Abstract
Objective:
In volumetric-modulated arc therapy (VMAT) prostate stereotactic body radiotherapy (SBRT), dose coverage of the planning target volume (PTV) becomes challenging when the sparing of rectum, bladder and urethra is strictly pursued. Our current 35-Gy-in-five-fraction plans only assure 33.2 Gy to ≥95% PTV ( ≥ 95%). Looking for an improved , increased near-maximum target dose (D2%) and prostate–rectum spacer insertion were tested.
Methods:
For 11 patients, two VMAT plans, with D2% ≤ 37.5 Gy (Hom) or D2% ≤ 40.2 Gy (Het), on each of two CT studies, before or after spacer insertion, were computed. All plans assured ≥95%, and <1 cm3 of rectum, bladder and urethra receiving ≥35 Gy. By hypothesis testing, several dose–volume metrics for target coverage and rectal sparing were compared across the four groups of plans. The impact of spacer insertion on the fractions of rectum receiving more than 18, 28 and 32 Gy () was further tested by linear correlation analysis.
Results:
By hypothesis testing, the increased D2% was associated with improvements in target coverage, whereas spacer insertion was associated with improvements in both target coverage and rectal . By linear correlation analysis, spacer insertion was related to the reductions in rectal for X ≥ 28 Gy.
Conclusion:
A slightly increased D2% or the use of spacer insertion was each able to improve . Their combined use assured ≥ 98% to all our patients. Spacer insertion was further causative for improvements in rectal sparing.
Advances in knowledge:
For VMAT plans in prostate SBRT, the distinct dosimetric usefulness of increased D2% and of the use of spacer insertion were validated in terms of target coverage and rectal sparing.
INTRODUCTION
Based on recent publications,1–6 stereotactic body radiotherapy (SBRT) represents an emerging safe and effective treatment option for selected patients with prostate cancer. Nowadays, prostate SBRT is mainly performed in five fractions, with a prescribed dose (Dp) generally equal to about 35 Gy.1–4 In a recent multi-institutional Phase I–II trial,5,6 Dp was escalated in five fractions up to 50 Gy. Both schedules seem associated with excellent preliminary outcomes and with acceptable levels of early/late toxicities, although a slight prevalence of genitourinary (GU) over rectal grade (G) ≤3 toxicities was reported, which should suggest for a general inclusion of the urethra, as in the studies by Boike et al5 and Kim et al,6 among the critical organs at risk (OARs) in the planning process.
The use of an endorectal balloon has been suggested as mandatory to reduce rectal toxicities in the 50-Gy-in-five-fraction schedule.5,6 Nevertheless, the endorectal balloon could be a limiting factor both to the patient, for the potential referred discomfort, and to the department, for the increased complexity in the daily treatment workflow. An alternative is represented by the transperineal insertion of a self-absorbable hydrogel as prostate–rectum interface spacer, which was reported as safe and effective in rectal dose sparing for intensity-modulated radiotherapy (IMRT) plans,7–10 and stable over a 39-daily-fractions treatment.10 In such studies, owing to the adoption of standard fractionation, soft constraints on the high dose to the rectum as a minimum dose to 25% of rectal volume (D25%) <70 Gy (i.e. D25% <90% Dp),7 or a rectal fractional volume receiving not less than 70 Gy (V70) <20% (i.e. < 20%),9,10 were used.
In a prospective Phase I–II study on prostate SBRT in low- to intermediate-risk patients,3,4 a Dp of 35 Gy was delivered in five fractions, while assuring 95% Dp to at least 95% of the planning target volume (PTV), ≥ 95%, and <1 cm3 of rectum and bladder to receive 35 Gy ( < 35 Gy), to minimize the risk of rectal and GU grade ≥3 toxicities. As a development of that study,3 the same < 35 Gy constraint was here extended to the urethral planning at risk volume [urethral-(PRV)]. A noteworthy fact is that in intensity-modulated treatments, a target dose heterogeneity within D98% ≥ 95% Dp and D2% ≤ 107% Dp is generally pursued, likely from combining the recommendations on target dose heterogeneity from the International Commision on Radiation Units and Measurements (ICRU),11 with ICRU volumetric definitions of near-minimum and near-maximum target doses.12 However, according to our experience, when 35-Gy-in-five-fraction prostate SBRT is planned by volumetric-modulated arc therapy (VMAT), the extension of the < 35 Gy constraint from rectum and bladder only, to the urethral-PRV also, makes ≥ 98%—equivalent to D98% ≥ 95% Dp—as a generally not achievable goal. Therefore, our current clinical protocol accepts plans assuring ≥ 95% only, with D2% ≤ 107% Dp. Looking for an improvement in target dose coverage, we searched for solutions to increase the target–OARs distances, the dose gradients at the target–OARs interfaces, or both. The former suggestion, which we translated into the adoption of a prostate–rectum hydrogel spacer, was originated by the reported increase in target dose coverage for IMRT plans by the use of a rectal spacer.7,9 The latter suggestion, which we translated into a slightly increased accepted target D2%—from 107% Dp (37.5 Gy) to 115% Dp (40.2 Gy)—was derived from the observation for static fields13 that an increased target dose heterogeneity can be associated with increased dose gradients at target boundaries.
The primary end point of this study, focused on 35-Gy-in-five-fraction prostate VMAT-SBRT, was to test if the adoption of a rectal spacer, and/or of a slightly increased level of accepted target dose heterogeneity (D2%), might improve our target dose coverage (i.e. ), while maintaining the same < 35 Gy constraint on rectum, bladder and urethral-PRV. As the secondary end point, the expected rectal dose sparing from the adoption of the rectal spacer in our case series was tested.
METHODS AND MATERIALS
Patients and spacer
In this plan comparison study, 11 patients (median age 73 years, range 62–78 years) with prostate adenocarcinoma, low and intermediate risks according to the National Comprehensive Cancer Network (NCCN), already included in a Phase I–II approved study by our institutional review board (IRB) and treated at Sacro Cuore Don Calabria Hospital in 2014, were further analyzed. To get a 90% statistical power in detecting, by one-side t-test, a unitary variation (i.e. 1%) from between the conceived groups of plans, the sample size (i.e. number of patients, n) was determined from the expression (n > 9σ2), where σ2 is the sample variance of the difference in between compared plans. The patients received a first CT simulation (NoSpc) before the transperineal insertion of 10 ml of SpaceOAR® (Augmenix Inc., Waltham, MA) hydrogel (polyethylene glycol gel) under transrectal ultrasound guidance, posteriorly to Denonvilliers' fascia according to the published reports.7,9,10 Concomitantly with spacer insertion, four gold seeds were also transperineally implanted by a urologist into the prostatic gland, as internal markers for image-guided patient set-up.14 A second CT simulation (Spc) was performed on average 1 week after the insertion of both the spacer and the gold seeds,15 a long enough time interval to allow the reabsorption of the potentially resulting oedema.
CT scans were performed, at 120 kVp with 3 mm of reconstructed slice thickness, on a wide bore system (Somatom Definition AS®; Siemens AG, Erlangen, Germany). In the same day of each CT simulation, both susceptibility-weighted (SWI) MRI, which allows the detection of the internal markers, and T2 weighted turbo spin echo (TSE) MRI were also performed. After SWI and CT data sets were rigidly registered by aligning the four internal markers, the co-registered T2 weighted TSE MRI data set was then used to assess the boundaries of the prostate, the urethra, the penile bulb and the rectal spacer, if present, and, furthermore, to verify the on-CT contoured rectum. On such fused CT-MRI studies, at mid-gland slice, the distance dpr from the posterior edge of the prostate to the inner rectal wall was measured. Patients were positioned supine, with a support (Kneefix®; Civco Medical Solutions, Coralville, IA) for the legs, and prepared to have a full bladder, by drinking half a litre of water 30 min before the procedure, and an empty rectum, by enema the day before and the morning of the procedure.
Treatment planning
The clinical target volumes (CTVs) were contoured by two physicians, with each patient exclusively followed by one of the two physicians, and included the prostate with (3 patients) or without (8 patients) the seminal vesicles according to the NCCN low or intermediate stage. Our approach to patient daily set-up verification consists of the combined use of a couple of stereoscopic portal images (ExacTrac®; BrainLab Inc., Munchen, Germany), which we first register on the four internal markers, and of a cone beam CT (OBI®; Varian Inc., Palo Alto, CA), which we then use for soft tissue alignment verification and deformation review. The computed pre-treatment translational and rotational shifts are then applied to a six-degrees-of-freedom robotic couch (PerfectPitch®; Varian Inc.). During treatment, after the first of the two arcs is completed, a further verification by two stereoscopic portal images is quickly performed. Based on the estimates of our interfraction and intrafraction set-up uncertainties, the expansion margin from CTV to PTV was chosen equal to 5 mm in all directions, except 3 mm posteriorly. Such a recipe for the expansion margin is consistent with previous reports on prostate IMRT, not only when tracking of intrafraction prostate motion is performed1,2,16,17 but also with pre-treatment set-up verification only.3 The expansion margin from urethra to urethral-PRV was 3 mm in all directions. While the rectum was contoured as a solid structure, from the anus to the rectosigmoid junction, a 3-mm thick rectal wall was downwards extracted from the contoured rectum for evaluation only. A summary of the volumes of CTV, PTV and of the overlaps between PTV and rectum, bladder and urethral-PRV (OVLrectum, OVLbladder and OVLPRV-u) is reported in Table 1. From a single planner, RapidArc® (Varian Inc.), VMAT treatments by two 360° arcs with 10-MV flattening filter-free (FFF) photon beam from a TrueBeam® (Varian Inc.) linac were optimized (PRO®, v.10.0.28; Varian Inc.). FFF photon beams were used because, as a result of their higher maximum dose rate up to a factor of four with respect to photon beams with flattening filter, they are associated with reduced treatment times and, hence, with a reduced risk of intrafraction organ motion.18 For planning approval, all plans had to satisfy < 35 Gy to the three critical OARs (rectum, bladder and urethral-PRV). To avoid conflicting planning aims between dose coverage of PTV and dose sparing of its overlapping regions with the adjacent critical OARs, ICRU Report 83 (par. 5.3.2 “Overlapping volumes and conflicting planning aims”),12 firstly recommends the use of subdivision of the volumes and secondly the use of relaxed dose objectives. In this study, we adopted a mixed approach, as described below and in Table 2. The PTV was divided in its three overlaps with the critical OARs (OVLrectum, OVLbladder and OVLPRV-u), and in the remainder sub-PTV, defined as the whole PTV subtracted of the above three overlapping regions. Two types of plans, distinguished in terms of near-maximum accepted target dose, were conceived. For the homogeneous (Hom) plans, a dose of 35 Gy was prescribed as a mean dose of the sub-PTV. The sub-PTV dose constraints were a near-minimum dose (D98%) ≥33.2 Gy (=95% 35 Gy) and a near-maximum dose (D2%) ≤37.5 Gy (=107% 35 Gy). For the heterogeneous (Het) plans, a simultaneous dose boosting at the 37.5 Gy level on the sub-PTV was conceived. In details, for Het plans, a dose of 37.5 Gy was prescribed as a mean dose of the sub-PTV, while the sub-PTV dose constraints were a near-minimum dose (D98%) ≥35 Gy (≅93% 37.5 Gy), where a relaxed constraint was adopted, and a near-maximum dose (D2%) ≤40.2 Gy (=107% 37.5 Gy). To the three overlaps, OVLrectum, OVLbladder and OVLPRV-u, both Hom and Het plans were required to assure < 35 Gy, as constraint on the near-maximum dose, and D98% ≥32 Gy, as relaxed constraint on the near-minimum dose. Further necessary constraints for planning approval, for any type of plan (Hom, Het) on any CT study (NoSpc, Spc), were ≥ 95% to the whole PTV, < 35%, < 10%, < 5% for rectum and <20 Gy for the femoral heads. Therefore, the Het plans, although their larger mean PTV dose and D2%, were approved only if satisfying the same dose–volume constraints to the OARs which were requested for the Hom plans. Being then at equal maximum tolerated risk to the OARs, in this study, the Het plans are considered as an heterogeneous variant of the 35-Gy-in-five-fraction Hom plans, with which they can be compared in terms of both whole PTV dose coverage, at the 33.2 Gy dose level, and rectal dose sparing, by , and . For each patient, the same set of initial optimization goals, except for targets when passing from Hom to Het plans, was used. Dose calculations were performed by AAA® (Varian Inc.) algorithm v. 10.0.28 with a dose calculation grid size equal to 2 mm and by including CT-based heterogeneity corrections. Finally, the Hom-Spc plans were effectively used for patients' treatment, differently from the other plan types, according to an IRB approved Phase I–II study in which the patients were recruited.
Table 1.
Individual volumes of target and overlapping structures contoured on both NoSpc and Spc CT studies
| Patient number |
NoSpc CT |
Spc CT |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTV (cm3) | PTV (cm3) | OVLrectum (cm3) | OVLbladder (cm3) | OVLPRV-u (cm3) | CTV (cm3) | PTV (cm3) | OVLrectum (cm3) | OVLbladder (cm3) | OVLPRV-u (cm3) | |
| 1 | 48.4 | 95.2 | 2.3 | 4.8 | 2.7 | 54.6 | 111.1 | 0.0 | 5.2 | 2.8 |
| 2 | 42.1 | 81.5 | 1.3 | 7.4 | 2.7 | 44.1 | 83.8 | 0.1 | 0.9 | 2.8 |
| 3 | 65.9 | 127.9 | 1.1 | 0.7 | 1.6 | 69.3 | 130.7 | 0.0 | 0.9 | 1.9 |
| 4 | 51.6 | 101.6 | 1.8 | 8.1 | 5.9 | 45.2 | 87.1 | 0.0 | 2.3 | 5.1 |
| 5 | 136.4 | 222.4 | 5.1 | 8.7 | 13.2 | 145.7 | 233.2 | 2.2 | 11.2 | 13.4 |
| 6 | 59.3 | 118.6 | 3.5 | 6.0 | 2.8 | 54.6 | 108.0 | 0.0 | 2.9 | 2.6 |
| 7 | 81.7 | 161.7 | 5.3 | 8.2 | 3.3 | 71.0 | 147.6 | 0.1 | 6.9 | 2.5 |
| 8 | 58.4 | 112.8 | 0.9 | 4.9 | 4.2 | 60.5 | 94.4 | 0.0 | 0.5 | 3.2 |
| 9 | 112.9 | 183.4 | 2.5 | 10.0 | 4.2 | 110.2 | 182.9 | 0.0 | 10.1 | 4.2 |
| 10 | 48.3 | 93.3 | 2.6 | 7.3 | 5.6 | 44.2 | 84.4 | 0.0 | 1.7 | 3.0 |
| 11 | 46.6 | 91.5 | 2.1 | 0.8 | 5.4 | 45.5 | 95.1 | 0.7 | 2.1 | 5.2 |
| Mean | 68.3 | 126.3 | 2.6 | 6.1 | 4.7 | 67.7 | 123.5 | 0.3 | 4.0 | 4.2 |
| Standard deviation | 30.4 | 44.6 | 1.5 | 3.1 | 3.1 | 32.3 | 47.6 | 0.7 | 3.8 | 3.2 |
CTV, clinical target volume; NoSpc, CT scan before spacer insertion; OVLbladder, overlap between PTV and bladder; OVLPRV-u, overlap between PTV and urethral-PRV; OVLrectum, overlap between PTV and rectum; PRV, planning at risk volume; PTV, planning target volume; Spc, CT scan after spacer insertion.
Table 2.
Necessary dose–volume constraints for planning approval of Hom (D2% ≤ 37.5 Gy) and Het (D2% ≤ 40.2 Gy) plans
| Volume | Hom plans | Any plan | Het plans |
|---|---|---|---|
| sub-PTV | D98% ≥ 33.2 Gy | D98% ≥ 35 Gy | |
| Dmean = 35 Gy | Dmean = 37.5 Gy | ||
| D2% ≤ 37.5 Gy | D2% ≤ 40.2 Gy | ||
| PTV | ≥ 95% | ||
| D2% ≤ 37.5 Gy | D2% ≤ 40.2 Gy | ||
| OVLrectum, OVLbladder, OVLPRV-u | < 35 Gy | ||
| D98% ≥ 32 Gy | |||
| Rectuma | < 35 Gy | ||
| < 5% | |||
| < 10% | |||
| < 35% | |||
| Femoral heads | < 20 Gy |
Dmean, mean dose of the structure; Dn%, minimum dose to n% of the structure; OVLbladder, overlap between PTV and bladder; OVLPRV-u, overlap between PTV and urethral-PRV; OVLrectum, overlap between PTV and rectum; PRV, planning at risk volume; PTV, planning target volume; sub-PTV, planning target volume minus OVLrectum, OVLbladder, and OVLPRV-u; , percentage of PTV structure receiving ≥m (Gy); , percentage of rectal structure receiving ≥m (Gy).
For plans computed on a CT study which was scanned after the insertion of a rectal spacer, a <1 cm3 extension for OVLrectum may result. In this case, the < 35 Gy constraint is applied to the whole rectum. As the extensions of OVLbladder and OVLPRV-u are always >1 cm3, the use of the < 35 Gy constraint to the overlaps assures the fulfilment of the same constraint to the whole organ.
Statistical comparisons
As a preliminary test on the appropriateness of our use of 10 ml of injected spacer, we verified if dpr ≥ 7.5 mm, firstly, and a ≥25% reduction in rectal , secondly, were each assured in at least 90% of our patients as a result of spacer insertion. The chosen thresholds for both the prostate–rectum separation and the related reduction in rectal dose involvement were based on the previous results for IMRT plans.10 Then, over our 11 patients, the values of D2%, D98%, D50%, and , for target dose coverage, and of V18, V28 and V32 from both rectal (r) and rectal-wall (rw) volumes, for rectal dose involvement, were computed for each plan, thus defining four multiparameter samples (Hom-NoSpc, Het-NoSpc, Hom-Spc and Het-Spc). According to ICRU Report 83,7 from such parameters, the homogeneity index (HI)—(D98% − D2%)/D50%— and the conformity index (CI)—, by assuming 95% as the reference isodose level (i.e. CI = )—were also estimated. Each sample of each parameter was first tested for normality of distribution by Lilliefors test. Then, according to the results of such preliminary test, by a non-parametric Wilcoxon signed-rank test, or by a parametric one-tail t-test, the samples of (D98%, D50%, HI, CI, , , , , , , , ) values from the four plan types (Hom-NoSpc, Het-NoSpc, Hom-Spc and Het-Spc) were compared. We further hypothesized that spacer insertion might improve rectal sparing, at least in the high-dose tail (i.e. D ≥ 28 Gy), as a result of the reduced overlap between rectum and PTV. At this purpose, we first computed the variations in (, and ), Δ (X = 18, 28 and 32), between Spc and NoSpc plans of the same type in terms of maximum allowed D2%, and the corresponding variations in the fractional overlaps with PTV of rectum (Δ). The existence of a linear correlation between such variations was then estimated by the Pearson–Bravais linear correlation coefficient, and the hypothesis of no correlation against the alternative that there is a non-zero correlation was tested by a Student's t distribution for a transformation of the correlation. All computations, at the 0.05 level for statistical significance, were performed by the intrinsic routines lillietest, for normality, ranksum and ttest, for statistical hypothesis testing, and corr, for linear correlation, from MATLAB language v. R2011b (MathWorks®, Natick, MA).
RESULTS
Anatomic volumes
No significant differences (two-tail t-test) between the contoured OAR volumes from the two samples of CT studies, before and after spacer insertion, were found for both rectum [(70 ± 25) cm3 (NoSpc) vs (61 ± 16) cm3 (Spc)] (p = 0.298) and bladder [(227 ± 169) cm3 (NoSpc) vs (273 ± 188) cm3 (Spc)] (p = 0.558). The same comparison for the PTV volumes (Table 1) did not produce statistically significant differences (p = 0.886), which is consistent with a negligible intraobserver variability from the two radiation oncologists who contoured the underlying CTV structure. Furthermore, no significant differences resulted for the overlaps between PTV and bladder (p = 0.179), or between PTV and urethral-PRV (p = 0.752). By contrast, the variation in the overlap between PTV and rectum (Δ) was found as statistically significant (p = 0.0001). Furthermore (one-tail t-test), as a result of spacer insertion, mean [standard deviation (SD)] dpr values were increased from 5.3 (1.8) to 14.5 (3.9) mm (p = 0.000002), while a dpr ≥ 7.5 mm was achieved in all patients (minimum dpr values were increased from 3 to 8 mm). Both latter results, about (Δ) and dpr, are consistent with a correctly inserted spacer.
Statistical hypothesis testing
The mean values, and corresponding SD, that we computed for each of the variables (D2%, D98%, D50%, HI, CI, , , , , , , , ), where Dn% is referred to the whole PTV and the apices r and rw stand for rectal and rectal wall respectively, and for each of the four plan types (Hom-NoSpc, Hom-Spc, Het-NoSpc and Het-Spc) are reported in Table 3.
Table 3.
Mean (standard deviation) values for the dose–volume metrics of the four plan types
| Plan types | D2% (Gy) | D98% (Gy) | D50% (Gy) | HI | CI | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hom-NoSpc | 36.2 (0.2) | 32.8 (0.2) | 35.0 (0.1) | 0.095 (0.007) | 0.99 (0.01) | 96.2 (0.9) | 49.9 (3.8) | 4.1 (1.7) | 8.6 (3.1) | 23.9 (7.8) | 8.2 (3.5) | 13.7 (5.1) | 25.5 (7.6) |
| Hom-Spc | 36.2 (0.2) | 33.0 (0.1) | 35.0 (0.0) | 0.090 (0.007) | 1.01 (0.02) | 97.2 (0.5) | 51.5 (2.7) | 0.9 (1.2) | 2.5 (2.8) | 10.9 (6.7) | 1.6 (2.4) | 3.7 (4.0) | 13.8 (6.7) |
| Het-NoSpc | 39.1 (0.2) | 33.2 (0.3) | 37.5 (0.1) | 0.155 (0.009) | 1.12 (0.02) | 98.1 (0.6) | 87.4 (3.2) | 4.6 (1.7) | 9.0 (2.9) | 23.9 (6.1) | 9.5 (3.8) | 14.7 (4.9) | 26.3 (6.6) |
| Het-Spc | 38.9 (0.2) | 33.7 (0.4) | 37.6 (0.1) | 0.140 (0.013) | 1.14 (0.03) | 99.0 (0.6) | 91.9 (2.7) | 1.2 (1.6) | 2.8 (3.0) | 11.4 (6.9) | 1.8 (2.5) | 4.3 (4.4) | 14.4 (6.7) |
CI, , conformity index; Dn%, minimum dose to n% of the PTV structure; Het, plans with D2% ≤ 40.2 Gy; HI, (D98% − D2%)/D50%, homogeneity index; Hom, plans with D2% ≤ 37.5 Gy; NoSpc, plans computed on CT scanned before spacer insertion; PTV, planning target volume; Spc, plans computed on CT scanned after spacer insertion; , percentage of PTV structure receiving ≥m (Gy); , percentage of rectal structure receiving ≥m (Gy); , percentage of rectal-wall structure receiving ≥m (Gy).
For plan comparison purposes, the five combinations—Hom-Spc vs Hom-NoSpc, Het-Spc vs Het-NoSpc, Het-NoSpc vs Hom-NoSpc, Het-Spc vs Hom-Spc, Het-Spc vs Hom-NoSpc—were conceived. In Table 4, the resulting p-values by comparing the parameters (D98%, D50%, HI, CI, , , , , , , , ) across the above five combinations of plans are reported, by normal values (Student's one-tail t-test) or italic values (Wilcoxon signed-rank test) characters. The p-values from the comparisons of D50%, HI, CI and between Het and Hom plans (<0.0005) are not reported in Table 4, since the variations from such parameters were the obvious consequence of a different dose prescription.
Table 4.
p-values from comparisons of target and rectal dose–volume metrics across five combinations of plan types
| Compared plan types | D98% | D50% | HI | CI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hom-Spc vs Hom-NoSpc | 0.002 | 0.175 | 0.961 | 0.002 | 0.014 | 0.076 | 0.0006 | 0.0006 | 0.0003 | 0.0005 | 0.00003 | 0.00006 |
| Het-Spc vs Het-NoSpc | 0.002 | 0.002 | 0.997 | 0.0009 | 0.049 | 0.001 | 0.002 | 0.002 | 0.0001 | 0.0005 | 0.00002 | 0.0002 |
| Het-NoSpc vs Hom-NoSpc | 0.0002 | <10−11 | 0.264 | 0.470 | 0.511 | 0.293 | 0.671 | 0.601 | ||||
| Het-Spc vs Hom-Spc | 0.00006 | 0.00008 | 0.717 | 0.870 | 0.563 | 0.792 | 0.628 | 0.580 | ||||
| Het-Spc vs Hom-NoSpc | <10−5 | 0.00008 | 0.004 | 0.002 | 0.0004 | 0.00005 | 0.00008 | 0.0009 |
Normal characters, p-values from one-tail t-test; italic characters, p-values from Wilcoxon signed-rank test.
CI, , conformity index; Dn%, minimum dose to n% of the PTV structure; Het, plans with D2% ≤ 40.2 Gy; HI, (D98% − D2%)/D50%, homogeneity index; Hom, plans with D2% ≤ 37.5 Gy; NoSpc, plans computed on CT scanned before spacer insertion; PTV, planning target volume; Spc, plans computed on CT scanned after spacer insertion; , percentage of PTV structure receiving ≥m (Gy); , percentage of rectal structure receiving ≥m (Gy); , percentage of rectal-wall structure receiving ≥m (Gy).
Firstly, by comparing plans of the same type in terms of maximum allowed D2%—Hom-Spc vs Hom-NoSpc or Het-Spc vs Het-NoSpc—spacer insertion significantly improved the sparing of rectum and rectal wall, for both Hom and Het plans. For , in particular, mean (SD) values were reduced from 4.1% (1.7%) to 0.9% (1.2%) in Hom plans (p = 0.0006) and from 4.6% (1.7%) to 1.2% (1.6%) in Het plans (p = 0.002). Furthermore, a ≥25% reduction in was achieved in all of our patients (minimum reductions were 34.1% for Hom plans and 37.1% for Het plans, respectively). This, together with the above reported 100% of patients with dpr ≥ 7.5 mm, suggests that our use of the spacer was appropriate.10 Target dose coverage was also improved in terms of D98% and , for both Hom and Het plans, and in terms of D50% and , for Het plans only. On the other hand, no significant variations were observed in D50% and in for Hom plans, consistently with the 35 Gy prescribed dose as a mean dose of the sub-PTV. Although plan HI was not significantly affected from spacer insertion, small but significant variations in plan CI resulted with spacer insertion for both Hom (p = 0.002) and Het (p = 0.0009) plans.
Secondly, by comparing plans at equal spacer insertion status—Het-NoSpc vs Hom-NoSpc or Het-Spc vs Hom-Spc—the increased maximum allowed D2% significantly improved target dose coverage in terms of D98% and , for both NoSpc and Spc plans. Instead, as expected, no significant rectal dose sparing was computed for both rectum and rectal wall.
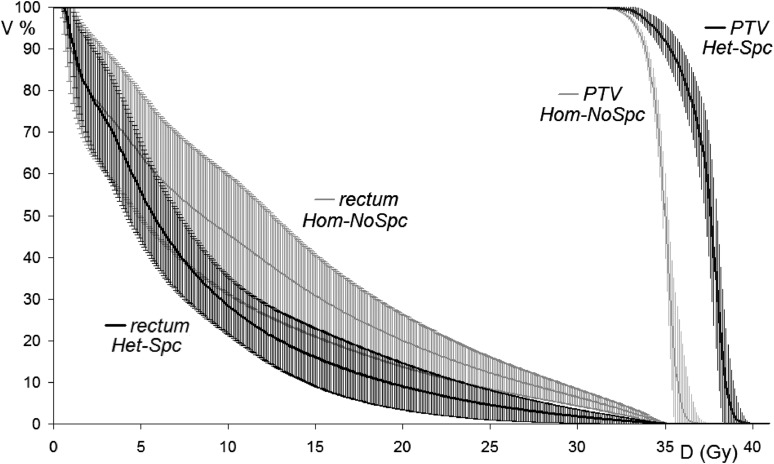
Finally, by directly comparing Het-Spc vs Hom-NoSpc plans, the combined effect of spacer insertion and increased D2% resulted in significant differences for all the considered parameters. In Figure 1, such comparison is further shown in terms of average dose–volume histograms, with ±1 SD error bars, for both rectum and PTV.
Figure 1.
Average dose–volume histograms, with error bars equal to ±1 standard deviation, for rectum and prostate planning target volume (PTV) by comparing Het-Spc vs Hom-NoSpc plans: the enlargement of the therapeutic window by the combined use of spacer insertion and increased accepted D2% is evident. D2%, minimum dose to 2% of PTV; Het, plans with D2% ≤ 40.2 Gy; Hom, plans with D2% ≤ 37.5 Gy; NoSpc, plans computed on CT scanned before spacer insertion; Spc, plans computed on CT scanned after spacer insertion.
Linear correlation analysis
The results from the previous paragraph suggested that, when passing from NoSpc to Spc plans of the same type in terms of maximum allowed D2%, the observed improvements in rectal sparing for the high-dose tail (i.e. D ≥ 28 Gy) might be determined from the reduced overlap between rectum and PTV (Δ) as a result of spacer insertion. Therefore, to support the existence of some correlation between the above variations, we performed linear correlation analysis in terms of Pearson–Bravais linear correlation coefficient, r. As a result, Δ significantly correlated with rectal , for both Hom [r = 0.777 (p = 0.008)] and Het plans [r = 0.626 (p = 0.05)], and with [r = 0.654 (p = 0.04)], for Hom plans. Instead, no significant linear correlation was observed between Δ and . Thus, spacer insertion seems to be causative in improving rectal dose sparing, at least in the high-dose tail (i.e. ≥80% Dp), in our sample of patients.
DISCUSSION
Data continue to emerge on several potential advantages of extreme hypofractionation compared with various other treatment strategies for localized prostate cancer. While other studies have demonstrated the effectiveness of the spacer in rectal dose sparing for IMRT plans optimized for standard fractionation,7–10 the present study is the first to assess the dosimetric impact of the combined use of both spacer insertion and slightly increased accepted target D2% in plans conceived for five-fraction schedules.
With regard to the effects of spacer insertion on rectal dose involvement, V18, V28 and V32, for both rectum and rectal wall, were significantly reduced after spacer insertion, for both Hom and Het plans. Furthermore, a significant linear correlation was observed between Δ (i.e. the variation of the overlap between PTV and rectum), whose extent is determined from the insertion or not of the spacer, and both rectal and . We then believe that such combined results confirm the existence of a causal relationship between spacer insertion and improved rectal dose sparing, at least in the high-dose tail (i.e. ≥80% Dp). This is consistent with previous reports on IMRT plans in standard fractionation, where the soft rectal constraints D25% <70 Gy (i.e. D25% <90% Dp)7 or V70 <20% (i.e. < 20%)9,10 had been pursued. As an added value from this study, the validity of the above causal relationship has been extended to five-fractions VMAT-SBRT plans, where the hard rectal constraint <35 Gy (i.e. < Dp) had to be satisfied.
The use of spacer insertion determined a significant improvement in target dose coverage () for both Hom and Het plans, as suggested from hypothesis testing (Table 4). However, when the average amount of such improvement is estimated by comparing Hom-Spc [ = 97.2 (0.5)] vs Hom-NoSpc [ = 96.2 (0.9)] plans, i.e. by isolating the contribute of spacer insertion from the effect of increased D2%, it was still not enough to assure the fulfilment of the desired ≥ 98% condition.
With regard to the effects of the increased accepted D2%, we think that the correspondingly increased dose gradients at target boundaries constitute a finite resource which can be distributed, by the optimization, between the two opposing goals of target coverage and adjacent critical structures sparing. In our plans, by stressing both Hom and Het ones to assure < 35 Gy to the three critical OARs, we devoted the most of this resource to improve target dose coverage. As a result, the improvements in by the use of a slightly increased accepted target D2%, Het-NoSpc [98.1 (0.6)], were enough to assure “on average” the desired ≥ 98% condition. Furthermore, we were then not surprised of the observed absence of any improvement on rectal dose involvement from comparing Het vs Hom plans, at equal spacer insertion status. Consistently with such premises, although the significant improvements in both and D98%, target dose coverage was mostly improved in terms of dose accumulation—i.e. D50%, —in PTV subregions far from our three critical OARs. The about 2.5-Gy computed increase in D50% (Table 3) for Het vs Hom plans, for both Spc and NoSpc CT studies, as a result of the different prescribed doses as mean dose of the sub-PTV, might be associated with an increase in the local tumour control probability (TCP). This would not be in contrast with the stated aim of the present study: to improve target dose coverage, with the implicit goal to improve TCP, without increased risk of toxicity for rectum, bladder and urethra. As development of the present study, a TCP-based plan-ranking analysis of the here presented plans, founded on the algorithms described in the study by Stavreva et al,19 is in progress.
In summary, in the Introduction section, we stated that the use of the < 35 Gy constraint for rectum, bladder and urethral-PRV, makes ≥ 98% as a generally not achievable goal from Hom-NoSpc plans [ = 96.2 (0.9)] for 35-Gy-in-five-fraction prostate VMAT-SBRT. The primary end point of this study was the development of a planning/treatment technique which improved our target dose coverage, by assuring ≥ 98%, while maintaining the < 35 Gy constraint to the three critical OARs. With this regard, the improvements in by the use of the spacer alone, Hom-Spc [97.2 (0.5)], were not enough. By contrast, the improvements from the use of a slightly increased accepted target D2%, Het-NoSpc [98.1 (0.6)], were “on average” enough. Finally, the combined use of both spacer insertion and increased accepted D2%, Het-Spc [99.0 (0.6)], assured ≥ 98% to all our patients.
The main limitations of this study are related to the fact that we compared plans which were independently optimized on the same CT data set, Het vs Hom, or even on different CT studies, Spc vs NoSpc. We believe that we have reasonably treated the former problem (Het vs Hom) by the involvement of a single planner, which performed the twin optimizations on the same volumes with the same initial optimization objectives to the OARs for each patient. According to the use of different CT studies (Spc vs NoSpc), we are aware of potential bias from variations in the contoured anatomic structures. However, thanks to our efforts in patients' preparation to CT simulation, we did not observe any statistically significant difference both in the whole bladder, rectum and PTV volumes, both in the overlaps with PTV from bladder and urethral-PRV in the two, Spc and NoSpc, CT studies.
Finally, we recognize that the use of 1 cm3 as threshold volume of rectum, bladder and urethral-PRV to receive 35 Gy is the first limiting factor to the improvement of our present level of target dose coverage (i.e. ≥ 95%). If no relevant incidence of rectal or GU grade ≥3 toxicities will result from our ongoing Phase I–II study, from which the here presented Hom-Spc plans were taken, we will further improve our target dose coverage by simply allowing for a slightly larger volume (e.g. 2 or 3 cm3) of rectum and bladder to receive 35 Gy.
CONCLUSION
The main aim of this study was the optimization of a VMAT technique for prostate SBRT to improve the target dose coverage (). According to the here presented results and discussed limitations, a general improvement in target coverage was associated with both a slightly increased accepted D2%, and the use of spacer insertion. The latter was further causative for improvements in rectal sparing. Therefore, the combined use of both spacer insertion and increased accepted D2% significantly improved both rectal sparing and our value, which passed from 96.2% (±0.9%) to 99.0% (±0.6%).
Contributor Information
Ruggero Ruggieri, Email: ruggieri.ruggero@gmail.com.
Stefania Naccarato, Email: stefania.naccarato@gmail.com.
Pavel Stavrev, Email: pavel.stravev@libero.it.
Nadejda Stavreva, Email: nadejda.stavreva@libero.it.
Sergio Fersino, Email: sergio.fersino@libero.it.
Niccolò Giaj Levra, Email: niccolo.giajlevra@libero.it.
Rosario Mazzola, Email: rosario.mazzola@libero.it.
Pietro Mancosu, Email: pietro.mancosu@libero.it.
Marta Scorsetti, Email: marta.scorsetti@libero.it.
Filippo Alongi, Email: filippo.alongi@libero.it.
REFERENCES
- 1.King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013; 109: 217–21. doi: 10.1016/j.radonc.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 2.Katz AJ, Kang J. Stereotactic body radiotherapy as treatment for organ confined low- and intermediate-risk prostate carcinoma, a 7-year study. Front Oncol 2014; 4: 240. doi: 10.3389/fonc.2014.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alongi F, Cozzi L, Arcangeli S, Iftode C, Comito T, Villa E, et al. Linac based SBRT for prostate cancer in 5 fractions with VMAT and flattening filter free beams: preliminary report of a phase II study. Radiat Oncol 2013; 8: 171. doi: 10.1186/1748-717X-8-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scorsetti M, Alongi F, Clerici E, Comito T, Fogliata A, Iftode C, et al. Stereotactic body radiotherapy with flattening filter-free beams for prostate cancer: assessment of patient-reported quality of life. J Cancer Res Clin Oncol 2014; 140: 1795–800. doi: 10.1007/s00432-014-1732-1 [DOI] [PubMed] [Google Scholar]
- 5.Boike TP, Lotan Y, Cho LC, Brindle J, DeRose P, Xie XJ, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol 2011; 29: 2020–6. doi: 10.1200/JCO.2010.31.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DW, Cho LC, Straka C, Christie A, Lotan Y, Pistenmaa D, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014; 89: 509–17. doi: 10.1016/j.ijrobp.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 7.Weber DC, Zilli T, Vallee JP, Rouzaud M, Miralbell R, Cozzi L. Intensity modulated proton and photon therapy for early prostate cancer with or without transperineal injection of a polyethylene glycol spacer: a treatment planning comparison study. Int J Radiat Oncol Biol Phys 2012; 84: e311–18. doi: 10.1016/j.ijrobp.2012.03.028 [DOI] [PubMed] [Google Scholar]
- 8.Susil RC, McNutt TR, DeWeese TL, Song D. Effects of prostate-rectum separation on rectal dose from external beam radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76: 1251–8. doi: 10.1016/j.ijrobp.2009.07.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinkawa M, Corral NE, Caffaro M, Piroth MD, Holy R, Djukic V, et al. Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. Radiother Oncol 2011; 100: 436–41. doi: 10.1016/j.radonc.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 10.Song DY, Herfarth KK, Uhl M, Eble MJ, Pinkawa M, van Triest B, et al. A multi-institutional clinical trial of rectal dose reduction via injected polyethylene-glycol hydrogel during intensity modulated radiation therapy for prostate cancer: analysis of dosimetric outcomes. Int J Radiat Oncol Biol Phys 2013; 87: 81–7. doi: 10.1016/j.ijrobp.2012.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Commission on Radiation Units and Measurements. ICRU Report 62: Prescribing, recording, and reporting photon beam therapy (supplement to ICRU Report 50). Bethesda, MD; 1999. [Google Scholar]
- 12.International Commission on Radiation Units and Measurements. ICRU Report 83: prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). J ICRU 2010; 10: 1–92. doi: 10.1093/jicru/ndq003 [DOI] [Google Scholar]
- 13.Richter A, Baier K, Meyer J, Wilbert J, Krieger T, Flentje M, et al. Influence of increased target dose inhomogeneity on margins for breathing motion compensation in conformal stereotactic body radiotherapy. BMC Med Phys 2008; 8: 5. doi: 10.1186/1756-6649-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy MJ. Fiducial-based targeting accuracy for external-beam radiotherapy. Med Phys 2002; 29: 334–44. doi: 10.1118/1.1448823 [DOI] [PubMed] [Google Scholar]
- 15.van der Heide UA, Kotte AN, Dehnad H, Hofman P, Lagenijk JJ, van Vulpen M. Analysis of fiducial marker-based position verification in the external beam radiotherapy of patients with prostate cancer. Radiother Oncol 2007; 82: 38–45. doi: 10.1016/j.radonc.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 16.Chen NL, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown university experience. Radiat Oncol 2013; 8: 58. doi: 10.1186/1748-717X-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu QJ, Li T, Yuan L, Yin FF, Lee WR. Single institution’s dosimetry and IGRT analysis of prostate SBRT. Radiat Oncol 2013; 8: 215. doi: 10.1186/1748-717X-8-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reggiori G, Mancosu P, Castiglioni S, Alongi F, Pellegrini C, Lobefalo F, et al. Can volumetric modulated arc therapy with flattening filter free beams play a role in stereotactic body radiotherapy for liver lesions? A volume-based analysis. Med Phys 2012; 39: 1112–18. doi: 10.1118/1.3679858 [DOI] [PubMed] [Google Scholar]
- 19.Stavreva N, Nahum A, Markov K, Ruggieri R, Stavrev P. Analytical investigation of the possibility of parameter invariant TCP-based radiation therapy plan ranking. Acta Oncol 2010; 49: 1324–33. doi: 10.3109/0284186X.2010.517782 [DOI] [PubMed] [Google Scholar]