Abstract
Purpose
Inflammatory breast cancer (IBC) is an aggressive subtype of breast cancer for which treatments vary, so we sought to identify factors that affect the receipt of guideline-concordant care.
Methods
Patients diagnosed with IBC in 2004 were identified from the Breast and Prostate Cancer Data Quality and Patterns of Care Study, containing information from cancer registries in seven states. Variation in guideline-concordant care for IBC, based on National Comprehensive Cancer Network (NCCN) guidelines, was assessed according to patient, physician, and hospital characteristics.
Results
Of the 107 IBC patients in the study without distant metastasis at the time of diagnosis, only 25.8% received treatment concordant with guidelines. Predictors of non-concordance included patient age (≥70 years), non-white race, normal body mass index (BMI 18.5–25 kg/m2), patients with physicians graduating from medical school >15 years prior, and smaller hospital size (<200 beds). IBC patients survived longer if they received guideline-concordant treatment based on either 2003 (p=0.06) or 2013 (p=0.06) NCCN guidelines.
Conclusions
Targeting factors associated with receipt of care that is not guideline-concordant may reduce survival disparities in IBC patients. Prompt referral for neoadjuvant chemotherapy and postoperative radiation therapy is also crucial.
Keywords: inflammatory breast cancer, breast cancer, guideline, healthcare disparities, epidemiology
1. INTRODUCTION
Inflammatory breast cancer (IBC) is an aggressive and lethal form of locally advanced breast cancer, with median overall survival being less than 4 years [1–4]. IBC is also a relatively rare subtype, comprising approximately 1–5% of all breast cancers among women in the United States [5–9]. Even though IBC accounts for a low percentage of breast cancer cases, it accounts for 7% of all breast cancer deaths since IBC patients have poorer survival than non-IBC breast cancer patients [5,10–12]. IBC patients tend to be younger than other breast cancer patients, with a median age at diagnosis of 57 years compared to 62 for all breast cancers combined [7,8]. Diagnosis of IBC is made clinically based on diffuse erythema, edema, and fine dimpling (peau d’orange) [2,13]. Other associated findings include skin thickening, nipple inversion, increased breast density, and stromal coarsening [13]. Underlying palpable masses are not always seen and are not required for diagnosis [14]. The clinical appearance of inflammation is due to the lymphatic obstruction that results from the tumor emboli invading the dermal lymphatic vessels in the breast [15]; this inflammation does not appear to result from the infiltration of lymphocytes or other inflammatory mediators [16–18].
Treatment for IBC is typically multi-modal [12], but outcomes remain worse than for stage-matched non-IBC breast cancer patients. Currently the National Comprehensive Cancer Network (NCCN) recommends neoadjuvant chemotherapy with an anthracycline-based regimen with or without taxanes followed by mastectomy and axillary lymph node dissection. Optimal dose and fractionation of radiotherapy is a topic of disagreement among experts. Tamoxifen and other hormone therapies may also be considered for women with hormone receptor-positive tumors. In the past, attempts to treat IBC with surgery alone or surgery combined with radiation therapy resulted in median overall survival times of less than 15 months and local recurrence rates as high as 50% [19]. Results from a large retrospective single-institution study of patients with IBC performed over a 20-year period demonstrated that initial treatment with an anthracycline-based regimen followed by local therapy resulted in 5 - and 10-year survival rates of 40% and 33%, respectively [20].
Because IBC is such a rare breast cancer subtype, studies have been challenged to demonstrate patterns of IBC treatment and whether treatment differs based on various patient, physician, or institution characteristics. It is imperative to understand the factors behind variation in the treatment of IBC. Using a multi-state, population-based sample of IBC patients, we examined factors that may have potentially affected receipt of care that was concordant with NCCN guidelines and how the receipt of guideline-concordant care affected survival.
2. MATERIALS AND METHODS
2.1 Patient Population and Data Sources
The Breast and Prostate Cancer Data Quality and Patterns of Care Study (POC-BP), a comprehensive patterns of care study from the National Program of Cancer Registries of the Centers for Disease Control and Prevention (CDC), collected information on breast and prostate cancer cases diagnosed in 2004 in seven states (California, Georgia, Kentucky, Louisiana, Minnesota, North Carolina, and Wisconsin). Cancer registry data were supplemented by re-abstracting hospital records and obtaining information about human epidermal growth factor receptor 2 (HER2) status, adjuvant treatment, and comorbidity from physicians and outpatient facilities and linkages with secondary files such as census data or information on hospitals and physicians. Of the 9,142 POC-BP breast cancer patients, 170 (1.9%) had inflammatory breast cancer (defined as T4d, N0–3, M0 using the 6th edition American Joint Committee on Cancer tumor-node-metastasis system for staging). 63 IBC cases with distant metastatic disease were excluded because their patterns of care would be markedly different (e.g. more likely to receive palliative care), leaving 107 cases for analysis.
2.2 Concordant Care
Definitions of “concordant care” were based on NCCN Clinical Practice Guidelines. In 2003, the following regimen was considered “guideline concordant” for the treatment of IBC: anthracycline neoadjuvant chemotherapy; mastectomy; radiation to the chest wall and supraclavicular nodes; and hormone therapy if estrogen receptor (ER)- or progesterone receptor (PR)-positive (NCCN Guidelines, 2003). In 2013, the following regimen was considered “guideline concordant” for IBC: taxane neoadjuvant chemotherapy plus trastuzumab if HER2+; anthracycline neoadjuvant chemotherapy unless TC (docetaxel and cyclophosphamide) or TCH (docetaxel, carboplatin, and trastuzumab) is used; mastectomy; radiation to the chest wall and supraclavicular nodes; hormone therapy if ER/PR-positive (NCCN Guidelines, 2013). Therefore, the only major change from 2003 to 2013 was the neoadjuvant chemotherapy guideline. We evaluated survival based on receipt of concordant care at the time of the study based on 2003 guidelines as well as receipt of care concordant with current 2013 guidelines. We analyzed overall concordance (the combination of all treatment modalities) as well concordance with individual treatment modalities.
2.3 Covariates of Interest
Racial/ethnic information was obtained from medical records and categorized into the following groups: non-Hispanic white, non-Hispanic black, Hispanic, and other. Insurance status at diagnosis was categorized into five groups as follows: private, Medicaid, Medicare/other public, none, and unknown. The private insurance group also included cases with Medicare with supplemental private insurance. Other public insurance comprised women with TRICARE, other military insurance, Veterans Affairs, or Indian Health Service coverage. The Medicaid group also included women on Medicare with Medicaid eligibility and other government programs. Body mass index (BMI) at the time of diagnosis was categorized into three groups: 18.5–24.9 kg/m2 (normal), 25–30 kg/m2 (overweight), and >30 kg/m2 (obese). Zero cases had BMI <18.5 kg/m2. Area socioeconomic measures were constructed from 2000 U.S. census data linked to the census tract of the patient’s residence at the time of diagnosis. Poverty and education at the residential census tract level were categorized into less than 20% (low poverty) versus ≥20% of persons (high poverty) with an income below the federal poverty level, and less than 25% (high education) versus ≥25% (low education) of adults (≥25 years old) with less than a high-school education, as done previously [21]. Another ecological measure included percent of the population that was in an urban area (100% urban, 100% rural, urban/rural mix). Comorbidities were assessed using the Adult Comorbidity Evaluation (ACE)-27, which includes conditions relevant to cancer treatment choice and outcome, and the severity of these conditions that were present at or before diagnosis [22,23]. Overall comorbidity index (none, low, moderate, or severe) was allotted based on the comorbidity with the highest level of decompensation.
The year of medical school graduation and primary specialty were obtained for all physicians from the Medicare Physician Identification and Eligibility Registry (MPIER) File, maintained by the US Centers for Medicare and Medicaid Services.
Institutional Commission on Cancer (CoC) status was determined based on the facility in which the patient received breast cancer surgery regardless of the location of other treatments because the surgeon usually determines the initial course of treatment and since most referrals for adjuvant therapy are made by a surgeon [24]. Information on hospital teaching status and number of beds was obtained from the American Hospital Directory.
For survival analysis, vital status and underlying cause of death were obtained from linkages with state death certificate files and National Death Index from the US National Center for Health Statistics. Person-time follow-up was calculated from the date of diagnosis through either the date of death or 5 years after diagnosis (1826 days), whichever occurred first. Patients were classified as having a breast cancer-related death if the death certificate indicated the underlying cause of death as C50 breast cancer (International Classification of Diseases ICD-10).
2.4 Statistical Analysis
We assessed the association of the covariates of interest with receipt of guideline-concordant care using χ2 tests. Kaplan-Meier survival curves were drawn to compare patients based on guideline concordance. Log-rank tests were calculated to compare all-cause and breast cancer-specific survival curves. All significance tests were two-sided; p-values less than 0.05 were considered statistically significant. All statistics were weighted by the sampling fractions used by each state registry for the respective sampling stratum to represent the source population. SAS procedures for survey data were used [25]. Due to the small sample size, multivariable analysis of predictors of guideline concordance was not performed.
3. RESULTS
3.1 Guideline Concordance
Table 1 displays the characteristics of the 107 IBC patients that were included in this analysis. The average age at diagnosis was 57.8 years. 74.0% of patients were white, and 37.6% of patients were obese. Most patients (90.9%) had some form of insurance.
Table 1.
General characteristics of inflammatory breast cancer patients, NPCR POC-BP, 2004
| Characteristic | Number (N=107) | Weighted* % |
|---|---|---|
| Age at diagnosis, years | ||
| <50 | 33 | 27.8 |
| 50–59 | 31 | 32.6 |
| 60–69 | 20 | 22.5 |
| ≥70 | 23 | 17.0 |
| Race/ethnicity | ||
| White, non-Hispanic | 56 | 74.0 |
| Black, non-Hispanic | 40 | 19.1 |
| Hispanic | 9 | 6.2 |
| Other | 2 | 0.7 |
| Body mass index (kg/m2) | ||
| 18.5–24.9 | 16 | 13.3 |
| 25–30 | 20 | 19.2 |
| >30 | 41 | 37.6 |
| Unknown | 30 | 29.9 |
| Participating State | ||
| California | 18 | 19.8 |
| Georgia | 34 | 22.4 |
| Kentucky | 5 | 7.7 |
| Louisiana | 18 | 8.1 |
| Minnesota | 9 | 7.8 |
| North Carolina | 16 | 26.3 |
| Wisconsin | 7 | 7.9 |
| Patient health insurance | ||
| Private | 51 | 52.1 |
| Medicare only/public | 27 | 24.5 |
| Medicaid | 22 | 14.4 |
| No insurance or unknown | 7 | 9.1 |
| Poverty (census tract) | ||
| Low (<20% of residents below poverty) | 74 | 80.2 |
| High (≥20% of residents below poverty) | 32 | 19.4 |
| Education (census tract) | ||
| High (≥20% adults with a high school degree) | 55 | 63.8 |
| Low (<20% adults with a high school degree) | 51 | 35.8 |
Abbreviation: NPCR POC-BP, National Program of Cancer Registries Breast and Prostate Cancer Data Quality and Patterns of Care Study.
Percentages weighted to reflect the sampling design.
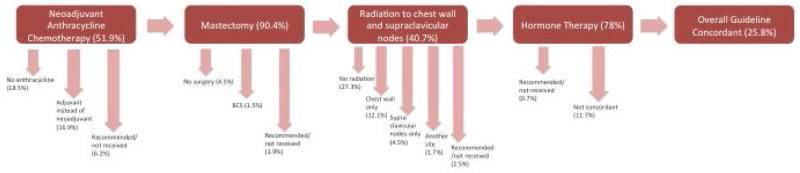
Figure 1 demonstrates the NCCN guidelines for treating IBC and where IBC patients deviated from this course. IBC is generally treated with neoadjuvant chemotherapy, mastectomy, radiation to the chest wall and supraclavicular nodes, and appropriate hormonal therapy. The advent of neoadjuvant chemotherapy in IBC treatment has improved surgery and locoregional control of the disease [26–28]. Only 25.8% of IBC patients received guideline-concordant treatment based on 2003 NCCN guidelines (Supplemental Table 1). 90.4% were concordant for surgery, 51.9% for chemotherapy, 40.7% for radiation, and 78% for hormone therapy if hormone receptor-positive (Supplemental Table 1). For chemotherapy, the timing of treatment (e.g. neoadjuvant or adjuvant) was taken into consideration for guideline concordance. Of the 40 patients that did not receive guideline-concordant care, 13 received an anthracycline in the adjuvant setting rather than neoadjuvant, while 27 did not receive any anthracycline therapy. Of the patients not receiving guideline-concordant radiation therapy, most did not receive any radiation at all, and some received radiation only to the chest wall or supraclavicular nodes but not both.
Figure 1.
Flow diagram demonstrating the NCCN guideline-concordant treatment regimen for inflammatory breast cancer in 2004. Arrows indicate where patients diverted if they were not concordant for the indicated treatment modalities. Patients for whom treatment data were not available for a certain modality are included in the percentages but are not displayed in the figure. BCS = breast conserving surgery (alias lumpectomy).
IBC tumors are more commonly HER2-positive compared to other breast cancers [29]. Of the 107 IBC patients, 41 were HER2-positive. 16 of these HER2-positive IBC patients received trastuzumab, 7 in the neoadjuvant and 9 in the adjuvant setting.
We examined whether guideline-concordant care differed based on different patient, physician, and institutional characteristics (Table 2). Regarding overall concordance (i.e. concordant care for all treatment modalities), 28.7% of patients under the age of 50 received guideline concordant care compared to 23.8% of patients 50–59 years, 49.7% of patients 60–69 years, and 8.4% patients aged ≥70 years. Furthermore, 34.2% of white patients received guideline-concordant care compared to 15.2% of black patients (p=0.03). IBC patients with normal BMI values were less likely to receive guideline-concordant care (p=0.003); 13.0% of patients with a BMI between 18.5 and 25, 22.5% of patients with a BMI between 25 and 30, and 47.2% of patients with a BMI over 30 received concordant care. There were no significant differences in receipt of concordant care based on census-tract poverty or education. Receipt of concordant care did not vary significantly with comorbidity index (p=0.85); while the sample size was small, the results suggest that women with severe comorbidities may be less likely to receive guideline concordant surgery, chemotherapy, or radiation.
Table 2.
Receipt of NCCN guideline-concordant treatment among inflammatory breast cancer patients based on patient, physician, and institution characteristics, CDC POC-BP, 2004*
| Overall | Surgery | Chemotherapy | Radiation | Hormone Therapy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | Number | % | Number | % | ||
| Total | 107 | 22 | 93 | 54 | 35 | 86 | |||||
| Patient Characteristics | |||||||||||
| Age at diagnosis | P=0.19 | ||||||||||
| <50 | 33 | 6 | 28.7 | 29 | 92.9 | 20 | 62.5 | 13 | 54.9 | 28 | 88.8 |
| 50–59 | 31 | 7 | 23.8 | 28 | 95.9 | 16 | 49.9 | 11 | 39.3 | 25 | 82.7 |
| 60–69 | 20 | 8 | 49.7 | 19 | 98.5 | 13 | 74.0 | 9 | 70.8 | 16 | 88.9 |
| ≥70 | 23 | 1 | 8.4 | 17 | 72.2 | 5 | 24.8 | 2 | 14.5 | 17 | 85.0 |
| Race | P=0.03 | ||||||||||
| White, NH | 56 | 15 | 34.2 | 51 | 94.5 | 31 | 56.0 | 23 | 54.5 | 45 | 86.0 |
| Black, NH | 40 | 6 | 15.2 | 33 | 83.0 | 19 | 54.6 | 11 | 26.3 | 33 | 84.8 |
| Hispanic | 9 | 0 | 0.0 | 8 | 94.1 | 3 | 52.7 | 0 | 0.0 | 7 | 93.8 |
| Other | 2 | 1 | 50.4 | 1 | 50.4 | 1 | 50.4 | 1 | 50.4 | 1 | 100.0 |
| Body mass index (kg/m2) | P=0.003 | ||||||||||
| 18.5–24.9 | 16 | 1 | 13.0 | 14 | 94.9 | 5 | 25.8 | 4 | 52.3 | 13 | 89.4 |
| 25–30 | 20 | 5 | 22.5 | 16 | 88.5 | 11 | 53.2 | 7 | 34.7 | 15 | 62.2 |
| >30 | 41 | 12 | 47.2 | 38 | 96.8 | 24 | 69.1 | 18 | 59.6 | 36 | 95.1 |
| Unknown | 30 | 4 | 13.6 | 25 | 86.3 | 14 | 53.1 | 6 | 30.5 | 22 | 91.0 |
| Insurance | P=0.63 | ||||||||||
| Private | 51 | 13 | 28.6 | 47 | 92.6 | 26 | 53.0 | 19 | 43.3 | 40 | 85.4 |
| Medicare | 27 | 4 | 27.2 | 22 | 93.2 | 12 | 50.5 | 7 | 56.6 | 22 | 95.8 |
| Medicaid | 22 | 3 | 16.6 | 17 | 82.3 | 10 | 53.9 | 6 | 28.9 | 18 | 69.9 |
| None/Unknown | 7 | 2 | 49.2 | 7 | 100.0 | 6 | 83.8 | 3 | 55.5 | 6 | 93.7 |
| Census-tract poverty | P=0.42 | ||||||||||
| <20% below poverty | 74 | 17 | 29.9 | 67 | 93.9 | 37 | 53.8 | 26 | 47.1 | 57 | 84.2 |
| ≥20% below poverty | 32 | 5 | 22.8 | 25 | 83.7 | 16 | 62.4 | 9 | 41.7 | 28 | 94.5 |
| Census-tract education | P=0.91 | ||||||||||
| <25% w/o HS degree | 55 | 15 | 29.3 | 51 | 94.1 | 30 | 51.8 | 23 | 47.6 | 43 | 84.7 |
| ≥25% w/o HS degree | 51 | 7 | 27.7 | 41 | 88.1 | 23 | 62.1 | 12 | 43.0 | 42 | 88.9 |
| Comorbidity index | P=0.85 | ||||||||||
| None | 38 | 9 | 33.6 | 35 | 97.6 | 20 | 56.7 | 13 | 50.1 | 32 | 86.6 |
| Mild | 43 | 8 | 23.8 | 37 | 87.4 | 24 | 58.6 | 12 | 36.5 | 32 | 86.1 |
| Moderate | 19 | 4 | 33.4 | 17 | 96.2 | 8 | 51.8 | 9 | 64.9 | 15 | 82.3 |
| Severe | 7 | 1 | 18.2 | 4 | 71.8 | 2 | 33.0 | 1 | 18.2 | 7 | 100.0 |
| Physician Characteristics | |||||||||||
| Time from graduation | |||||||||||
| Surgeon | P=0.02 | ||||||||||
| <15 years | 18 | 5 | 51.2 | 17 | 96.4 | 10 | 74.0 | 8 | 63.9 | 14 | 89.8 |
| >15 years | 39 | 10 | 31.5 | 39 | 100.0 | 24 | 62.0 | 15 | 52.2 | 33 | 91.4 |
| Unknown | 39 | 7 | 16.8 | 37 | 97.7 | 20 | 47.4 | 12 | 38.8 | 31 | 76.5 |
| Radiation oncologist | P=0.37 | ||||||||||
| <15 years | 16 | 8 | 60.1 | 16 | 100.0 | 11 | 72.2 | 12 | 86.0 | 15 | 100.0 |
| >15 years | 32 | 10 | 38.1 | 30 | 96.4 | 23 | 74.6 | 16 | 56.8 | 28 | 89.7 |
| Unknown | 17 | 4 | 33.0 | 17 | 100.0 | 11 | 65.7 | 7 | 64.0 | 15 | 94.0 |
| Chemotherapy oncologist | P=0.72 | ||||||||||
| <15 years | 15 | 3 | 31.3 | 11 | 83.1 | 8 | 73.9 | 4 | 36.5 | 15 | 100.0 |
| >15 years | 58 | 15 | 36.4 | 53 | 95.8 | 35 | 64.6 | 23 | 53.8 | 46 | 87.8 |
| Unknown | 19 | 4 | 24.8 | 19 | 100.0 | 11 | 56.6 | 7 | 54.7 | 16 | 89.7 |
| Hospital Characteristics | |||||||||||
| CoC Accredited | P=0.50 | ||||||||||
| Yes | 57 | 14 | 32.6 | 51 | 91.4 | 33 | 57.6 | 22 | 49.2 | 46 | 89.8 |
| No | 38 | 6 | 22.8 | 33 | 93.0 | 16 | 54.0 | 9 | 36.7 | 31 | 79.1 |
| Teaching status | P=0.62 | ||||||||||
| Teaching | 48 | 9 | 31.7 | 43 | 94.9 | 30 | 65.5 | 14 | 39.3 | 36 | 81.1 |
| Non-teaching | 45 | 10 | 24.3 | 40 | 89.7 | 18 | 43.0 | 16 | 51.3 | 38 | 91.2 |
| Size (number of beds) | P=0.02 | ||||||||||
| <200 | 24 | 2 | 6.0 | 18 | 70.7 | 5 | 13.2 | 5 | 27.9 | 18 | 85.0 |
| 200–299 | 12 | 4 | 44.5 | 12 | 100.0 | 7 | 75.0 | 5 | 62.0 | 10 | 84.2 |
| 300–399 | 15 | 2 | 8.0 | 15 | 100.0 | 8 | 39.7 | 5 | 49.9 | 11 | 79.5 |
| ≥400 | 42 | 11 | 38.2 | 38 | 95.2 | 28 | 70.3 | 15 | 43.1 | 35 | 88.2 |
Abbreviations: CoC, Commission on Cancer; NH, non-Hispanic; NPCR POC-BP, National Program of Cancer Registries Breast and Prostate Cancer Data Quality and Patterns of Care Study.
Percentages weighted to reflect the sampling design. Chemotherapy oncologist refers to the physician that determined the chemotherapy regimen; it was not a medical oncologist in all cases. Time from graduation refers to graduation from medical school. Calculation of chi-square test p-values excluded categories of unknown status. For race, p-value compares non-Hispanic White to all others.
When analyzing differences based on physician characteristics (Table 2), we found that IBC patients were less likely to receive overall guideline-concordant care for all treatment modalities if their surgeon had completed their medical degrees more than 15 years prior (p=0.02). Receipt of guideline-concordant care was not related to time from graduation for the physicians administering radiation or chemotherapy (p=0.37 and p=0.72, respectively).
Results suggested that IBC patients who had their surgery at a small (less than 200 beds) hospital were less likely to receive guideline concordant care overall (p=0.02). 32.6% of IBC patients seen at CoC-accredited hospitals received guideline-concordant care compared to 22.8% of patients seen at hospitals not accredited by CoC (p=0.50). Furthermore, 31.7% of IBC patients seen at a teaching hospital received guideline-concordant care compared to 24.3% of patients seen at a non-teaching hospital (p=0.62).
3.2 Survival after IBC Diagnosis
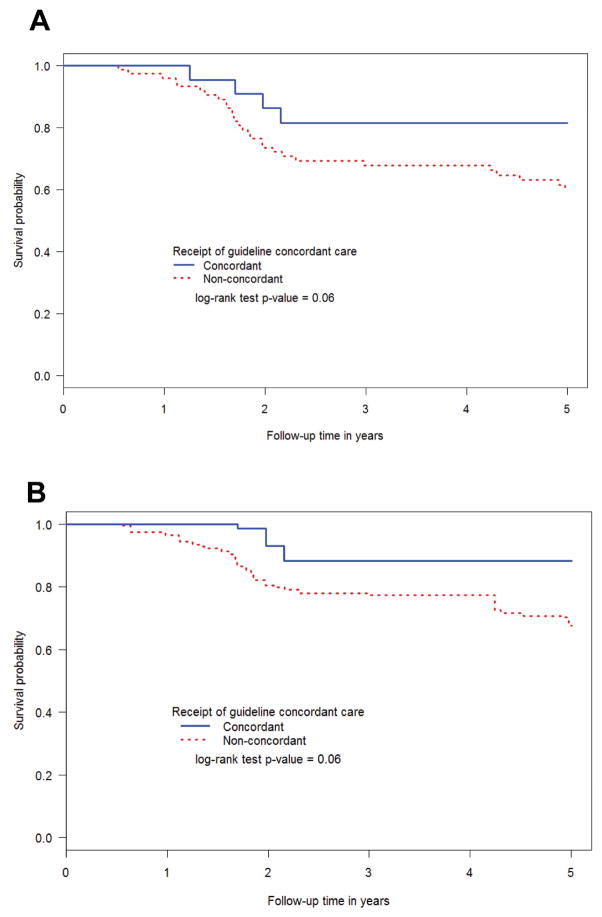
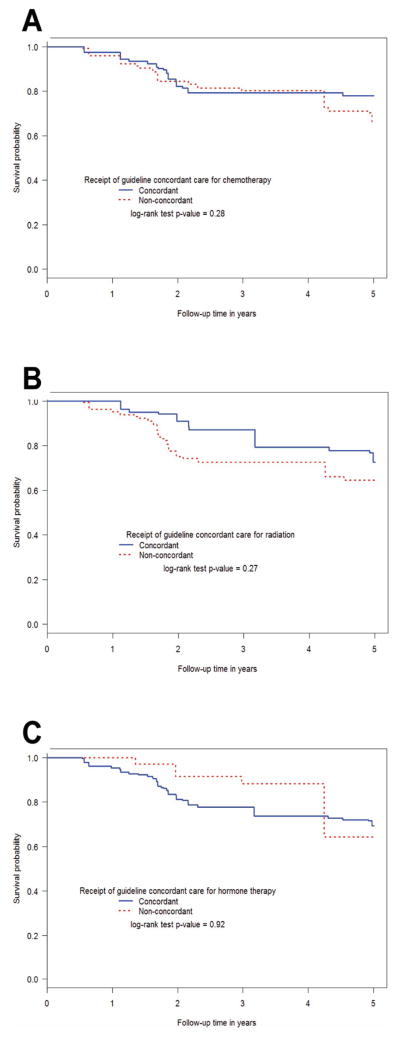
We compared the survival among IBC patients based on guideline concordance for radiation, chemotherapy, and hormone therapy. We assessed both breast cancer-specific survival (Figure 2) and overall survival (Supplementary Figure 1) using the Kaplan-Meier method. For breast cancer-specific survival, IBC patients survived longer if they received guideline-concordant treatment based on 2003 NCCN guidelines (Figure 2A; p=0.06). We also assessed whether patients receiving care that is now concordant with 2013 NCCN guidelines had longer breast cancer-specific survival. The only difference between 2003 and 2013 guidelines was the addition of taxane and trastuzumab (if HER2+) for neoadjuvant therapy and possible substitutions for anthracycline neoadjuvant therapy. These were all available in 2004 when these patients were treated. Indeed, we found that IBC patients receiving such care appeared to have longer survival (Figure 2B; p=0.06). When examining guideline concordance for the individual treatment modalities, there was no significant difference in breast cancer-specific survival when comparing IBC patients that did or did not receive guideline-concordant chemotherapy (p=0.28), radiation (p=0.27), or hormone therapy (p=0.92, Figure 3). Likewise, no significant differences in all-cause survival were observed when examining guideline concordance in the individual treatment modalities (Supplementary Figures 2, 3, and 4). We did not compare patients based on receipt of guideline-concordant surgery because nearly all patients (90.4%) received guideline-concordant mastectomy.
Figure 2.
Breast cancer-specific survival among inflammatory breast cancer patients based on receipt of care concordant with (A) 2003 or (B) 2013 NCCN guidelines using the Kaplan-Meier method. NPCR POC-BP, 2004–2009.
Figure 3.
Breast cancer-specific survival among inflammatory breast cancer patients based on whether they did or did not receive guideline-concordant care based on 2003 NCCN guidelines for (A) chemotherapy, (B) radiation, and (C) hormone therapy using the Kaplan-Meier method. NPCR POC-BP, 2004–2009.
4. DISCUSSION
IBC is a one of the rarest yet most lethal types of breast cancer. To our knowledge, this study provides the most comprehensive analysis of the receipt of guideline-concordant care in IBC patients. Understanding of this disease has been limited by the different definitions used to classify IBC (i.e. clinical, pathological, epidemiologic) as well as the lack of effective treatments. Treatment decisions are complicated, as evidenced by our finding that only 25.8% of IBC patients were concordant with NCCN guidelines. Reasons for this lack of concordance are unclear and multifactorial, but may reflect a nihilistic attitude amongst providers about the prognosis of patients with IBD. Here we report that patients who received care that followed 2003 NCCN guidelines had significantly improved survival. Similarly, a recent study demonstrated that IBC patients with stage III disease had an overall median survival of 66 months, while the subgroup that received multimodal therapy had a median survival of 107 months [12], a survival rate that compares favorably to other malignancies commonly treated with curative intent.
Despite the publishing of guidelines and recommendations, receipt of guideline-concordant care varies based on many factors. In our series, black race, low BMI, longer time since graduation for the surgeon, and small hospital size were associated with decreased likelihood of receiving guideline-concordant care. Furthermore, patients over 70 years old and with higher comorbidity indices were less likely to receive guideline concordant care, although these were not statistically significant. These findings are largely consistent with previous breast cancer studies, but they are important to report for IBC given the paucity of studies on this population. For example, we found that black IBC patients were less likely to receive guideline-concordant care, and previous studies have shown similar racial disparities in receipt of guideline-concordant care for breast cancer [23,30–32]. Specific to IBC, black women tend to be diagnosed at a younger age and also have worse outcomes compared to their white counterparts [33]. One explanation for the racial disparities identified in our data series is that minority status and socioeconomic status are strongly correlated, and poorer patients generally have limited access to care, limited treatment options, and difficulty with treatment compliance [31]. A second explanation is that mistrust in the medical delivery system is greater among minorities [34].
With regard to age, in older women comorbid conditions and lessened ability to tolerate treatment (i.e. performance status) could have impacted treatment decisions. Previous studies have also shown that increased patient age is associated with non-concordant treatment for cancer [23,35,36]. The 2003 NCCN guidelines state that there is insufficient data to make chemotherapy recommendations for those over 70, and such decisions need to be made on an individual basis; therefore, the standard of care was less clear for these older patients. Furthermore, there is generally a lack of women over 70 that participate in clinical trials [37]. Regarding comorbidities, a recent analysis of patients with early stage breast cancer from this same data set similarly found that increased comorbidity burden correlated with decreased likelihood of receiving guideline-concordant care [23].
IBC patients were less likely to receive guideline concordant care if their surgeon had completed their medical degree more than 15 years prior. This may suggest that some physicians are not keeping up to date with NCCN guidelines, or may be favoring therapy choices based on clinical experience. A previous report of physician survey data showed that a greater proportion of younger physicians rated themselves as “heavy users” of clinical guidelines compared to older physicians [38]. Based on the finding that 16.9% of patients received adjuvant rather than neoadjuvant chemotherapy, it is possible that the surgeon was not promptly referring the patient to medical oncology prior to surgery. Given the data demonstrating improved survival with neoadjuvant chemotherapy in IBC [28], timely referral to medical oncology is essential for IBC patients. Similarly, there was very low concordance with administration of radiation therapy. Optimal radiation technique has been associated with improved local control for patients with IBC, and series reporting the most favorable outcomes for this population have utilized tri-modality therapy (chemotherapy, surgery and radiation) [39–41].
When analyzing differences based on hospital characteristics, we found that IBC patients treated at a small (less than 200 beds) hospital were less likely to receive guideline concordant care overall. Small hospitals rarely see IBC patients and therefore are not as familiar with the NCCN guidelines for IBC. Previous studies have demonstrated improved outcomes for patients treated at higher volume centers [42–44].
A major strength of this study is its large-scale population-based design, including data from seven states and from individuals of all ages, making the sample size larger than most other IBC studies. Additional strengths are the inclusion of National Program of Cancer Registries that did not participate in previous patterns of care studies and the inclusion of comprehensive treatment information. One limitation is the relatively small number of IBC cases, which precluded multivariate analyses; however, all previous IBC research has had to deal with this challenge.
5. CONCLUSION
In conclusion, this is the only study to our knowledge that analyzed disparities in the receipt of guideline-concordant care in IBC patients based on patient, physician, and hospital characteristics. Our data suggest the need for efforts to improve concordance with NCCN guidelines for treating IBC. In addition, results strongly suggest that IBC patients may benefit from seeking care from board-certified specialists with experience at this rare disease and recent training. Prompt referral for neoadjuvant chemotherapy and post-operative radiation therapy is also crucial. In the absence of new, more effective therapies, interventions targeted to physicians are warranted to reduce variation in the treatment of IBC patients and to improve survival for these women.
Supplementary Material
Acknowledgments
The data used for this publication were collected by the CDC National Program of Cancer Registries Patterns of Care Study for Female Breast and Prostate Cancers through cooperative agreements with the participating state cancer registries including grant U01DP000261 to JFW. RAD is supported by the University of Wisconsin Medical Scientist Training Program (T32GM008692). This article was written on behalf of the Patterns of Care Study BP Group. This study was also supported in part by NIH grant P30CA014520. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
CONFLICT OF INTEREST
S.A.S. has stock ownership in Pfizer.
AUTHORSHIP CONTRIBUTIONS
Conception and design: JFW, ATD
Collection and assembly of data: JFW, ATD, JMH, RAD
Data analysis and interpretation: All authors
Manuscript writing: All authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Low JA, Berman AW, Steinberg SM, Danforth DN, Lippman ME, Swain SM. Long-term follow-up for locally advanced and inflammatory breast cancer patients treated with multimodality therapy. J Clin Oncol. 2004;22:4067–4074. doi: 10.1200/JCO.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 2.Merajver SD, Iniesta MD, Sabel MS. Inflammatory breast cancer. 4. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 3.Dawood S, Merajver SD, Viens P, Vermeulen PB, Swain SM, Buchholz TA, Dirix LY, Levine PH, Lucci A, Krishnamurthy S, Robertson FM, Woodward WA, Yang WT, Ueno NT, Cristofanilli M. International expert panel on inflammatory breast cancer: Consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22:515–523. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine PH, Steinhorn SC, Ries LG, Aron JL. Inflammatory breast cancer: The experience of the surveillance, epidemiology, and end results (seer) program. J Natl Cancer Inst. 1985;74:291–297. [PubMed] [Google Scholar]
- 5.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: The surveillance, epidemiology, and end results program at the national cancer institute. J Natl Cancer Inst. 2005;97:966–975. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wingo PA, Jamison PM, Young JL, Gargiullo P. Population-based statistics for women diagnosed with inflammatory breast cancer (united states) Cancer Causes Control. 2004;15:321–328. doi: 10.1023/B:CACO.0000024222.61114.18. [DOI] [PubMed] [Google Scholar]
- 7.Anderson WF, Schairer C, Chen BE, Hance KW, Levine PH. Epidemiology of inflammatory breast cancer (ibc) Breast Dis. 2005;22:9–23. doi: 10.3233/bd-2006-22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang S, Parker SL, Pham T, Buzdar AU, Hursting SD. Inflammatory breast carcinoma incidence and survival: The surveillance, epidemiology, and end results program of the national cancer institute, 1975–1992. Cancer. 1998;82:2366–2372. [PubMed] [Google Scholar]
- 9.Stocks LH, Patterson FM. Inflammatory carcinoma of the breast. Surg Gynecol Obstet. 1976;143:885–889. [PubMed] [Google Scholar]
- 10.Chang S, Alderfer JR, Asmar L, Buzdar AU. Inflammatory breast cancer survival: The role of obesity and menopausal status at diagnosis. Breast Cancer Res Treat. 2000;64:157–163. doi: 10.1023/a:1006489100283. [DOI] [PubMed] [Google Scholar]
- 11.Fouad TM, Kogawa T, Liu DD, Shen Y, Masuda H, El-Zein R, Woodward WA, Chavez-MacGregor M, Alvarez RH, Arun B, Lucci A, Krishnamurthy S, Babiera G, Buchholz TA, Valero V, Ueno NT. Overall survival differences between patients with inflammatory and noninflammatory breast cancer presenting with distant metastasis at diagnosis. Breast Cancer Res Treat. 2015 doi: 10.1007/s10549-015-3436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matro JM, Li T, Cristofanilli M, Hughes ME, Ottesen RA, Weeks JC, Wong YN. Inflammatory breast cancer management in the national comprehensive cancer network: The disease, recurrence pattern, and outcome. Clin Breast Cancer. 2014 doi: 10.1016/j.clbc.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tardivon AA, Viala J, Corvellec Rudelli A, Guinebretiere JM, Vanel D. Mammographic patterns of inflammatory breast carcinoma: A retrospective study of 92 cases. Eur J Radiol. 1997;24:124–130. doi: 10.1016/s0720-048x(96)01137-0. [DOI] [PubMed] [Google Scholar]
- 14.Bonnier P, Charpin C, Lejeune C, Romain S, Tubiana N, Beedassy B, Martin PM, Serment H, Piana L. Inflammatory carcinomas of the breast: A clinical, pathological, or a clinical and pathological definition? Int J Cancer. 1995;62:382–385. doi: 10.1002/ijc.2910620404. [DOI] [PubMed] [Google Scholar]
- 15.Ellis DL, Teitelbaum SL. Inflammatory carcinoma of the breast. A pathologic definition. Cancer. 1974;33:1045–1047. doi: 10.1002/1097-0142(197404)33:4<1045::aid-cncr2820330422>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Bièche I, Lerebours F, Tozlu S, Espie M, Marty M, Lidereau R. Molecular profiling of inflammatory breast cancer: Identification of a poor-prognosis gene expression signature. Clin Cancer Res. 2004;10:6789–6795. doi: 10.1158/1078-0432.CCR-04-0306. [DOI] [PubMed] [Google Scholar]
- 17.Kleer CG, van Golen KL, Merajver SD. Molecular biology of breast cancer metastasis. Inflammatory breast cancer: Clinical syndrome and molecular determinants. Breast Cancer Res. 2000;2:423–429. doi: 10.1186/bcr89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen PP. Rosen’s breast pathology. 3. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 19.Zucali R, Uslenghi C, Kenda R, Bonadonna G. Natural history and survival of inoperable breast cancer treated with radiotherapy and radiotherapy followed by radical mastectomy. Cancer. 1976;37:1422–1431. doi: 10.1002/1097-0142(197603)37:3<1422::aid-cncr2820370325>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA, Theriault RL, Strom EA, Wasaff BJ, Asmar L, Frye D, Hortobagyi GN. Combined-modality treatment of inflammatory breast carcinoma: Twenty years of experience at m. D. Anderson cancer center. Cancer Chemother Pharmacol. 1997;40:321–329. doi: 10.1007/s002800050664. [DOI] [PubMed] [Google Scholar]
- 21.Wu XC, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, Fleming ST, Morris CR, Huang B, Trentham-Dietz A, Lipscomb J. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30:142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 22.Piccirillo JF, Creech C, Zequeira R, Anderson S, Johnston AS. Inclusion of comorbidity into oncology data registries. 1999;26:66–70. [Google Scholar]
- 23.Kimmick G, Fleming ST, Sabatino SA, Wu XC, Hwang W, Wilson JF, Lund MJ, Cress R, Anderson RT Group CfDCaPNPoCRPoCS. Comorbidity burden and guideline-concordant care for breast cancer. J Am Geriatr Soc. 2014;62:482–488. doi: 10.1111/jgs.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siminoff LA, Zhang A, Saunders Sturm CM, Colabianchi N. Referral of breast cancer patients to medical oncologists after initial surgical management. Med Care. 2000;38:696–704. doi: 10.1097/00005650-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 25.SAS. Sas institute: Sas/stat 9.2 user’s guide: Survey data analysis. Cary, NC: SAS Institute; 2009. pp. 25–194. [Google Scholar]
- 26.Schick P, Goodstein J, Moor J, Butler J, Senter KL. Preoperative chemotherapy followed by mastectomy for locally advanced breast cancer. J Surg Oncol. 1983;22:278–282. doi: 10.1002/jso.2930220415. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, Howell A, Costa SD, Beuzeboc P, Untch M, Blohmer JU, Sinn HP, Sittek R, Souchon R, Tulusan AH, Volm T, Senn HJ. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: Review and recommendations. J Clin Oncol. 2003;21:2600–2608. doi: 10.1200/JCO.2003.01.136. [DOI] [PubMed] [Google Scholar]
- 28.Dawood S, Cristofanilli M. Ibc as a rapidly spreading systemic disease: Clinical and targeted approaches using the neoadjuvant model. J Natl Cancer Inst Monogr. 2015;2015:56–59. doi: 10.1093/jncimonographs/lgv017. [DOI] [PubMed] [Google Scholar]
- 29.Turpin E, Bièche I, Bertheau P, Plassa LF, Lerebours F, de Roquancourt A, Olivi M, Espié M, Marty M, Lidereau R, Vidaud M, de Thé H. Increased incidence of erbb2 overexpression and tp53 mutation in inflammatory breast cancer. Oncogene. 2002;21:7593–7597. doi: 10.1038/sj.onc.1205932. [DOI] [PubMed] [Google Scholar]
- 30.Freedman RA, Virgo KS, He Y, Pavluck AL, Winer EP, Ward EM, Keating NL. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117:180–189. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 31.Bhargava A, Du XL. Racial and socioeconomic disparities in adjuvant chemotherapy for older women with lymph node-positive, operable breast cancer. Cancer. 2009;115:2999–3008. doi: 10.1002/cncr.24363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy GP, Lipscomb J, Gillespie TW, Goodman M, Richardson LC, Ward KC. Variations in guideline-concordant breast cancer adjuvant therapy in rural georgia. Health Serv Res. 2015;50:1088–1108. doi: 10.1111/1475-6773.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang S, Buzdar AU, Hursting SD. Inflammatory breast cancer and body mass index. J Clin Oncol. 1998;16:3731–3735. doi: 10.1200/JCO.1998.16.12.3731. [DOI] [PubMed] [Google Scholar]
- 34.Bickell NA, Weidmann J, Fei K, Lin JJ, Leventhal H. Underuse of breast cancer adjuvant treatment: Patient knowledge, beliefs, and medical mistrust. J Clin Oncol. 2009;27:5160–5167. doi: 10.1200/JCO.2009.22.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giordano SH, Hortobagyi GN, Kau SW, Theriault RL, Bondy ML. Breast cancer treatment guidelines in older women. J Clin Oncol. 2005;23:783–791. doi: 10.1200/JCO.2005.04.175. [DOI] [PubMed] [Google Scholar]
- 36.Vinod SK, Sidhom MA, Delaney GP. Do multidisciplinary meetings follow guideline-based care? J Oncol Pract. 2010;6:276–281. doi: 10.1200/JOP.2010.000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.(EBCTCG) EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 38.Kenefick H, Lee J, Fleishman V. Improving physician adherence to clinical practice guidelines: Barriers and strategies for change [Google Scholar]
- 39.Vinh-Hung V, Voordeckers M, Van de Steene J, Soete G, Lamote J, Storme G. Omission of radiotherapy after breast-conserving surgery: Survival impact and time trends. Radiother Oncol. 2003;67:147–158. doi: 10.1016/s0167-8140(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 40.Bristol IJ, Woodward WA, Strom EA, Cristofanilli M, Domain D, Singletary SE, Perkins GH, Oh JL, Yu TK, Terrefe W, Sahin AA, Hunt KK, Hortobagyi GN, Buchholz TA. Locoregional treatment outcomes after multimodality management of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2008;72:474–484. doi: 10.1016/j.ijrobp.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liauw SL, Benda RK, Morris CG, Mendenhall NP. Inflammatory breast carcinoma: Outcomes with trimodality therapy for nonmetastatic disease. Cancer. 2004;100:920–928. doi: 10.1002/cncr.20083. [DOI] [PubMed] [Google Scholar]
- 42.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: Importance in quality of cancer care. J Clin Oncol. 2000;18:2327–2340. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- 43.Scharl A, Göhring UJ. Does center volume correlate with survival from breast cancer? Breast Care (Basel) 2009;4:237–244. doi: 10.1159/000229531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barocas DA, Mitchell R, Chang SS, Cookson MS. Impact of surgeon and hospital volume on outcomes of radical prostatectomy. Urol Oncol. 2010;28:243–250. doi: 10.1016/j.urolonc.2009.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.