Abstract
Many debilitating psychiatric conditions, including drug addiction, are characterized by poor decision making and maladaptive risk-taking. Recent research has begun to probe this relationship to determine how brain mechanisms mediating risk-taking become compromised after chronic drug use. Currently, however, the majority of work in this field has used male subjects. Given the well-established sex differences in drug addiction, it is conceivable that such differences are also evident in risk-based decision making. To test this possibility, male and female adult rats were trained in a “Risky Decision making Task” (RDT), in which they chose between a small, “safe” food reward and a large, “risky” food reward accompanied by an increasing probability of mild footshock punishment. Consistent with findings in human subjects, females were more risk averse, choosing the large, risky reward significantly less than males. This effect was not due to differences in shock reactivity or body weight, and risk-taking in females was not modulated by estrous phase. Systemic amphetamine administration decreased risk-taking in both males and females; however, females exhibited greater sensitivity to amphetamine, suggesting that dopaminergic signaling may partially account for sex differences in risk-taking. Finally, although males displayed greater instrumental responding for food reward, reward choice in the RDT was not affected by satiation, indicating that differences in motivation to obtain food reward cannot fully account for sex differences in risk-taking. These results should prove useful for developing targeted treatments for psychiatric conditions in which risk-taking is altered and that are known to differentially affect males and females.
Keywords: risk, decision-making, sex differences, reward
Introduction
Maladaptive risk-taking in the face of punishment is a feature of many neuropsychiatric diseases. In particular, elevated risk-taking is closely associated with drug addiction, in that it is evident in chronic drug users (Bechara et al., 2001; Bornovalova, Daughters, Hernandez, Richards, & Lejuez, 2005; Gowin, Mackey, & Paulus, 2013) and is predictive of relapse vulnerability (Bowden-Jones, McPhillips, Rogers, Hutton, & Joyce, 2005; De Wilde, Verdejo-Garcia, Sabbe, Hulstijn, & Dom, 2013; Moeller et al., 2001). Given this relationship, there is considerable interest in understanding the behavioral and neural mechanisms of risk-taking and how they become compromised by chronic drug use. To date, much of the research investigating relationships between risk-taking and addiction has been conducted in male subjects. However, addiction in men and women differs considerably (Bobzean, DeNobrega, & Perrotti, 2014). While men have higher rates of drug use and dependence, women develop dependence more rapidly and are more vulnerable to relapse (Anglin, Hser, & McGlothlin, 1987; Brady & Randall, 1999; Griffin, Weiss, Mirin, & Lange, 1989; Ignjatova & Raleva, 2009; Robbins, Ehrman, Childress, & O’Brien, 1999). These differences have also been borne out in preclinical models of addiction. For example, female rats acquire cocaine self-administration more rapidly, are more willing to work for drug reinforcers, and reinstate drug-seeking at higher rates compared to male rats (Carroll, Morgan, Lynch, Campbell, & Dess, 2002; Hu, Crombag, Robinson, & Becker, 2004; Jackson, Robinson, & Becker, 2006; Lynch, 2006; Lynch & Carroll, 1999).
Emerging evidence has revealed that males and females also differ in some forms of risky decision making (R. van den Bos, Davies, et al., 2013; R. van den Bos, Homberg, & de Visser, 2013; R. van den Bos, Jolles, van der Knaap, Baars, & de Visser, 2012). For example, in a rodent gambling task, male rats are quicker to choose the more advantageous long-term option than females. In addition, female rats are more sensitive to losses and less sensitive to rewards than males (R. van den Bos et al., 2012). To summarize, the bulk of the existing literature suggests that females tend to be more risk-averse than males; however, the decision-making tasks that have been used to investigate sex differences to date do not incorporate the potential for explicit punishment or adverse consequences that is encountered in many real-world decisions, including those involving drug use. To model this type of decision making, our laboratory developed a rat model of risky decision making (the “Risky Decision making Task”), which incorporates both reward and risk of explicit punishment (delivery of a mild footshock) (Mitchell, Vokes, Blankenship, Simon, & Setlow, 2011; Simon, Gilbert, Mayse, Bizon, & Setlow, 2009; Simon et al., 2011). In previous work with this model, we have shown that male rats decrease their choice of the risky option as the risk of punishment increases (Orsini, Trotta, Bizon, & Setlow, 2015; Simon et al., 2009). Further, we have shown that high levels of risk-taking predict greater cocaine intake and that chronic cocaine self-administration causes long-lasting increases in risk-taking (Mitchell et al., 2014). It is unclear, however, if under drug-naïve conditions, females perform comparably to males when faced with the risk of adverse consequences. The experiments described below were designed to determine whether there are sex differences in decision making involving risk of explicit punishment.
Materials and Methods
Subjects
Male and female Long-Evans rats (n = 16 male; n = 16 female; 60 days old; Charles River Laboratories, Raleigh, NC) were individually housed and kept on a 12 hour light/dark cycle (lights on at 0700) with access to water and food ad libitum except as noted. Rats were trained in two cohorts, each with n = 8 male and n = 8 female. Prior to the start of behavioral testing, rats were handled 2–3 times a week to habituate them to the experimenters. During all behavioral procedures, rats were food restricted to 85% of their free-feeding weight. This was determined by calculating 85% of their free-feeding weight measured just prior to food restriction (one week prior to the commencement of behavioral testing). Prior to restriction, males weighed 304.6 ± 2.1 g (mean ± SEM) and females weighed 215.6 ± 3.3 g. To account for growth during periods of restriction, rats’ target weights were adjusted upward by 5 g/week. Rats were tested five days/week between 0900 and 1200. Animal procedures were conducted in accordance with the University of Florida Institutional Animal Care and Use Committee and followed guidelines of the National Institutes of Health.
Apparatus
Behavioral sessions were conducted in eight computer-controlled behavioral test chambers (Coulbourn Instruments). Each chamber was housed in a sound-attenuating cabinet and was outfitted with a recessed food pellet delivery trough with a photobeam to detect nosepokes into the trough and a 1.12 W lamp to illuminate the trough, into which 45 mg grain-based food pellets (Test Diet; 5TUM) could be delivered. The trough was located 2 cm above the floor in the center of the front wall of the chamber. Two retractable levers were located to the left and right of the food trough and were positioned 11 cm above the floor of the chamber. A 1.12 W houselight was mounted on the rear wall of the sound-attenuating cabinet. The floor of the chamber consisted of stainless steel rods coupled to a shock generator (Coulbourn Instruments), which delivered scrambled footshocks. An activity monitor was positioned above each test chamber to monitor locomotor activity throughout each behavioral session. This monitor consisted of an array of infrared (body heat) detectors focused over the entire test chamber. Movement in the test chamber (in x, y, or z planes) was defined as a relative change in the infrared energy falling on the different detectors. The test chambers were interfaced with a computer running Graphic State 3.0 software (Coulbourn Instruments), which controlled task event delivery and data collection. The same test chambers were used for both males and females, and chambers were cleaned with a dilute chlorhexidine solution after each rat. Rats in each cohort were run in two squads of 8 rats each, with the order of male and female rats tested in each chamber counterbalanced across squads.
Behavioral Procedures
Overview of Experimental Design
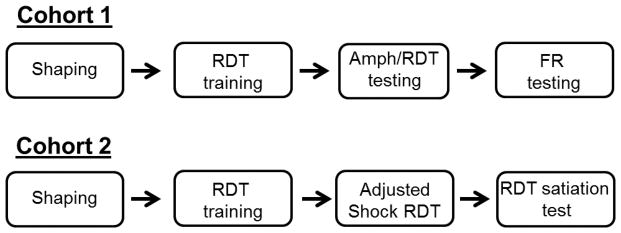
The experimental design is depicted in Figure 1. Upon arrival, rats were trained in the Risky Decision Making Task (RDT) for 25–34 days, at which point stable behavior emerged. In the first cohort of rats (n=8 male and 8 female), vaginal swabs were taken from females immediately after each session to track their estrous cycle over the course of testing. The same cohort was then used to test the effects of acute amphetamine administration on RDT performance. In this set of experiments, the shock intensity was kept constant across all rats (0.25–0.3 mA). Finally, these rats were tested on various fixed ratio (FR) schedules of lever pressing for food reward. As with RDT training, vaginal swabs were taken from each female after each FR session. During this time, rats were run on each FR schedule for as long as it took to ensure that each phase of the estrous cycle was represented.
Figure 1. Schematic of experimental design.
In the first cohort of rats, males and females were initially shaped on the various components of the task, after which they were trained in the RDT until stable performance emerged. Rats then received systemic administration of amphetamine (0, 0.3, 1.0, 1.5 mg/kg) 10 min prior to RDT testing. Finally, they were tested on various fixed ratio (FR) schedules of lever pressing for food reward. As with the first cohort, the second cohort of rats was shaped and trained in the RDT until stable behavior emerged. Shock intensities in the RDT were then adjusted for body weight (1.0 mA/kg); rats were tested in the RDT with adjusted shock intensities until behavioral stability. Finally, rats were then given free access to food for 24 hours followed by testing in the RDT.
Once stable performance emerged in the second cohort of rats (n=8 male and 8 female), shock intensity was then titrated for each rat according to its body weight (1.0 mA/kg). Rats were trained using these procedures until stable behavior was obtained (18 sessions). All rats were then returned to the same shock intensity (0.25 mA) and tested in the RDT for five days. At this point, rats were given free access to food for 24 hours followed by testing in the RDT.
Risky Decision-Making Task
Prior to training on the RDT, rats were shaped to perform the various task components (nosepoking into the food trough and lever pressing for food delivery) as described previously (Mitchell et al., 2011; Simon et al., 2009; Simon & Setlow, 2012). Each daily session in the RDT was 60 min in duration and consisted of five 18-trial blocks. Each 40 s trial began with illumination of the houselight and the food trough light. A nosepoke into the food trough extinguished the trough light and triggered extension of either a single lever (forced choice trial) or both levers simultaneously (free choice trial). If rats failed to nosepoke within 10 s, both the house and trough lights were extinguished and the trial was scored as an omission. A press on one lever resulted in a small, “safe” food reward (1 food pellet) whereas a press on the other lever resulted in a large, “risky” food reward (2 pellets) that was accompanied by a possible 1 s footshock contingent on a pre-set probability that was specific to each trial block. For all experiments except that in which shock intensity was titrated by body weight, the intensity was kept lower (0.25 – 0.30 mA) than in previous studies with only male rats (Mitchell et al., 2011; Shimp, Mitchell, Beas, Bizon, & Setlow, 2015; Simon et al., 2009; Simon et al., 2011). This was done to avoid floor effects on choice behavior in female rats, as we initially hypothesized that females would be more sensitive to the shock than males. Rats began training in the RDT at a shock intensity of 0.25 mA, which increased to 0.3 mA by the time behavior had stabilized (25 days for cohort 1 and 34 days for cohort 2). The probability of footshock was set at 0% in the first block of trials and increased across successive blocks (25%, 50%, 75% and 100%, respectively). The large food reward was delivered upon every choice of the “risky” lever, irrespective of shock delivery. The left/right positions of the “safe” and “risky” levers were counterbalanced across rats, but for each rat, the lever identities remained the same throughout the course of testing. Each block of trials began with 8 forced choice trials in which the shock contingencies in effect for that block were established (4 presentations of each lever, randomly presented), and were followed by 10 free choice trials. If rats failed to lever press in 10 s, the levers were retracted and the lights extinguished and the trial was scored as an omission. Food delivery was accompanied by re-illumination of the houselight and food trough light, which were extinguished after collection of the food pellets or after 10 s, whichever occurred sooner. On the forced choice trials (in which only one lever was present) the probability of shock following a press on the large reward lever was dependent across the four trials in each block. For example, in the 25% risk block, one and only one of the four forced choice trials (randomly selected) always resulted in shock, and in the 75% risk block, three and only three of the four forced choice trials always resulted in shock. In contrast, the probability of shock on the free choice trials (in which both levers were present) was independent, such that the probability of shock on each trial was the same, irrespective of shock delivery on previous trials in that block.
Fixed Ratio Schedules of Reinforcement
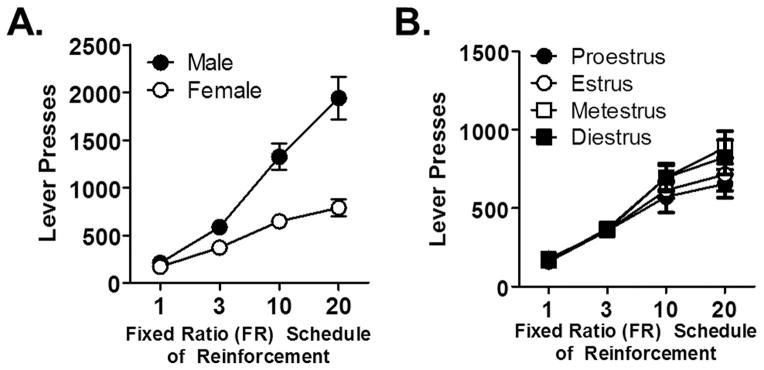
To assess motivation to obtain food reward, rats were given the opportunity to press a single lever for food delivery (1 pellet) under fixed ratio (FR) schedules (FR1, FR3, FR10, and FR20). Because previous work has demonstrated that estrous phase influences responding for rewards (Anker & Carroll, 2011; Dreher et al., 2007; Sakaki & Mather, 2012), rats were tested on each FR schedule until each estrous phase for each female was represented for a particular ratio. Thus, rats were tested on FR1 for 6 days, on FR3 for 9 days, on FR10 for 12 days and on FR 20 for 9 days. If an estrous phase occurred more than once within a FR schedule, the lever presses for each FR session of that schedule were averaged for that estrous phase. Each FR session (30 min) occurred once a day and sessions were presented in ascending order.
Estrous cycle assessments
In female rats, ovulation occurs every 4–5 days and begins with the met-estrus phase (14–18 hours), in which follicles develop in the ovaries. This is followed by the di-estrus phase, which lasts 60–70 hours, and then the pre-ovulatory, or pro-estrus phase, lasting 12 hours. As estradiol increases during the pro-estrus phase, the female enters into the estrus phase, which is the period of sexual receptivity and ovulation. To determine whether estrous cycle influences behavior in the RDT and FR schedules of reinforcement, all females were smeared with vaginal lavages immediately after each behavioral session. Samples were placed on microscope slides and examined with light microscopy (5X). Each of the four phases of the estrous cycle was identified using specific criteria (Marcondes, Bianchi, & Tanno, 2002): 1) pro-estrus: the majority of cells were nucleated epithelial cells 2) estrus: the majority of cells were cornified epithelial cells 3) met-estrus: cells contained both cornified epithelial cells and leukocytes and 3) di-estrus: majority of cells were leukocytes, with a few nucleated epithelial cells.
Drugs
To assess the effects of acute amphetamine on risk-taking in male and female rats, d-amphetamine sulfate (0, 0.3, 1.0 and 1.5 mg/kg in 0.9% saline vehicle; Drug Supply Program at the National Institute on Drug Abuse) was administered systemically 10 min prior to RDT test sessions every other day using a within-subjects, Latin square design as in our previous work (Mitchell et al., 2011; Simon et al., 2011).
Data analysis
Raw data files were extracted from Graphic State software and compiled using a custom macro written for Microsoft Excel (Dr. Jonathan Lifshitz, University of Kentucky). Compiled data were analyzed using SPSS 22.0. Choice performance in each block of the RDT was measured as the percentage of free choice trials (out of the total number of completed trials, excluding omissions) on which a rat chose the large reward in each trial block. To determine stable behavioral performance on the RDT, a repeated measures analysis of variance (ANOVA; RDT session X trial block) was conducted on free choice trials from 5 consecutive sessions. Stable performance was defined as the absence of either a main effect of session or interaction between session and trial block in this analysis (Simon & Setlow, 2012; Winstanley, Theobald, Cardinal, & Robbins, 2004). Effects of sex on free choice trials (averaged across 5 consecutive sessions of stable performance) were determined using a two-factor repeated measures ANOVA with trial block as within-subjects factor and sex as the between-subjects factor. Choice response latencies were measured as the interval between lever extension and lever press, excluding trials on which rats failed to press the lever altogether (omissions). Forced choice trials were used to assess response latencies because of insufficient data from free choice trials (i.e., some rats chose one lever exclusively on some blocks of free choice trials). In addition, latencies on the forced choice trials provided a measure of incentive motivation for the rewards that was relatively uncontaminated by comparative reward values or decision processes (Giertler, Bohn, & Hauber, 2003; Orsini, Trotta, et al., 2015; Schoenbaum, Setlow, Saddoris, & Gallagher, 2003; Shimp et al., 2015). Effects of sex on forced choice trial latencies were analyzed using a three-factor repeated measures ANOVA (sex X trial block X lever identity). Baseline locomotor activity was measured by averaging activity (in arbitrary units) across all intertrial intervals (in which no lights or levers were present). Shock reactivity was defined as activity during the 1 s shock delivery periods, averaged across the entire test session. The effects of amphetamine on risk-taking were analyzed using a three-factor repeated measures ANOVA (sex X drug dose X trial block). Lever pressing in the FR schedules of reinforcement was analyzed using a two-factor repeated measures ANOVA (sex X FR schedule), with post-hoc comparisons conducted on each FR schedule.
Results
Effects of sex on risky decision making
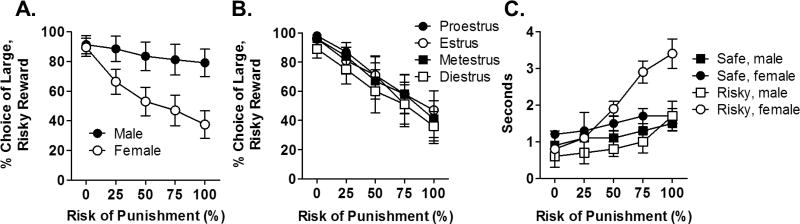
Rats in both cohorts were trained in the RDT for 25–34 days, at which point choice performance was stable [day, F (4, 120) = 0.80, p = 0.53; block, F (4, 120) = 19.16, p < 0.001; day X block, F (16, 480) = 1.15, p = 0.31)] in both males and females [day X block X sex, F (16, 480) = 1.33, p = 0.71]. At the time of stable RDT performance, males weighed (mean ± SEM) 303 ± 2.5 g and females weighed 222 ± 1.9 g. Final RDT performance was assessed during the last 5 (stable) days of training (Fig 2A). There was a significant main effect of sex [F (1, 30) = 5.20, p = 0.03] such that females chose the large, risky reward significantly less than males. In addition, there was a significant sex X block interaction [F (4, 120) = 6.90, p < 0.001]: male rats predominantly chose the large, risky reward across all blocks whereas females displayed a steep reduction in choice of the large, risky reward across trials blocks. Importantly, there was a significant main effect of block in both males and females when analyzed separately [males: F (4, 60) = 2.7, p = 0.04; females: F (4, 60) = 17.1, p < 0.001], indicating that both sexes were sensitive to the increasing risk of punishment during the task. Interestingly, the observed sex difference was evident as early as the first five days of training in the task [main effect of sex, F (1, 30) = 6.7, p = 0.01; block X sex interaction, F (1, 30) = 8.0, p =0.01]. Although performance at this point was not yet stable, these data suggest that the differences in risk-taking were not likely due to differences between males and females in acquisition of the task contingencies. Note that the high levels of preference for the large reward in males in this experiment compared to those in our previous work (Mitchell et al., 2011; Shimp et al., 2015; Simon et al., 2009; Simon et al., 2011) were likely due to the relatively low shock intensity (see Methods). Consistent with their risk-averse behavior, females made significantly more omissions than males in the RDT [main effect of sex, [Table 1; F (1, 30) = 14.02, p < 0.01]. However, there were no significant differences between males and females in their reactivity to shock delivery [Table 1; sex, F (1, 23) = 1.22, p = 0.28; sex X block, F (3, 69) = 0.65, p = 0.59]. In contrast, males were more active during inter-trial intervals than females, as there was a main effect of sex [Table 1; F (1, 30) = 7.5, p = 0.01] and a significant sex X block interaction [F (4, 120) = 2.7, p = 0.03] on locomotor activity. To determine whether females’ performance in the RDT was influenced by estrous cycle, vaginal samples were taken each day following the RDT session. A repeated measures ANOVA revealed no differences in RDT performance between phases of the estrous cycle [cycle, F (3, 21) = 0.72, p = 0.55; cycle X block, F (12, 84) = 0.70, p = 0.74; Fig 2B]. Finally, we assessed whether choice behavior in the second squad was affected by rats of the opposite sex in the first squad having been in the test chambers previously. There was neither a significant sex X squad interaction [F (1, 28) = 0.22, p = 0.64] nor a significant sex X squad X block interaction [F (4, 112) = 0.22, p = 0.93]. Together, these data demonstrate that females are significantly more risk averse than males, but that their choice behavior is unaffected by estrous cycle.
Figure 2. Performance in the risky decision making task in males and females.
(A) Male rats chose the large, risky reward significantly more than female rats. (B) Risky decision-making performance in females did not differ across the estrous cycle. (C) Response latencies for the small, safe reward were not different between males and females. In contrast, females had significantly longer latencies to respond for the large, risky reward. Data (mean ± SEM percent choice of the large, risky reward or mean ± SEM seconds) are averaged over the final five RDT test sessions, at which point all rats displayed stable performance.
Table 1. Trial omissions, locomotor activity and shock reactivity in the Risky Decision-Making Task in males and females.
In the Risky Decision-Making Task (RDT) with the same shock magnitudes for all rats, females omitted significantly more than males and showed less locomotor activity than males. When shocks were adjusted for body weight, females still omitted significantly more trials than males. This difference in trial omissions was also observed across all doses of amphetamine. At the 1.0 and 1.5 mg/kg doses of amphetamine, females displayed significantly less locomotor activity during shock delivery than males. Finally, there was a significant sex X dose interaction on shock reactivity, such that females showed significantly less locomotor activity during shock delivery relative to males at the highest dose of amphetamine.
| Session | % Omitted Trials | Locomotion (locomotor units/ITI) | Shock reactivity (locomotor units/shock) |
|---|---|---|---|
| Risky Decision-Making Task with equal shock magnitude | |||
| Male | 3.4 (1.9)* | 16.6 (1.7)* | 2.6 (0.2) |
| Female | 15.2 (2.5)* | 11.3 (0.9)* | 2.3 (0.3) |
| Risky Decision-Making Task with adjusted shocks | |||
| Male | 0.3 (0.2)* | 18.6 (2.7) | N/A |
| Female | 10.1 (4.1)* | 13.6 (2.5) | N/A |
| Amphetamine | |||
| Vehicle | |||
| Male | 5.8 (4.5) | 17.5 (2.9) | 2.8 (0.3) |
| Female | 18.0 (5.3) | 14.2 (1.8) | 2.1 (0.3) |
| 0.3 mg/kg | |||
| Male | 4.5 (3.2)* | 21.2 (3.2) | 2.5 (0.3) |
| Female | 16.3 (4.4)* | 17.2 (2.1) | 2.2 (0.4) |
| 1.0 mg/kg | |||
| Male | 7.3 (3.6)* | 23.8 (2.9) | 2.5 (0.3)* |
| Female | 31 (6.8)* | 24.7 (4.0) | 1.2 (0.3)* |
| 1.5 mg/kg | |||
| Male | 14.8 (3.4)* | 25.8 (2.1) | 2.1 (0.4)* |
| Female | 66.8 (9.4)* | 22.6 (2.4) | 0.3 (0.2)*† |
Asterisks indicate a significant difference between males and females.
A cross indicates a significant sex X dose interaction.
To further assess differences between males and females in risk-taking behavior, we analyzed latencies to press each lever during forced choice trials (Fig 2C). While there was no main effect of lever [small, safe versus large, risky; F (1, 25) < 0.01, p = 0.95], there was a significant main effect of sex [F (1, 25) = 26.04, p < 0.01] as well as significant lever X sex [F (1, 25) = 4.90, p = 0.04] and lever X block X sex [F (4, 100) = 8.0, p < 0.01] interactions. Further analyses of latencies for each lever separately revealed that while there were no differences between males and females in their latencies to press the small, safe lever [sex, F (1, 30) = 2.29, p = 0.14; sex X block, F (4, 120) = 0.34, p = 0.85], females had significantly longer latencies to press the large, risky lever than males [sex, F (1, 25) = 20.06, p < 0.01; sex X block, F (4, 100) = 12.11, p < 0.01]. These longer latencies in females are consistent with their risk-averse behavioral profile and further indicate that females find the risky choice less valuable than the small, safe choice compared to males.
Effect of shock titration for body weight on risky decision making in males and females
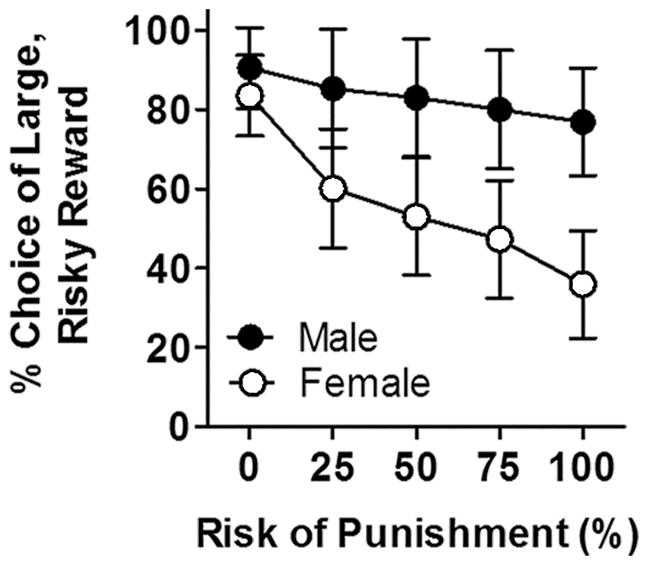
One interpretation of the greater risk aversion in females is that they perceive the shock differently than males due to their smaller body weights. The fact that there was no significant correlation between body weight and mean percent choice of the large, risky reward (controlling for sex, r = 0.18, p = 0.35) argues against this possibility [see also (Mitchell et al., 2014)]. To address this issue more directly, however, shock intensities were adjusted as a function of body weight (1.0 mA/kg) every other day in the second cohort of rats (Cooper, Goings, Kim, & Wood, 2014). Rats were tested in the RDT using these procedures until stable behavior emerged (18 sessions; Fig 3). At this point, males weighed (mean ± SEM) 299.6 ± 2.9 g and females weighed 223 ± 3.7 g. While there was not a main effect of sex [F (1, 14) = 2.21, p = 0.16), there was a significant sex X block interaction [F (4, 56) = 2.65, p = 0.04], such that females chose the small, safe lever significantly more than males as the risk of punishment increased. When choice behavior was analyzed separately for males and females, there was a main effect of block in females [F (4, 28) = 6.41, p < 0.01], although the main effect of block in males did not reach statistical significance [F (4, 28) = 2.40, p = 0.07]. Consistent with their more risk-averse profile of behavior, females also made significantly more omissions than males [F (1, 14) = 5.87, p = 0.03; Table 1), though there was not a sex X block interaction [F (4, 56) = 0.23, p = 0.92]. Finally, in contrast to the RDT with equal shock magnitudes across all rats, there were no significant differences in locomotor activity between males and females [sex, F (1, 14) = 1.81, p = 0.20; sex X block, F (4, 56) = 1.43, p = 0.88]. Considered together, these data argue that sex differences in risk-taking are not due to body weight differences in the perception/sensation of the punishment (shock) associated with the large reward.
Figure 3. Performance in the risky decision making task with shock intensities adjusted for body weight.
Male rats chose the large, risky reward significantly more than female rats when shock intensities were adjusted for each rat according to body weight (1.0 mA/kg). Data (mean ± SEM percent choice of the large, risky reward) are averaged over the final five sessions in this version of the task, at which point rats displayed stable performance.
Effects of pre-feeding on risky decision making in males and females
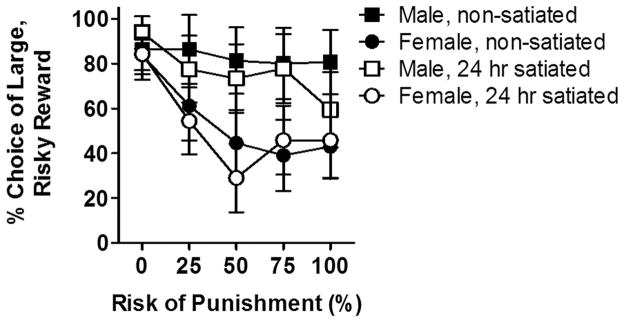
It is possible that the greater choice of the large, risky reward in males was due to greater motivation for the food reward. To address this possibility, the second cohort of rats was re-trained to stability (using the same shock intensity for all rats), and then given free access to food for 24 h prior to testing in the RDT. At the time of testing, males weighed (mean ± SEM) 310.9 ± 3.6 g and females weighed 229 ± 2.3 g. Our laboratory has previously shown that this manipulation does not alter choice behavior in the RDT in male rats, such that rats still decrease their choice of the large, risky reward as risk of punishment increases (Simon et al., 2009). Consistent with this prior work, there was no effect of satiation on choice performance [F (1, 14) = 1.79, p = 0.20; Fig 4], nor were there significant sex X satiation [F (1, 14) = 0.38, p = 0.55] or sex X satiation X block interactions [F (4, 56) = 1.03, p = 0.40]. When performance was analyzed separately for each sex, there was neither a main effect of satiation [males, F (1, 7) = 2.19, p =0.18; females, F (1, 7) = 0.23, p = 0.65] nor a significant interaction between satiation and block [males, F (1, 28) = 2.32, p = 0.08; females, F (1, 28) = 0.65, p =0.63]. However (and consistent with our previous work), there was a non-significant trend toward an increase in omissions due to satiation in both males and females [F (1, 14) = 4.81, p = 0.05]. There was also a main effect of sex on omissions due to satiation [F (1, 14) = 6.44, p = 0.02] as females omitted significantly more than males when satiated. There was, however, no sex X satiation interaction [F (1, 14) = 0.10, p = 0.76].
Figure 4. Effects of satiation on performance in the risky decision making task.
There was no effect of twenty-four hour free feeding on choice of the large, risky reward in male and female rats. Data (mean ± SEM percent choice of the large, risky reward) are averaged across rats during each of the two test sessions.
Effects of sex on lever pressing for food reward
As another test of food motivation, all rats were tested on a series of ascending fixed ratio schedules of reinforcement (FR1, FR3, FR10 and FR20). In addition, we collected vaginal samples from females after each session to determine whether lever pressing was influenced by estrous cycle. Rats were tested on each FR schedule until each phase of the estrous cycle had a corresponding FR test session. At the time of FR testing, males weighed (mean ± SEM) 313.3 ± 10.8 g on FR1, 328.3 ± 7.8 g on FR3, 343.1 ± 3.3 g on FR 10 and 350.7 ± 2.9 g on FR20. Females weighed 259.3 ± 6.1 g on FR1, 256.8 ± 4.2 g on FR3, 265.2 ± 3.4 g on FR10 and 276.5 ± 6.6 g on FR20. Males pressed significantly more than females on each FR schedule (ps < 0.05; Fig 5A). However, there was no FR schedule X cycle interaction [F (9, 54) = 0.99, p = 0.46; Fig 5B], indicating that estrous cycle did not influence instrumental responding under these conditions. Even though males and females differed in lever pressing for food, there were no significant correlations between numbers of lever presses on any of the FR schedules and average choice performance in the RDT (controlling for sex; FR1, r = −0.24, p = 0.39; FR3, r = 0.27, p = 0.33; FR10, r = 0.22, p = 0.44; FR20, r = 0.22, p = 0.43). The lack of such relationships argues against the possibility that differences in FR performance (and corresponding differences in food motivation) account for the sex differences observed in risk-taking. Finally, there were no significant correlations between body weight (at the time of each FR test) and FR performance [FR1, r = 0.35, p = 0.20; FR3, r = 0.26, p = 0.34; FR10, r = 0.47, p = 0.08; FR20, r = 0.28, p = 0.31].
Figure 5. Instrumental responding for food reward in males and females.
(A) Rats were tested under a series of fixed ratio (FR) schedules of lever pressing for food (FR1, FR3, FR10 and FR20). Males lever pressed significantly more than females on each FR schedule. (B) In females, responding across the FR schedules did not differ across phases of the estrous cycle. Data are represented as the mean ± SEM number of lever presses at each FR schedule.
Effects of amphetamine on risky decision making in males and females
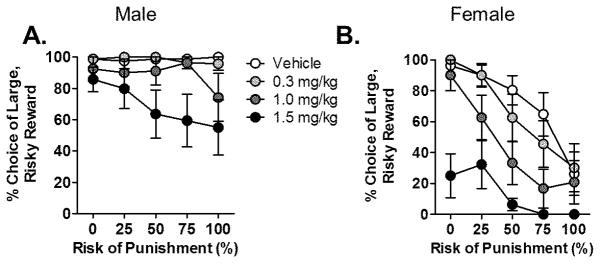
Our laboratory has previously shown that amphetamine dose-dependently decreases risk-taking behavior in males (Mitchell et al., 2011; Simon et al., 2009). To determine whether amphetamine differentially modulates risk-taking behavior in females, we administered amphetamine (0.3, 1.0, 1.5 mg/kg) or saline vehicle 10 minutes prior to test sessions in the RDT. At the time of these tests, males weighed 310.7 ± 3.3 g and females weighed 229.7 ± 3.3 g. Consistent with our previous findings, amphetamine decreased risk-taking [F (3, 42) = 23.39, p < 0.001; Fig 6], with the greatest effect occurring at the highest dose. Although the dose X sex interaction only neared significance [F (3, 42) = 2.34, p = 0.09], there was a significant dose X sex X block interaction [F (12, 168) = 3.11, p = 0.001] indicating that different doses of amphetamine affected males and females differently across the five blocks in the RDT. Separate analyses of each dose of amphetamine independently (compared with vehicle) revealed that while there was no effect of the low dose of amphetamine in males [F (1, 7) = 0.15, p =0.71] or females [F (1, 7) = 1.00, p = 0.35], there was a significant effect of the medium dose of amphetamine on choice behavior in females [F (1, 7) = 17.29, p < 0.01; Fig. 6B], but not males [F (1, 7) = 1.50, p = 0.26; Fig. 6A]. The highest dose of amphetamine significantly affected choice behavior in both males [F (1, 7) = 6.38, p = 0.04] and females [F (1, 7) = 53.48, p < 0.01]. Amphetamine also dose-dependently increased the number of omissions [F (3, 42) = 16.12, p < 0.001; Table 1], an effect that was greater in females than in males [sex, F (1, 14) = 27.69, p < 0.001; dose X sex, F (3, 42) = 7.32, p < 0.001]. In contrast, amphetamine dose-dependently increased locomotor activity [F (3, 42) = 15.85, p < 0.001] to the same extent in males and females [sex, F (1, 14) = 0.48, p = 0.50; sex X dose, F (3, 42) = 1.16, p = 0.34]. Finally, amphetamine dose-dependently decreased locomotor activity during delivery of footshock [F (3, 42) = 10.61, p < 0.001], an effect that was significantly more pronounced in females than males [sex, F (1, 14) = 9.52, p = 0.01; sex X dose, F (3, 42) = 3.66, p = 0.02]. Collectively, these data show that amphetamine decreases risk-taking in both males and females, but that females are more sensitive to the effects of the drug than males.
Figure 6. Effects of amphetamine on performance in the risky decision making task in males and females.
(A) Systemic amphetamine decreased choice of the large, risky reward in male rats only at the highest dose. (B) Systemic amphetamine dose-dependently decreased choice of the large, risky reward in female rats. Data are represented as the mean ± SEM percent choice of the large, risky reward.
Discussion
Emerging evidence has shown that sex differences are present in some forms of decision making in rodents, such as a rat version of the Iowa Gambling Task and delay discounting tasks (Koot, van den Bos, Adriani, & Laviola, 2009; Perry, Nelson, Anderson, Morgan, & Carroll, 2007; R. van den Bos et al., 2012). The present data reveal that these sex differences extend to decision-making processes involving risk of explicit punishment. These differences were not readily accounted for by differences in body weight affecting punishment sensation, nor could they fully be explained by differences in reward motivation. These findings are significant in that they begin to identify and characterize the extent to which males and females differ in a behavioral phenotype associated with addiction as well as other psychiatric disorders (e.g., anorexia nervosa, bipolar disorder) (Bechara et al., 2001; Gowin et al., 2013; Kaye, Wierenga, Bailer, Simmons, & Bischoff-Grethe, 2013; Orsini, Moorman, Young, Setlow, & Floresco, 2015; van Enkhuizen et al., 2014).
The higher level of risk-taking observed in male relative to female rats is in line with evidence in human subjects showing that males engage in more risky behavior than females (Cross, Copping, & Campbell, 2011; Killgore, Grugle, Killgore, & Balkin, 2010). For example, males exhibit more reckless driving than females and make up the largest proportion of homicide offenders in the United States. Consistent with the current findings, females, in contrast, have been shown to display greater avoidance behavior (Sheynin, Moustafa, Beck, Servatius, & Myers, 2015). Using both human and animal models of avoidance behavior, previous work has shown that females are also slower to extinguish these avoidance behaviors than males (Beck, Jiao, Pang, & Servatius, 2010; Sheynin, Beck, Servatius, & Myers, 2014). There are several possible explanations that could account for these differences in behavior. In a recent review, Cross, Coping and Campbell (2011) describe several theories that may account for sex differences in impulsivity, which can also be applied to understanding such differences in risk-based decision making. In particular, males may be more risk-taking because they may be less sensitive to punishment and more sensitive to reward compared to females. Conversely, females may be more risk-averse because they may be more sensitive to punishment. Evolutionarily, these traits might ensure greater reproductive success in both sexes. Punishment hyposensitivity and increased reward sensitivity might allow males to compete with other males for mates and secure resources for themselves and their offspring. Reproductive success in females, however, would require them to avoid harm to themselves and their offspring and inhibit behavior that could put them in danger. Differences in risk-taking between males and females, observed both within this study and in the real world, may reflect these different evolutionary strategies and may in fact predispose them to different psychiatric disorders (e.g., anorexia nervosa, which is associated with high levels of risk aversion, is much more prevalent in females than males (Hudson, Hiripi, Pope, & Kessler, 2007; Kaye et al., 2013).
Another possible account for the increased choice of the large, risky reward in males is that they are more motivated to obtain food than females. Indeed, when tested on fixed ratio schedules of reinforcement, male rats lever pressed significantly more than females across all schedules. In contrast, in another control experiment, we found that pre-feeding the rats on standard home cage chow for 24 hours prior to testing, which should cause satiation, did not differentially affect choice performance in males and females in the RDT. This experiment suggests that differences in hunger between males and females did not account for differences in RDT performance; however, while the food pellets used in the RDT and the home cage chow had the same nutrient composition, the rats may still have perceived them as different foods [and thus may have experienced only sensory-specific satiety (Zeeb & Winstanley, 2013)]. An experiment in which rats were satiated on the food pellets used in the RDT would help to address this issue. In addition to the evidence from the satiation control experiment, there were no differences in latencies to choose the small reward in the RDT between males and females, suggesting that there were no baseline differences in incentive motivation for the food reward. Consistent with this interpretation, a prior study found no sex differences in lever pressing for food on a progressive ratio schedule of reinforcement (van Hest, 1988), which, arguably, may be a better measure of food motivation than fixed ratio responding. Despite these arguments against motivation for food accounting for the sex differences in risk-taking, it is still challenging to rule out this possibility completely. We are currently exploring the use of modified versions of the RDT that employ different reinforcers (e.g., water) that may be less sensitive to sex differences in food motivation.
It is interesting to note that in the Iowa Gambling Task (IGT), a gambling-based decision-making task, males tend to choose the less risky, but more advantageous long-term option and females tend to choose the more risky but long-term disadvantageous option (R. van den Bos, Homberg, et al., 2013). These findings seem to contrast with those in the current study in which males showed greater risk taking than females. There are several possible ways in which to reconcile these differences. First, the risks associated with the net gain/large reward in each task are different: in the RDT, the risk is that of physical punishment whereas in the IGT, it is the risk of lost opportunity (see Orsini, Moorman, Young, Setlow & Floresco, in press for discussion of this issue). Second, the differences in sex effects in these tasks could be due to the use of different strategies used to solve the tasks. In contrast to the RDT, which is conducted across multiple sessions and likely reflects informed choice, the IGT is conducted in a single session and, thus, is thought to assess the process by which a subject gathers information about task contingencies and learns the most advantageous strategy (R. van den Bos et al., 2012). Previous work has shown that females require more trials in the IGT than males before developing a preferential strategy (Bolla, Eldreth, Matochik, & Cadet, 2004; R. van den Bos, Davies, et al., 2013; R. van den Bos, den Hiejer E., Vlaar S., Houx BB., 2007; R. van den Bos, Homberg, et al., 2013). This could appear as though females are more “risky”; however, it could also reflect the fact that they spend more time in the “information gathering” phase in which they are evaluating all available options. Hence, rather than being more risky in their IGT performance, females may simply be using a different strategy than males to determine the best option. Consistent with this idea, in the current study, females took longer (29 days) than males (17 days) to reach stable performance in the RDT. It is therefore possible that, like in the IGT, males and females use different strategies in the RDT to make adaptive decisions.
Neuroimaging studies have suggested that sex differences in risky decision making are due to specific differences in brain structure and function. In particular, it appears that regions of the orbitofrontal cortex are differentially engaged in males and females during performance of the IGT (Bolla et al., 2004) such that male participants have greater activity in the lateral orbitofrontal cortex (lOFC) than females. In the Risky Gains Task, another risk-based decision-making task, the lOFC in females, but not males, is dynamically engaged during feedback periods, and this activity appears to regulate their subsequent choice (Lee, Chan, Leung, Fox, & Gao, 2009). For example, there was a negative correlation between percent signal change in the lOFC and risky choices that were preceded by punishment, and a positive correlation between percent signal change in the lOFC and risky choices preceded by risky, but unpunished, choices. Recently, our laboratory established a role for the lOFC in decision making in the face of explicit punishment, such that this region is important for the ability to accurately calculate punishment probabilities to guide choice behavior (Orsini, Trotta, et al., 2015). Therefore, risk-taking differences between males and females may be a result of differential engagement of the lOFC during different parts of the decision-making process (e.g., choice points versus feedback periods). Future experiments are aimed at investigating this possibility.
Interestingly, estrous cycle had no effect on risk-taking behavior in females. This is perhaps surprising given the wealth of evidence documenting changes in behavioral responses to drugs of abuse across the estrous cycle (Becker, 1999; Evans, Haney, & Foltin, 2002; Festa & Quinones-Jenab, 2004; Jackson et al., 2006; Justice & de Wit, 1999; Quinones-Jenab, Ho, Schlussman, Franck, & Kreek, 1999; White, Justice, & de Wit, 2002). Despite this, evidence regarding an influence of estrous cycle in decision making in drug-naïve conditions is mixed. In humans, IGT performance is not affected by menstrual cycle (Reavis & Overman, 2001; R. van den Bos, den Hiejer E., Vlaar S., Houx BB., 2007). In contrast, intertemporal choice (delay discounting) varies across the menstrual cycle in humans (Smith, Sierra, Oppler, & Boettiger, 2014), and estradiol modulates effort-based decision making in rats (Uban, Rummel, Floresco, & Galea, 2012). Together with the current findings, these data suggest that the effects of ovarian hormones on decision making may depend on the specific decision factors (reward delay or effort vs. risk of punishment) involved in the task. In addition, given the interactions between ovarian hormones and drugs of abuse, it is possible that while the estrous cycle may not affect risk-based decision making in drug-naïve conditions, it may influence decision-making after chronic drug use. Moreover, it is conceivable that in the current study, performance in the RDT during one stage of the estrous cycle could have affected performance at another stage in the cycle. It will be of interest in future studies to more directly address the role of ovarian hormones in risky decision making (e.g., by comparing RDT performance in ovariectomized female rats with ovariectomized female rats with hormone replacement). Indeed, in support of a role for gonadal hormones in risky decision making, a recent study by Cooper et al. (2014) showed that administration of testosterone increases choice of the large, risky reward in the RDT in male rats.
Previous work in our laboratory showed that amphetamine decreases risk-taking in the RDT (Simon et al., 2009). In the current study, we replicated this finding in males and observed that amphetamine had comparable effects in females. Interestingly, females seemed to be more sensitive to the effects of amphetamine than males: whereas only the 1.5 mg/kg dose decreased risk-taking in males, both the 1.0 and 1.5 mg/kg doses decreased risk-taking in females. Moreover, females omitted significantly more trials than males at all doses of amphetamine. Thus, in the RDT, systemic amphetamine seems to have exacerbated the risk-averse phenotype observed in females. Our laboratory showed previously that the effects of acute amphetamine on risk-taking are attenuated by a dopamine D2 (but not D1) receptor antagonist and mimicked by a D2 (but not D1) receptor agonist (Simon et al., 2011). Further, acute administration of the selective serotonin reuptake inhibitor citalopram has no effect on risky choice in the RDT (Setlow, Mitchell, Vera & Weiss, 2012). The role of noradrenergic signaling in RDT performance is as of yet untested [although see (Montes, Stopper, & Floresco, 2015)], but the findings to date suggest that amphetamine’s effects are due at least in part to actions on dopamine signaling.
The mechanisms by which an amphetamine-induced increase in dopamine availability might attenuate risk-taking are not clear. Amphetamine can potentiate the impact of aversive stimuli (such as footshock punishment) on choice behavior, and aversive cues can elicit dopamine release in the nucleus accumbens shell (Badrinarayan et al., 2012; Killcross, Everitt, & Robbins, 1997); however amphetamine can similarly potentiate the impact of appetitive stimuli on choice behavior, and appetitive cues are also effective in eliciting accumbens dopamine release (Cacciapaglia, Saddoris, Wightman, & Carelli, 2012; Day, Jones, Wightman, & Carelli, 2010; Saddoris et al., 2015; Sugam, Day, Wightman, & Carelli, 2012). A potential resolution may be found in recent data showing that dopamine is released in the nucleus accumbens prior to successful (but not unsuccessful) avoidance of a shock in a signaled avoidance task (Oleson, Gentry, Chioma, & Cheer, 2012), suggesting that elevated dopamine signaling is necessary for avoiding adverse outcomes (see Orsini et al. in press for further discussion). Irrespective of amphetamine’s mechanisms of action, the present data raise the possibility that differences in sensitivity to dopamine neurotransmission in males and females may partially account for the observed sex differences in risk-taking. Such a conclusion could be viewed as somewhat surprising, however, as estradiol modulates dopaminergic function (Becker, 1999; Bobzean et al., 2014; Thompson, 1999), yet there were no discernable effects of estrous phase on risk-taking in females. Future studies are warranted to determine whether other endocrine mechanisms independent of the estrous cycle mediate the increased sensitivity to amphetamine in females.
It would appear that the greater risk aversion in females is contradictory to patterns of drug use observed in females. For example, females escalate drug use and progress from drug use to dependence more rapidly, and are more likely to relapse, compared to their male counterparts (Bobzean et al., 2014; Lynch, 2006). Given that higher levels of risk-taking in drug-naïve male rats predict greater subsequent cocaine self-administration (Mitchell et al., 2014), these data might predict that females would show greater risk-taking than males. One way to reconcile this discrepancy is to consider that females may display greater risk-taking after initiating drug use. We found previously that in addition to the predictive relationship between risk-taking and cocaine self-administration, cocaine self-administration causes a further increase in risk-taking in male rats (Mitchell et al., 2014). It is possible that females may make this transition more quickly than males, which could account for their rapid progression from drug use to dependence. It is as of yet unknown whether sex differences in risk-taking are evident following chronic drug use, but future experiments are aimed at addressing this question as it may help to explain why females are more vulnerable to relapse than males.
In summary, the findings from these studies indicate that males and females differ in decision making involving risk of punishment, with males exhibiting greater risk-taking than females. These results have important implications not only for addiction, but also for other psychiatric diseases characterized by maladaptive increases [e.g., attention deficit hyperactivity disorder (Ernst et al., 2003)] or decreases [e.g., anorexia nervosa (Kaye et al., 2013)] in risk-taking. Future work will attempt to uncover the mechanisms mediating these differences in risk-taking, with the hope of developing more targeted treatments for psychiatric conditions that affect males and females differentially.
Acknowledgments
We thank the Drug Supply Program at NIDA for kindly providing D-amphetamine, Ms. Bonnie McLaurin and Ms. Shannon Wall for technical assistance, and Dr. Linda Bean for assistance with estrous cycle determination. Supported by NIH DA024671 and DA036534 (BS) and a McKnight Brain Institute Fellowship and a Thomas H. Maren Fellowship (CAO).
References
- Anglin MD, Hser YI, McGlothlin WH. Sex differences in addict careers. 2. Becoming addicted. Am J Drug Alcohol Abuse. 1987;13(1–2):59–71. doi: 10.3109/00952998709001500. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J Neurosci. 2012;32(45):15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beck KD, Jiao X, Pang KC, Servatius RJ. Vulnerability factors in anxiety determined through differences in active-avoidance behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):852–860. doi: 10.1016/j.pnpbp.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64(4):803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, DeNobrega AK, Perrotti LI. Sex differences in the neurobiology of drug addiction. Exp Neurol. 2014;259:64–74. doi: 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cereb Cortex. 2004;14(11):1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Experimental and Clinical Psychopharmacology. 2005;13(4):311–318. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- Bowden-Jones H, McPhillips M, Rogers R, Hutton S, Joyce E. Risk-taking on tests sensitive to ventromedial prefrontal cortex dysfunction predicts early relapse in alcohol dependency: a pilot study. J Neuropsychiatry Clin Neurosci. 2005;17(3):417–420. doi: 10.1176/appi.neuropsych.17.3.417. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22(2):241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Cacciapaglia F, Saddoris MP, Wightman RM, Carelli RM. Differential dopamine release dynamics in the nucleus accumbens core and shell track distinct aspects of goal-directed behavior for sucrose. Neuropharmacology. 2012;62(5–6):2050–2056. doi: 10.1016/j.neuropharm.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161(3):304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Cooper SE, Goings SP, Kim JY, Wood RI. Testosterone enhances risk tolerance without altering motor impulsivity in male rats. Psychoneuroendocrinology. 2014;40:201–212. doi: 10.1016/j.psyneuen.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol Bull. 2011;137(1):97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol Psychiatry. 2010;68(3):306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilde B, Verdejo-Garcia A, Sabbe B, Hulstijn W, Dom G. Affective Decision-Making Is Predictive of Three-Month Relapse in Polysubstance-Dependent Alcoholics. European Addiction Research. 2013;19(1):21–28. doi: 10.1159/000339290. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104(7):2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, Bolla K. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160(6):1061–1070. doi: 10.1176/appi.ajp.160.6.1061. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46(5):509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Giertler C, Bohn I, Hauber W. The rat nucleus accumbens is involved in guiding of instrumental responses by stimuli predicting reward magnitude. Eur J Neurosci. 2003;18(7):1993–1996. doi: 10.1046/j.1460-9568.2003.02904.x. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Mackey S, Paulus MP. Altered risk-related processing in substance users: Imbalance of pain and gain. Drug Alcohol Depend. 2013;132(1–2):13–21. doi: 10.1016/j.drugalcdep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46(2):122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29(1):81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatova L, Raleva M. Gender difference in the treatment outcome of patients served in the mixed-gender program. Bratisl Lek Listy. 2009;110(5):285–289. [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145(1):67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36(2):110–120. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross AS, Everitt BJ, Robbins TW. Symmetrical effects of amphetamine and alpha-flupenthixol on conditioned punishment and conditioned reinforcement: Contrasts with midazolam. Psychopharmacology. 1997;129(2):141–152. doi: 10.1007/s002130050174. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Grugle NL, Killgore DB, Balkin TJ. Sex differences in self-reported risk-taking propensity on the Evaluation of Risks scale. Psychol Rep. 2010;106(3):693–700. doi: 10.2466/PR0.106.3.693-700. [DOI] [PubMed] [Google Scholar]
- Koot S, van den Bos R, Adriani W, Laviola G. Gender differences in delay-discounting under mild food restriction. Behav Brain Res. 2009;200(1):134–143. doi: 10.1016/j.bbr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Lee TM, Chan CC, Leung AW, Fox PT, Gao JH. Sex-related differences in neural activity during risk taking: an fMRI study. Cereb Cortex. 2009;19(6):1303–1312. doi: 10.1093/cercor/bhn172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14(1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62(4A):609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology (Berl) 2011;218(4):703–712. doi: 10.1007/s00213-011-2363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Beas BS, Morgan D, Bizon JL, Setlow B. Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacology. 2014;39(4):955–962. doi: 10.1038/npp.2013.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21(4):193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Montes DR, Stopper CM, Floresco SB. Noradrenergic modulation of risk/reward decision making. Psychopharmacology (Berl) 2015;232(15):2681–2696. doi: 10.1007/s00213-015-3904-3. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Gentry RN, Chioma VC, Cheer JF. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J Neurosci. 2012;32(42):14804–14808. doi: 10.1523/JNEUROSCI.3087-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neurosci Biobehav Rev. 2015 doi: 10.1016/j.neubiorev.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Trotta RT, Bizon JL, Setlow B. Dissociable Roles for the Basolateral Amygdala and Orbitofrontal Cortex in Decision-Making under Risk of Punishment. J Neurosci. 2015;35(4):1368–1379. doi: 10.1523/JNEUROSCI.3586-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007;86(4):822–837. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101(1):15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Reavis R, Overman WH. Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behav Neurosci. 2001;115(1):196–206. doi: 10.1037/0735-7044.115.1.196. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53(3):223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biol Psychiatry. 2015;77(10):903–911. doi: 10.1016/j.biopsych.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Mather M. How reward and emotional stimuli induce different reactions across the menstrual cycle. Soc Personal Psychol Compass. 2012;6(1):1–17. doi: 10.1111/j.1751-9004.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39(5):855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Setlow B, Mitchell MR, Vera K, Weiss VG. Effects of Acute Administration of Drugs Targeting Serotonergic Neurotransmission in a Rat Model of Risky Decision-making. Poster presented at the annual meeting of the Society for Neuroscience; New Orleans, LA. 2012. Oct, [Google Scholar]
- Sheynin J, Beck KD, Servatius RJ, Myers CE. Acquisition and extinction of human avoidance behavior: attenuating effect of safety signals and associations with anxiety vulnerabilities. Front Behav Neurosci. 2014;8:323. doi: 10.3389/fnbeh.2014.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin J, Moustafa AA, Beck KD, Servatius RJ, Myers CE. Testing the role of reward and punishment sensitivity in avoidance behavior: a computational modeling approach. Behav Brain Res. 2015;283:121–138. doi: 10.1016/j.bbr.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp KG, Mitchell MR, Beas BS, Bizon JL, Setlow B. Affective and cognitive mechanisms of risky decision making. Neurobiol Learn Mem. 2015;117:60–70. doi: 10.1016/j.nlm.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34(10):2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Setlow B. Dopaminergic modulation of risky decision-making. J Neurosci. 2011;31(48):17460–17470. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Setlow B. Modeling risky decision making in rodents. Methods Mol Biol. 2012;829:165–175. doi: 10.1007/978-1-61779-458-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Sierra Y, Oppler SH, Boettiger CA. Ovarian cycle effects on immediate reward selection bias in humans: a role for estradiol. J Neurosci. 2014;34(16):5468–5476. doi: 10.1523/JNEUROSCI.0014-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugam JA, Day JJ, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine encodes risk-based decision-making behavior. Biol Psychiatry. 2012;71(3):199–205. doi: 10.1016/j.biopsych.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TL. Attenuation of dopamine uptake in vivo following priming with estradiol benzoate. Brain Res. 1999;834(1–2):164–167. doi: 10.1016/s0006-8993(99)01508-5. [DOI] [PubMed] [Google Scholar]
- Uban KA, Rummel J, Floresco SB, Galea LA. Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacology. 2012;37(2):390–401. doi: 10.1038/npp.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R, Davies W, Dellu-Hagedorn F, Goudriaan AE, Granon S, Homberg J, Adriani W. Cross-species approaches to pathological gambling: a review targeting sex differences, adolescent vulnerability and ecological validity of research tools. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2454–2471. doi: 10.1016/j.neubiorev.2013.07.005. [DOI] [PubMed] [Google Scholar]
- van den Bos R, den Hiejer E, Vlaar S, Houx BB. Exploring gender differences in decision-making using the Iowa Gambling Task. In: EJE, editor. Psychology of decision making in education, behavior, and high risk situations. Hauppage, NY (USA): Nova Science Publishers Inc; 2007. pp. 207–226. [Google Scholar]
- van den Bos R, Homberg J, de Visser L. A critical review of sex differences in decision-making tasks: focus on the Iowa Gambling Task. Behav Brain Res. 2013;238:95–108. doi: 10.1016/j.bbr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- van den Bos R, Jolles J, van der Knaap L, Baars A, de Visser L. Male and female Wistar rats differ in decision-making performance in a rodent version of the Iowa Gambling Task. Behav Brain Res. 2012;234(2):375–379. doi: 10.1016/j.bbr.2012.07.015. [DOI] [PubMed] [Google Scholar]
- van Enkhuizen J, Henry BL, Minassian A, Perry W, Milienne-Petiot M, Higa KK, Young JW. Reduced dopamine transporter functioning induces high-reward risk-preference consistent with bipolar disorder. Neuropsychopharmacology. 2014;39(13):3112–3122. doi: 10.1038/npp.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hest A, van Haaren F, van de Poll NW. The behavior of male and female Wistar rats pressing a lever for food is not affected by sex differences in food motivation. Behav Brain Res. 1988;(27):215–221. doi: 10.1016/0166-4328(88)90118-0. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73(4):729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24(20):4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA. Functional disconnection of the orbitofrontal cortex and basolateral amygdala impairs acquisition of a rat gambling task and disrupts animals’ ability to alter decision-making behavior after reinforcer devaluation. J Neurosci. 2013;33(15):6434–6443. doi: 10.1523/JNEUROSCI.3971-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]