Abstract
Small molecule inhibitors of the phosphatidylinositol 3-kinase (PI3K), Akt and mTOR pathway currently in the clinic produce a paradoxical reactivation of the pathway they are intended to suppress. Furthermore, fresh experimental evidence with PI3K antagonists in melanoma, glioblastoma and prostate cancer shows that mitochondrial metabolism drives an elaborate process of tumor adaptation culminating with drug resistance and metastatic competency. This is centered on reprogramming of mitochondrial functions to promote improved cell survival and to fuel the machinery of cell motility and invasion. Key players in these responses are molecular chaperones of the Heat Shock Protein 90 (Hsp90) family compartmentalized in mitochondria, which suppress apoptosis via phosphorylation of the pore component, Cyclophilin D, and enable the subcellular repositioning of active mitochondria to membrane protrusions implicated in cell motility. An inhibitor of mitochondrial Hsp90s in preclinical development (Gamitrinib) prevents adaptive mitochondrial reprogramming and shows potent anti-tumor activity in vitro and in vivo. Other therapeutic strategies to target mitochondria for cancer therapy include small molecule inhibitors of mutant isocitrate dehydrogenase (IDH) IDH1 (AG-120) and IDH2 (AG-221) which opened new therapeutic prospects for high-risk AML patients. A second approach of mitochondrial therapeutics focuses on agents that elevate toxic ROS levels from a leaky electron transport chain, nevertheless the clinical experience with these compounds, including a quinone derivative, ARQ 501, and a copper chelator, elesclomol (STA-4783) is limited. In light of these evidences, we discuss how best to target a resurgence of mitochondrial bioenergetics for cancer therapy.
BACKGROUND
Rewiring of mitochondrial function in tumors
Unlike normal cells that oxidize pyruvate in the mitochondrial respiratory chain to produce bioenergy (ATP), tumors rely on a “fermentative”, glycolytic metabolism that converts glucose to pyruvate and then lactate in the cytosol, irrespective of oxygen availability (1). Recognized almost a century ago, this “Warburg effect” is now considered a hallmark of cancer (2), independent of stage or genetic makeup. Why tumors with their high biosynthetic needs rely on an energetically inefficient metabolism is not entirely clear. However, the role of glycolysis in generation of biomass to support cell proliferation (1), dampening the production of toxic ROS from mitochondria (3), and adaptation to an hypoxic microenvironment (4), have all been implicated as drivers of metabolic rewiring.
This compounds additional evidence that, at least in certain tumors, disabling mitochondrial respiration favors disease progression. Accordingly, loss-of-function mutations in oxidative phosphorylation genes produce a pro-oncogenic, pseudo-hypoxic state (5), inactivation of tumor suppressors, for instance p53 (6), or activating mutations in the Ras oncogene (7) stimulates glycolysis at the expense of oxidative phosphorylation, and stabilization of Hypoxia-Inducible Factor-1 (HIF1), a master regulator of oxygen homeostasis, dampens mitochondrial respiration to promote glycolysis (8). Irrespective, a rewired tumor metabolism is clearly important for disease outcome, conferring aggressive traits of metastatic competency and drug resistance (4). Not surprisingly based on these findings, mitochondrial function has been dubbed as a “tumor suppressor” (9), restoring oxidative phosphorylation was proposed as a therapeutic target (10), and agents that inhibit glycolysis have entered clinical testing in cancer patients (see below).
On the other hand, the dichotomy between glycolysis and oxidative phosphorylation in cancer bioenergetics may not be as rigid as previously thought. In fact, we know that mitochondria remain fully functional in most tumors (11), oxidative phosphorylation still accounts for a large fraction of ATP produced in cancer (12), and even under conditions of severe hypoxia, cytochromes are fully oxidized to support cellular respiration (13). This biochemical evidence fits well with a flurry of functional data that point to oxidative phosphorylation as an important cancer driver (Table 1). Accordingly, mitochondrial respiration contributes to oncogene-dependent transformation (14) and metabolic reprogramming (15), supports energy-intensive mechanisms of protein translation in tumors (16), maintains cancer “stemness” (17), favors malignant repopulation after oncogene ablation (18), and promotes the emergence of drug resistance (19) (Table 1 summarizes the mitochondrial pathways involved in cancer and the effect of targeting such pathways). In addition, there is evidence that oxidative phosphorylation may be required to support tumor cell motility (20) and metastasis (21), potentially under conditions of stress or limited nutrient availability. Mechanistic aspects of how tumors may regulate oxidative phosphorylation have also come into better focus, pointing to a key role of protein folding quality control maintained by mitochondria-localized Heat Shock Protein-90 (Hsp90) chaperones (22), as well as organelle proteases (23), in mitochondrial homeostasis in tumors. In this context, Hsp90 chaperones predominantly accumulate in mitochondria of tumors, but not most normal tissues, to preserve the folding and activity of key regulators of permeability transition, electron transport chain, citric acid cycle, fatty acid oxidation, amino acid synthesis and cellular redox status. Inhibition of this pathway has profound implications for tumor cells. While complete inhibition of mitochondrial Hsp90s activates massive tumor cell death by apoptosis and other mechanisms, suboptimal, non-toxic inhibition of this pathway induces a phenotype of acute cellular starvation with reduced bioenergetics output, phosphorylation of nutrient-sensing AMPK-activated kinase (AMPK) and inhibition of mTORC1, which in turn triggers an unfolded protein response and induction of autophagy. This compensatory response promotes survival and maintains a proliferative advantage in genetically disparate tumors, correlating with worse outcome in lung cancer patients. Several groups have recently demonstrated the importance of OxPhos and protein folding in the mitochondria for metastatic dissemination in mice models. For instance, targeting OxPhos by siRNA knockdown of mtHsp90 led to 80% reduction in breast cancer metastasis to bone (20). Furthermore, OxPhos impairment after shRNA silencing of the mitochondrial biomass factor PGC-1α inhibited breast cancer metastasis to lung by 70–90% (21). On the contrary, activation of autophagy by expression of a constitutively active mutant of AMPK or ULK1 impaired lung cancer metastasis to liver (60–80% reduction) (20).
Table 1. Mitochondrial pathways that contribute to cancer.
The main mitochondrial functions implicated in cancer are presented along the molecular pathways involved. Where available, the effect of targeting these mitochondrial pathways in tumorigenesis, tumor growth or metastasis is presented. PDK, pyruvate dehydrogenase kinase; NFAT, Nuclear factor of activated T-cells; ROS, reactive oxygen species; ALT2, mitochondrial alanine aminotransferase; GLS, glutaminase; GPI, glucose 6-phosphate isomerase; CIII, electron transport chain complex III; OxPhos, oxidative phosphorylation; TCA, tricarboxylic acid; mTORC1, mammalian target of rapamacyn complex 1; 4E-BPs, Eukaryotic translation initiation factor 4E binding proteins; IMP2, insulin-like growth factor 2 mRNA-binding protein 2; NDUFS3/7, NADH dehydrogenase (ubiquinone) iron-sulfur protein 3 and 7; NDUF3, NADH dehydrogenase (ubiquinone) 1α complex assembly factor 3; PGC1α, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
| function | genes/pathway | targeted by | effect on tumors | cancer type | Ref. |
|---|---|---|---|---|---|
| apoptosis resistance | PDK-dependent metabolic-electrical remodeling involves NFAT, mitochondria ROS and K+ channels Kv1.5 | DCA (PDK inhibitor) | ↓xenograft growth by 50–85% vs vehicle | various | 10 |
| oncogene-dependent transformation | K-Ras V12, glutamine metabolism (ALT2, GLS) pentose phosphate pathway (GPI), CIII (Rieske) ROS/ERK | LSL-Kras G12D x TFAMfl/fl | ↓tumor load by 70–80% vs Kras alone | lung | 14 |
| metabolic reprogramming | c-Myc, miR-23a/b, mitochondrial glutaminase (GLS), glutamine catabolism and transport (ASCT2), TCA cycle | 15 | |||
| protein translation | mTORC1/4E-BPs translation of mt-mRNAs (including the components of complex V, mt ribosomal proteins and TFAM) | 16 | |||
| cancer stemness | IMP2, OxPhos (NDUFS3, NDUFS7, NDUF3) | IMP2 shRNA | ↑survival of mice injected with gliomaspheres by 60–160% (IMP2 sh vs control sh) | glioblastoma | 17 |
| malignant repopulation | K-Ras, p53, mitochondrial OxPhos, b-oxidation, biogenesis (PGC1a) | oligomycin (ETC inhibitor) | ↑ maximal survival of mice bearing regressed tumors by >300% | pancreatic ductal adenocarcinoma | 18 |
| drug resistance | H3K4-demethylase JARID1B, mitochondrial OxPhos | Vemurafenib (V600E Braf inhibitor) + phenformin (biguanide hypoglycemic agent) | ↓xenograft growth by 50–60% vs single vemurafenib | melanoma | 19 |
| drug resistance | mtHsp90, Akt2 translocation to mitochondria, CypD | BEZ235 (PI3K antagonist) + Gamitrinib (mtHsp90 inhibitor) | ↑ median survival of mice bearing GBM by >40% vs monotherapy | glioblastoma | 35 |
| tumor cell motility | mtHsp90, AMPK, mTOR, ULK1/FIP200/FAK | mtHsp90 siRNA CA. AMPK or ULK1 expression |
↓breast cancer metastasis to bone by 80% ↓lung cancer metastasis to liver by 60–80% |
various | 20 |
| metastasis | PGC-1α, mitochondrial biogenesis, OxPhos | PGC-1α shRNA | ↓breast cancer metastasis to lung by 70–90% | various | 21 |
Mitochondria and tumor adaptation
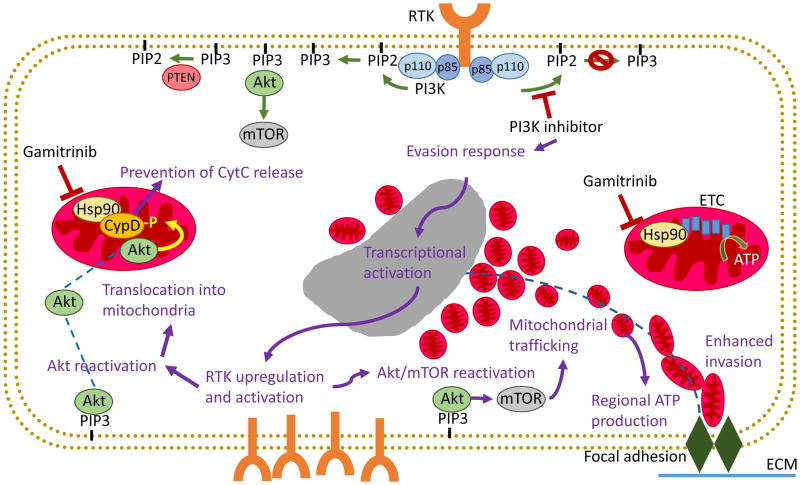
Against this more composite backdrop, where tumor metabolism dynamically integrates both glycolysis and oxidative phosphorylation, new evidence has uncovered an unexpected role of mitochondria in tumor adaptation to molecular therapy. We have known for some time that exposure of tumors to small molecule inhibitors of the phosphatidylinositol 3-kinase (PI3K) (24), Akt and mTOR pathway currently in the clinic, produce a paradoxical reactivation of the pathway they are intended to suppress (25–27). What was not known was the impact (if any) of this response on disease behavior. Now, more recent studies demonstrated that molecular therapy with PI3K antagonists induced transient metabolic quiescence with reduced oxygen and glucose consumption rates, while promoting the redistribution of active Akt, mostly Akt2 from cytosol to mitochondria (28). Once in mitochondria, Akt2 readily phosphorylated cyclophilin D (CypD) (28) a structural component of the permeability transition pore (29), resulting in inhibition of apoptosis and treatment resistance (Figure 1) (28). Indeed, CypD−/− cells reconstituted with a non-phosphorylatable CypD mutant cDNA were sensitized to cell death (40–50% increase in cell killing compared to control). However, this was not the only adaptive response initiated by PI3K therapy in tumors. In fact, treatment with PI3K antagonists induced extensive morphological changes in mitochondria of transformed cells, with formation of elongated organelles that infiltrated the cortical cytoskeleton (60–150% increase in cortical mitochondria compared to control) and localized in proximity of membrane protrusions associated with cell motility (Figure 1) (30). This process of subcellular mitochondrial trafficking required active oxidative phosphorylation and organelle dynamics, but not ROS production (30). In turn, these repositioned, “cortical” mitochondria provided an efficient, “regional” energy source to fuel the machinery of cell motility, supporting heightened turnover of focal adhesion complexes (150–300% increase in the rate of formation of new focal adhesion complexes), increased membrane lamellipodia dynamics (30–50% increase in size of cell protrusions) and enhanced random cell motility. Overall, this culminated with paradoxical increased tumor cell migration (80–110% increase in speed of migration and 65–85% increase in distance traveled) and invasion (100–300% increased 2D invasion of prostate cancer cells; 50–100% increase invasion of 3D glioblastoma spheroids) after PI3K therapy compared to controls (Figure 1) (30).
Figure 1. Molecular therapy induces adaptive mitochondrial reprogramming in cancer.
Activation of the PI3K pathway leads to formation of PIP3 and recruitment of Akt to the cell membrane, where it can be phosphorylated and activated. (Left) Exposure to small molecule PI3K antagonists currently in the clinic promotes the Hsp90-dependent recruitment of active Akt2 from cytosol to mitochondria, and Akt-phosphorylation of the permeability pore component CypD resulting in apoptosis inhibition. (Right) Exposure to PI3K therapy also induces the oxidative phosphorylation-dependent redistribution of mitochondria to the cortical cytoskeleton in proximity with focal adhesion complexes implicated in cell motility, and providing an efficient, regional energy source to fuel tumor cell motility and invasion. PI3K therapy reprogramming can be blocked by combination with the mtHsp90 inhibitor Gamitrinib. ECM, extracellular matrix; ETC, electron transport chain; RTK, receptor tyrosine kinase.
The implications of these findings may be far-reaching. Supported by compelling experimental evidence (31), there have been high expectations that therapeutic targeting of the PI3K pathway could fulfill the goals of “personalized” cancer medicine. The reality in the clinic was different, as these agents showed limited activity, short-lived patient gains and measurable toxicity (24). The new findings described above (28, 30) may explain, at least in part, this unfavorable outcome, and should caution against the use of PI3K antagonists as monotherapy in the clinic. From a mechanistic standpoint, these results highlight a new, powerful role of mitochondrial metabolic reprogramming in tumor adaptation, modulating treatment response and paradoxical acquisition of metastatic competency.
CLINICAL-TRANSLATIONAL ADVANCES
Altogether, the reshaped research landscape summarized above points to mitochondria as a hub of tumor responses, and important therapeutic target in cancer (32). This concept has been successfully pursued with the clinical development of modulators of apoptosis, a process regulated at the outer mitochondrial membrane (29). But the idea of targeting mitochondrial metabolism, let alone mechanisms of mitochondrial adaptation for cancer therapeutics is still in its infancy (32).
Even therapeutic efforts to disable well-established glycolytic pathways in tumors (11, 33, 34) have relied on a relatively small portfolio of drug candidates. As an example, early stage clinical trials pursued inhibition of hexokinases (HK) isoenzymes, with the goal of preventing the first reaction of glycolysis. One such HK inhibitors in the clinic is Lonidamine (TH-070), a derivative of indazole-3-carboxylic acid. A Phase II trial in 35 patients with ovarian cancer showed an 80% objective response rate (ORR) of TH-070 in combination with paclitaxel and cisplatin (35). A Phase II trial in 31 patients with NSCLC who were treated with TH-070 in combination with cisplatin, epidoxorubicin and vindesine showed 89% of patients had either a partial remission (PR) or stable disease (SD) (35). Despite this early promising trials, TH-070 development was terminated after disappointing results in two randomized Phase III trials (35).
A second agent that prevents glycolysis, 2-deoxyglucose (2-DG), a non-metabolizable glucose analog was also pursued in the clinic. However, dose escalation Phase I trials in patients with castrate-resistant prostate cancer and other advanced solid tumors resulted in asymptomatic QTc prolongation that limited further drug evaluation (35).
An independent approach involved therapeutic inhibition of pyruvate dehydrogenase kinase (PDK1) with the small molecule antagonist dichloroacetate (DCA). PDK1 is a recognized therapeutic target in cancer (36) given its ability to phosphorylate and inhibit the Pyruvate Dehydrogenase Complex (PDC), thus preventing the decarboxylation of pyruvate to Acetyl-CoA and its subsequent entry in the tricarboxylic acid cycle (37). Clinical trials (Phase I) with DCA in glioma patients revealed manageable tolerability and hints of objective responses (38). Although a phase II trial of DCA in non-small cell lung cancer was stopped early due to treatment-related deaths and lack of clinical benefit (39), encouraging preclinical results were reported for the combination of DCA plus 5-FU and cisplatin in gastric cancer (35). DCA is being currently evaluated in patients with head and neck cancer, glioblastoma and other solid tumors.
Therapeutic strategies to target mitochondria for cancer therapy are at an even earlier stage of development. A promising area is the ongoing development of small molecule inhibitors of mutant isocitrate dehydrogenase (IDH) isoforms, including IDH2 that localizes to mitochondria. Gain-of-function IDH mutations identified in gliomas (40), acute myelogenous leukemias (AML) (41), and perhaps operative in other cancer types, promote the accumulation of 2-hydroxyglutarate (2-HG). This is an oncometabolite that deregulates chromatin remodeling enzymes, resulting in epigenetic silencing of tumor suppressor loci and differentiation block in AML (34). Early trials with mutant IDH1 (AG-120) and IDH2 (AG-221) inhibitors produced impressive objective responses in 60% of AML patients (including complete responses with or without platelet response) (35), opening new therapeutic prospects for high-risk (>60 years of age) AML patients. A second approach of mitochondrial therapeutics focused on agents that elevate toxic ROS levels from a leaky electron transport chain. The clinical experience with these compounds, including a quinone derivative, ARQ 501, and a copper chelator, elesclomol (STA-4783) is limited. In single-agent Phase I trials, treatment with ARQ 501 was well tolerated in patients with head and neck cancer and other advanced solid tumors. In a Phase II trial in patients with unresectable pancreatic adenocarcinoma, the combination of ARQ 501 and gemcitabine resulted in stable disease in 65% of patients after being on trial for 8 weeks (35). Unfortunately, the early clinical activity of elesclomol in combination with paclitaxel in a randomized phase II trial in melanoma (42), showing more than 200% increase in progression free survival compared to paclitaxel alone, was not confirmed in a subsequent phase III trial (43).
Against this backdrop, an agent originally designed in our laboratory, Gamitrinib (44), may provide a first-in-class, “mitochondriotoxic” activity, conceptually distinct from the above strategies. Gamitrinib was generated to target abundant pools of Hsp90 and its structurally related chaperone, TRAP-1 present in mitochondria, selectively of tumor cells (45). Gamitrinib relies on a combinatorial structure where the Hsp90 ATPase inhibitory module of 17-allylaminogeldanamycin (17-AAG), a first-generation Hsp90 inhibitor, is linked to the mitochondria-targeting moiety of triphenylphosphonium (44). This enables fast and efficient accumulation of Gamitrinib in mitochondria, with virtual no inhibition of cytosolic Hsp90, whereas none of the first or second-generation Hsp90 antagonists currently in the clinic had the ability to accumulate in mitochondria (44). Due to this subcellular selectivity, Gamitrinib did not affect known Hsp90 client proteins in the cytosol, for instance Akt and Chk1 levels, but induced acute mitochondrial dysfunction with depolarization of inner membrane potential and release of cytochrome c in the cytosol, two molecular prerequisites of apoptosis. In contrast, none of the additional derivatives of 17-AAG as well as purine- and isoxazole resorcinol–based Hsp90 antagonists (17-AAG; hydroquinone derivative of 17-AAG, IPI-504; purine analog BIIB021; or isoxazole NVP-AUY922 Hsp90 inhibitors) affected mitochondrial integrity (44). Consistent with these findings, targeting mitochondrial Hsp90s resulted in catastrophic and irreversible collapse of mitochondrial functions (44), disabled Complex II-dependent oxidative phosphorylation (22), and induced acute mitochondrial permeability transition (46), producing potent anticancer activity in localized and disseminated xenograft and genetic tumor models (47). Treatment of mice bearing lung cancer xenografts with Gamitrinib produced a 50–80% of tumor growth inhibition compared to vehicle (44). Gamitrinib combined with TRAIL suppressed the growth of established glioblastomas in mice by 70–85% (46). In the Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model, Gamitrinib prevented the formation of localized prostate tumors of neuroendocrine or adenocarcinoma origin, as well as metastatic prostate cancer to abdominal lymph nodes and liver (47). Intriguingly, targeting the mitochondrial quality control protease, ClpP also produced robust anticancer activity (50–60% reduction of xenograft growth) in recent preclinical studies, reinforcing the importance of mitochondrial protein folding in tumor maintenance (23). There is a compelling rationale to think that these organelle-localized chaperones may provide promising targets for cancer therapy. First, their activity likely improves protein folding quality control in mitochondria (48), an ideal mechanism to buffer the risk of proteotoxic stress typical of highly bioenergetically active (tumor) cells. Second, a proteomics screen of mitochondrial molecules that require Hsp90 for folding uncovered key regulators of virtually every organelle function (22), suggesting that disabling this pathway may globally compromise organelle homeostasis. Third, mitochondrial Hsp90 chaperones have been shown to directly sustain tumor cell invasion and metastasis by dampening the activation of autophagy and the unfolded protein response (20).
The preclinical development of Gamitrinib in anticipation of human testing is now progressing as an academic endeavor. In addition to general issues of formulation (like its parent compound, 17-AAG, Gamitrinib is water-insoluble) and dosing, critical questions of tolerability for normal tissues that are dependent on mitochondrial respiration are of top priority, even though preclinical data have suggested a manageable toxicity profile in mice. Of key relevance are also opportunities for combination therapy with cytotoxics or molecular agents (46). In this context, the unique “mitochondriotoxic” mechanism of action of Gamitrinib (44) may be ideally suited to counter therapy-induced tumor adaptation as a driver of disease progression (28, 30). Recent evidence seems to validate this premise, as sub-therapeutic concentrations of Gamitrinib reversed mitochondrial reprogramming induced by PI3K antagonists, blocked the recruitment of mitochondria to the cortical cytoskeleton in these settings, and suppressed tumor cell invasion (20, 30), and metastasis (47). This may create tangible opportunities for repurposing agents that have shown limited activity in the clinic, as Gamitrinib potently synergized with PI3K therapy in a high-throughput drug combination screen, converting a transient, cytostatic effect into potent, cytotoxic anticancer activity (28, 30). In these experiments, the combination Gamitrinib and a PI3K/mTOR inhibitor (BEZ235) extended animal survival in a glioblastoma model (vehicle: median survival = 28.5 days; Gamitrinib+PI3Ki: median survival = 40 days, P = 0.003), compared with single-agent treatment (PI3Ki: median survival = 32 days, P = 0.02; Gamitrinib: median survival = 35 days, P = 0.008).
CONCLUDING REMARKS
Despite a wealth of mechanistic evidence, the exploitation of tumor metabolism for cancer therapy is at an early stage of development. Despite safety concerns for normal tissues, the initial clinical experience with metabolism-modifying drugs has not uncovered major toxicities, confirming the different wiring of metabolic pathways in normal versus transformed cells. A perspective that this process hinges exclusively on glycolysis has been updated by more recent observations, which identified a central role of mitochondrial reprogramming in tumor maintenance, drug resistance and metastatic competency. Together, this suggests a more integrated view of tumor metabolism, where glycolysis and oxidative phosphorylation cooperate in a dynamic interplay shaped by the selective pressure of a chronically hypoxic, nutrient-depleted and therapy-exposed microenvironment. Clearly, this new level of complexity poses fresh challenges to pinpoint which bioenergetics pathway(s) may be best suitable for therapeutic intervention, and further heightens the impact of tumor adaptation as an important barrier to durable responses in the clinic. In this context, agents like Gamitrinib that disable broad mechanisms of adaptive mitochondrial reprogramming may open unique therapeutic prospects in drug-resistant tumors, and effectively repurpose otherwise modestly efficacious molecular therapy.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants P01CA140043, R01CA78810, and CA190027 (to D.C. Altieri) and F32CA177018 (to M.C. Caino); a Challenge Award from the Prostate Cancer Foundation (to D.C. Altieri and M.C. Caino); and the Office of the Assistant Secretary of Defense for Health Affairs through the Prostate Cancer Research Program under award no. W81XWH-13-1-0193 (to D.C. Altieri).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–83. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 5.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–3. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 7.Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–9. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–13. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857–66. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–37. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 12.Moreno-Sanchez R, Marin-Hernandez A, Saavedra E, Pardo JP, Ralph SJ, Rodriguez-Enriquez S. Who controls the ATP supply in cancer cells? Biochemistry lessons to understand cancer energy metabolism. Int J Biochem Cell Biol. 2014;50:10–23. doi: 10.1016/j.biocel.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DF, Rumsey WL, Green TJ, Vanderkooi JM. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem. 1988;263:2712–8. [PubMed] [Google Scholar]
- 14.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–93. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–5. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita M, Gravel SP, Chenard V, Sikstrom K, Zheng L, Alain T, et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 2013;18:698–711. doi: 10.1016/j.cmet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Janiszewska M, Suva ML, Riggi N, Houtkooper RH, Auwerx J, Clement-Schatlo V, et al. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26:1926–44. doi: 10.1101/gad.188292.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–32. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23:811–25. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caino MC, Chae YC, Vaira V, Ferrero S, Nosotti M, Martin NM, et al. Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J Clin Invest. 2013;123:2907–20. doi: 10.1172/JCI67841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chae YC, Angelin A, Lisanti S, Kossenkov AV, Speicher KD, Wang H, et al. Landscape of the mitochondrial Hsp90 metabolome in tumours. Nat Commun. 2013;4:2139. doi: 10.1038/ncomms3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole A, Wang Z, Coyaud E, Voisin V, Gronda M, Jitkova Y, et al. Inhibition of the mitochondrial protease ClpP as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2015;27:864–76. doi: 10.1016/j.ccell.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–56. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2012;109:2718–23. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–57. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh JC, Siegelin MD, Vaira V, Faversani A, Tavecchio M, Chae YC, et al. Adaptive mitochondrial reprogramming and resistance to PI3K therapy. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju502. pii: dju502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tait SW, Green DR. Mitochondria and cell signalling. J Cell Sci. 2012;125:807–15. doi: 10.1242/jcs.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caino MC, Ghosh JC, Chae YC, Vaira V, Rivadeneira DB, Faversani A, et al. PI3K therapy reprograms mitochondrial trafficking to fuel tumor cell invasion. Proc Natl Acad Sci U S A. 2015;112:8638–43. doi: 10.1073/pnas.1500722112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 32.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–64. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 33.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sborov DW, Haverkos BM, Harris PJ. Investigational cancer drugs targeting cell metabolism in clinical development. Expert Opin Investig Drugs. 2015;24:79–94. doi: 10.1517/13543784.2015.960077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700–8. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saunier E, Benelli C, Bortoli S. The pyruvate dehydrogenase complex in cancer: An old metabolic gatekeeper regulated by new pathways and pharmacological agents. Int J Cancer. 2015 doi: 10.1002/ijc.29564. [DOI] [PubMed] [Google Scholar]
- 38.Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra4. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 39.Garon EB, Christofk HR, Hosmer W, Britten CD, Bahng A, Crabtree MJ, et al. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2014;140:443–52. doi: 10.1007/s00432-014-1583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huse JT, Aldape KD. The evolving role of molecular markers in the diagnosis and management of diffuse glioma. Clin Cancer Res. 2014;20:5601–11. doi: 10.1158/1078-0432.CCR-14-0831. [DOI] [PubMed] [Google Scholar]
- 41.McKenney AS, Levine RL. Isocitrate dehydrogenase mutations in leukemia. J Clin Invest. 2013;123:3672–7. doi: 10.1172/JCI67266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Day S, Gonzalez R, Lawson D, Weber R, Hutchins L, Anderson C, et al. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J Clin Oncol. 2009;27:5452–8. doi: 10.1200/JCO.2008.17.1579. [DOI] [PubMed] [Google Scholar]
- 43.O’Day SJ, Eggermont AM, Chiarion-Sileni V, Kefford R, Grob JJ, Mortier L, et al. Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J Clin Oncol. 2013;31:1211–8. doi: 10.1200/JCO.2012.44.5585. [DOI] [PubMed] [Google Scholar]
- 44.Kang BH, Plescia J, Song HY, Meli M, Colombo G, Beebe K, et al. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J Clin Invest. 2009;119:454–64. doi: 10.1172/JCI37613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–70. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 46.Siegelin MD, Dohi T, Raskett CM, Orlowski GM, Powers CM, Gilbert CA, et al. Exploiting the mitochondrial unfolded protein response for cancer therapy in mice and human cells. J Clin Invest. 2011;121:1349–60. doi: 10.1172/JCI44855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang BH, Siegelin MD, Plescia J, Raskett CM, Garlick DS, Dohi T, et al. Preclinical characterization of mitochondria-targeted small molecule hsp90 inhibitors, gamitrinibs, in advanced prostate cancer. Clin Cancer Res. 2010;16:4779–88. doi: 10.1158/1078-0432.CCR-10-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chae YC, Caino MC, Lisanti S, Ghosh JC, Dohi T, Danial NN, et al. Control of tumor bioenergetics and survival stress signaling by mitochondrial HSP90s. Cancer Cell. 2012;22:331–44. doi: 10.1016/j.ccr.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]