Abstract
Previous studies have reported that blood viscosity is significantly increased following exercise. However, these studies measured both pre- and post-exercise blood viscosity at 37 °C even though core and blood temperatures would be expected to have increased during the exercise. Consequently, the effect of exercise-induced hyperthermia on mitigating change in blood viscosity may have been missed. The purpose of this study was to isolate the effects of exercise-induced hemoconcentration and hyperthermia, as well as determine their combined effects, on blood viscosity. Nine subjects performed 2 h of moderate-intensity exercise in the heat (37 °C, 40% rH), which resulted in significant increases from pre-exercise values for rectal temperature (37.11 ± 0.35 °C to 38.76 ± 0.13 °C), hemoconcentration (hematocrit = 43.6 ± 3.6% to 45.6 ± 3.5%), and dehydration (Δbody weight = −3.6 ± 0.7%). Exercise-induced hemoconcentration significantly (P < 0.05) increased blood viscosity by 9% (3.97 to 4.30 cP at 300 s−1) while exercise-induced hyperthermia significantly decreased blood viscosity by 7% (3.97 to 3.70 cP at 300 s−1). However, when both factors were considered together, there was no overall change in blood viscosity (3.97 to 4.03 cP at 300 s−1). The effects of exercise-induced hemoconcentration, increased plasma viscosity, and increased red blood cell aggregation, all of which increased blood viscosity, were counterbalanced by increased RBC deformability (e.g., RBC membrane shear elastic modulus and elongation index) caused by the hyperthermia. Thus, blood viscosity remained unchanged following prolonged moderate-intensity exercise in the heat.
Key Terms: red blood cell aggregation, hyperthermia, plasma viscosity, red blood cell deformability
INTRODUCTION
During the last three decades numerous studies (Martin et al. 1985, Brun et al. 1994, Kingwell et al. 1997, Bouix et al. 1998, Hitosugi et al. 2004, Tripette et al. 2010, Ahmadizad et al. 2011) have reported a 5–15% increase in blood viscosity following various types of exercise. The increase in blood viscosity was usually attributed to exercise-induced hemoconcentration as blood viscosity is influenced by hematocrit (Hct) (de Simone et al. 1990, Pries et al. 1992, Chong-Martinez et al. 2003). The rise in Hct is induced by several mechanisms including fluid shifts due to increases in blood pressure, dehydration, release of sequestered red blood cells (RBCs) from the spleen, and water trapping in working skeletal muscle (Brun et al. 1998, Ahmadizad et al. 2006, Tripette et al. 2010). Other hemorheological factors, such as changes in plasma viscosity, RBC deformability, and RBC aggregation can also contribute to changes in blood viscosity during exercise (Galea and Davidson 1985, Neuhaus and Gaehtgens 1994). However, it should be noted that in an effort to isolate the effect of hemoconcentration on blood viscosity, the pre- and post-exercise blood samples in these studies were measured at identical temperatures (usually 37 °C). Such methodology discounts that prolonged exercise almost always elevates core and blood temperature several degrees above the resting temperature (Gonzalez-Alonso et al. 1999, 2015). At a constant Hct, an increase in temperature reduces blood viscosity (Rand et al. 1964, Snyder 1971, Cinar et al. 2001). Thus, past studies that measured pre- and post-exercise blood viscosity at identical, normothermic temperatures (e.g., 37 °C), did not replicate actual in vivo conditions and, therefore, likely missed the effect of exercise-induced hyperthermia on mitigating change in blood viscosity. This is important as exercise increases the energetic demand and rate of oxygen delivery and utilization (Baskurt and Meiselman 2003). Consequently, the blood rheological properties that influence oxygen transport and distribution during exercise are extremely important to better understand the mechanisms for aerobic exercise performance (Yalcin et al. 2003).
In light of the above, the purpose of the current study was to determine the effects of exercise-induced hemoconcentration and hyperthermia on blood rheology and the factors that determine blood viscosity (plasma viscosity, RBC deformability and RBC aggregability). We hypothesized that, while exercise-induced hemoconcentration would increase blood viscosity and hyperthermia would decrease blood viscosity, their combined effects would counterbalance each other such that there would be no overall change in blood viscosity during prolonged exercise in the heat.
METHODS
Subjects
Subjects for the study were nine healthy volunteers (four males, five females) all of whom had been regularly participating in physical activity. They had a mean ± SD age, weight, and maximal oxygen uptake (V̇O2max) of 28 ± 10 y, 64.3 ± 7.9 kg, and 44 ± 5 ml·kg−1·min−1, respectively. The study was approved by the San Diego State University Institutional Review Board in accordance with the Declaration of Helsinki and signed informed consent was obtained from each subject prior to participation.
At the subjects’ initial laboratory visit, V̇O2max was measured with a graded treadmill test performed to volitional exhaustion with oxygen uptake measured each min by a calibrated metabolic measurement cart (Parvo Medics, Sandy, UT). All subjects had a respiratory exchange ratio of > 1.1 at exhaustion. During their second laboratory visit, subjects performed a 2 h walking bout at approximately 35% of their V̇O2max in an environmental chamber (37 °C, 40% rH). Prior to reporting to the laboratory, subjects were encouraged to drink plenty of fluids and refrain from exercise during the previous 12 h. An initial blood sample was collected via venipuncture from the antecubital space into a vacutainer with EDTA. Dry, semi-nude body weight was measured to the nearest 0.1 kg. In addition, each subject self-inserted a flexible temperature probe 10 cm past the anal sphincter. While in a thermoneutral room (approximately 23 °C), rectal temperature was recorded to the nearest 0.01 °C. During the 2 h exercise bout, fluids were not allowed in order to provoke dehydration and hemoconcentration. Rectal temperature was continuously monitored during exercise and recovery. After completion of the exercise bout, a post-exercise blood sample was collected and dry body weight was again measured. Blood samples were refrigerated until hematological and rheological analyses were performed within 12 hours of collection (Uyuklu et al. 2009).
Hematocrit
Hct was reported as the average of duplicate readings from both pre- and post-exercise blood samples. The blood was separated with a microhematocrit centrifuge and the values determined with a hematocrit reader. Hct readings had and an error of < 1%.
Blood viscosity
Blood viscosity was measured using a computerized cone-plate (4 cm diameter and 2°-cone cell) rheometer AR-G2 (TA Instruments, New Castle, DE, USA). Blood viscosities were measured at 11 shear rates ranging from 10 and 1000 s−1. Blood viscosity at 300 s−1, which is the typical shear rate in the brachial artery during prolonged submaximal exercise (Padilla et al. 2011, Simmons et al. 2010), was calculated by linear interpolation from the 250 and 400 s−1 values. Blood viscosity was measured in triplicate for each sample first at the rectal temperature recorded for the subject at the time of the pre-exercise blood draw and again at the rectal temperature recorded at the post-exercise blood draw. Sample temperature was manipulated with a rheometer Peltier plate (cone and plate, and controlled to ± 0.1 °C). Sample evaporation was minimized by using a solvent trap in conjunction with the appropriate geometry. The solvent trap includes an evaporation blocker mechanism as the solvent trap cover creates a thermally stable vapor barrier that eliminates any solvent loss during the measurements. The solvent trap that covers the cone, plate and sample, holds deionized water in a well around the sample at the temperature of the measurement. Although water in the solvent trap well evaporates to the vapor pressure equilibrium the sample does not evaporate, as the air around it is at equilibrium with the vapor pressure of water at each temperature. Rheometer general calibration was completed with standard-viscosity liquids provided by Cannon Instrument Company (State College, PA), where the viscosity measured with the rheometer were found to be within 3% of the standard viscosity values (for shear rates between 10 and 1000 s−1). Additionally, the rheometer performs calibration of the magnetic bearings via a rotational force mapping without the spindle, and between samples the rheometer calibrates for friction and spindle inertia. The AR-G2 rheometer has a torque range of 0.01 to 200 mN·m−1. Torque measurements were performed at each shear rate during three consecutive periods of 120 s each. If torque drifted more than 10% with each period, the torque measurement for that period was discarded. The data reported are the averages of three replicate measurements.
Plasma viscosity
Plasma viscosity was measured after centrifugation of the blood (5 min at 2000 rpm), using only clean supernatant solution, with a computerized cone-plate viscometer (LVDV-2 plus, Brookfield Engineering Laboratories; Middleboro, MA) and a CP-40 spindle. Plasma viscosities were measured at 11 shear rates ranging from 10 and 1000 s−1. Plasma viscosity at 300 s−1, which is the typical shear rate in the brachial artery during prolonged submaximal exercise (Padilla et al. 2011, Simmons et al. 2010), was calculate by linear interpolation from the 250 and 400 s−1 values. The viscometer was calibrated using Brookfield viscosity standard fluids where the viscosity measured with the rheometer were found within 5% of the standard viscosity values. Plasma viscosity was measured in triplicate for each sample, first at the pre-exercise rectal temperature recorded for the subject and again at the post-exercise temperature.
Red blood cell aggregation
The degree of RBC aggregation was determined from duplicate measurements on a 20 μl blood sample with a photometric rheoscope (Myrenne Aggregometer®, Germany) (Lee et al. 2007, Elmer et al. 2011), which gives the indices of RBC aggregation ‘M’ during stasis after shearing at 600 s−1. Briefly, this device measures the changes in light transmission, which are observed when sheared RBC suspensions are abruptly brought to a full stop. The decrease in the optical signal reflects the formation of RBC aggregates. The aggregation time is the reciprocal of the initial slope (calculated between 0.5 and 2 s after the shear has stopped). The aggregation index is a measurement of the extent of erythrocyte aggregation and is the relative surface area above the curve calculated over the first 10 s. The Myrenne Aggregometer was housed in an acrylic box with temperature control, and aggregation was measured at each subject’s pre- and post-exercise temperature. The system was allowed 5 min to equilibrate to the new temperature and the temperature of the aggregometer and cover slides was measured using an IR thermometer Dewalt (DCT414S1, Baltimore, MD). Samples were pre-warmed using a heated water-bath. Pre-warmed samples were transferred to a preheated cover slide and placed in the aggregometer. All sample manipulations were completed inside the heated acrylic box. The hematocrit of post-exercise blood samples was not corrected to match the pre-exercise values to better approximate the in vivo exercise condition. The instrument computes an aggregation index proportional to the area under the light transmission curve, which reflects the degree of aggregation attained by the end of the 10 s period. The aggregometer reports two aggregation indexes depending on the rotation speed of the cone, M (at stasis) and M1 (at 3 s−1). Repeated measurements with a blood sample, with and without aggregation, showed an error of <5%.
Micropipette aspiration
Cells were suspended in phosphate buffered saline (PBS at pH = 7.4) and washed twice (3,500 rpm, 10 min). Washed cells were then resuspended and placed in a small glass chamber attached to an isothermal stage regulated to the pre- or post-exercise rectal temperature recorded when the blood sample was collected (Sensortec Inc., Clifton, NJ). Micropipettes (A.M. Systems, Everett, WA) were pulled (P-97, Sutter Instruments, San Rafael, CA) to an internal diameter of ~2 μm and an opening angle of approximately 9°. The micropipette was attached to a pneumatic micromanipulator (Narishige, Japan). Aspiration pressure was induced hydrostatically. Measurements were completed at high magnification (100X LUMPFL; Olympus) using a video system (Cohu, San Diego, CA). Subsequent aspiration length as a function of aspiration pressure was measured off-line from the recorded images. Red blood cell (RBC) membrane shear elastic modulus was calculated based on the relationship between aspiration pressure and membrane tongue length, as previously reported (Evans, 1983). Pipettes for cell aspiration was tested using 7 μm microbeads (Bangs Laboratories, Fisher, IN) with a membrane shear elastic modulus of 10 mdyn·cm−2 in a PBS solution. Only pipettes with repeatability and agreement (membrane shear elastic modulus for microbeads within less than 10% from manufacture value) were used in the study.
Elongation index
The RBC elongation index (EI) indicates the cell deformation for a given shear stress applied (Johnson, 1989; Baskurt et al., 2009). EI was measured by suspending 0.1 mL of washed packed cells in 20 mL of deformability buffer at both the subject’s pre- and post-exercise rectal temperature. Measuring RBC deformability with this methodology has been previously reported (Yalcin et al., 2014). The buffer was prepared using 50 g of dextran (Sigma-Aldrich, St. Louis, MO, 500 kDa), 34 mL of distilled water and 10 mL of OptiPrep (Nycomed Pharma, Asker, Norway) mixed into 200 mL of PBS yielding an isotonic buffer with a Newtonian viscosity of 19.6 cP (at 10 – 1000 s−1, slope not different from zero) and a pH of 7.4. Cell suspension was passed through cylindrical microcapillaries with a 20 μm internal diameter (Polymicro Technologies, Phoenix, AR). Microchannels were attached to an isothermal stage regulated to the pre- or post-exercise temperature on an inverted microscope (Olympus IMT-2). Images were acquired using a digital camera and laser (425 nm, 5 mW, Power Technology, Alexander, AR). The extent of cell deformation was determined from the diffraction image created by the laser beam. The images were processed off-line (Cell Profiler software, www.cellprofiler.org). The EI was calculated as (L1−L2)/(L1+L2) where L1 and L2 are the length and width of the diffraction pattern (Baskurt et al., 2009). A typical test starts by setting a hydrostatic pressure head connected to the microchannel and the initial priming of the microcapillaries includes vacuum connection to the waste exit. While the blood is flowing through the microchannel, the emitted laser beam traverses the diluted RBC suspension and it is diffracted by the flowing RBCs. The diffraction pattern is captured by the camera and the EI is measured as the isointensity curve in the diffraction pattern and fitted with an ellipse-shape to obtain L1 and L2. Shear stress was calculated assuming that the microcapillary is a straight, cylindrical tube with rigid walls. Thus, shear stress defined by the Poisseuille equation could be calculated as: shear stress = 32 μQ/(π· d3), where Q is the mean volumetric flow rate, μis the viscosity of the diluted RBC suspension, and d the vessel diameter. Shear stress was maintained constant via preserving hydrostatic pressure in the diluted RBC suspension reservoir. Shear stress was confirmed via measurement of flow rate into the waste chamber. Mass flow into the waste container was measured using a precision scale calibrated with the diluted RBC suspension density measured using a 10 mL Gay-Lussac pycnometer.
Measurements were performed at 50 dynes·cm−2, as at this shear stress RBC aggregation does not affect the measurement (Yip et al., 1983). System operation and algorithm optimization was confirmed used 7 μm non-deformable (microbeads silica) and 7 μm deformable microbeads (Bangs Laboratories).
Statistical analysis
Pre- and post-exercise Hct, rectal temperature, and dry body weight were compared using paired t-tests. Each rheological variable presented in Table 1 was analyzed using a two-way repeated measure ANOVA with Bonferroni pairwise comparisons. An alpha level of 0.05 was used to establish significance.
Table 1.
Effects of exercise-induced hemoconcentration and hyperthermia on various blood rheology parameters. Each was assessed before and after exercise to account for hemoconcentration caused by dehydration. In addition, each paramenter was measured and reported at each subject’s normothermic (pre-exercise measurement) and hyperthermic (post-exercise measurement) core temperature. Values are means ± SD.
| Pre-exercise | Post-exercise | |||
|---|---|---|---|---|
|
| ||||
| normothermic | hyperthermic | normothermic | hyperthermic | |
| blood viscosity (cP)* | 3.97 ± 0.25 | 3.69 ± 0.251 | 4.33 ± 0.291 | 4.03 ± 0.272,3 |
| plasma viscosity (cP)* | 1.14 ± 0.05 | 1.09 ± 0.061 | 1.27 ± 0.071 | 1.22 ± 0.081,2,3 |
| RBC aggregation (AU) | 21.29 ± 1.90 | 19.59 ± 2.111 | 25.86 ± 1.971 | 23.18 ± 1.651,2,3 |
| RBC shear modulus (m dyn·cm−2) | 5.48 ± 0.22 | 4.74 ± 0.181 | 5.69 ± 0.20 | 4.92 ± 0.171,3 |
| Elongation index (AU)** | 0.33 ± 0.01 | 0.36 ± 0.021 | 0.38 ± 0.011 | 0.41 ± 0.011,2,3 |
reported at a shear rate of 300 s−1
reported at 50 dyn·cm−2
different than pre-exercise normothermic (p < 0.05)
different than pre-exercise hyperthermic (p < 0.05)
different than post-exercise normothermic (p < 0.05)
RESULTS
Exercise for 2 h at a moderate intensity in the heat produced significant hyperthermia, hemoconcentration, and dehydration in all subjects. The mean pre-exercise rectal temperature was 37.11 ± 0.35 °C and increased post-exercise to 38.76 ± 0.13 °C (P < 0.05). Likewise, the mean pre-exercise Hct was 43.6 ± 3.6% and increased to 45.6 ± 3.5% (P < 0.05). Lastly, the exercise produced a significant 3.6% decrease in body weight from 64.3 ± 7.9 kg to 62.0 ± 7.6 kg (P < 0.05).
Blood viscosity
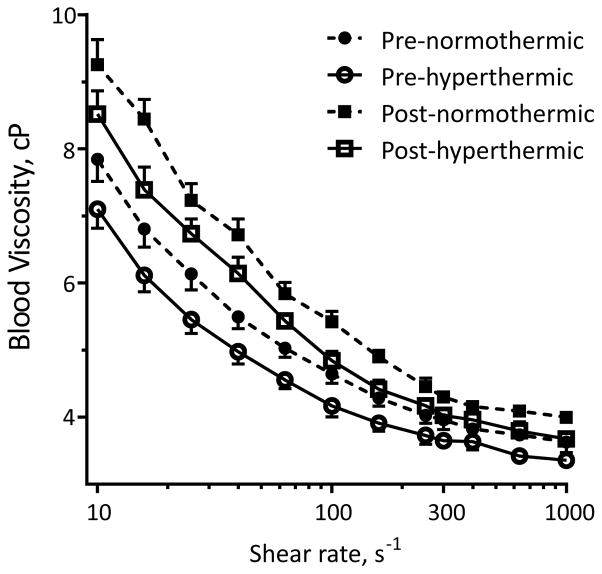
Results of the blood viscosity measurements at 11 different shear rates are shown in Figure. 1. Pre- and post-exercise blood samples were measured at each subject’s pre-exercise and post-exercise temperature. As expected, since blood is a non-Newtonian fluid, blood viscosity during all four conditions was inversely related to shear rate. In addition, both hyperthermia and hemoconcentration affected blood viscosity at all shear rates. Specifically, hemoconcentration decreased blood viscosity while hyperthermia increased blood viscosity. For example, as shown in Table 1, the mean blood viscosity at 300 s−1, which is the typical shear rate in the brachial artery during prolonged, submaximal exercise (Padilla et al. 2011, Simmons et al. 2010) measured at pre-exercise temperature was 3.97 ± 0.25 cP, and decreased to 3.69 ± 0.25 cP when measured at the corresponding post-exercise temperature (P < 0.05). Post-exercise blood viscosity (4.33 ± 0.29 cP) at pre-exercise temperature was significantly higher than the pre-exercise blood viscosity at pre-exercise temperature (P < 0.05). However, post-exercise blood viscosity at post-exercise temperature (4.03 ± 0.27 cP) was not different from the pre-exercise blood viscosity at pre-exercise temperature. Thus, the exercise-induced hemoconcentration increased blood viscosity by 9%, hyperthermia caused a 7% decrease, and the combined effects of hemoconcentration and hyperthermia had no effect on blood viscosity.
Figure 1.
Pre-and post-exercise, normothermic and hyperthermic blood viscosity (mean ± SD) at the 11 different shear rates.
Plasma viscosity
As plasma is a Newtonian fluid, only data at 300 s−1 are shown in Table 1. The pre-exercise plasma viscosity (at 300 s−1) at pre-exercise temperature was 1.14 ± 0.05 cP, and significantly decreased to 1.09 ± 0.06 cP when measured at post-exercise temperature (P < 0.05). The post-exercise plasma viscosity (at 300 s−1) at pre-exercise temperature was 1.27 ± 0.07 cP, and significantly decreased to 1.22 ± 0.08 cP when measured at post-exercise temperature. The post-exercise plasma viscosities at pre-exercise and post-exercise temperature were significantly higher than the pre-exercise plasma viscosity at pre-exercise temperature (P < 0.05). Thus, exercise-induced hemoconcentration increased plasma viscosity while hyperthermia decreased viscosity. Overall, the combined effects of hemoconcentration and hyperthermia increased plasma viscosity.
Red blood cell aggregation
Results of the RBC aggregation measurements are shown in Table 1. The pre-exercise normothermic AI was 21.29 ± 1.90 AU that decreased to 19.59 ± 2.11 AU when measured at the hyperthermic temperature (P < 0.05). The post-exercise normothermic AI (25.86 ± 0.18 AU) was higher than when measured at the hyperthermic temperature (23.18 ± 1.65 AU) (P < 0.05) and both post-exercise AI measurements were higher than the pre-exercise normothermic AI (P < 0.05). Thus, exercise-induced hemoconcentration increased the AI by 21% and hyperthermia decreased the AI by 8%. Collectively, the exercise-induced hemoconcentration and hyperthermia increased the AI by 9%.
RBC membrane shear elastic modulus
RBC membrane shear elastic modulus results are shown in Table 1. The Hct independent measurement of RBC membrane elastic modulus via micropipette aspiration indicated that exercise did not affect the red cell membrane mechanical properties. However, increased temperature equally increased the RBC membrane shear elastic modulus at both pre- and post-exercise. The pre-exercise RBC membrane shear elastic modulus increased by 14% after increasing the temperature to the post-exercise temperature (5.48 ± 0.22 to 4.74 ± 0.18 mdyn·cm−2; P < 0.05). Similarly, the post-exercise RBC membrane shear elastic modulus increased by 10% as temperature was increased from the pre- to post-exercise temperature (5.48 ± 0.22 to 4.92 ± 0.17 mdyn·cm−2; P < 0.05).
Elongation index
The Hct independent elongation index results are shown in Table 1. The pre-exercise elongation index, at 50 dyn·cm−2, was 0.33 ± 0.01 at the pre-exercise temperature and increased to 0.36 ± 0.02 at the post-exercise temperature (P < 0.05). The post-exercise elongation index was 0.38 ± 0.01 at the pre-exercise temperature and increased to 0.41 ± 0.01 at the post-exercise temperature. Both exercise and hyperthermia appear to have affected the Hct independent elongation index as the pre- and post-exercise elongation indexes differed at the pre- and post-exercise temperatures, respectively (P < 0.05). Therefore, both exercise-induced hemoconcentration and hyperthermia independently increased the elongation index by 15% and 9%, respectively, while their combined effects increased the elongation index by 32%.
DISCUSSION
The principal finding of this study was that there was no change in blood viscosity following prolonged, moderate-intensity exercise in the heat after accounting for the effect of hyperthermia. Previous studies measured pre- and post-exercise blood viscosity at the same temperatures (37 °C). As seen in Table 1, when the pre- and post-exercise blood viscosities (at 300 s−1) were measured at the same normothermic temperature (approximately 37 °C), blood viscosity increased significantly by 9%, an increase brought about by the exercise-induced hemoconcentration. This magnitude of increase is similar to that reported in other studies that measured changes in blood viscosity following prolonged, sub-maximal exercise (Vandewalle et al. 1988, Brun et al. 1994, Kingwell et al. 1997, Connes et al. 2009, Tripette et al. 2010, Ahmadizad et al. 2011). However, we also found that the effect of exercise-induced hyperthermia decreased blood viscosity by 7%. Taken together, the effects of hemoconcentration and hyperthermia counterbalanced each other in that there was no overall change in blood viscosity after prolonged, moderate-intensity exercise. To our knowledge, this is the first time such a finding has been reported in the literature. Moreover, the moderate increase in core temperature seen during exercise could be hypothesized to be beneficial as it attenuates the increase in blood viscosity brought about by hemoconcentration. This would assist capillary blood flow and reduce the chance for thrombus formation (Cinar et al. 2001, Cabrales 2007). It should be noted that the proposed counterbalancing effect of hyperthermia on blood viscosity most likely occurs predominately in the exercising limbs. The reason being is that it has been clearly shown by Gonzalez-Alonso et al. (1999, 2015) that blood temperature approximates core temperature in exercising human limbs, but not in nonexercising limbs.
Vessel size, pressure gradient, and the viscosity of blood determine blood flow in the circulatory system, although the hemodynamic importance of blood viscosity remained unstudied until recently. Blood viscosity fluctuates dramatically in response to changes in Hct and temperature. Although relatively moderate and linear increments occur with small changes in Hct or temperature, large increases in Hct produce exponential changes in viscosity. Conversely increases in temperature reduce viscosity. Except for the Hct and plasma protein concentration, temperature affects all factors that determine blood fluidity (the inverse of viscosity). RBC deformability is enhanced by increases in temperature, as indicated by the reduced RBC membrane elastic shear module and increased elongation index. Its membrane is the only solid element in the human RBC and is the source of RBC elasticity (Nash and Wyard 1981). Erythrocytes are remarkably deformable and weakly elastic (Meiselman et al. 1978, Platt et al. 1978, Waugh and Hochmuth 1987, Vertessy and Syeck 1989). Since its lipid bilayers are fluid in plane and do not appreciably resist or recover from shear deformation, they expand and shrink reversibly (elastically) in response to variations in temperature. However, plasma viscosity decreases with increasing temperature (Platt et al. 1978). Although relatively moderate and linear decreases in plasma viscosity occur with a moderate increase in temperature, these changes have a significant effect on blood viscosity since blood is a suspension of cells in plasma. Thus, at normal hematocrits, a decrease in plasma viscosity will decrease whole blood viscosity. Because of the non-Newtonian flow properties of blood, plasma becomes increasingly more or less viscous when adjacent portions move more slowly in respect to each other.
Blood is a two-phase suspension of formed elements, namely RBCs, white blood cells, and platelets, suspended in plasma. The viscosity of blood is determined primarily by four physiologic variables; 1) Hct, 2) plasma viscosity, 3) RBC aggregation, and 4) RBC deformability (Baskurt and Meidelman 2003, Brun et al. 1998, El-Sayed 1998). In the current study, each of these four determinants was measured before and after exercise and at two different temperatures matched to each subject’s core temperature recorded at the time of the blood draws. This allowed us to isolate and determine the individual effects of exercise-induced hemoconcentration and exercise-induced hyperthermia on each of the four primary determinants of blood viscosity.
Hematocrit
Moderate-intensity exercise in the heat for 2 h resulted in a 3.6% reduction in body weight that caused Hct to increase from 43.6% to 45.6%. This magnitude of exercise-induced hemoconcentration is consistent with past studies (Costill and Fink 1974, Vandewalle et al. 1988) that used prolonged exercise to induce dehydration. Similarly, others have examined the relationship between blood viscosity and Hct both in vivo (Rand et al. 1964, Cinar et al. 2001) and in vitro (Martin et al. 1985). This relationship is exponential when observed across a wide range of Hct values (10 to 90%). However, in the normal physiologic range (30 to 50%) the relationship is nearly linear and the two variables are strongly correlated (r = 0.84) (Johnson 1989, Baskurt and Meiselman 2003). Within this range, blood viscosity increases about 4% for every one unit increase in Hct (Baskurt and Meiselman 2003). Therefore, the two unit increase in Hct in our subjects would be predicted to have increased blood viscosity by 8%, which is in close agreement with the 9% increase we observed in the post-exercise sample when measure at the pre-exercise temperature (Table 1).
Plasma viscosity
A review of the literature supports that both exercise and temperature affect plasma viscosity. Most studies report that plasma viscosity increased between 5 and 15% following exercise, which is similar to our observation (Connes et al. 2004, Connes et al. 2013). The post-exercise plasma viscosity (at 300 s−1) at pre-exercise temperature increased by 12% from the pre-exercise plasma viscosity at pre-exercise temperature. However, post-exercise plasma viscosity at post-exercise temperature was reduced by 5% compared to pre-exercise plasma viscosity at pre-exercise temperature. Since core temperature increased by 1.8 °C, there was a 3% decrease in plasma viscosity (at 300 s−1) per degree Celsius increase in temperature. This observation agrees closely with the findings of Harkness (1971) who reported a 2 to 3% decrease per degree Celsius change. When sedentary or endurance-trained subjects performed short-duration maximal or submaximal exercise, plasma viscosity increased by up to 5–15% at the end of exercise when both samples were measured at the pre-exercise temperature (Brun et al. 1994, Connes et al. 2004). Erroneously, this increase has been used to explain other blood rheological changes, including the reported increase of blood viscosity. An increased plasma viscosity has been attributed to the rise in plasma protein content, such as albumin, fibrinogen, and globulins, and, at least in part, to water loss.
RBC aggregation
The literature has been equivocal regarding the effect of exercise on RBC aggregation. Previous studies have reported no change (Gurcan et al. 1998), a decrease (Connes et al. 2009), or an increase (Kayatekin et al. 2010, Simmonds et al. 2013) in RBC aggregation during or following exercise. The reason for such a discrepancy is not yet understood; however, the explanation may involve methodological differences in: 1) subject fitness level (sedentary vs. endurance trained), 2) exercise intensity and duration, 3) measurement technique to determine RBC aggregation, and 4) whether aggregation indices were measured on native or standardized Hct (Connes et al. 2013). A clearer picture emerges though when reviewing studies that used a design similar to the current study. Studies that incorporated prolonged exercise (>20 min) with increased hemoconcentration and in which RBC aggregation was measured at the native and not a standardized Hct reported significant increases in aggregation. For example, Simmonds et al. (2013) exercised subjects for 24 min in which there was a three unit increase in Hct and they observed that several indices of RBC aggregation increased by up to 58%. Likewise, after subjects performed 30 min of moderate intensity exercise that caused an in increased hemoconcentration, RBC aggregation times had decreased significantly indicating that RBC aggregation had increased during the exercise (Bruix et al. 1998). Our results support these reports that prolonged, submaximal exercise resulting in increased hemoconcentration will increase RBC aggregation. As seen in Table 1, the post-exercise normothermic AI was higher than the pre-exercise normothermic AI. Furthermore, we also observed that increased temperature decreased RBC aggregation that, to our understanding, has not been previously reported in the literature. The exact mechanism by which exercise increases RBC aggregation is uncertain, although at least two causes seem plausible. First, prolonged submaximal exercise that produces significant hemoconcentration most likely also increases plasma fibrinogen concentration (Martin et al. 1985). Since fibrinogen is necessary for RBC aggregation (Simmonds et al 2013), a hemoconcentration-induced increase in plasma fibrinogen concentration would initiate the increase in RBC aggregation seen following prolonged exercise. Second, an increased cell-to-cell contact time that resulted from exercise-induced increases in Hct may also be responsible for increasing RBC aggregation. Support for this hypothesis comes from Simmonds et al. (2013) who measured RBC aggregation in post-exercise blood samples at both native and standardized (40%) Hct. They observed that RBC aggregation indices were increased only in the native, hemoconcentrated blood samples. When samples were diluted to a standardize Hct of 40%, the increase in RBC aggregation was obscured and did not change statistically. This suggests that exercise-induced hemoconcentration, as was produced in the current study, is necessary to increase RBC aggregation. Such a hypothesis may help to explain the divergent results in the literature concerning the effect of exercise on RBC aggregation. This is an area needing further investigation.
RBC deformability
One of the ways we studied RBC deformability was the assessment of RBC membrane shear elastic modulus. Several studies (Waugh and Evans 1979, Sung and Chien 1992, Connes et al. 2009) have shown that increases in temperature enhanced RBC deformability. For example, Waugh and Evans (1979) reported that RBC membrane elastic modulus, as measured by the micropipette aspiration technique, was significantly decreased by approximately 20% when temperature was increased from 23 °C to 41 °C. More recently, Park et al. (2008) observed that the RBC elastic modulus, when measured at 37 °C, decreased from 6.2 μN·m−1 to 4.9 μN·m−1 when measured again at 41 °C. This was a 5% reduction per degree Celsius increase in temperature and agrees favorably with our data in Table 1. Our subjects had a 1.65 °C increase in core temperature that resulted in a reduction of 7% per degree Celsius in RBC membrane elastic modulus. The effects of acute exercise on RBC deformability have been examined in several studies, with most reporting a decrease (Platt et al. 1978, Galea and Davidson 1985, Brun et al. 1994, Gurcan et al. 1998, Yalcin et al. 2000). This decrease may help to prevent an increase in blood viscosity during exercise.
The exact mechanism by which increases in temperature alter RBC deformability is not completely understood. In an attempt to address this issue, Park et al. (2008, 2010) used diffraction phase microscopy to measure instantaneous nanometer level fluctuations in RBC membrane thickness at both 37 °C and 41 °C. At 41 °C, they found a 53% increase in membrane fluctuations than at 37 °C. To explain this change, they postulated that membrane fluctuations result from structural changes in the phospholipid membrane and spectrin network as a consequence of being heated. Specifically, an increased temperature may result in more spontaneous dissociations of the spectrin network where it junctions with the phospholipid bilayer. This reduced tension within the cytoskeleton ultimately decreases membrane stiffness and improves RBC deformability.
Another assessment of RBC deformability commonly used is the elongation index (EI) measured by laser diffraction ektacytometry, which is generally considered the gold standard (Vent-Schmidt et al. 2015). The EI measures the ability of the entire RBC to adopt a new configuration when subjected to mechanical forces, and a larger EI indicates greater RBC deformability. However, there is no consensus about how exercise affects EI, as studies have reported a decrease (Brun et al. 1998) or an increase (Wahl et al. 2012, Vent-Schmidt et al. 2015) in EI during exercise. The explanation for the differing effects of exercise on EI may be related to the hemoglobin-oxygen saturation attained during exercise as well as the fitness level of subjects (Connes et al. 2004, 2009). Studies in which subjects performed maximal exercise that caused exercise-induced hypoxemia (EIH) with more than a 4% decrease in blood oxygen saturation observed either no change or a decrease in EI (Brun et al. 1998, Connes et al. 2004). Conversely, studies that used lower intensity, submaximal exercise that did not cause EIH, or a significant rise in blood lactate concentration, reported an increase in EI (Connes et al 2004, 2009). For example, Connes et al. (2009) found that EI increased 7–8% during light-to-moderate intensity exercise, which were supported by the findings in the current study. Furthermore, our findings have extended the seminal work of Connes et al. (2009) in which we observed that exercise and temperature independently increase EI. Table 1 shows that prolonged, submaximal exercise increased EI by 15%, while the exercise-induced hyperthermia increased EI by 9%. The effects of exercise and increased temperature appear to be additive as EI after exercise increased 24% from the pre-exercise normothermic blood. The increase in EI is in agreement with the findings of Gurcan et al. (1998) who reported a 22% increase in RBC deformability following prolonged moderate-intensity exercise. More recently, Suhr et al. (2012) investigated the mechanism responsible for the exercise-induced increase in RBC deformability. Subjects ran for 1 h on a treadmill and both EI and RBC nitric oxide synthase (NOS) were significantly increased. Their data provided strong evidence that exercise-induced shear stress activates RBC-NOS via the PI3/Akt kinase pathway, which would increase NO production inside RBCs and promote greater RBC deformability (i.e., increased EI). This was confirmed by the demonstration that pharmacological inhibition of the PI3/Akt pathway with wortmannin led to simultaneous decreases in RBC-NOS activation, NO production, and RBC deformability (Suhr et al. 2012). Enhanced RBC deformability may increase tissue perfusion and oxygenation during exercise (Parthasarathi et al. 1999, Hambrecht et al. 2000, Connes et al. 2009).
It is important to note that the two indices of RBC deformability measured in the current study, EI and the RBC membrane shear elastic modulus, are both independent of exercise-induced changes in Hct, plasma viscosity, and RBC aggregation. Hyperthermia brought about changes in both EI and the RBC membrane shear elastic modulus that increased RBC deformability, which is the major factor counterbalancing the exercise-induced increases in Hct, plasma viscosity, and RBC aggregation.
Lastly, most studies when measuring the effects of exercise on blood viscosity reported the results at a given shear rate. However, as seen in Fig 1. blood is a shear-thinning fluid and its viscosity is inversely related to shear rate. It is well known that shear rate increases during exercise (Padilla et al. 2011, Simmons et al. 2011). Thus, it has been reported by Connes et al. (2013) that during exercise hemoconcentration increases blood viscosity, while the increase in shear rate decreases blood viscosity which counterbalances each other out. Coupling this fact, with the results of the current study, a more complete picture starts to immerge. Thus, it appears that during exercise, hemoconcentration increases blood viscosity, while increases in shear rate and temperature both decrease blood viscosity, so that a net reduction in blood viscosity occurs. For example, in the current study using the data presented in Fig 1. assuming a resting shear rate of 50 s−1 (Padilla et al. 2011, Simmons et al. 2011) the mean pre-normothermic blood viscosity was 5.31 ± 0.73 cP. Again using Fig. 1 during prolonged submaximal exercise assuming the shear rate increased to 300 s−1 (Padilla et al. 2011, Simmons et al. 2011) the mean post-hyperthermia blood viscosity would significantly decrease to 3.69 ± 0.60 cP. Thus, the combined effects of increased shear rate coupled with exercise-induced hyperthermia, could theoretically decrease blood viscosity by 31%, in spite of the exercise-induced hemoconcentration.
According to the Poiseuille law, blood viscosity determines vascular resistance. From an exercise physiology perspective, increased blood viscosity has been suspected to negatively impact on performance. Indeed, significant correlations have been reported between resting viscosity and indices of physical fitness such as endurance time (Brun et al. 1998). However, our experimental results demonstrated that blood viscosity remains unaltered during exercise, in fact, vascular resistance can decrease and tissue perfusion increases, because as exercise increases blood flow, it also increases wall shear stress and stimulate nitric oxide (NO) production by endothelial cells to create a vasodilatory compensation. Connes et al. (2013) recently studied the relationships between the changes in blood viscosity, vascular resistance, NO production and vascular hindrance in healthy sportsmen performing submaximal cycling exercise. They observed a positive correlation between exercise and the increase in NO production. Further studies are needed to address understand the responses of the cardiovascular system (macro and microcirculation) in parallel to blood rheology during exercise. Others have reported destruction of erythrocytes during exercise, which can reduced Hct post-exercise, lead to anemia (e.g. sports anemia) (Chong-Martenez et al. 2003, Church et al. 2002 ), and release hemoglobin out of the RBCs scavenging NO and producing vasoconstriction. Smith et al. (2012) previously suggested that exercise-induced oxidative stress increases intravascular hemolysis. The decrement in hemolysis after enhancement of the antioxidant defense may indicate the contribution of oxidative stress to intravascular hemolysis in exercise.
Limitations
Technical and logistic constrains prevented the immediate post-collection analysis of hemorheological measurements. Uyukle et al. (2009) reported at 4°C, RBC aggregation was stable up to 12 hours. In the present study all samples were stored for less than 12 h, and the time between pre- and post-exercise sample analysis was minutes apart. However, RBC aggregation was measured at uncorrected hematocrit for the post-exercise samples, which could influence the higher aggregation measured in the post-exercise samples compared to pre-exercise.
In conclusion, we demonstrated that when hyperthermia was accounted for by measuring the post-exercise blood sample at the actual in vivo core temperature and not at a set temperature, as past studies have done, there is no change in blood viscosity during prolonged moderate-intensity exercise in the heat. The exercise-induced hemoconcentration, increased plasma viscosity, and increased RBC aggregation, all of which increased blood viscosity, were counterbalanced by increased RBC deformability (e.g., RBC membrane shear elastic modulus and elongation index) caused by the hyperthermia. A potential advantage of the hyperthermic effects on blood viscosity is that it may improve blood flow at a time in which the body is highly metabolically active.
New Findings.
Exercise-induced hemoconcentration, increased plasma viscosity, and increased blood aggregation, all of which increased blood viscosity, were counterbalanced by increased RBC deformability (e.g., RBC membrane shear elastic modulus and elongation index) caused by the hyperthermia. Thus, blood viscosity remained unchanged following prolonged moderate-intensity exercise in the heat.
Acknowledgments
This work was supported by NIH grants from the Heart Lung and Blood Institute, P01-HL110900, R01-HL52684, and R56-HL123015.
References
- Ahmadizad S, El-Sayed MS, MacLaren DP. Effects of water intake on the responses of haemorheological variables to resistance exercise. Clin Hemorheol Microcirc. 2006;35:317–327. [PubMed] [Google Scholar]
- Ahmadizad S, Moradi A, Nikookheslat S, Ebrahimi H, Rahbaran A, Connes P. Effects of age on hemorheological responses to acute endurance exercise. Clin Hemorheol Microcirc. 2011;49:165–174. doi: 10.3233/CH-2011-1466. [DOI] [PubMed] [Google Scholar]
- Baskurt OK, Hardeman MR, Uyuklu M, Ulker P, Cengiz M, Nemeth N, Shin S, Alexy T, Meiselman HJ. Parameterization of red blood cell elongation index--shear stress curves obtained by ektacytometry. Scan J Clin Lab Invest. 2009;69:777–788. doi: 10.3109/00365510903266069. [DOI] [PubMed] [Google Scholar]
- Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thrombos Hemost. 2003;29:435–450. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- Bouix D, Peyreigne C, Raynaud E, Monnier JF, Micallef JP, Brun JF. Relationships among body composition, hemorheology and exercise performance in rugbymen. Clin Hemorheol Microcirc. 1998;19:245–254. [PubMed] [Google Scholar]
- Brun JF, Khaled S, Raynaud E, Bouix D, Micallef JP, Orsetti A. The triphasic effects of exercise on blood rheology: which relevance to physiology and pathophysiology? Clin Hemorheol Microcirc. 1998;19:89–104. [PubMed] [Google Scholar]
- Brun JF, Micallef JP, Orsetti A. Hemorheologic effects of light prolonged exercise. Clin Hemorheol. 1994;14:807–818. [Google Scholar]
- Cabrales P. Effects of erythrocyte flexibility on microvascular perfusion and oxygenation during acute anemia. Am J Physiol. 2007;293:H1206–1215. doi: 10.1152/ajpheart.00109.2007. [DOI] [PubMed] [Google Scholar]
- Chong-Martinez B, Buchanan TA, Wenby RB, Meiselman HJ. Decreased red blood cell aggregation subsequent to improved glycaemic control in Type 2 diabetes mellitus. Diabet Med. 2003;20:301–306. doi: 10.1046/j.1464-5491.2003.00926.x. [DOI] [PubMed] [Google Scholar]
- Church TS, Lavie CJ, Milani RV, Kirby GS. Improvements in blood rheology after cardiac rehabilitation and exercise training in patients with coronary heart disease. Am Heart J. 2002;143:349–355. doi: 10.1067/mhj.2002.119758. [DOI] [PubMed] [Google Scholar]
- Cinar Y, Senyol AM, Duman K. Blood viscosity and blood pressure: role of temperature and hyperglycemia. Am J Hypertens. 2001;14:433–438. doi: 10.1016/s0895-7061(00)01260-7. [DOI] [PubMed] [Google Scholar]
- Connes P, Bouix D, Durand F, Kippelen P, Mercier J, Prefaut C, Brun JF, Caillaud C. Is hemoglobin desaturation related to blood viscosity in athletes during exercise? Int J Sports Med. 2004;25:569–574. doi: 10.1055/s-2004-821118. [DOI] [PubMed] [Google Scholar]
- Connes P, Simmonds MJ, Brun JF, Baskurt OK. Exercise hemorheology: classical data, recent findings and unresolved issues. Clin Hemorheol Microcirc. 2013;53:187–199. doi: 10.3233/CH-2012-1643. [DOI] [PubMed] [Google Scholar]
- Connes P, Tripette J, Mukisi-Mukaza M, Baskurt OK, Toth K, Meiselman HJ, Hue O, Antoine-Jonville S. Relationships between hemodynamic, hemorheological and metabolic responses during exercise. Biorheology. 2009;46:133–143. doi: 10.3233/BIR-2009-0529. [DOI] [PubMed] [Google Scholar]
- Costill DL, Fink WJ. Plasma volume changes following exercise and thermal dehydration. J Appl Physiol. 1974;37:521–525. doi: 10.1152/jappl.1974.37.4.521. [DOI] [PubMed] [Google Scholar]
- de Simone G, Devereux RB, Chien S, Alderman MH, Atlas SA, Laragh JH. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation. 1990;81:107–117. doi: 10.1161/01.cir.81.1.107. [DOI] [PubMed] [Google Scholar]
- El-Sayed MS. Effects of exercise and training on blood rheology. Sports Med. 1998;26:281–292. doi: 10.2165/00007256-199826050-00001. [DOI] [PubMed] [Google Scholar]
- Elmer J, Cabrales P, Wang Q, Zhang N, Palmer AF. Synthesis and biophysical properties of polymerized human serum albumin. Biotech Progress. 2011;27:290–296. doi: 10.1002/btpr.531. [DOI] [PubMed] [Google Scholar]
- Evans EA. Bending elastic modulus of red blood cell membrane derived from buckling instability in micropipet aspiration tests. Biophys J. 1983;43:27–30. doi: 10.1016/S0006-3495(83)84319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea G, Davidson RJ. Hemorrheology of marathon running. Int J Sports Med. 1985;6:136–138. doi: 10.1055/s-2008-1025826. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Calbet JA, Nielsen B. Metabolic and thermodynamic responses to dehydration-induced reductions in muscle bllod flow in exercising humans. J Physiol. 1999;520:577–589. doi: 10.1111/j.1469-7793.1999.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Calbet JAL, Boushel R, Helge JW, Sondergaard H, Munch-Andersen T, van Hall G, Mortensen SP, Secher NH. Blood temperature and perfusion to exercising and non-exercicing human limbs. Ex Physiol. 2015;45:1–15. doi: 10.1113/EP085383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurcan N, Erbas D, Ergen E, Bilgehan A, Dundar S, Aricioglu A, Dikmenoglu N. Changes in blood haemorheological parameters after submaximal exercise in trained and untrained subjects. Physiol Res. 1998;47:23–27. [PubMed] [Google Scholar]
- Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA. 2000;283:3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- Harkness J. The viscosity of human blood plasma; its measurement in health and disease. Biorheology. 1971;8:171–193. doi: 10.3233/bir-1971-83-408. [DOI] [PubMed] [Google Scholar]
- Hitosugi M, Kawato H, Nagai T, Ogawa Y, Niwa M, Iida N, Yufu T, Tokudome S. Changes in blood viscosity with heavy and light exercise. Med Sci Law. 2004;44:197–200. doi: 10.1258/rsmmsl.44.3.197. [DOI] [PubMed] [Google Scholar]
- Johnson RM. Ektacytometry of red blood cells. Methods Enzymol. 1989;173:35–54. doi: 10.1016/s0076-6879(89)73004-4. [DOI] [PubMed] [Google Scholar]
- Kayatekin BM, Ozcaldiran B, Aksu I, Topcu A, Ustuntas AEOA, Bediz CS. Effects of swimming on erythrocyte rheological properties. Biol Sport. 2010;27:99–103. [Google Scholar]
- Kingwell BA, Berry KL, Cameron JD, Jennings GL, Dart AM. Arterial compliance increases after moderate-intensity cycling. Am J Physiol. 1997;273:H2186–2191. doi: 10.1152/ajpheart.1997.273.5.H2186. [DOI] [PubMed] [Google Scholar]
- Lee BK, Alexy T, Wenby RB, Meiselman HJ. Red blood cell aggregation quantitated via Myrenne aggregometer and yield shear stress. Biorheology. 2007;44:29–35. [PubMed] [Google Scholar]
- Martin DG, Ferguson EW, Wigutoff S, Gawne T, Schoomaker EB. Blood viscosity responses to maximal exercise in endurance-trained and sedentary female subjects. J Appl Physiol. 1985;59:348–353. doi: 10.1152/jappl.1985.59.2.348. [DOI] [PubMed] [Google Scholar]
- Meiselman HJ, Evans EA, Hochmuth RM. Membrane mechanical properties of ATP-depleted human erythrocytes. Blood. 1978;52:499–504. [PubMed] [Google Scholar]
- Nash GB, Wyard SJ. Erythrocyte membrane elasticity during in vivo ageing. Biochim Biophys Acta. 1981;643:269–275. doi: 10.1016/0005-2736(81)90072-9. [DOI] [PubMed] [Google Scholar]
- Neuhaus D, Gaehtgens P. Haemorrheology and long term exercise. Sports Med. 1994;18:10–21. doi: 10.2165/00007256-199418010-00003. [DOI] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Bracjial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Ex Physiol. 2011;96:1019–1027. doi: 10.1113/expphysiol.2011.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Best CA, Auth T, Gov NS, Safran SA, Popescu G, Suresh S, Feld MS. Metabolic remodeling of the human red blood cell membrane. Proc Natl Acad Sci USA. 2010;107:1289–1294. doi: 10.1073/pnas.0910785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Diez-Silva M, Popescu G, Lykotrafitis G, Choi W, Feld MS, Suresh S. Refractive index maps and membrane dynamics of human red blood cells parasitized by Plasmodium falciparum. Proc Natl Acad Sci USA. 2008;105:13730–13735. doi: 10.1073/pnas.0806100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathi K, Lipowsky HH. Capillary recruitment in response to tissue hypoxia and its dependence on red blood cell deformability. Am J Physiol. 1999;277:H2145–2157. doi: 10.1152/ajpheart.1999.277.6.H2145. [DOI] [PubMed] [Google Scholar]
- Platt HA, Chuba JV, Kaplan HS. Initial studies of the temperature-viscosity relationship of human plasma and serum. Biorheology. 1978;15:29–35. [PubMed] [Google Scholar]
- Pries AR, Neuhaus D, Gaehtgens P. Blood viscosity in tube flow: dependence on diameter and hematocrit. Am J Physiol. 1992;263:H1770–1778. doi: 10.1152/ajpheart.1992.263.6.H1770. [DOI] [PubMed] [Google Scholar]
- Rand PW, Lacombe E, Hunt HE, Austin WH. Viscosity of normal human blood under normothermic and hypothermic conditions. J Appl Physiol. 1964;19:117–122. doi: 10.1152/jappl.1964.19.1.117. [DOI] [PubMed] [Google Scholar]
- Simmonds MJ, Connes P, Sabapathy S. Exercise-induced blood lactate increase does not change red blood cell deformability in cyclists. PloS One. 2013;8:e71219. doi: 10.1371/journal.pone.0071219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol. 2010;110:389–397. doi: 10.1152/japplphysiol.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Stout JR, Kendall KL, Fukuda DH, Cramer JT. Exercise-induced oxidative stress: the effects of beta-alanine supplementation in women. Amino Acids. 2012;43:77–90. doi: 10.1007/s00726-011-1158-x. [DOI] [PubMed] [Google Scholar]
- Snyder GK. Influence of temperature and hematocrit on blood viscosity. Am J Physiol. 1971;220:1667–1672. doi: 10.1152/ajplegacy.1971.220.6.1667. [DOI] [PubMed] [Google Scholar]
- Suhr F, Brenig J, Muller R, Behrens H, Bloch W, Grau M. Moderate exercise promotes human RBC-NOS activity, NO production and deformability through Akt kinase pathway. PloS One. 2012;7:e45982. doi: 10.1371/journal.pone.0045982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung KL, Chien S. Influence of temperature on rheology of human erythrocytes. Chin J Physiol. 1992;35:81–94. [PubMed] [Google Scholar]
- Tripette J, Loko G, Samb A, Gogh BD, Sewade E, Seck D, Hue O, Romana M, Diop S, Diaw M, Brudey K, Bogui P, Cisse F, Hardy-Dessources MD, Connes P. Effects of hydration and dehydration on blood rheology in sickle cell trait carriers during exercise. Am J Physiol. 2010;299:H908–914. doi: 10.1152/ajpheart.00298.2010. [DOI] [PubMed] [Google Scholar]
- Vandewalle H, Lacombe C, Lelievre JC, Poirot C. Blood viscosity after a 1-h submaximal exercise with and without drinking. Int J Sports Med. 1988;9:104–107. doi: 10.1055/s-2007-1024988. [DOI] [PubMed] [Google Scholar]
- Vent-Schmidt J, Waltz X, Pichon A, Hardy-Dessources MD, Romana M, Connes P. Indirect viscosimetric method is less accurate than ektacytometry for the measurement of red blood cell deformability. Clin Hemorheol Microcirc. 2015;59:115–121. doi: 10.3233/CH-131727. [DOI] [PubMed] [Google Scholar]
- Vertessy BG, Steck TL. Elasticity of the human red cell membrane skeleton. Effects of temperature and denaturants. Biophys J. 1989;55:255–262. doi: 10.1016/S0006-3495(89)82800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyuklu M, Cengiz M, Ulker P, Hever T, Tripette J, Connes P, Nemeth N, Meiselman HJ, Baskurt OK. Effects of storage duration and temperature of human blood on red blood cell deformability and aggreation. Clin Hemorheol Microcir. 2009;41:269–278. doi: 10.3233/CH-2009-1178. [DOI] [PubMed] [Google Scholar]
- Wahl P, Bloch W, Mester J, Born DP, Sperlich B. Effects of different levels of compression during sub-maximal and high-intensity exercise on erythrocyte deformability. Eur J Appl Physiol. 2012;112:2163–2169. doi: 10.1007/s00421-011-2186-7. [DOI] [PubMed] [Google Scholar]
- Waugh R, Evans EA. Thermoelasticity of red blood cell membrane. Biophys J. 1979;26:115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh RE, Hochmuth RM. Mechanical equilibrium of thick, hollow, liquid membrane cylinders. Biophys J. 1987;52:391–400. doi: 10.1016/S0006-3495(87)83227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin O, Bor-Kucukatay M, Senturk UK, Baskurt OK. Effects of swimming exercise on red blood cell rheology in trained and untrained rats. J Appl Physiol. 2000;88:2074–2080. doi: 10.1152/jappl.2000.88.6.2074. [DOI] [PubMed] [Google Scholar]
- Yalcin O, Erman A, Muratli S, Bor-Kucukatay M, Baskurt OK. Time course of hemorheological alterations after heavy anaerobic exercise in untrained human subjects. J Appl Physiol. 2003;94:997–1002. doi: 10.1152/japplphysiol.00368.2002. [DOI] [PubMed] [Google Scholar]
- Yalcin O, Ortiz D, Tsai AG, Johnson PC, Cabrales P. Microhemodynamic aberrations created by transfusion of stored blood. Transfusion. 2014;54:1015–1027. doi: 10.1111/trf.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip R, Mohandas N, Clark MR, Jain S, Shohet SB, Dallman PR. Red cell membrane stiffness in iron deficiency. Blood. 1983;62:99–106. [PubMed] [Google Scholar]