Abstract
Previous studies showed that the anterior cingulate cortex (ACC) plays a role in selective visual attention. The current study further examined the role of the ACC in attention using a visual cuing task with task-relevant and task-irrelevant stimuli. On every trial, two stimuli were presented on the touchscreen; one was task-relevant and the other was task-irrelevant. Rats were trained to attend to the task-relevant stimulus over the task-irrelevant stimulus to determine which side of the touchscreen should be selected for reward. After the rats were well-trained, cannulae targeting the ACC were implanted bilaterally for infusions of PBS or muscimol. When the ACC was functionally intact, high task performance was correlated with the anticipatory touches toward the reward; rats touched the stimulus proximal to the correct side more often, regardless of its task-relevancy. Analysis of the pre-surgery training data showed that rats developed anticipatory touches during training. Linear discriminant analyses of the touches also showed that the touches predict rats’ choices in trials. With muscimol infusions, choice accuracy was impaired and the anticipatory touches toward the correct response location were less frequent. A control experiment, in which there were no irrelevant stimuli, showed no effects of ACC inactivation on choice accuracy or anticipatory touches. These results indicate that the rat ACC plays a critical role in reducing distraction from irrelevant stimuli as well as in guiding attention toward the goal locations.
Keywords: anterior cingulate, visual attention, memory, rodent, touchscreen
Introduction
Limitations on cognitive resources necessitate information filtering through selective attention. It has been suggested that visual attention selects goal-relevant targets over goal-irrelevant distractors for later processing (Corbetta & Shulman, 2002; Treisman, 1969). The findings of human imaging studies (Ramos-Quiroga et al., 2013; Rossi, Pessoa, Desimone, & Ungerleider, 2009; Suzuki & Gottlieb, 2013), as well as monkey physiological investigations (Desimone & Duncan, 1995; Everling, Tinsley, Gaffan, & Duncan, 2006; Panagiotaropoulos, Deco, Kapoor, & Logothetis, 2012; Schafer & Moore, 2011), indicate that the prefrontal and cingulate cortices are at the center of the functional network underlying attention and related processes (Corbetta & Shulman, 2002; Dalley, Cardinal, & Robbins, 2004; Duncan & Owen, 2000; Kastner & Ungerleider, 2000; Miller & Cohen, 2001). Behavioral, neural, and mathematical evidence have argued for significant roles of the anterior cingulate cortex (ACC) in attention in terms of error detection and conflict monitoring. Several studies specifically suggest that ACC can resolve attentional conflict by orienting and boosting attention toward task-relevant events (Orr & Weissman, 2009; Weissman, Giesbrecht, Song, Mangun, & Woldorff, 2003; Weissman, Gopalakrishnan, Hazlett, & Woldorff, 2005; Weissman, Mangun, & Woldorff, 2002).
Studies using functional imaging in human subjects have showed that the dorsal ACC is critical when there is conflict between the target (or goal behavior) and distractor stimuli (M. M. Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Carter et al., 1998; Carter, Botvinick, & Cohen, 1999). Similar findings have been found in monkey electrophysiology studies as well (Bush et al., 2002; Shen et al., 2014). Specifically, in a recent study by Shen et al. (2014), the authors argued that primate ACC unit firing conveys inhibitory control of attentional focus, the prevention of bottom-up distraction, and choice implementation by encoding errors in attentional tasks. Studies using rodents have also suggested a significant role of the ACC in inhibiting distracting information (Bussey, Muir, Everitt, & Robbins, 1997; Cardinal et al., 2002, 2003; Ng, Noblejas, Rodefer, Smith, & Poremba, 2007). In those studies, rats were trained to attend to a specific target stimulus (or dimension) over distractor stimuli to obtain reward. Lesions of the ACC significantly impaired task performance in learning (Bussey et al., 1997), retrieval (Cardinal et al., 2002, 2003), or generalization (Ng et al., 2007).
The studies introduced so far have emphasized the role of the ACC in cognitive control, but the specific functions differed across the various behavioral paradigms; some studies focused more on the inhibition of distractors (Bussey et al., 1997; Cardinal et al., 2002, 2003; Ng et al., 2007), whereas others emphasized conflict monitoring or resolution (M. M. Botvinick et al., 1999; Carter et al., 1998, 1999). In addition, computation models suggest an “actor” mechanism, which adaptively regulates behavior by feedback from the environment (Holroyd & McClure, 2015; Rushworth, Buckley, Behrens, Walton, & Bannerman, 2007; Walton, Croxson, Behrens, Kennerley, & Rushworth, 2007). To test each hypothesis within a single study as well as to examine any additional functions of the ACC, we developed a rodent version of a visual cuing task with task-irrelevant stimuli. We used an infrared touchscreen apparatus that has previously supported robust visual discrimination and categorization in rats to examine the effects of task-irrelevant stimuli on visual discrimination behavior (Brooks et al., 2013; Wasserman, Castro, & Freeman, 2012) (Fig. 1A). By combining this behavioral approach with reversible inactivation methods, each specific function of the ACC was tested in the current study.
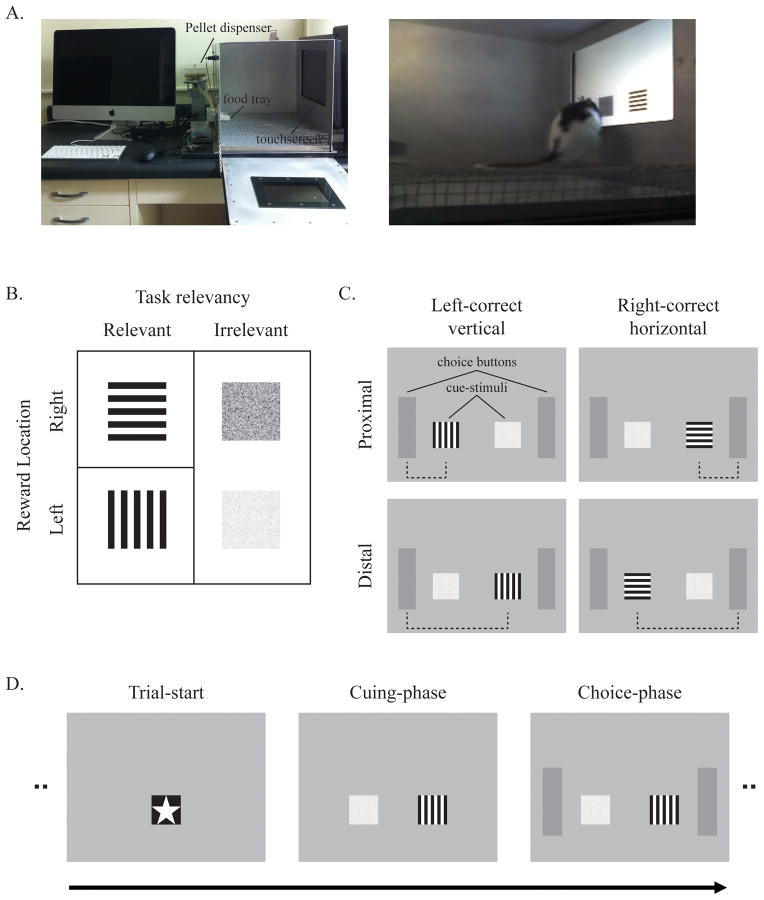
Figure 1.
Image of testing apparatus, visual stimuli, task design, and sample trial sequence. A. A touchscreen was attached to the right wall of the chamber and the rat touches the stimuli appearing on the screen. A small food pellet was delivered via a rotary pellet feeder to the food tray installed on the left wall. All devices were controlled by a custom written program in Matlab running on an iMac. B. Two distinctive orientation patches (horizontal and vertical) and two different brightness patches (light and dark) were used in the task. Only task-relevant orientation patches signaled to the rat which side was to be touched to obtain reward. C. “Visual stimuli” were composed of one of the relevant target stimuli and one of the irrelevant distractor stimuli. The task-relevant stimulus appeared either proximal or distal to the correct response location. D. Every trial started with the appearance of the trial-initiation stimulus in the center of the screen. After a single touch, the visual stimuli appeared on the screen and the rat was required to touch either of them five times to trigger the presentation of the choice buttons on both sides of the screen. When the rat touched one of the choice buttons, a reward was administered on correct trials and correction trials were given on incorrect trials, except during testing sessions.
Our rats were trained to discriminate task-relevant stimuli from the task-irrelevant stimuli to determine the location of the rewarded response. To track attention during the visual discrimination, rats were required to touch the cue-stimuli five times and the touch coordinates were recorded. After observing the visual stimuli, the rats were required to touch either the left or right choice buttons for food reward. By changing the locations of the task-relevant and task-irrelevant stimuli on different trials, the spatial response conflict was manipulated. On half of the trials, the task-relevant stimuli appeared proximal to the reward location while the task-irrelevant ones appeared distally. On the other half of the trials, the task-relevant stimuli appeared distal to the reward location while the task-irrelevant stimuli appeared proximally. In other words, the spatial locations of the task-relevant stimuli and the rewarded response locations were congruent in the proximal condition, whereas they were incongruent in the distal condition. Thus, in the distal condition (but not the proximal condition), the rats were required to change spatial attention more abruptly toward the opposite side for reward after the visual discrimination (Fig. 1B–D).
After the task had been learned, either PBS or muscimol was infused into the ACC during test sessions using an ABBA design. Inactivation-related changes in choice accuracy, response latency, and touch coordinates were analyzed across the infusions. Touch coordinates were further examined with a linear discriminant analysis (LDA) classifier. Finally, the rats were tested again with the same infusion schedule while they were performing a control task in which no task-irrelevant stimuli appeared.
Materials and Methods
Subjects
Subjects were 9 male Long-Evans rats (300 – 320 g). They were individually housed in standard rodent cages on a 12 h light/dark cycle, with all training and test sessions given during the light phase. Rats were food-deprived to 85% of free-feeding weight, but they were able to access water ad libitum. All procedures were approved by the Institutional Care and Use Committee at the University of Iowa.
Handling/shaping
After rats arrived in the animal colony, a 1-week acclimation period was given in which they had ad libitum access to food and water. Food was then restricted and the rats were individually handled daily. After the rats acclimated to handling, they were placed on a lab cart for 10 min where several 45-mg food pellets (MLab Rodent Tablet 45 mg, TestDiet Inc., IN) were scattered. The handling/cart session was repeated until the rats consumed all the pellets within 10 min without defecating or urinating on the cart, which typically took 7 days.
Rats were individually shaped to touch a visual stimulus on a touchscreen in a training chamber to receive a single food pellet (see below for details about the apparatus and visual stimuli). Once the rat touched the stimulus presented on the center of the screen, a gray rectangle (choice button in main experiments) appeared on either the left or right side of the screen. After the rat touched the gray rectangle, a single pellet was delivered into a food tray. While the rat was consuming the food, the trial-initiation stimulus reappeared on the center of the screen for the next trial. Rats were trained to a criterion of 80 shaping trials in 1 hr. The session was terminated when either the rats finished all the trials or 1 hr had elapsed. Shaping sessions continued until the rats finished 80 trials in 30 min on 2 consecutive d. It took 7–10 d to shape the rats.
Behavioral apparatus
Rats were trained in a touchscreen-outfitted chamber (Fig. 1A) described in previous studies (Brooks et al., 2013; Wasserman et al., 2012). The chamber was 36 (w) × 41 (d) × 36 (h) cm in size. Inside the chamber, a metal mesh grid (34 (w) × 39 (d) × 5 (h) cm in size) was placed slightly above the floor. Rats’ behaviors were observed and recorded through a transparent window (13.5 (w) × 10 (h) cm in size) in the chamber door. An aluminum food tray (6.5 (w) × 13 (d) × 4.5 (h) cm in size) was at the base of the wall opposite the touchscreen. A rotary pellet feeder (Med Associates Inc., Georgia, VT, model ENV-203IR) delivered a 45-mg food pellet into the food tray. A small light bulb was installed at the top of the back wall and was illuminated throughout the behavioral sessions. An infrared touchscreen (15-inch, EloTouch Systems, Fremont, CA) on one wall was positioned in front of an LCD flat screen monitor (NEC, Melville, NY, Model 1550V). The touchscreen, LCD screen monitor, light bulb, and rotary feeder were controlled by an Apple iMac computer (Apple, Cupertino, CA, Model iMac 10.1) via a relay controller (National Control Devices, Osceola, MO, Model RS-232). A customized program written in MATLAB (Mathworks, Natick, MA) using the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997) controlled all devices (e.g., visual stimulus presentation and behavioral recording via touch technology) (Fig. 1A). White noise was provided during shaping, training, and test sessions to mask any extraneous noise.
Visual stimuli
All visual stimuli were 150 × 150 pixels in size and appeared on a uniform gray background (1024 × 768 pixel resolution, RGB: 192 192 192) (Fig. 1B). A white star on a black square background (150 × 150 pixel) was the trial-initiation stimulus (Fig. 1D). Grey rectangles (100 × 350 pixel, RGB: 127 127 127) appeared on both left and right sides of the screen to serve as choice buttons.
Visual cuing task with task-irrelevant stimuli
In the visual cuing task with task-irrelevant stimuli, every trial started with the appearance of the trial-initiation stimulus in the center of the screen (Fig. 1D). Rats could initiate each trial in a self-paced manner by touching the trial-initiation stimulus once. Next, two visual stimuli appeared side-by-side in the center of the screen. The rats had to touch the visual stimuli, regardless of their task-relevancy, a total of five times to activate the choice buttons on the left- and right-sides of the screen. The multiple-touch requirement on the cue-stimuli is necessary for the initial learning because it forces the rat to process the visual stimuli. Also, this requirement enables us to track how the rat’s attention is deployed to the task-relevant and task-irrelevant stimuli as well as its anticipation of the rewarded response location while it is performing the task. Four visually distinctive stimuli were used; two grating stimuli that differed in orientation (horizontal vs. vertical) and other two stimuli that differed in brightness (white vs. black). One dimension (orientation or brightness) defined the task-relevant stimulus and the other defined the task-irrelevant stimulus (counterbalanced across the rats). Each relevant stimulus indicated the correct response location to produce a reward (left vs. right) (Fig. 1B & 1C). On each trial, one orientation stimulus and one brightness stimulus were pseudo-randomly chosen in a counterbalanced manner to create the trial stimuli. The two stimuli appeared side-by-side in the center of the screen. Regardless of its local position, the task-relevant stimulus had to be attended to over the task-irrelevant stimulus to determine which choice button should be touched to obtain food reward. Relevant and irrelevant stimuli appeared in these left and right positions equally often throughout the training session. Both relevant and irrelevant stimuli appeared equally throughout the session in a counterbalanced manner, so 8 trials (local positions of the stimuli (2) × relevant stimuli (2) × irrelevant stimuli (2)) made up each block, for 10 blocks (80 trials). In every 8-trial block, the trial order was randomized. Both the task-relevancy of the stimuli (orientation vs. brightness) and the correct-side for food reward (left vs. right) were counterbalanced across the rats. Depending on the type of task-relevant stimulus presented, regardless of its local position (proximal vs. distal), rats had to touch either the left or right choice button to obtain food reward (Fig. 1C & Fig. 4). Once the rats touched one of the choice buttons, the trial was terminated and the next trial started after a 3-s intertrial interval; food reward was dropped into the food tray when rats made the correct choice response. If the incorrect choice button was touched, then the rats received correction trials until the correct response was made. Once a rat’s accuracy was 80% or greater for 2 consecutive days, the correction trials were deleted for the remaining training and testing sessions.
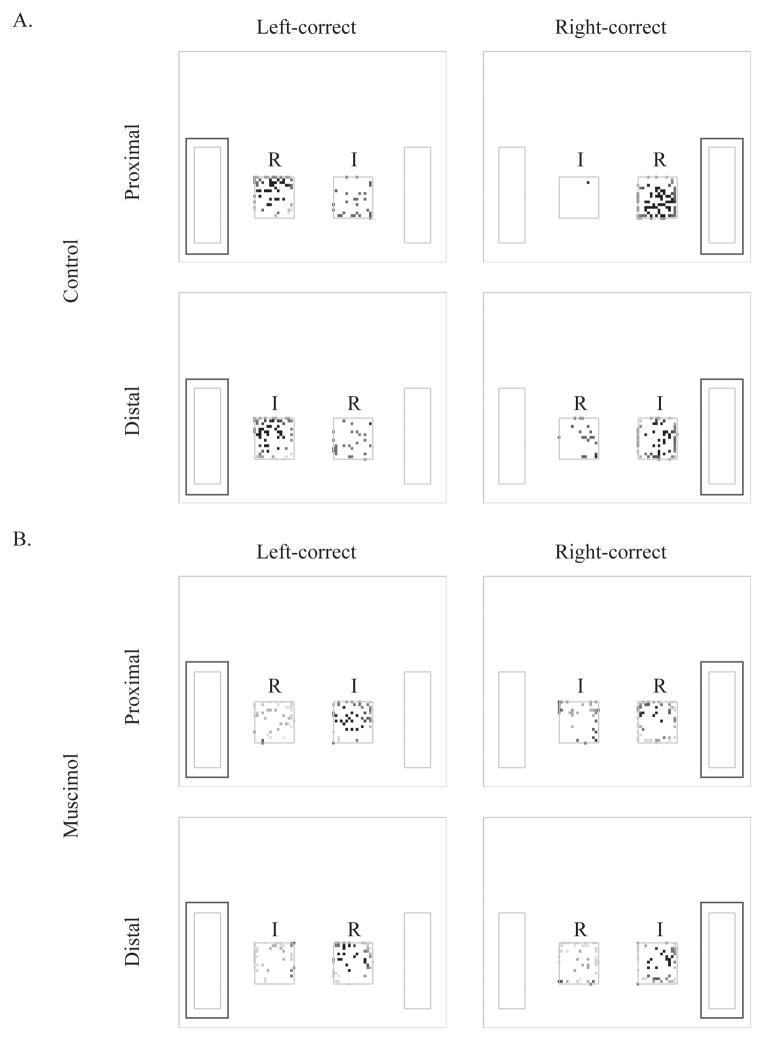
Figure 4.
Touches of the visual stimuli during control and muscimol sessions. Touches of the visual stimuli were mapped in terms of correct side and local positions of the task-relevant stimuli. A. When the ACC was functionally intact, touches were highly concentrated on the visual stimulus near the correct response location regardless of task-relevancy. B. When the ACC was inactivated, touches on the visual stimulus near the correct response location were dispersed onto both stimuli. Note that the data shown came from PBS and muscimol sessions of the same rat. Frequency of touches was colored in the blue-red (light-dark) dimension; reddish (dark) color indicates high and bluish (light) color represents low numbers of touches. The labels “R” and “I” in each cell indicate relevant and irrelevant visual stimuli, respectively. Green (gray) rectangles surrounding the choice buttons indicate the correct side for obtaining reward in a given condition. The letters and green (gray) rectangles were not visible to the rat during training or testing.
Control task
In the control task, all of the procedures were the same as in the visual cuing task with task-irrelevant stimuli, except that no task-irrelevant stimuli were given throughout the session. Two identical task-relevant stimuli were presented during the cuing-phase on control task trials (Fig. 8A). In pilot experiments, we used a control task in which a single task-relevant stimulus, without a task-irrelevant stimulus, appeared in the center of the screen, but the rats failed to activate the choice buttons. The rats kept touching the locations where the two training stimuli had previously appeared instead of touching the center location. As a result, we modified the control task to have presentations of two identical task-relevant stimuli side-by-side, which required the rats to abide by the same behavioral rules for producing food reward.
Figure 8.
Significant correlations held between LDA classifier performance and task accuracy in the testing and pre-learning sessions. In both pre- (A) and post-surgery sessions (C), touches of the visual stimuli were used to train a LDA classifier to predict rats’ choices. Classifier performance was conservatively estimated with leave-one-out-cross-validation. Classifier performance was significantly impaired during the muscimol infusion periods (A). Also, classifier performance linearly improved across the pre-learning stages (C). The correlation between classifier performance and task accuracy showed that classifier performance was positively correlated with task accuracy. Task performance can be reliably predicted by touches of the visual stimuli (B & D). Error bars show the standard error of the mean.
Surgery
After the rats met the pre-surgical behavioral criterion (80% or better for 2 consecutive days), two guide cannulae (26G, 4-mm long; Plastics One, Roanoke, VA) coupled with stylets (2-mm protrusion from the tip of the guide cannula) were implanted bilaterally targeting the anterior cingulate cortex (ACC). Under isoflurane (1–5%) anesthesia, two holes for the cannulae were drilled through the skull: 2.0 mm anterior to bregma and 0.7 mm lateral to midline. The cannulae were then inserted 2.3 mm ventral to the skull surface with 10° angle toward periphery and secured to the skull by bone cement (Zimmer, Warsaw, IN) and screws. After surgery, the rats were placed on a heating pad to prevent hypothermia and were monitored until they were fully awake and mobile.
Drug infusion procedure
The general procedure for muscimol infusion has been described in previous studies (Halverson & Freeman, 2010). The stylet was removed from the guide cannula and replaced with a 30-ga infusion cannula that extended 2.0 mm from the tip of the guide cannula. The infusion cannula was connected to polyethylene tubing (PE 50; 110–120 cm), which was connected to a 10-μL gas-tight syringe (Hamilton). The syringe was placed in an infusion pump (Harvard Apparatus), and 0.25 μL of phosphate-buffered saline (PBS, pH 7.4) or muscimol (2.0 mmol, pH 7.4) was infused over 2 min at a rate of 8.0 μL/h. Both before and after the infusion, 1 min was given to stabilize the infusion needle in the target area. All test sessions began 35 min after the infusion. After each infusion session, a 1d break was given to minimize residual drug effects.
Histology
Histological verifications of the cannula positions were done after the rats completed all behavioral tests. Rats were anesthetized with sodium pentobarbital and then perfused with ~300 ml PBS and ~300 ml 10% formalin. Brains were removed and stored in 10% formalin and 30% sucrose at 4°C for 3 days before sectioning. Coronal sections (50 μm) of the brain were collected using a sliding microtome (Thermo Fisher Scientific, Waltham, MA). Brain sections were then stained with thionin (Sigma-Aldrich, St. Louis, MO).
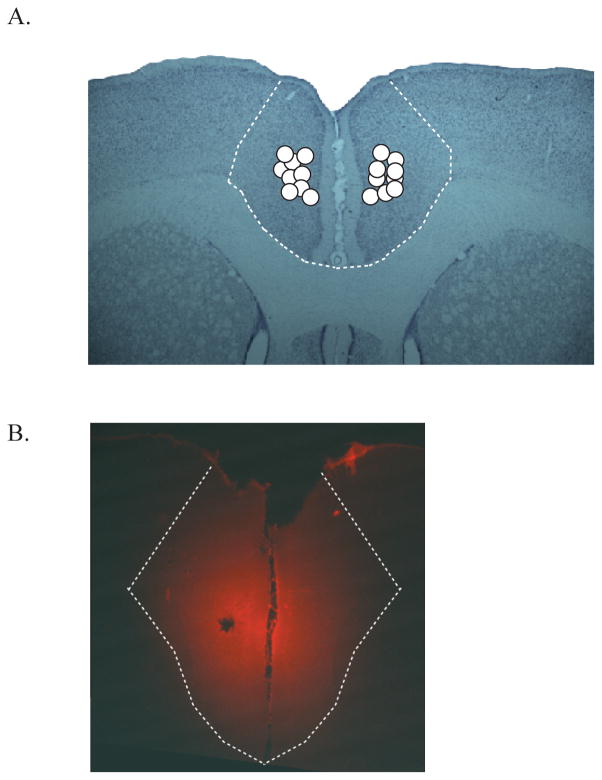
To verify the diffusion range of muscimol during the test sessions, the same amount (.25 μl per hemisphere) of fluorescent muscimol (BODIOY TMR-X Muscimol; Molecular Probes, Eugene, OR) was injected before the perfusion. The unstained sections were then examined with a red fluorescent filter. However, it is important to note that the molecular weight of fluorescent muscimol is about six times greater than the muscimol used in this study (Allen et al., 2008), so the spread of fluorescent muscimol may be underestimated in Figure 2B.
Figure 2.
Verification of cannula tips and estimation of muscimol diffusion in the ACC. A. Position of cannula tips from all rats were marked in anterior cingulate cortex. B. Diffusion of fluorescent muscimol within the ACC. White dotted line represents the anatomical boundary of the ACC.
Touch recording
An infrared touch screen (1024 × 769 pixel resolution) in front of the LCD monitor recorded touches during the session. The initial breakage of the infrared field was regarded as a touch. Custom-written Matlab code calculated touch coordinates as well as touch time to determine both accuracy and latency within trials. Rats were free to touch any location on the touch screen, but only touches within the specific areas of interest counted for transitions between trial phases. For example, touches within the trial initiation stimulus (a white star on the black background; 150 × 150 px2 at the center) were only recorded during the trial-start phase. Touches outside the trial initiation stimulus did not trigger the cuing stimuli to be presented. Until the rat touched one of the cuing stimuli five times, touches within the two separate cuing stimuli areas (150 × 150 px2 for each) were recorded during the cuing-phase. In the same way, both left and right choice button areas (100 × 135 px2 for each side) were only valid to be touched during the choice-phase. No other touches were recorded. Also, there was no time limitation in each phase (Fig. 1D).
Behavioral measurement
Touch coordinates within trials were analyzed depending on what the task-relevant cue stimulus was (i.e., vertical vs. horizontal) as well as where it appeared (i.e., left vs. right). Five touches during the cuing-phase were individually recorded as either task-relevant or task-irrelevant depending on where the task-relevant cue stimulus appeared. For example, if the vertical orientation stimulus appeared on the right side of the screen, then all of the touches on the left cue stimulus were task-irrelevant whereas the touches on the right cue stimulus were task-relevant. The ratio between task-relevant and task-irrelevant touches in each trial was calculated. For the control data, the left-to-right touch ratio was calculated because there were no irrelevant stimuli. Touch ratio values were examined across the sessions or correlated with accuracy. A single touch during the choice-phase was used to determine accuracy within trials. For example, the trials in which the choice touches fell on the left choice button area were regarded as correct if the vertical orientation stimulus was given; the other trials where the touches fell on the right choice button area were scored as incorrect (vice versa for horizontal orientation stimulus). Latency was defined as the time interval between the fifth touch on the cue stimulus and the first touch on the choice button.
Touch maps
Touches during the cuing-phase were plotted on heat maps to visualize touch frequency in different conditions. Specifically, the 150 × 150 pixel2 area of the cue stimulus was binned into 16 × 16 grids. Then touches within each grid were calculated and smoothed using the adaptive binning method (Skaggs, McNaughton, Gothard, & Markus, 1993).
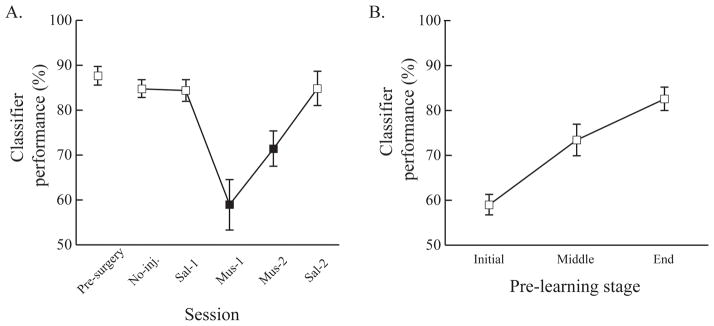
Linear discriminant analysis (LDA) classifier
To examine whether touches during the cuing-phase anticipated the upcoming location of the rewarded response, an LDA classifier was used. Touch ratio values were used to train an LDA classifier to predict whether the rat would choose left or right choice buttons across trials. LDA classifier performance was evaluated across both injection schedules to assess the ACC inactivation effects (Fig. 7A) and the pre-learning sessions (Fig. 7B). Leave-one-out-cross-validation was used in evaluating LDA classifier performance to avoid overestimation (Stone, 1974).
Figure 7.
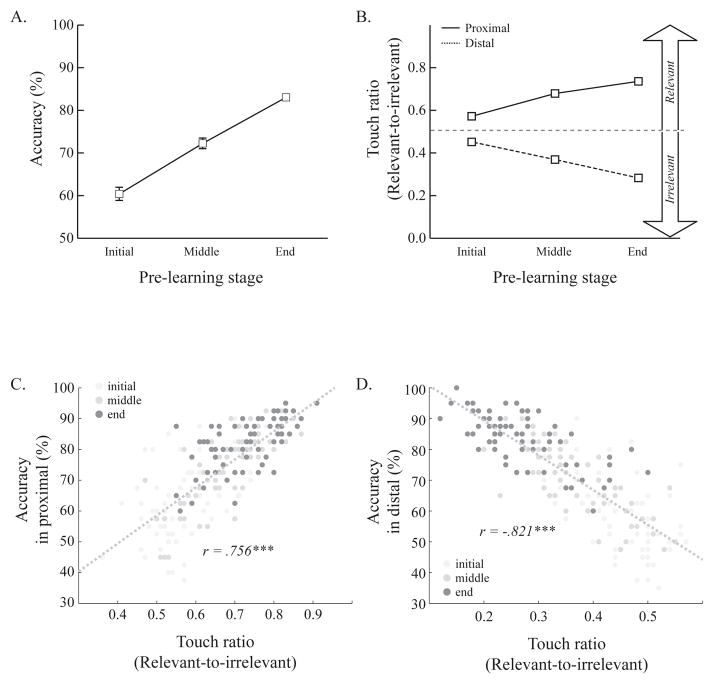
Development of anticipatory touches of the visual stimulus near the correct response location during learning. Numbers of training sessions for each rat were divided into three pre-learning stages to examine how touch ratio values changed with training. A. Accuracy significantly increased across the pre-learning stages. B. Touch ratio values from both proximal and distal conditions diverged across the pre-learning stage. Rats touched both visual stimuli equally at the beginning of the training, but they increasingly touched the visual stimulus near the correct response location as learning progressed. The dotted line indicates chance level. C & D. More touches of the stimulus near the correct response location were significantly correlated with accuracy in both proximal and distal conditions. Darker colors represent later stages of pre-learning. Error bars show the standard error of the mean.
Statistical analyses
Accuracy, latency, and touches were the dependent variables in the current study. All of these measures were mainly analyzed with repeated-measures ANOVA with injection/training session as factors. Post-hoc pairwise comparisons using Tukey-HSD (Honest Significant Difference) test (alpha = 0.05) were conducted afterwards if there were significant main effects or interactions. To observe the relationship between touch ratio and task accuracy, Pearson’s bivariate correlation analysis was used. Both touch ratio and accuracy from each task-relevant cue location condition were tested separately.
Results
Rats were trained on the visual cuing task with task-irrelevant stimuli until they reached a pre-surgery criterion (equal to or greater than 80% correct to both orientation stimuli, 2 d in a row). The median number of days to reach criterion was 29 d (range = 11 – 35 d). Once rats fully recovered from surgery, they were retrained until again reaching criterion (median = 4 d; range: 3 – 12 d). After they re-passed the criterion (no-inj), ACC infusions started; rats were tested with either PBS or muscimol (2.0 mmol) infusions. PBS was infused for the first 2 sessions (sal-1), muscimol was infused for the next 4 sessions (mus-1 and mus-2), and PBS was infused again for the last 2 sessions (sal-2). All test sessions began 35 min after the infusion. After each infusion session, a 1-day break was given to minimize residual drug effects. Throughout the analyses, 2 days of testing sessions were binned into 1 block to increase statistical power, except for the correlational analyses.
Cannula placements
Figure 2 is a schematic illustration indicating the placement of cannula tips into the target area. Specifically, the cannula tip locations were found between 2.0 – 2.3 mm anterior to bregma, 0.2 – 1.0 mm lateral to midline, and 0.8 – 3.1 mm ventral to the skull surface. No distinction was made between cingulate cortex area 1 and 2. We verified that all infusion cannula tips were placed within ACC. In addition, the fluorescent photomicrograph shows that the muscimol infusions were well localized within the ACC.
ACC inactivation impairs performance on the visual cuing task with task-irrelevant stimuli
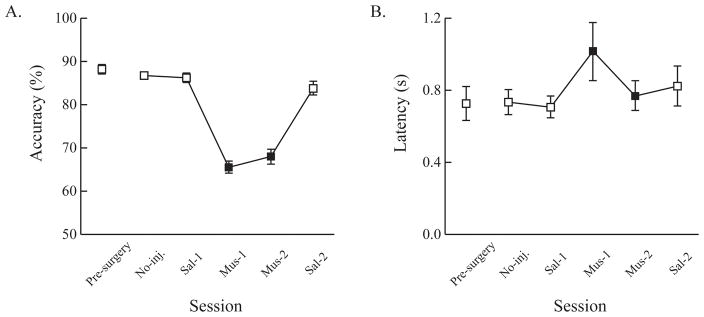
Overall accuracy for the visual cuing task with task-irrelevant stimuli across sessions with different drug infusions was examined (Fig. 3A). Rats showed more than 85% correct responding in the visual cuing task before and after surgery. The PBS infusion did not impair choice accuracy. However, when muscimol was infused into ACC, choice accuracy was impaired; this impairment persisted as long as the ACC was inactivated. When PBS was infused again, the performance level recovered to the level when PBS was first infused. These observations were confirmed by an ANOVA with session as a factor, which yielded a highly significant main effect of session (F(5, 40) = 61.47, p < 0.001). Post-hoc pairwise comparisons showed that choice accuracy was significantly lower during the muscimol sessions compared to the no infusion or PBS sessions (all ps < 0.05). Choice accuracy did not differ between inactivation sessions (p > 0.05). Comparisons among pre-surgery and saline sessions were not significantly different either (all ps > 0.05).
Figure 3.
Changes in behavioral measures across drug infusion schedules. Once rats passed the post-surgery criterion, both overall and conditional accuracy and latency were measured and plotted across the infusion schedules. Rats were tested when either no drug infusion was made (No-inj.) or PBS (Sal-1 and Sal-2) or muscimol were infused (Mus-1 and Mus-2) into the ACC. Filled squares indicate when the ACC was inactivated by muscimol whereas open squares indicate when the ACC was not inactivated. Accuracy was significantly impaired when muscimol was infused into the ACC (A). Latency measures were similar across sessions (B). Error bars show the standard error of the mean.
Unlike choice accuracy, latency was similar across all sessions. An ANOVA with session as the factor showed that the main effect of session was not significant (F(5, 40) = 1.84, p = 0.13). Overall latency was slightly higher during muscimol infusion sessions, but it did not significantly differ from the control sessions (all ps > 0.05) (Fig. 3B). The comparisons within neither muscimol nor control sessions were statistically significant (all ps > 0.05).
ACC plays a role in anticipatory touches toward the correct response location
Rats were trained to touch the visual stimuli five times to make the choice buttons appear on the sides of the screen. The coordinates of touches to the visual stimuli were recorded across trials. Every session’s touches were plotted and the frequencies were color-mapped (red for high and blue for low) in terms of the correct response side (where the correct choice button would next appear) and the local position of the task-relevant stimulus. Touch-maps during control and muscimol sessions from a single rat are presented in Figure 4.
In control sessions, when PBS was infused into the ACC, the rat touched the task-relevant stimulus more often than the task-irrelevant stimulus in the proximal condition in which the task-relevant cue stimulus appeared closer to the reward location. However, the rat touched the task-irrelevant stimulus more often than the task-relevant stimulus in the distal condition. In other words, the rat primarily touched the visual stimulus near the side where the correct choice button was next to appear, regardless of task-relevancy (Fig. 4A). The anticipatory response patterns toward the correct choice button were weakened when ACC was inactivated by muscimol. The touches were more evenly distributed across the two stimuli, regardless of the local position of the stimulus, although the rat still seemed to touch the visual stimulus near the correct side somewhat more than the other side (Fig. 4B).
The touch ratio between relevant and irrelevant visual stimuli was calculated in both the proximal and distal conditions to quantify the above observations. The touch ratio was computed by dividing the number of touches on the task-relevant stimulus by the total numbers of touches on both visual stimuli. Thus, the touch ratio will be 1.0 when the rat touches the task-relevant stimulus alone and the number will be 0.0 when rat touches the task-irrelevant stimulus alone. The number will be 0.5 when the rat touches both relevant and irrelevant stimuli equally often. After the touch ratio was obtained, the values were scatter-plotted with choice accuracy in both the proximal and distal conditions to examine the relationship between touch ratio and choice accuracy.
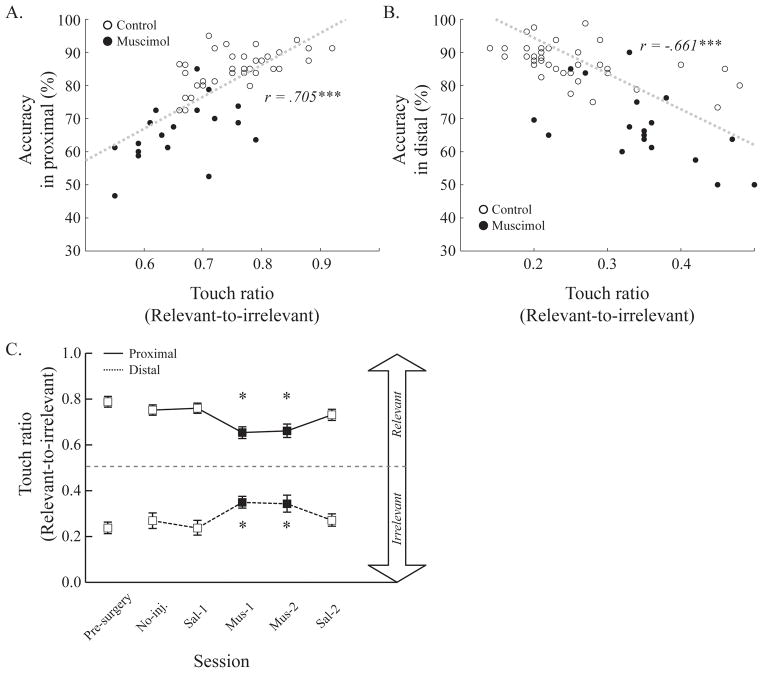
The results showed a statistically significant positive correlation in the proximal condition (r = 0.71, p < 0.001; Fig. 5A) and a statistically significant negative correlation in the distal condition (r = −0.66, p < 0.001; Fig. 5B). So, the higher the accuracy, the more often the rats touched the stimulus in the location proximal to the correct choice button, regardless of the stimulus being relevant or irrelevant—that is why the above correlation was positive when the relevant stimulus is in the proximal location, but negative when it was in the distal location. Correlations between touch ratio and accuracy were significant in both the proximal and distal conditions in both the control and the muscimol groups (ps < 0.05). These results indicate that the more likely the rats were to touch the visual stimulus near the location of the upcoming correct choice button, the more accurately they performed the visual cuing task.
Figure 5.
Disruption of anticipatory touches of the visual stimulus near the correct response location during ACC inactivation. Touches during the cuing-phase were quantified and then plotted with task accuracy in both proximal (A) and distal conditions (B). A touch ratio near 0.5 indicates that rats touched both visual stimuli almost equally often. Touch ratios closer to 1.0 in the proximal condition and touch ratios closer to 0.0 in the distal condition indicate that rats touched the visual stimulus near the correct response location. Touch ratio values were significantly changed across the infusion schedules. In both proximal and distal conditions, biased touches toward the correct side during control sessions (open circles) were decreased during muscimol sessions (filled circle) (C). Dotted line indicates chance level. Error bars show the standard error of the mean.
The changes in touch ratio were further examined as a function of the testing session (Fig. 5C). In the proximal condition, the touch ratio was high before the muscimol infusion, but it fell during the muscimol infusion period. Then, the touch ratio recovered to the previous level in the last PBS session. In the distal condition, the touch ratio remained low before the muscimol infusion. The touch ratio increased during ACC inactivation and it then dropped to the previous level in the last PBS session. A two-way ANOVA with task-relevant stimulus position and session as factors supported the above observations. There was a significant main effect of task-relevant stimulus position (F(1, 40) = 185.04, p < 0.001) as well as a significant interaction with session (F(5, 40) = 12.39, p < 0.001). The main effect of session was not significant (F(5, 40) = 0.38, p = 0.86). In addition, post-hoc pairwise comparisons showed that touch ratio values were significantly decreased or increased during muscimol infusion periods compared to control sessions in both the proximal and distal conditions (ps < 0.05), respectively. The magnitude of the change in the touch-ratio during muscimol infusions was not significantly different in the two task-relevant stimulus positions (paired-sample t-test, t(8) = 0.70, p = 0.50). However, the touch ratio values during muscimol sessions were still significantly different from chance (one-sample t-test, all ps < 0.001), indicating that the rats touched the stimulus that was proximal to the correct response more often than the distal stimulus even during ACC inactivation. These results suggest that touches were more concentrated on the stimulus near the correct response regardless of the position of the task-relevant stimulus when the ACC functioned normally. When the ACC was inactivated by muscimol, although touches to the proximal stimulus were still more frequent than to the distal stimulus, touches were more evenly divided, with many more occurring to the opposite side from the correct response. Thus, anticipation of the upcoming correct response location was impaired by ACC inactivation.
Conflict monitoring and actor mechanisms of the ACC
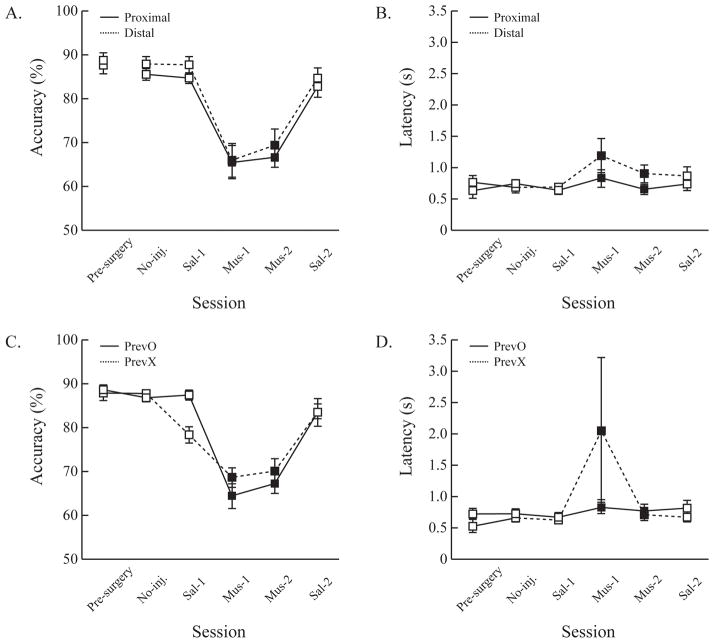
The results so far showed that the ACC is critical in the current visual cuing task. When the ACC was inactivated by muscimol, accuracy was impaired due to the failure to ignore task-irrelevant stimuli. However, one could argue that the impairment might come from different sources. As Botvinick and colleagues have proposed (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick et al., 1999; Carter et al., 1998, 1999), the spatial conflict between the task-relevant cue location and the correct reward location would not be resolved when the ACC was inactivated. To test this alternative explanation, both the accuracy and the latency across the injection schedules were analyzed by task-relevant cue location condition (i.e., proximal vs. distal). If conflict monitoring/resolution is the main function of the ACC, then greater impairment should be found in the distal condition than in the proximal condition because the task-relevant cue stimuli appear on the opposite side of the screen from the correct reward locations, so there would be greater spatial conflict to be resolved in the distal condition. The results showed, however, that no significant difference was found between proximal and distal conditions. From pre-surgery criterion performance to the last PBS infusion session, accuracy in the proximal condition was comparable to the distal condition. During muscimol inactivation, accuracy was equally impaired. A repeated-measures ANOVA confirmed these observations by showing that neither the main effect of task-relevant cue location (F(1, 8) = 0.23, p = 0.64) nor the interaction (F(5, 40) = 0.26, p = 0.93) was statistically significant. Only the main effect of session was significant due to the impairment during the muscimol infusions (F(5, 40) = 60.64, p < 0.001). Post-hoc pairwise comparisons showed that accuracy during muscimol sessions was comparable (p > 0.05), but was significantly lower than during the control sessions (ps < 0.05)(Fig. 6A). Latency was additionally analyzed to test the congruency effects. The same analysis on the latency data showed that there was a significant main effect of the task-relevant cue location (F(1, 8) = 27.31, p < 0.001), but neither a main effect of session (F(5, 40) = 1.75, p = 0.15) nor interaction (F(5,408) = 1.328, p = 0.27) was statistically significant. Although the results indicate that the rats responded faster in the proximal condition than in the distal condition in general, faster latency in the proximal condition is not directly related to the ACC inactivation (Fig. 6B).
Figure 6.
Behavioral changes across drug infusion schedules by the task-relevant cue location (proximal vs. distal)(A & B) and by whether the previous trial was correct (PrevO vs. PrevX)(C & D). Across the injection schedules, the performance impairment during the muscimol sessions (filled squares) was comparable between proximal and distal as well as between PrevO and PrevX (solid and dotted lines, respectively). Error bars show the standard error of the mean.
Another alternative explanation of the impairment is that the ACC failed to regulate decisions by the feedback from the task (Holroyd & McClure, 2015; Walton et al., 2007). To test the actor mechanism of the ACC, accuracy was analyzed by the correctness of the previous trials. If the main function of the ACC is to adaptively regulate behavior by feedback, then performance when the previous trial was correct (PrevO) should differ from when the previous trial was incorrect (PrevX). The results showed that accuracy was similar throughout the injection schedules, except during the first saline infusion; more errors were found in the PrevX condition than in the PrevO. A repeated-measures ANOVA with session and previous trial correctness as factors showed that there was a significant main effect of session (F(5, 40) = 42.441, p < 0.001). However, both the main effect of previous trial correctness (F(1, 8) = 0.11, p = 0.75) and the interaction (F(5, 40) = 2.16, p = 0.08) were not statistically significant. Again, the main effect of session was mainly due to the impairment during the muscimol injections; accuracy in Mus-1 was comparable to Mus-2 (p > 0.05), but significantly lower than all control sessions (ps < 0.05). Among the control sessions, none of the comparisons was statistically significant (ps > 0.05) (Fig. 6C). The same analysis on the latency data showed that the main effects of session (F(5, 40) = 0.51, p = 0.76), the previous trial correctness (F(1, 8) = 0.91, p = 0.37), and the interaction (F(5, 40) = 0.80, p = 0.56) were not statistically significant. The results indicate that the impairment during the muscimol sessions was not due to a failure for rat to regulate their decisions by response-produced feedback (Fig. 6D).
Development of anticipatory touches toward the correct response location during learning
Pre-surgery data were analyzed to assess how anticipatory touches toward the correct response location were acquired as the rats learned the visual cuing task. Because the total number of pre-learning sessions differed across the rats, the pre-learning sessions were trisected per each rat and then the data were collapsed by three different stages of the pre-learning (initial, middle, and end) (Fig. 7A). There were quantitative differences in accuracy across the pre-learning stages. Initially, accuracy was slightly above 60%, it increased to 72% in the middle stage, and it then reached 83% by the end stage. A one-way ANOVA with pre-learning stage as a factor confirmed these observations; there was a significant main effect of pre-learning stage (F(2, 16) = 49.78, p < 0.001). Post-hoc pairwise comparisons also showed that the change across pre-learning stages were significant (ps < 0.05). These results indicate that the division of pre-learning sessions reliably reflected acquisition of the visual cuing task.
Across the pre-learning stages, touch ratio values were calculated in both the proximal and distal conditions (Fig. 7B). In both conditions, touch ratio values were near 0.5 in the initial stage of learning, indicating that touches were fairly evenly divided between the stimuli. However, as learning progressed, the values diverged; touch ratio values increased in the proximal condition and decreased in the distal condition. As the rats learned the visual cuing task, they touched the stimulus that appeared near the correct response more in both conditions. A two-way ANOVA with pre-learning stage and local positions of the stimulus as factors confirmed this interpretation. There was a significant main effect of the local positions of the stimulus (F(1, 16) = 57.86, p < 0.001) and an interaction (F(2, 16) = 60.79, p < 0.001). There were no significant effects of pre-learning stage (F(2, 16) = 0.73, p = 0.50). These results indicate that rats touched both stimuli equivalently in the initial part of training, but they developed anticipatory responding toward the correct response location as they learned where they needed to touch to obtain reward.
In addition, the relationship between task accuracy and touch ratio was examined in both the proximal and distal conditions (Fig. 7C and 7D). As expected from the divergence of the touch ratios across the pre-learning stages, touch ratio values were significantly correlated with choice accuracy. Specifically, high touch ratios tended to occur with high task accuracy and low touch ratios tended to occur with low accuracy in the proximal condition (r = 0.76, p < 0.001). The opposite pattern in the distal condition was also statistically significant (r = −0.82, p < 0.001). Significant correlations were also found in both the proximal and distal conditions for each pre-learning stage (ps < 0.001). Again, the results indicate that touches of the stimulus become directed toward the correct response as learning progressed.
Touches on the visual stimuli were reliable predictors of choices when the ACC functioned normally
We tested how well the rats’ left or right choice responses could be predicted by touches of the visual stimuli and whether that prediction would be affected by ACC inactivation using LDA classifier. Touch ratio values were used to train an LDA classifier to predict whether the rat would choose left or right response buttons. Classifier performance was evaluated by leave-one-out-cross-validation to avoid overestimation (Stone, 1974).
The analysis on the post-surgery test data showed that the classifier performed well in predicting the rats’ choices on trials when the ACC functioned normally. Classifier performance was higher than 80% across the sessions. However, when the ACC was inactivated by muscimol, classifier performance was impaired. A one-way ANOVA with session as a factor showed a highly significant main effect of session (F(5, 40) = 12.59, p < 0.001). Post-hoc pairwise comparisons showed that classifier performance was significantly impaired during muscimol sessions compared to control sessions (all ps < 0.05). Furthermore, classifier performance was similar among the control sessions and among the muscimol sessions (Fig. 8A). The same LDA was conducted for pre-surgery training data. The results showed that classifier performance increased linearly across the pre-learning stages and this linear increase was statistically significant (F(2, 16) = 39.63, p < 0.001; all ps from post-hoc pairwise comparisons < 0.05) (Fig. 8B). These results indicate that the rats’ choices could be predicted by the patterns of touches on the visual stimuli as long as the ACC was functionally intact.
No ACC engagement when irrelevant stimuli are absent
The above results suggest that the ACC is crucial for performing the visual cuing task with task-irrelevant stimuli and for performing anticipatory touches toward the correct response location. To confirm that the above impairments were due to the rats’ failure to dampen the effects of distracting irrelevant stimuli during the ACC inactivation and not to a general impairment in performing a visual discrimination, a control task was employed. To have the same response requirement in terms of spatial location in the control task, two identical task-relevant stimuli were presented side-by-side; the task-irrelevant stimuli were absent throughout the sessions (Fig. 9A). After the rats finished all of the procedures related to the visual cuing task, eight out of the nine rats were tested in the control experiment. The same infusion schedules and protocols were used for the control sessions.
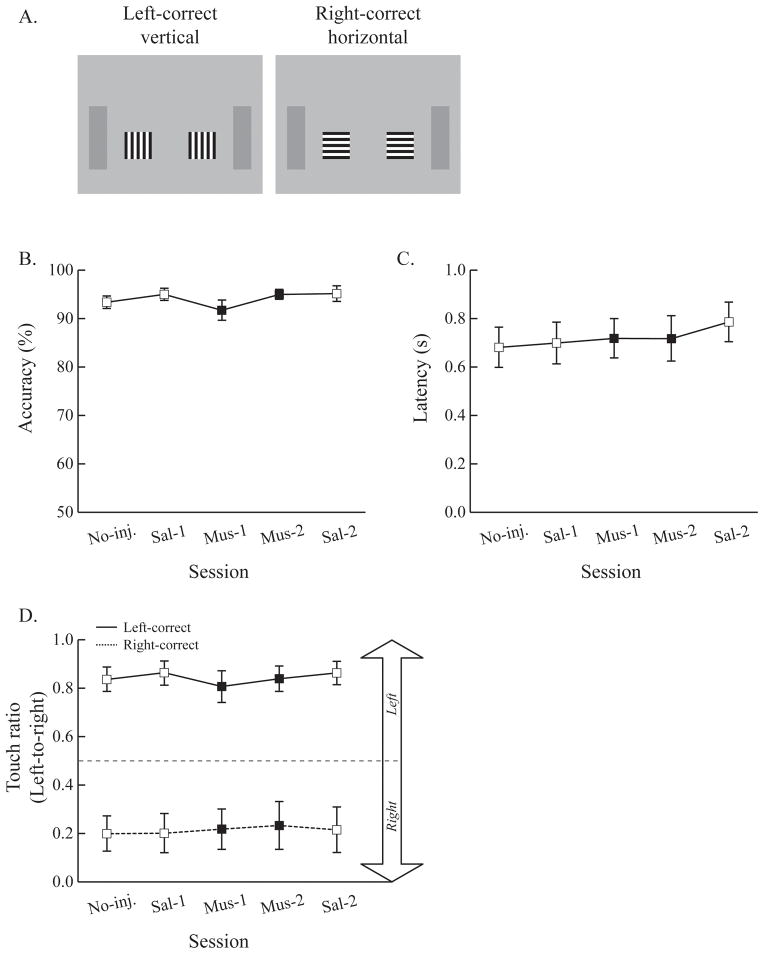
Figure 9.
No effects of ACC inactivation on task performance or anticipatory touches toward the correct response location were observed when the task-irrelevant stimuli were deleted. A. Design of the control experiment where only task-relevant stimuli were presented. B & C. Both accuracy and latency were compared across sessions. Muscimol infusion did not impair task performance. D. Touch ratio values in both left- and right-correct conditions were calculated and plotted. Again, the touch ratio was 0.5 when rats touched both left and right stimuli equally often. Without distraction from the task-irrelevant stimuli, touches of the visual stimulus near the correct choice were not affected by ACC inactivation. Error bars show the standard error of the mean.
Rats performed well on the control task from the beginning of testing. Accuracy was high during both PBS and muscimol sessions (Fig. 9B). A one-way ANOVA with session as a factor showed that the main effect of session was not statistically significant (F(4, 28) = 2.32, p = 0.08). The results were the same for latency; latency was constant across the sessions (F(4, 28) = 1.67, p = 0.19) (Fig. 9C). These results indicate that ACC inactivation did not impair visual discrimination performance when the task-irrelevant stimulus was not presented.
The touch ratio was also calculated for the control experiment data (Fig. 9D). Touch ratio values were calculated in terms of left- and right-correct conditions. Again, the touch ratio value would be 0.5 if rats touched the left and right stimuli equally often, but the value would be 1.0 or 0.0 if rats only touched one of the stimuli. Touch ratio values were near or above 0.8 in the left-correct condition throughout the testing sessions. Even during the muscimol infusion periods, the values did not decrease to less than 0.8. No changes in the values were observed in right-correct condition either; the touch ratio values were always near or slightly above 0.2 and they did not fluctuate across sessions. A two-way ANOVA with correct condition and session as factors confirmed these observations. There was a main effect of correct condition (F(1, 7) = 79.46, p < 0.001), but the main effect of session (F(4, 28) = 0.931, p = 0.46) and the interaction (F(4, 28) = 1.92, p = 0.14) of the two factors were not statistically significant. The control results suggest that the inactivation of the ACC did not impair either task performance or anticipatory touches when there was no task-irrelevant stimulus.
Discussion
We used an infrared touchscreen apparatus to train rats in a visual cuing task in which relevant and irrelevant stimuli were presented simultaneously on each trial. After the rats had learned the task, we gave them test sessions with muscimol inactivation of the ACC or infusions of the vehicle. In addition to choice accuracy and latency, we quantified rats’ touches on the visual stimuli before the choice buttons were made available in order to measure anticipatory responses toward the upcoming correct response location. Muscimol inactivation of the ACC produced an impairment in visual cuing task choice accuracy when irrelevant stimuli were presented, but not when irrelevant stimuli were omitted. Muscimol inactivation also impaired rats’ anticipatory touches toward the correct response location as well as the LDA classifier’s performance.
ACC is necessary for reducing the effects of visual distractors
The visual cuing task with task-irrelevant stimuli required the rats to attend to the task-relevant stimuli and not be distracted by the task-irrelevant stimuli. When the ACC functioned normally, rats successfully attended to the task-relevant stimuli and correctly selected the reward-associated choice response. They did not seem to ignore the task-irrelevant stimuli, however, because they touched whichever stimulus—relevant or irrelevant—was nearest to the correct response location (see discussion below). Inactivation of the ACC impaired choice accuracy. The impairment could not be due to perceptual, motivational, or locomotor deficits or to forgetting the general task procedures, because muscimol inactivation did not impair the rats’ performance during the control experiment in which the task-irrelevant stimuli were absent. These findings suggest that inactivation of the ACC impaired performance on the visual cuing task by causing a deficit in reducing distraction produced by the task-irrelevant stimuli.
In an earlier study by Bussey et al. (1997), the rats were given lesions of the ACC and then trained on visual discrimination tasks using a touchscreen apparatus. The ACC lesions impaired acquisition of the 8-pair concurrent visual discrimination (simultaneous presentation) task, but not a single-pair simultaneous discrimination or a visuospatial conditional discrimination task. Because the single-pair discrimination task in the Bussey et al. study was similar to the current visual cuing task with distractors, one might argue that the current results conflict with the earlier findings. Although rats were required to pay attention to the target over the distractor in order to obtain reward in both tasks, there were several differences between the studies. First, Bussey et al. measured deficits in learning whereas the current study assessed discrimination based on an already acquired memory. Second, different from Bussey et al., the goal-location for the reward was spatially dissociated from the target in the current study. In the earlier study, the rats were required to directly touch the target stimulus. In the current study, by contrast, the rats were required to touch the correct choice button to obtain the reward after they had discriminated the target from the distractor. Third, there were two values of the target and distractor stimuli in the current study (e.g., horizontal and vertical for the orientation stimuli and light and dark for the brightness stimuli); however, there was only a single stimulus for the target and the distractor in Bussey et al. and both the target and the distractor were always fixed. In the current visual cuing task, either horizontal or vertical stimuli served as targets whereas either light or dark stimuli served as distractors. Combinations of those stimuli resulted in four possible pairs in a discrimination session, so the current task would be more similar to an 8-pair concurrent discrimination than a single-pair discrimination task. Therefore, the current findings are generally consistent with the previous study by Bussey et al. in emphasizing the role of ACC in visual discrimination with distractors.
Notably, the current study specifically proposed that the role of ACC was to reduce distraction by task-irrelevant stimuli rather than to associate stimulus and reward. Bussey et al. concluded that the deficit in acquisition of the 8-pair concurrent discriminations with ACC lesions was caused by a deficit in learning stimulus-reward associations. They suggested that the lower mnemonic load and interference in the single-pair discrimination did not prevent the ACC from forming the stimulus-reward association. However, the impairment in performance of the current visual cuing task with distractors was not caused by a deficit in stimulus-reward associations because the stimulus-reward associations in the control task (i.e., the association between two task-relevant stimuli and reward) were well learned before muscimol testing began and there was no accuracy impairment during ACC inactivation. Therefore, we conclude that the deficit in the cuing task performance with ACC inactivation was due to rats’ inability to reduce distraction by task-irrelevant stimuli.
Touches on the task-relevant and task-irrelevant stimuli reflect prospective memory for the correct response location rather than encoding of the stimuli themselves
Touches of the task-relevant and task-irrelevant stimuli were highly concentrated on the stimulus that appeared near the correct response location, before the choice buttons were actually presented. During muscimol inactivation, touches were more evenly divided between the relevant and irrelevant visual stimuli. This finding suggests that touches on the task-relevant and task-irrelevant stimuli reflected prospective memory for the upcoming correct response location (Wasserman, 1985) rather than the meaning or encoding of the visual stimuli themselves. If the touches were more directly related to the meaning of the visual stimuli, then they would have been focused on and tracked the task-relevant stimuli in both the proximal and distal conditions. It is possible that the touches reflected the encoding of the visual stimuli rather than the prospectively correct response location earlier during learning (Fig. 6B) and the touch ratio did not predict reward location well at this stage (Fig. 7B). However, once the rats acquired the visual cuing task, touches on the task-relevant and task-irrelevant stimuli diverged toward both extremes (0.0 and 1.0). ACC inactivation, however, affected anticipatory responding for the upcoming correct choice location as well as choice accuracy during the choice period (i.e., after the choice buttons were presented).
Inactivation of the ACC did not produce a complete deficit in anticipatory responding in the visual cuing task and had no effect at all in the control task, which suggests that the ACC is not the sole area determining anticipatory touches toward the correct response location in the current task. Other neural structures in the rostral prefrontal cortex, parietal cortex, basal ganglia, or hippocampus might be important in behavioral guidance toward the correct response location (Chafee & Goldman-Rakic, 2000; Kennedy & Shapiro, 2009; MacDonald, Cohen, Stenger, & Carter, 2000; Tanji & Hoshi, 2001; Wise & Murray, 2000; Young & Shapiro, 2011). Those areas could therefore partially compensate for the loss of ACC function during inactivation sessions in the visual cuing task and could mediate anticipatory behavior in the control task.
The absence of spatial congruency effects
In the current visual cuing task, task-relevant stimuli appeared either proximal or distal to the correct response locations. Spatial congruency was manipulated by placing the task-relevant stimulus on either the same (proximal) or the opposite side (distal) as the correct response. Therefore, it could be assumed that rats were required to attend to the task-relevant stimulus as well as resolve the conflict between where-to-attend and where-to-respond as in Simon-effect studies with humans or monkeys (Botvinick et al., 1999; Carter et al., 1998, 1999; Riehle, Kornblum, & Requin, 1997; Simon & Wolf, 1963). However, the results showed that choice accuracy as well as choice latency was similarly impaired in both the proximal and distal conditions with no congruency effects being observed.
It is possible that the spatial incompatibility in the distal condition would not be sufficient to produce conflict for several reasons. First, there was no response accuracy or speed requirement in the current study. Rats were allowed to spend as much time as they wished in completing trials. There would therefore be enough time for rats to resolve the spatial conflict before they executed the choice response. Second, the peripheral vision of rodents has a wider viewpoint than humans or nonhuman primates, so the stimulus-response incompatibility might not be large enough to trigger a spatial conflict. The distance between the task-relevant stimulus and the correct location for reward in the distal condition may be farther than the current setup to trigger spatial conflict in peripheral vision. Third, the ratio between proximal (congruent) and distal (incongruent) trials was equal throughout the session. Previous studies suggest that the ratio between congruent and incongruent trials is important to have congruency or conflict effects because fewer incongruent trials prevents the incongruent condition from becoming familiarized or informative which leads to conflict adaptation (Botvinick et al., 2001; Lindsay & Jacoby, 1994; Lowe & Mitterer, 1982; Mordkoff, 2012). Fourth, the task was fully learned before the test. The expectedness or information of the distal condition might be too saturated to induce the congruency effects. Fifth, different from earlier findings, the rat’s viewpoint was not fixed in the current study. Rats were allowed to move freely within the chamber, so depending on their locations in the chamber, spatial congruency may or may not exist. For example, when the rats lingered on the left side of the chamber in the right-correct distal condition, where-to-attend and where-to-respond shared the same direction in space, there would be no spatial conflict between the task-relevant stimulus and the response location for the reward. In an earlier study (Courtière, Hardouin, Burle, Vidal, & Hasbroucq, 2007), it was experimentally demonstrated that rats also display a Simon-effect as in human and nonhuman primate studies. Rats were placed in the chamber in which a speaker was attached to each of the left and right walls. Rats were supposed to press the lever at the center of the chamber until the sound came from one of the speakers. Depending on the sound pitch (low vs. high); the rats were required to go to either the congruent or incongruent side of the chamber to obtain reward. Both mean reaction time and accuracy deteriorated in the incongruent condition. Instead of using visual stimuli, the study used the auditory stimuli from two different-ends to control the spatial components of where-to-attend and where-to-respond. More rigorous controls of the spatial components might be necessary in the current paradigm (e.g., fixate the body in the center of the screen during the cuing phase) to produce a Simon-effect.
Conclusions
Rats were trained in a visual cuing task with task-irrelevant stimuli and the role of the ACC was tested with reversible inactivation. Task accuracy was significantly impaired when the ACC was inactivated. Analysis of touch locations before the choice responses were made showed that anticipatory touches toward the correct response location were also dependent upon the ACC. The results further showed that the touch locations before the presentation of the choice buttons reliably predicted the final response location. In the control task, in which the task-irrelevant stimuli were removed, ACC inactivation did not cause an impairment in accuracy or in anticipatory touches toward the correct response location. Overall, the results indicate that the rodent ACC is crucial in selecting the target stimulus and reducing distraction from irrelevant stimuli as proposed in earlier human and nonhuman primate studies (Weissman et al., 2005, 2002). The increased distraction by task-irrelevant stimuli during ACC inactivation resulted in impaired prospective coding of the correct response location. These findings suggest that rodent models may be suitable for visual cognitive neuroscience research.
Acknowledgments
This work was supported by National Institutes of Health grant MH080005 (J. H. F.) and Stuit Fellowship funds (J. H. F.). We thank Prof. Richard Hazeltine for helpful discussion, Ka H. Ng and Adam B. Steinmetz for technical assistance, and Jonathan Schacherer for shaping and training the rats.
References
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. Journal of Neuroscience Methods. 2008;171(1):30–38. doi: 10.1016/j.jneumeth.2008.01.033. http://doi.org/10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. http://doi.org/10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Brooks DI, Ng KH, Buss EW, Marshall AT, Freeman JH, Wasserman EA. Categorization of photographic images by rats using shape-based image dimensions. Journal of Experimental Psychology. Animal Behavior Processes. 2013;39(1):85–92. doi: 10.1037/a0030404. http://doi.org/10.1037/a0030404. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. http://doi.org/10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behavioral Neuroscience. 1997;111(5):920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Everitt BJ. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behavioral Neuroscience. 2002;116(4):553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Marbini HD, Toner AJ, Bussey TJ, Robbins TW, Everitt BJ. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behavioral Neuroscience. 2003;117(3):566–587. doi: 10.1037/0735-7044.117.3.566. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences. 1999;10(1):49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior Cingulate Cortex, Error Detection, and the Online Monitoring of Performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. http://doi.org/10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. Journal of Neurophysiology. 2000;83(3):1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. http://doi.org/10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Courtière A, Hardouin J, Burle B, Vidal F, Hasbroucq T. Simon effect in the rat: a new model for studying the neural bases of the dual-route architecture. Behavioural Brain Research. 2007;179(1):69–75. doi: 10.1016/j.bbr.2007.01.012. http://doi.org/10.1016/j.bbr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews. 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. http://doi.org/10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. http://doi.org/10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Tinsley CJ, Gaffan D, Duncan J. Selective representation of task-relevant objects and locations in the monkey prefrontal cortex. The European Journal of Neuroscience. 2006;23(8):2197–2214. doi: 10.1111/j.1460-9568.2006.04736.x. http://doi.org/10.1111/j.1460-9568.2006.04736.x. [DOI] [PubMed] [Google Scholar]
- Gibson BM, Wasserman EA, Frei L, Miller K. Recent advances in operant conditioning technology: a versatile and affordable computerized touchscreen system. Behavior Research Methods, Instruments, & Computers: A Journal of the Psychonomic Society, Inc. 2004;36(2):355–362. doi: 10.3758/bf03195582. [DOI] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Ventral Lateral Geniculate Input to the Medial Pons Is Necessary for Visual Eyeblink Conditioning in Rats. Learning & Memory. 2010;17(2):80–85. doi: 10.1101/lm.1572710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, McClure SM. Hierarchical control over effortful behavior by rodent medial frontal cortex: A computational model. Psychological Review. 2015;122(1):54–83. doi: 10.1037/a0038339. http://doi.org/10.1037/a0038339. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. http://doi.org/10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proceedings of the National Academy of Sciences. 2009 doi: 10.1073/pnas.0903259106. http://doi.org/10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed]
- Lindsay DS, Jacoby LL. Stroop process dissociations: the relationship between facilitation and interference. Journal of Experimental Psychology. Human Perception and Performance. 1994;20(2):219–234. doi: 10.1037//0096-1523.20.2.219. [DOI] [PubMed] [Google Scholar]
- Lowe DG, Mitterer JO. Selective and divided Attention in a Stroop task. Canadian Journal of Psychology. 1982;36(4):684–700. doi: 10.1037/h0080661. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science (New York, NY) 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. http://doi.org/10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mordkoff JT. Observation: Three reasons to avoid having half of the trials be congruent in a four-alternative forced-choice experiment on sequential modulation. Psychonomic Bulletin & Review. 2012;19(4):750–757. doi: 10.3758/s13423-012-0257-3. http://doi.org/10.3758/s13423-012-0257-3. [DOI] [PubMed] [Google Scholar]
- Ng CW, Noblejas MI, Rodefer JS, Smith CB, Poremba A. Double Dissociation of Attentional Resources: Prefrontal Versus Cingulate Cortices. The Journal of Neuroscience. 2007;27(45):12123–12131. doi: 10.1523/JNEUROSCI.2745-07.2007. http://doi.org/10.1523/JNEUROSCI.2745-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr JM, Weissman DH. Anterior cingulate cortex makes 2 contributions to minimizing distraction. Cerebral Cortex (New York, NY: 1991) 2009;19(3):703–711. doi: 10.1093/cercor/bhn119. http://doi.org/10.1093/cercor/bhn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotaropoulos TI, Deco G, Kapoor V, Logothetis NK. Neuronal discharges and gamma oscillations explicitly reflect visual consciousness in the lateral prefrontal cortex. Neuron. 2012;74(5):924–935. doi: 10.1016/j.neuron.2012.04.013. http://doi.org/10.1016/j.neuron.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Ramos-Quiroga JA, Picado M, Mallorqui-Bague N, Vilarroya O, Palomar G, Richarte V, Casas M. The neuroanatomy of attention deficit hyperactivity disorder in adults: structural and functional neuroimaging findings. Revista de neurologia. 2013;56(Suppl 1):S93–S106. [PubMed] [Google Scholar]
- Riehle A, Kornblum S, Requin J. Neuronal correlates of sensorimotor association in stimulus-response compatibility. Journal of Experimental Psychology. Human Perception and Performance. 1997;23(6):1708–1726. doi: 10.1037//0096-1523.23.6.1708. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale. 2009;192(3):489–497. doi: 10.1007/s00221-008-1642-z. http://doi.org/10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Buckley MJ, Behrens TEJ, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Current Opinion in Neurobiology. 2007;17(2):220–227. doi: 10.1016/j.conb.2007.03.001. http://doi.org/10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Moore T. Selective attention from voluntary control of neurons in prefrontal cortex. Science (New York, NY) 2011;332(6037):1568–1571. doi: 10.1126/science.1199892. http://doi.org/10.1126/science.1199892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Ardid S, Kaping D, Westendorff S, Everling S, Womelsdorf T. Anterior Cingulate Cortex Cells Identify Process-Specific Errors of Attentional Control Prior to Transient Prefrontal-Cingulate Inhibition. Cerebral Cortex. 2014:bhu028. doi: 10.1093/cercor/bhu028. http://doi.org/10.1093/cercor/bhu028. [DOI] [PMC free article] [PubMed]
- Simon JR, Wolf JD. Choice reaction times as a function of angular stimulus-response correspondence and age. Ergonomics. 1963;6:99–105. [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. An information-theoretic approach to deciphering the hippocampal code. In: Hanson SJ, Cowan JD, Giles CL, editors. Advances in neural information processing systems. San Mateo, CA: Morgan Kaufman; 1993. pp. 1030–1037. [Google Scholar]
- Stone M. Cross-Validatory Choice and Assessment of Statistical Predictions. Journal of the Royal Statistical Society. Series B (Methodological) 1974;36(2):111–147. [Google Scholar]
- Suzuki M, Gottlieb J. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nature Neuroscience. 2013;16(1):98–104. doi: 10.1038/nn.3282. http://doi.org/10.1038/nn.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. Behavioral planning in the prefrontal cortex. Current Opinion in Neurobiology. 2001;11(2):164–170. doi: 10.1016/s0959-4388(00)00192-6. [DOI] [PubMed] [Google Scholar]
- Treisman AM. Strategies and models of selective attention. Psychological Review. 1969;76(3):282–299. doi: 10.1037/h0027242. [DOI] [PubMed] [Google Scholar]
- Walton ME, Croxson PL, Behrens TEJ, Kennerley SW, Rushworth MFS. Adaptive decision making and value in the anterior cingulate cortex. NeuroImage. 2007;36(Suppl 2):T142–154. doi: 10.1016/j.neuroimage.2007.03.029. http://doi.org/10.1016/j.neuroimage.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman EA. Animal memory. Hillsdale, NJ: Erlbaum; 1985. Prospection and retrospection as processes of animal short-term memory; pp. 53–75. [Google Scholar]
- Wasserman EA, Castro L, Freeman JH. Same-different categorization in rats. Learning & Memory (Cold Spring Harbor, NY) 2012;19(4):142–145. doi: 10.1101/lm.025437.111. http://doi.org/10.1101/lm.025437.111. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. NeuroImage. 2003;19(4):1361–1368. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cerebral Cortex (New York, NY: 1991) 2005;15(2):229–237. doi: 10.1093/cercor/bhh125. http://doi.org/10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Mangun GR, Woldorff MG. A role for top-down attentional orienting during interference between global and local aspects of hierarchical stimuli. NeuroImage. 2002;17(3):1266–1276. doi: 10.1006/nimg.2002.1284. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA. Arbitrary associations between antecedents and actions. Trends in Neurosciences. 2000;23(6):271–276. doi: 10.1016/s0166-2236(00)01570-8. [DOI] [PubMed] [Google Scholar]
- Young JJ, Shapiro ML. Dynamic Coding of Goal-Directed Paths by Orbital Prefrontal Cortex. The Journal of Neuroscience. 2011;31(16):5989–6000. doi: 10.1523/JNEUROSCI.5436-10.2011. http://doi.org/10.1523/JNEUROSCI.5436-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]