific antibody binding through surface Fc receptor, cells were preincubated with CD16/32 mouse BD Fc BlockTM (BD pharmingen). Fluorochrome-conjugated anti-mouse antibodies or PE or APC -conjugated HPV16 E7aa49-57 peptide loaded H-2 Db tetramer were added to the cells and incubated at 4°C for 1 hour. Cells were acquired with FACSCalibure flow cytometer and the data analyzed with CellQuest (Becton Dickinson Immunocytometry System, Mountain View, CA) or FlowJo (FlowJo, Ashland, OH) software.
Characterization of E7-specific CD8+ T cell activation by intracellular cytokine staining followed by flow cytometry analysis
To detect HPV16 E7-specific CD8+ T cell responses by IFN-γ intracellular staining, splenocytes or single vaginal tissue cells were isolated using methods described previously (27), then stimulated with HPV16 E7aa49-57 peptide (1 μg/ml) in the presence of GolgiPlug (BD Pharmingen, San Diego, CA) at 37°C overnight. The stimulated cells were stained with PE-conjugated rat anti-mouse CD8a monoclonal antibody. Cells were subjected to intracellular cytokine staining using the Cytofix/Cytoperm kit according to the manufacturer’s instructions. Intracellular IFN-γ was stained with FITC-conjugated rat anti-mouse IFN-γ monoclonal antibody. Flow cytometry analysis was performed using FACSCalibur and analyzed with either CellQuest or FlowJo software.
In vivo tumor treatment and imaging techniques
To establish orthotopic cervicovaginal cancers, 2×104 of TC-1 luc cells were injected into the vaginal wall of female wild-type or β7 integrin-deficient C57BL/6 mice. Three days later, mice were vaccinated with 50 μg of pNGVL4a-sig/E7(detox)/HSP70 DNA in 50 μL volume by either cervicovaginal vaccination (lateral wall of the cervicovaginal tract) or intramuscular injection. Seven days later, the mice were boosted with 1x107 PFU of TA-HPV in 50 μl volume by either cervicovaginal vaccination or intramuscular injection. Genital tumor growth was monitored once a week by bioluminescence using a Xenogen imaging system. Briefly, D-Luciferin was dissolved to 7.8 mg/mL in PBS, filter sterilized, and stored at −80°C. Mice were given D-Luciferin by i.p. injection (200μl/mouse, 75mg/kg) and anesthetized with isoflurane. In vivo bioluminescence imaging for luciferase expression was conducted on a cryogenically cooled IVIS system using Living Image acquisition and analysis software (Xenogen). Mice were placed onto the warmed stage inside the light-tight camera box with continuous exposure to 1%–2% isoflurane. Images were acquired 10 min after D-luciferin administration and imaged for 2 min. The levels of light from the bioluminescent cells were detected by the IVIS imager, integrated, and digitized. The region of interest from displayed images was designated around the vagina and quantified as total photon counts using Living Image 2.50 software (Xenogen).
Immunohistochemical staining
Cervicovaginal tissues were harvested en bloc from vaccinated mice seven days after the last immunization. Paraffin-embedded tissue sections were prepared by the Department of Pathology at Johns Hopkins University. The sections were deparaffinized in xylene, and heat-based antigen retrieval was performed for 30 minutes using target retrieval solution followed by peroxidase block. The sections were incubated with rat anti-mouse MAdCAM-1 antibody at 4°C overnight, followed by HRP-conjugated secondary rabbit anti-rat antibody at room temperature for 1 hour. The sections were then incubated with anti-Rabbit Labeled Polymer at room temperature for 30 minutes. DAB served as a chromogen, and Gill’s hematoxylin was used as a counterstain.
Adoptive transfer experiment
Splenocytes from intramuscular pNGVL4a-sig/E7(detox)/HSP70 DNA primed and cervicovaginal TA-HPV vaccinia virus boosted C57BL/6 mice, either wild-type or β7 integrin deficient, were labelled with 0.5 μM of CFSE. 4 × 107 of CFSE labelled splenocytes were then adoptively transferred into wild-type C57BL/6 mice through retro-orbital injection. One day after transfer, splenocytes and cervicovaginal cells were prepared from recipient mice. The cells were stained with PE-conjugated anti-mouse CD8a antibody and acquired with FACSCalibur. CD8+CFSE+ cells were analyzed with FlowJo software.
CD11c+ dendritic cell isolation
Single cells from spleen and vaginal tissue were prepared as described above. CD11c+ dendritic cells were positively isolated from single cells of spleen and vagina tissue using CD11c magnetic beads (Miltenyi Biotec, San Diego, CA) according to manufacturer’s protocol. CD11c+ cells were resuspended in complete RPMI medium, and counted.
In vitro dendritic cell/T cell co-cultures
Naïve OVA-specific OT-1 CD8+ T cells were isolated from Rag1 knockout OT-1 TCR transgenic mice and labeled with 0.5 μM of CFSE. 1 × 105 of these CFSE labelled OT-1 T cells were plated into each well of 48-well plate and mixed with purified CD11c+ DCs (described above) at a DC: T cell ratio of 1:5 in the presence of OVA protein (25 μg/mL) and IL-2 (20 IU/mL). As a positive control, retinoic acid was added to the cultures at the concentration of 50 ng/mL. After 4 days, the expression of α4β7 on proliferating OT-1 cells was evaluated by flow cytometry analysis. For the retinoic acid inhibition assays, the DC/T cell co-cultures were incubated in the presence of 1μM of RAR-β antagonist, LE540.
Statistical analyses
All data are expressed as mean ± standard deviation (S.D.) and are representative of at least two separate experiments. Comparisons between individual data points were made using Student’s t-test. The non-parametric Mann-Whitney test was used for comparing two different groups. All p values <0.05 were considered significant.
Results
Heterologous prime-boost vaccination with pNGVL4a-sig/E7(detox)/HSP70 DNA followed by TA-HPV elicits stronger systemic E7-specific CD8+ T cell responses compared to a homologous prime-boost regimen
Chen et al demonstrated the synergistic induction of HPV16 E7-specfic IFN-γ+ CD8+ T cell responses by heterologous prime-boost vaccination in C57BL/6 mice administered naked pcDNA3-sig/E7/LAMP-1 DNA intramuscularly and a recombinant vaccinia virus expressing sig/E7/LAMP-1 delivered intraperitoneally. We sought to confirm that a heterologous prime-boost strategy utilizing vaccines previously utilized in the clinic (pNGVL4a-sig/E7(detox)/HSP70 DNA and TA-HPV vaccinia virus) is also effective therapeutic vaccine approach. Three regimens were evaluated and compared to unvaccinated controls; (1) Naïve C57BL/6 mice (five per group) were vaccinated intramuscularly with pNGVL4a-sig/E7(detox)/HSP70 DNA at week 0 and 1, followed by a single TA-HPV vaccination at week 2, or (2) received homologous prime-boost vaccination comprising repeat vaccination with either pNGVL4a-sig/E7(detox)/HSP70 DNA alone, or (3) TA-HPV alone. The spleens were harvested from each group of immunized mice at week 3, and the fraction of splenocytes derived from each mouse comprising CD8+ T cells producing IFN-γ in response to E7 peptide stimulation for 24h was measured by intracellular cytokine staining (Figure 1). The heterologous prime-boost regimen generated a stronger E7-specfic IFN-γ+ CD8+ T cell response compared to repeat vaccination with either pNGVL4a-sig/E7(detox)/HSP70 DNA alone or TA-HPV alone.
Figure 1. Characterization of the E7-specific CD8+ T cell immune response using intracellular IFN-γ cytokine staining followed by flow cytometry analysis.
C57BL/6 mice (5 per group) were intramuscularly vaccinated with pNGVL4a-sig/E7(detox)/HSP70 DNA (50μg per mouse) prime followed six days later by TA-HPV (1x107 per mouse) boost, DNA prime followed by DNA boost, TA-HPV only or received no vaccination. One week after the last immunization, splenocytes were analyzed by flow cytometry. A. Data shown are from a representative flow cytometry analysis. The number in the right upper corner indicates the number of CD8+ IFN-γ+ E7-specific T cells in 105 total splenocytes. B. Bar graph. Values are shown as mean ± SD, *p<0.05, **p<0.01, ns, not significant.
Local vaccination with heterologous prime-boost vaccination generates a stronger E7-specific CD8+ T cell response in cervicovaginal tissues than peripheral vaccination
HPV-specific T cells must traffic to the genital tract to clear intraepithelial neoplasia of the vagina or cervix. Therefore we sought to elicit E7-specfic CD8+ T cells in cervicovaginal tissues of mice via local vaccination. After a single priming vaccination with pNGVL4a-sig/E7(detox)/HSP70 DNA, C57BL/6 mice were boosted 7 days later by TA-HPV administered either by cervicovaginal or intramuscular vaccination. One week after TA-HPV vaccination, systemic and local E7-specific CD8+ T cell responses were analyzed by flow cytometry after staining with E7 peptide-loaded H-2Db tetramer of splenocytes or cells recovered from the cervicovaginal tissues, respectively. As shown in Figures 2A and B, mice receiving either cervicovaginal or intramuscular heterologous prime-boost vaccination generated robust systemic E7-specific CD8+ T cell responses. Intramuscular delivery generated a significantly higher systemic E7-specific CD8+ T cell responses than cervicovaginal delivery. In contrast, mice that received the cervicovaginal heterologous prime-boost regimen generated significantly higher number of total number of CD8+ T cells as well as E7-specific CD8+ T cells in the cervicovaginal tract compared to mice vaccinated intramuscularly (Figures 2C-E). The total number of CD8+ T cells in the cervicovaginal tissues of mice that received intramuscular vaccination was not significantly different from naïve mice, indicating that intramuscular administration did not lead to increase of CD8+ T cells in the cervicovaginal tract, regardless of E7 specificity.
Figure 2. Characterization of E7-specific CD8+ T cells in splenocytes and cervicovaginal tract using E7 peptide-loaded H-2Db tetramer and intracellular IFN-γ staining.
C57BL/6 mice (5 per group) were vaccinated with pNGVL4a-sig/E7(detox)/HSP70 (50μg per mouse) prime followed 7 days later by TA-HPV (1x107 per mouse) boost with the regimen administered either within cervicovaginal tissues or intramuscularly. One week after the last immunization, splenocytes and cervicovaginal cells were prepared for the detection of total or HPV16 E7-specific CD8+ T cells by either tetramer or IFN-γ intracellular staining. Representative flow cytometry analysis by HPV16 E7 tetramer staining of splenocytes (A) and cervicovaginal cells (D). Summary of flow cytometry analysis of E7 tetramer+ CD8+ T cells in splenocytes (B), total CD8+ T cells (C) and E7 tetramer+ CD8+ T cells (E) in cervicovaginal tissues. Representative flow cytometry analysis by IFN-γ intracellular staining of splenocytes (F) and cervicovaginal cells (H). Summary of flow cytometry analysis of E7-specific IFN-γ –producing CD8+ T cells in splenocytes (G) and in cervicovaginal tissues (I). Values are shown as mean ± SD, *p<0.05, **p<0.01, ns, not significant.
We also characterized the presence of E7-specific CD8+ T cells in the spleen and cervicovaginal tract by intracellular cytokine staining for IFNy followed by flow cytometry analysis. In contrast to the measurement of total E7-specific CD8+ T cell responses by staining with E7 peptide-loaded H-2Db tetramer of splenocytes (Figure 2A and B), no significant difference in the systemic E7-specific IFN-γ+ CD8+ T cell response was observed upon assay by intracellular cytokine staining of peptide stimulated splenocytes from mice vaccinated with either the intramuscular or cervicovaginal heterologous prime-boost regimen (Figure 2F and G). However, cervicovaginal heterologous prime-boost vaccination induced a significantly higher (8-fold) E7-specific IFN-γ+ CD8+ T cell response in murine cervicovaginal tissue as compared to intramuscular administration (Figure 2H and I). Taken together, our data generated from two different immunologic assays indicates that compared to intramuscular administration, cervicovaginal vaccination with pNGVL4a-sig/E7(detox)/HSP70 DNA and TA-HPV generates significantly more E7-specific CD8+ T cell infiltrates in the cervicovaginal tissues, without compromising the generation E7-specific IFN-γ+ CD8+ T cell responses in the spleen.
We next examined whether it is required to administer both the pNGVL4a-sig/E7(detox)/HSP70 DNA priming vaccination and the TA-HPV boost ICV to induce both a robust systemic E7-specific CD8+ T cell response and its recruitment in to cervicovaginal tissues. For the induction of a systemic E7-specific CD8+ T cell response, intramuscular administration of the pNGVL4a-sig/E7(detox)/HSP70 DNA priming vaccination was more effective than cervicovaginal delivery before the cervicovaginal TA-HPV booster vaccination, and not significantly different than with intramuscular administration of the TA-HPV booster vaccination (Supplementary Figures 1A and B). This may reflect an improved DNA uptake and E7 presentation by muscle cells as compared with cervicovaginal tissues. Indeed, cervicovaginal vaccination with the pNGVL4a-sig/E7(detox)/HSP70 DNA did not effectively prime the E7-specific CD8+ T cell response to an intramuscular booster vaccination with TA-HPV (Supplementary Figures 1A and B).
When examining the numbers of E7-specific CD8+ T cells in the genital tract of the immunized mice (Supplementary Figures 1C and D), significantly higher number of E7-specific CD8+ T cells were observed in the cervicovaginal tract in the groups that received the cervicovaginal TA-HPV booster vaccination as compared with intramuscular vaccination, regardless of the route by which the DNA priming immunization was administered. Interestingly, after cervicovaginal vaccination boosting with TA-HPV, a higher number of E7-specific CD8+ T cells was detected in the genital tract of the intramuscular primed mice as compared with those primed with the pNGVL4a-sig/E7(detox)/HSP70 DNA by ICV administration (Supplementary Figures 1C and D). We speculate that this may reflect a higher overall response because of improved DNA vaccine delivery in muscle as compared to cervicovaginal tissues.
Cervicovaginal vaccination with pNGVL4a-sig/E7(detox)/HSP70 DNA and TA-HPV controls tumor in the genital tract better than intramuscular vaccination
The growth of luciferase-expressing TC-1 (TC-1 luc) tumor in the genital tract can be readily monitored by imaging of bioluminescence and it provides a model of HPV16+ high grade CIN/microinvasive cervical cancer. Therefore we assessed the impact of route of administration of the prime-boost vaccination regimen upon control of luciferase-expressing TC-1 tumor established in the genital tract. TC-1 luc cells were injected submucosally into the vaginal wall of C57BL/6 mice. One day later, groups (n=5) of these mice were immunized via either intramuscular or cervicovaginal administration with pNGVL4a-sig/E7(detox)/HSP70 DNA, and 7 days later with TA-HPV by the same route. Mice were monitored for tumor growth by luminescence imaging on day 3 and day 17 after tumor challenge. The imaging on day 3 confirmed that, as shown in Figure 3A and B, cervicovagional vaccination with pNGVL4a-sig/E7(detox)/HSP70 DNA and TA-HPV exhibited significantly better control of the TC-1 luc tumor, as measured by decreased luminescence in the genital tract, on day 17 compared to mice receiving intramuscular vaccination or no vaccination. Furthermore, most mice which had cervicovaginal vaccination survived past 80 days, whereas all control mice and those vaccinated intramuscularly died before 30 days (Figure 3C). This data indicates that cervicovaginal vaccination better controls HPV+ tumor in the genital tract and prolongs survival compared to vaccination through intramuscular injection.
Figure 3. Characterization of in vivo therapeutic antitumor effects generated by pNGVL4a-sig/E7(detox)/HSP70 prime and TA-HPV boost via ICV or IM vaccination.
C57BL/6 mice (5 per group) were challenged with luciferase-expressing TC-1 cells (2x104 per mouse) in the submucosa of the vagina. One day later, mice were immunized with pNGVL4a-sig/E7(detox)/HSP70 and 7 days later, mice were immunized with TA-HPV via ICV or IM. The signal in the vagina was monitored by luminescence on day 3 and day 17 after injection of TC-1 luciferase-expressing cells. A. Luminescence images of representative mice challenged with luciferase-expressing TC-1 tumor and treated according to the various treatment regimens. B. Bar graph showing luminescence intensity, values are shown as mean ± SD, *p<0.05, **p<0.01, ns, not significant. C. Kaplan-Meier survival analysis of mice in various treatment groups.
Cervicovaginal vaccination with pNGVL4a-sig/E7(detox)/HSP70 DNA then TA-HPV causes accumulation of α4β7+ and CCR9+ E7-specific CD8+ T cells in genital tissues
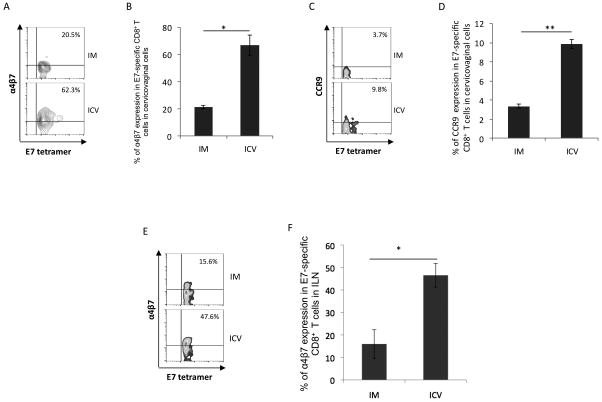
In order to determine whether the E7-specific CD8+ T cells induced by the prime-boost regimen were Trms attracted to the genital tract, we evaluated them for the expression of tissue-specific molecules α4β7 and CCR9. α4β7 is a mucosa-associated homing integrin, is known to be expressed by T cells in the cervicovaginal tract of humans (28), and functions by interacting with Mucosal Addressin Cell Adhesion Molecule-1 (MAdCAM-1) (for review see (29)). Also involved in the homing and retention of lymphocytes in mucosal tissue is the chemokine receptor CCR9 whose ligand is CCL25, which is commonly expressed in the epithelium of respiratory, gastrointestinal and urogenital tissues (for review see (23)). As shown in Figure 4A-D, naïve mice which were vaccinated in the cervicovaginal tissues with pNGVL4a-sig/E7(detox)/HSP70 DNA and TA-HPV one week later had a significantly higher percentage of E7-specific CD8+ T cells expressing α4β7 or CCR9 among all E7 tetramer positive cells in the cervicovaginal tract as compared to mice vaccinated intramuscularly with this regimen. Likewise, a greater fraction of E7-specific CD8+ T cells expressing α4β7 or CCR9 were detected in the regional iliac lymph node of mice vaccinated in cervicovaginal tissue as compared to those vaccinated intramuscularly (Figure 4E and F). These data indicate that intravaginal boost vaccination is more effective than intramuscular vaccination in generating antigen-specific CD8+ T cells that express α4β7 or CCR9 in the cervicovaginal tract.
Figure 4. Characterization of α4β7 and CCR9 expression by E7-specific CD8+T cells in cervicovaginal tissue and iliac lymph node.
C57BL/6 mice (5 per group) were vaccinated with pNGVL4a-sig/E7(detox)/HSP70 (50μg per mouse) prime followed six days later by TA-HPV (1x107 per mouse) boost either within cervicovaginal tissues or intramuscularly. One week after the second immunization, cervicovaginal and iliac lymph node cells were analyzed by flow cytometry using E7 peptide-loaded tetramer, antibody against α4β7 and CCR9. Representative flow cytometry analysis of α4β7 (A), CCR9 (C) in the cervicovaginal cells and α4β7 in the iliac lymph node cells (E) by E7-specific CD8+ T cells. Summary of flow cytometry analysis of α4β7 expression by E7-specific CD8+ T cells in cervicovaginal tissues (B), iliac lymph node (F) and CCR9 expression in cervicovaginal tissues (D). Values are shown as mean ± SD, *p<0.05, **p<0.01, ns, not significant.
Cervicovaginal vaccination with pNGVL4a-sig/E7(detox)/HSP70 DNA prime followed by TA-HPV boost induces expression of MAdCAM-1 in cervicovaginal tissues
Because MAdCAM-1 is an important addressin for nonlymphoid tissue-specific T cell homing programs (23), we sought to determine whether cervicovaginal vaccination can induce local expression of MAdCAM-1 on the cervicovaginal tissue. The genital tracts were excised from mice vaccinated either within cervicovaginal tissues or intramuscularly with pNGVL4a-sig/E7(detox)/HSP70 DNA followed by TA-HPV, and their tissues were fixed and analyzed by immunohistochemistry staining. As shown in Supplementary Figure 2, mice receiving cervicovaginal vaccination were associated with significantly higher expression as indicated by higher amount of positive brown staining of MAdCAM-1 within plump high endothelial venule cells in the cervicovaginal tissue compared to naïve mice or intramusclar vaccinated mice. This data suggests that cervicovaginal vaccination with this heterologous prime-boost regimen induces the expression of addressin molecules in high endothelial venules of the cervicovaginal tract, which may further assist in homing of antigen-specific CD8+ T cells by interacting with integrin α4β7.
Induction of antigen-specific CD8+ T cell therapeutic antitumor effect by cervicovaginal vaccination is dependent on α4β7
Since our ICV heterologous prime-boost regimen generated E7-specific CD8+ T cells that express α4β7 and induced increased expression of MAdCAM-1 in high endothelial venules of cervicovaginal tissue, we reasoned that α4β7 played a significant role in the recruitment of E7-specific CD8+ T cells to the genital tract. To test this, we administered the cervicovaginal heterologous prime-boost regimen in wild type mice and Itgb7−/− mice. As shown in Figure 5A and B, cervicovaginal vaccination generated similar number of E7-specific CD8+ T cells in the spleen in wild type mice compared to mice deficient of α4β7. However, cervicovaginal vaccination induced a significantly higher number of E7-specific CD8+ T cells in the cervicovaginal tissue of wild type mice compared to mice lacking α4β7 (Figure 5C and D). Furthermore, in experiments to treat genital TC-1 luc tumor, cervicovaginal heterologous prime-boost vaccinations effectively control tumor growth in wild type mice while α4β7 deficient mice showed significant tumor growth 17 days after tumor challenge (Figure 5E and F). In addition, as shown in Figure 5G, 80% of wild type mice receiving cervicovaginal vaccination survived past 60 days while all the α4β7 deficient mice died within 30 days after tumor challenge. These results suggest that α4β7 is responsible for the increase in local antigen-specific CD8+ T cells in the cervicovaginal tract. Furthermore, the findings suggest that it is not a direct killing effect by the vaccinia virus in the genital tract that is main mechanism responsible for local tumor control, but rather the recruitment of antigen-specific CD8+ T cells and immunologic control.
Figure 5. Integrin β7 is required for E7-specific CD8+ T cell homing to the cervicovaginal tissue and enhanced therapeutic antitumor effect.
Wild type ( WT) and Itgb7−/ − mice were vaccinated ICV with pNGVL4a-sig/E7(detox)/HSP70 (50μg per mouse) prime followed 7 days later by TA-HPV (1x107 per mouse) boost. One week after the last immunization, cervicovaginal tissues and splenocytes were analyzed by flow cytometry. To test the role of integrin β7 in the antitumor effect generated by vaccination, WT or Itgb7−/ − C57BL/6 mice (5 per group) were challenged with luciferase-expressing TC-1 cells (2x104 per mouse) in the submucosa of the vagina. One day later, mice were immunized with pNGVL4a-Sig/E7(detox)/HSP70 and 7 days later, mice were immunized with TA-HPV. The signal in the vagina was monitored by luminescence on day 3 and day 17 after injection of TC-1 luciferase-expressing cells. Representative flow cytometry analysis of splenocytes (A) and cervicovaginal cells (C). Summary of flow cytometry analysis of E7-specific CD8+ T cells in splenocytes (B) and in cervicovaginal tissues (D). Luminescence images of representative mice challenged with luciferase-expressing TC-1 tumor and treated according to the various treatment regimens (E). Summary of the tumor luminescence intensity (F). Kaplan-Meier survival analysis of mice in various treatment groups (G). Values are shown as mean ± SD, *p<0.05, **p<0.01, ns, not significant.
CD8+ T cells lacking α4β7 fail to traffic to the cervicovaginal tract
We sought to further assess the role of α4β7 in the traffic of CD8+ T cells into the cervicovaginal tract. Wild type and Itgb7−/ − mice were vaccinated within cervicovaginal tissues with the heterologous prime-boost regimen, and 7 days after the last immunization, lymphocytes were isolated from the spleens and labeled with CFSE. The CFSE-labeled T cells were adoptively transferred retro-orbitally into WT naïve mice, and one day after transfer, T cells in spleen and cervicovaginal tract were isolated and analyzed by flow cytometry. Expression of α4β7 on the isolated T cells was detected through anti-α4β7 antibody staining (Supplementary Figure 3A). As shown in Supplementary Figure 3B and C, there is no significant difference in the numbers of adoptively transferred wild type CD8+ T cells compared to the number of transferred α4β7 deficient CD8+ T cells in the spleen of the recipient mice. In contrast, a significantly higher number of adoptively transferred wild type CD8+ T cells compared to number of transferred α4β7 deficient CD8+ T cells were detected in the cervicovaginal tissues of the recipient mice (Supplementary Figure 3C and D). These data indicate that the expression of α4β7 is responsible for the trafficking of CD8+ T cells into the cervicovaginal tract, and that the increased number of E7-specific CD8+ T cells in the cervicovaginal tract observed is mainly contributed by T cell homing mediated by α4β7.
Cervicovaginal dendritic cells induce expression of α4β7 on CD8+ T cells via retinoic acid production
Previously, it has been shown that retinoic acid can induce expression of α4β7 on CD8+ T cells (30–33). It has also been reported that dendritic cells in the gut but not in the spleen express retinal dehydrogenase (RALDH), a class I aldehyde dehydrogenase (ALDH1), which is responsible for the production of retinoic acid (34). We hypothesized that dendritic cells in the cervicovaginal tract produce retinoic acid, which induces expression of α4β7 in CD8+ T cells that have differentiated to a tissue-resident phenotype. We co-cultured CD11c+ dendritic cells isolated from the spleen or the cervicovaginal tract with CFSE-labeled OT-1 cells at a ratio of 1:5 and stimulated with ovalbumin (OVA) protein and IL-2. Unstimulated OT-1 cells served as negative control, while stimulated OT-1 cells co-cultured with retinoic acid served as positive control. After 4 days, α4β7 expression on OT-1 cells was analyzed by flow cytometry. As shown in Figure 6A and B, a significantly higher number of OT-1 cells expressed α4β7 when co-cultured with dendritic cells isolated from the cervicovaginal tract compared to co-culturing with dendritic cells isolated from spleens. This data suggest that, under these conditions, dendritic cells from the cervicovaginal tract are capable of inducing α4β7 expression in CD8+ T cells whereas dendritic cells from the spleen are not. We then assessed the role of retinoic acid in the induction of α4β7 expression. LE540, a retinoic acid receptor inhibitor, was added to the OT-1 cell culture to block the effects of retinoic acid. Blockade of retinoic acid stimulation resulted in a reduction in the expression of α4β7 in activated OT-1 cells (Figure 6C and D). This result suggests that retinoic acid is responsible for triggering expression of α4β7 in CD8+ T cells, consistent with previous studies (30–33). Taken together, our data indicate that dendritic cells in the cervicovaginal tract can induce expression of α4β7 in CD8+ T cells in a retinoic acid dependent manner.
Figure 6. Induction of integrin α4β7 by vaginal DC and is mediated by retinoic acid.
CD11c+ dendritic cells were isolated from either the spleen or the cervicovaginal tract. These cells were then co-cultured with CFSE-labeled OT-1 cells at a ratio of 1:5 at the presence of OVA protein and IL-2 for 4 days. To inhibit retinoic acid receptor (RAR),a RAR-β inhibitor, LE540 was added into the cell culture. The cells were then stained with anti-mouse CD8 and α4β7. Representative flow cytometry analysis of α4β7 by OT-1 cells after stimulation with OVA protein pulsed dendritic cells from either spleen or vagina (A) or at the presence of RAR-β inhibitor, LE540 (C). Summary of the flow cytometry data of α4β7 expression by OT-1 cells after stimulation with OVA protein pulsed dendritic cells from either spleen or vagina (B) or at the presence of RAR-β inhibitor, LE540 (D).
Discussion
In the current study, we identified a heterologous prime-boost regimen that induces vaccine antigen-specific CD8+ T cell immune responses and therapeutic effect against an HPV16 E6/E7+ orthotopic tumor model in the cervicovaginal tract. Both the pNGVL4a-sig/E7(detox)/HSP70 naked DNA vaccine and TA-HPV have been used safely, either alone or together, in clinical trials (5, 8–13). Indeed, they are being tested in sequence in an ongoing clinical trial, in women with HPV16+ CIN2/3 (20). In our murine model, cervicovagional delivery elicits greater intensity of E7-specific CD8+ T cells than intramuscular administration of TA-HPV induction in cervicovaginal tissues. Furthermore, the enhanced local E7-specific CD8+ T cell responses generated by cervicovaginal route of vaccine boost extends to the E7 peptide vaccine system (Supplementary Figure 4). Furthermore, in mice bearing established E6/E7-expressing cervicovaginal tumors, cervicovaginal vaccination, presumably adjacent to the tumor, produces significantly better tumor control. Indeed, subcutaneous vaccination of the DNA prime followed by vaccinia vaccine boost also elicited a weaker E7-specific CD8+ T cell response compared to cervicovaginal vaccination (Suppplementary Figure 8). This result further supports the necessity of local administration of the vaccines in the cervicovaginal tract. Importantly, DNA prime is necessary to elicit the enhanced antitumor responses, as homologous prime-boost with only TA-HPV results in significantly weaker tumor control in mice compared to heterologous DNA prime followed by vaccinia boost (Supplementary Figure 5). Of note, Cervicovaginal administration of the DNA vaccine was not required; in fact, intramuscular priming generated a better response than ICV.
To assess whether CD4+ cells play a role in the observed enhancement of local E7-specific CD8+ T cell responses by our DNA prime followed by vaccinia boost regimen, we vaccinated CD4 T cells depleted mice with our vaccines. As shown in Supplementary Figure 6, CD4 depletion almost completely abolish the generation of E7-specific CD8+ T cells by the DNA prime followed by vaccinia boost vaccination in the spleen, draining lymph nodes, and the cervicovaginal tissues. These results indicate that CD4 cells play a critical role in eliciting E7-specific CD8+ T cell responses both locally and systemically.
The pNGVL4a-sig/E7(detox)/HSP70 naked DNA vaccine has previously been administered intramuscularly in clinical trials (8). While TA-HPV has previously been administered either intramuscularly (5), or intradermally (9, 10), the cervicovaginal route of administration has not been tested in humans. However, cervical administration of a recombinant E2 modified vaccinia Ankara (MVA) was well tolerated and was associated with lesion regression in a phase II trial with women with HPV disease (35). Taken together, the cervicovaginal route of administration of therapeutic HPV vaccinia virus vaccine such as TA-HPV may potentially be applicable to HPV associated lesions, particularly in advanced refractory cervical and head and neck cancer. However, recombinant vaccinia virus vaccine may potentially be risky for immunocompromised patients. Thus, for future clinical translation, such population should be excluded to minimize risk of general vaccinia infection.
The environment in the cervicovaginal tract is subjected to effects of the variation in hormones during cell cycle. In a previous study, the transfection efficiency of DNA vaccine administered through vaginal tract (lumen) using a micropipette followed by electroporation was indeed affected by hormonal differences (36). However, here we are directly injecting our DNA and vaccinia vaccines into the cervicovaginal tissue, thus the complication in absorption due to hormonal variation may not be applicable to our method of administration. Nevertheless, the full effect of hormonal variation on the immune responses generated by the cervicovaginal boost with TA-HPV vaccinia vaccine should be further investigated in future studies.
The E7-specific CD8+ T cells induced in the cervicovaginal tissues by cervicovaginal boost with TA-HPV express the mucosa-associated homing integrins α4β7 and CCR9, suggesting that these CD8 T cells are Trms. Using CD103 as a marker for Trm, we observed that there are slightly more CD103+ E7-specific Trm in the DLN and in the cervicovaginal tract compare to in the spleen (Supplementary Figure 7). Furthermore, CD103+ cells from cervicovaginal tract express higher amount of S1P1 proteins (Supplementary Figure 7). It has been shown that S1P1 can help with tissue retention of T cells (Ledgerwood et al). Thus the increased expression of S1P1 may contribute to the retention of the CD103+ T cells in the cervicovaginal tract, which may contribute to the antitumor effect against local cervicovaginal tumor. However, previously Skon et al. has reported that S1P1 transcriptional downregulation is required for establishment of tissue resident memory T cells in the spleen, salivary glands, and small intestine (37). In comparison, here we observed an increase in S1P1 expression in CD103+ E7-specific CD8+ T cells in the cervicovaginal tract. The difference in the expression level of S1P1 may be explained by the difference in the local tissue environment. Another potential explanation for the discrepancy of our data may be related to the timing of evaluating S1P1 expression on T cells following vaccination. Unlike the experimental method in Skon et al. which evaluates S1P1 mRNA expression through qPCR at least 4 weeks after treatment, we assess S1P1 protein level in just one week after the last vaccination by flow cytometry. The expression of S1P1 changes based on different location and phases of T cells activation and differentiation (38). S1P1 expression is high while the T cells are still in circulation, and as they traffic into the local tissue environment, cytokines milieu triggers the downregulation of S1P1 in T cells leading to their retention in the local environment and gradual differentiation into S1P1-Low Trms. We only assessd S1P1 protein level just one week after treatment and the downregulation of S1P1 may have not been fully initiated. It will be of interest to further characterize the expression kinetics of S1P1 in CD103+ T cell in the cervicovaginal tract following vaccination in future studies in order to generate more insight into the molecular mechanism of the generation of Trms in the cervicovaginal tract.
In addition, the expression of α4β7 is necessary for the observed enhancement of antigen-specific CD8+ T cell antitumor immune responses in cervicovaginal tissues elicited by our prime-boost regimen. Of note, ltgb7−/ − mice also lacks CD103. However, CD103 mainly plays a role for the retention of T cells in the epithelia whereas α4β7 is responsible for trafficking (39). Our results with ltgb7−/ − mice show that the enhanced local E7-specific CD8+ T cell response by our vaccination approach is mainly mediated by α4β7 (Figure 5). However, Itgb7 deficiency may also lead to impairment of the generation of Trms due to lacking CD103, and consequently a decrease in the retention of CD8+ T cells in the local environment. Finally, consistent with previous findings by Zeng et al (33), we demonstrated that the expression of α4β7 on CD8+ T cells can be mediated by stimulation with retinoic acid that is secreted by dendritic cells obtained from the cervicovaginal tissues. Taken together, these data suggest that, after a DNA priming vaccination, the administration of this recombinant HPV vaccinia vaccine via the cervicovaginal route may be more effective than intramuscular or subcutaneous injection in generating local cell-mediated immune responses for the control of HPV-associated diseases in cervicovaginal tract.
We showed that DCs from the cervicovaginal tract can induce the expression of α4β7 on CD8+ T cells, and that this effect is mediated by retinoic acid production. The current treatment regimen of heterologous prime followed by cervicovaginal boost may well have generated enhanced production of retinoic acid in the cervicovaginal DCs that induced expression of α4β7 by CD8+ T cells. This regimen was associated with the accumulation of both E7-specific and total CD8+ T cell populations in the cervicovaginal tissues, raising the possibility that it is non-specific inflammation associated with the vaccinia expression rather than the presence of the antigen that is driving this homing response. Other cell types, including stromal cells, epithelial cells, and macrophages have also been shown to produce retinoic acid (40–43). Whether these cells play a role in the generation of Trms in the cervicovaginal tissues following the heterologous prime-cervicovaginal boost vaccination regimen should be investigated in future studies. Furthermore, α4β7 and CCR9 have also been shown to interact with MAdCAM-1 and TECK, the CCR9 ligand, expressed in the small intestine (34).
Our current data are consistent with previous observations in terms of the efficacy of DNA-vaccinia prime-boost vaccination regimens in controlling HPV-associated disease in this preclinical model (14). Previously, we employed Vac-Sig/E7/LAMP-1 and Sig/E7/LAMP-1 DNA in various homologous and heterologous combinations in a preclinical model and found that priming with Sig/E7/LAMP-1 DNA and boosting with Vac-Sig/E7/LAMP-1 generated the strongest E7-specific CD8+ T cell responses (14). The encouraging preclinical data led to an ongoing clinical trial assessing the efficacy of pNGVL4a-sig/E7(detox)/HSP70 DNA prime followed by TA-HPV boost in patients with grade 3 cervical intraepithelial neoplasia using intrmuscular administration (20). Patients who were vaccinated peripherally, who had residual dysplasia post-vaccination, nonetheless had striking histologic and molecular changes in the lesions, including markedly increased intensity of CD8+ T cell infiltrates in both the stromal and epithelial compartments, compared to pre-vaccination. These infiltrates were organized in tertiary lymphoid structures, showed evidence of proliferation induced by cognate antigen, and expression of genes associated with immune activation and effector function. These findings suggest that, in humans, peripheral therapeutic vaccination can induce immune responses that localize in target lesions, and that analyses of immune responses at sites of antigen are likely to be much more informative than analyses of systemic responses.
In this model, cervicovaginal boost vaccination with TA-HPV is more effective in generating E7-specific CD8+ T cells in the cervicovaginal tract than intramuscular administration,(see Supplementary Figure 1), suggesting that future clinical trials should consider using cervicovagional administration for TA-HPV. In our study, we demonstrated cervicovaginal boost with TA-HPV generated CD8 T cells with a Trm phenotype in the cervicovaginal tract. Previously, it has been shown that cervicovaginal vaccination with heterotypic HPV pseudovirions induced antigen-specific T cells expressing CD103 (44). The induction or enhancement of existing Trm-mediated immunity may be advantageous in the context of HPV-associated disease. In other peripheral tissues, including skin and lung, tissue-resident memory T cells have been shown to provide a ‘first response’ to antigen, secreting cytokines that recruit circulating T cells (22, 45). In murine models, Trms in normal mucosa have been shown to be able to protect against mucosal challenge with HIV, while systemic CD8+ T cells were unable to provide protection (46). It is likely that analyses of tissue immune responses will provide insights that are not obvious in cells in circulation; clinical trials testing therapeutic HPV vaccination regimens should include monitoring immune responses whenever possible.
In summary, we showed that cervicovaginal administration of therapeutic HPV vaccines in mice with established tumors generates enhanced antigen-specific CD8+ T cells with the mucosal Trm phenotype as well as therapeutic effects, compared to intramuscular vaccination.
Supplementary Material
Statement of Translational Relevance.
It has been demonstrated in preclinical studies that heterologous vaccination regimens consisting of DNA vaccine priming, followed by boosting with viral vector constructs, elicit stronger effector responses than vaccination with either DNA alone or viral vectors alone. Recent clinical data from a trial testing a heterologous DNA-prime, recombinant vaccinia vector-based boost (TA-HPV) demonstrate tissue localization of effector immune responses following peripheral, intramuscular vaccination in the deltoid muscles prior to standard therapeutic resection. In our preclinical study, cervicovaginal boost vaccination with TA-HPV is more effective in generating E7-specific CD8+ T cell mediated tumor control in the cervicovaginal tract than intramuscular administration. Furthermore, cervicovaginal boost with TA-HPV generated CD8+ T cells with a Trm phenotype in the cervicovaginal tract. Because both the therapeutic HPV DNA vaccine and the HPV vaccinia vaccine have been tested in the clinical arena, our data support applying cervicovaginal boost with HPV vaccinia for future clinical investigation.
Acknowledgments
This work was supported by the National Institutes of Health/National Cancer Institute Cervical Cancer SPORE P50CA098252, Head and Neck Cancer SPORE P50 CA-DE019032, 2R01CA114425-06, and R01CA142691 grants.
Footnotes
Conflict of Interest: T.-C. Wu and Richard B.S. Roden are co-founders of and have an equity ownership interest in Papivax LLC. Also, they own Papivax Biotech Inc. stock options and are members of Papivax Biotech Inc.'s Scientific Advisory Board. Additionally, under a licensing agreement between Papivax Biotech Inc. and the Johns Hopkins University, Dr. Wu and Dr. Roden are entitled to royalties on an invention described in this article. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies
References
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of pathology. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Trimble CL, Piantadosi S, Gravitt P, Ronnett B, Pizer E, Elko A, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:4717–23. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yugawa T, Kiyono T. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Reviews in medical virology. 2009;19:97–113. doi: 10.1002/rmv.605. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Science translational medicine. 2014;6:221ra13. doi: 10.1126/scitranslmed.3007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh WK, Alvarez RD, Maldonado L, Wang C, Wu TC, Trimble C. Phase I evaluation of therapeutic HPV16 E7 vaccination before resection of HPV16 + CIN2/3. Gynecology Oncology. 2014;133:28–9. [Google Scholar]
- 7.Trimble C, Lin CT, Hung CF, Pai S, Juang J, He L, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21:4036–42. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 8.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:361–7. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–7. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann AM, Stern PL, Rankin EM, Sommer H, Nuessler V, Schneider A, et al. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:3676–85. [PubMed] [Google Scholar]
- 11.Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer research. 2003;63:6032–41. [PubMed] [Google Scholar]
- 12.Smyth LJ, Van Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, Sehr P, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:2954–61. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 13.Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, Baldwin PJ, et al. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2006;16:1075–81. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Wang TL, Hung CF, Pardoll DM, Wu TC. Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies of HPV-16 E7-expressing DNA vaccines. Vaccine. 2000;18:2015–22. doi: 10.1016/s0264-410x(99)00528-9. [DOI] [PubMed] [Google Scholar]
- 15.Hanke T, Neumann VC, Blanchard TJ, Sweeney P, Hill AV, Smith GL, et al. Effective induction of HIV-specific CTL by multi-epitope using gene gun in a combined vaccination regime. Vaccine. 1999;17:589–96. doi: 10.1016/s0264-410x(98)00238-2. [DOI] [PubMed] [Google Scholar]
- 16.Schneider J, Gilbert SC, Blanchard TJ, Hanke T, Robson KJ, Hannan CM, et al. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nature medicine. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 17.Sedegah M, Jones TR, Kaur M, Hedstrom R, Hobart P, Tine JA, et al. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7648–53. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanke T, Blanchard TJ, Schneider J, Hannan CM, Becker M, Gilbert SC, et al. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA boost vaccination regime. Vaccine. 1998;16:439–45. doi: 10.1016/s0264-410x(97)00226-0. [DOI] [PubMed] [Google Scholar]
- 19.Kent SJ, Zhao A, Best SJ, Chandler JD, Boyle DB, Ramshaw IA. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. Journal of virology. 1998;72:10180–8. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Center SKCC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Vaccine Therapy With or Without Imiquimod in Treating Patients With Grade 3 Cervical Intraepithelial Neoplasia. [cited 2013 April 18] Available from: http://clinicaltrials.gov/show/NCT00788164 NLM Identifier: NCT00788164; 2008. [Google Scholar]
- 21.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nature reviews Immunology. 2009;9:153–61. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 22.Cauley LS, Lefrancois L. Guarding the perimeter: protection of the mucosa by tissue-resident memory T cells. Mucosal immunology. 2013;6:14–23. doi: 10.1038/mi.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardelli-Haefliger D, Dudda JC, Romero P. Vaccination route matters for mucosal tumors. Science translational medicine. 2013;5:172fs4. doi: 10.1126/scitranslmed.3005638. [DOI] [PubMed] [Google Scholar]
- 24.Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, et al. Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors. Science translational medicine. 2013;5:172ra20. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer research. 1996;56:21–6. [PubMed] [Google Scholar]
- 26.Huang B, Mao CP, Peng S, He L, Hung CF, Wu TC. Intradermal administration of DNA vaccines combining a strategy to bypass antigen processing with a strategy to prolong dendritic cell survival enhances DNA vaccine potency. Vaccine. 2007;25:7824–31. doi: 10.1016/j.vaccine.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soong RS, Song L, Trieu J, Knoff J, He L, Tsai YC, et al. Toll-like Receptor Agonist Imiquimod Facilitates Antigen-Specific CD8+ T-cell Accumulation in the Genital Tract Leading to Tumor Control through IFNgamma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-14-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, et al. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol. 2010;185:7107–14. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Current molecular medicine. 2009;9:836–50. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Mima K, Hayashi H, Imai K, Kuroki H, Nakagawa S, Okabe H, et al. High CD44s expression is associated with the EMT expression profile and intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. Journal of hepato-biliary-pancreatic sciences. 2013;20:429–34. doi: 10.1007/s00534-012-0580-0. [DOI] [PubMed] [Google Scholar]
- 32.Svensson M, Johansson-Lindbom B, Zapata F, Jaensson E, Austenaa LM, Blomhoff R, et al. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal immunology. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng R, Oderup C, Yuan R, Lee M, Habtezion A, Hadeiba H, et al. Retinoic acid regulates the development of a gut-homing precursor for intestinal dendritic cells. Mucosal immunology. 2013;6:847–56. doi: 10.1038/mi.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruane D, Brane L, Reis BS, Cheong C, Poles J, Do Y, et al. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. The Journal of experimental medicine. 2013;210:1871–88. doi: 10.1084/jem.20122762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corona Gutierrez CM, Tinoco A, Lopez Contreras M, Navarro T, Calzado P, Vargas L, et al. Clinical protocol. A phase II study: efficacy of the gene therapy of the MVA E2 recombinant virus in the treatment of precancerous lesions (NIC I and NIC II) associated with infection of oncogenic human papillomavirus. Human gene therapy. 2002;13:1127–40. doi: 10.1089/104303402753812520. [DOI] [PubMed] [Google Scholar]
- 36.Kanazawa T, Takashima Y, Hirayama S, Okada H. Effects of menstrual cycle on gene transfection through mouse vagina for DNA vaccine. International journal of pharmaceutics. 2008;360:164–70. doi: 10.1016/j.ijpharm.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 37.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nature immunology. 2013;14:1285–93. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zajac AJ, Harrington LE. Tissue-resident T cells lose their S1P1 exit visas. Cellular & molecular immunology. 2014;11:221–3. doi: 10.1038/cmi.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadley GA, Higgins JM. Integrin alphaEbeta7: molecular features and functional significance in the immune system. Advances in experimental medicine and biology. 2014;819:97–110. doi: 10.1007/978-94-017-9153-3_7. [DOI] [PubMed] [Google Scholar]
- 40.Hammerschmidt SI, Friedrichsen M, Boelter J, Lyszkiewicz M, Kremmer E, Pabst O, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. The Journal of clinical investigation. 2011;121:3051–61. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molenaar R, Greuter M, van der Marel AP, Roozendaal R, Martin SF, Edele F, et al. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol. 2009;183:6395–402. doi: 10.4049/jimmunol.0900311. [DOI] [PubMed] [Google Scholar]
- 42.Bhat PV. Retinal dehydrogenase gene expression in stomach and small intestine of rats during postnatal development and in vitamin A deficiency. FEBS letters. 1998;426:260–2. doi: 10.1016/s0014-5793(98)00355-x. [DOI] [PubMed] [Google Scholar]
- 43.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature immunology. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 44.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. The Journal of clinical investigation. 2012;122:4606–20. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–31. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belyakov IM, Ahlers JD, Brandwein BY, Earl P, Kelsall BL, Moss B, et al. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. The Journal of clinical investigation. 1998;102:2072–81. doi: 10.1172/JCI5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.