Abstract
Background
Bone marrow mononuclear cells (BMMNCs) can counteract oxidative stress and inhibit the inflammatory response in focal ischemic stroke models. However, the effect of BMMNC transplantation on carotid atherosclerosis needs to be determined.
Methods
The carotid atherosclerotic plaque model was established in New Zealand White rabbits by balloon injury and 8 weeks of high-fat diet. Rabbits were randomized to receive an intravenous injection of autologous BrdU-labeled BMMNCs or an equal volume of phosphate-buffered saline. Plaques were evaluated for expression of proinflammatory and anti-inflammatory cytokines, antioxidant proteins, and markers of cell death.
Results
BMMNCs migrated into atherosclerotic plaque on the first day after cell transplantation. BMMNC-treated rabbits had smaller plaques and more collagen deposition than did the vehicle-treated controls on day 28 (p<0.05). BMMNC treatment significantly increased endothelial nitric oxide synthase and the antioxidant enzymes glutathione peroxidase and superoxide dismutase in plaques compared to vehicle treatment on day 7. BMMNC-treated rabbits also had lower levels of cleaved caspase-3 expression; lower levels of proinflammatory cytokines interleukin-1β, tumor necrosis factor-α, and matrix metalloproteinase 9; and higher levels of insulin-like growth factor-1 and its receptor (p<0.05).
Conclusions
Autologous BMMNC transplantation can suppress the process of atherosclerotic plaque formation and is associated with enhanced antioxidative effect, reduced levels of inflammatory cytokines and cleaved caspase-3, and increased expression of insulin-like growth factor-1 and its receptor. BMMNC transplantation represents a novel approach for the treatment of carotid atherosclerosis.
Keywords: Atherosclerosis, Bone marrow mononuclear cell, Inflammatory, Insulin-like growth factor, Oxidative stress
Introduction
As the population ages and the standard of living rises, carotid atherosclerosis has become an independent and important risk factor for the development of ischemic cerebrovascular disease [1]. In recent decades, atherosclerosis has become a leading cause of death and long-term disability in adults worldwide [1,2].
Carotid atherosclerosis begins with the accumulation of low-density lipoprotein (LDL) and an increase in reactive oxygen species (ROS) in intima [3]. The LDL particles are subjected to oxidative modifications through enzymatic attack by ROS in the intima and are transformed into oxidized LDL (OxLDL) [4]. In plaque, OxLDL increases O2− and H2O2 [5,6], which directly lead to apoptosis of endothelial cells (ECs) and smooth muscle cells (SMCs) and a reduction in endothelial nitric oxide synthase (eNOS) [7]. In addition, OxLDL significantly upregulates the expression of proinflammatory factors, including matrix metalloproteinase 9 (MMP-9), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) [8–10], thus causing an inflammatory response in plaque that promotes the development of atherosclerosis [11].
Under homeostatic conditions, scavenging systems are in place to limit ROS levels [12]. O2− may be converted by a family of superoxide dismutases (SODs) to H2O2, which can be removed from the system by catalase or glutathione peroxidase (GPx) [13,14]. However, atherosclerosis inhibits the expression of SODs and GPx [15,16]. Therefore, augmenting antioxidative pathways by upregulating SODs and GPx has been proposed as a potential method to decrease atherosclerosis progression.
Insulin-like growth factor 1 (IGF-1) has pleiotropic protective effects for most diseases and has been shown to play an indispensable role in reducing oxidative stress and delaying atherosclerotic plaque progression [17]. IGF-1 acts mainly through its receptor, IGF-1R [18], which prevents OxLDL-induced cell apoptosis and inflammatory responses [19]. Recently, reports have suggested that the transplantation of bone marrow mononuclear cells (BMMNCs) can upregulate endogenous IGF-1 level in the myocardial infarction model [20]. Therefore, we hypothesized that transplantation of autologous BMMNCs might also exert protective effects in carotid atherosclerosis plaques. In the current study, we assessed the effect of autologous BMMNC transplantation on carotid atherosclerosis plaque size and on plaque levels of anti-oxidant enzymes and proinflammatory cytokines and mediators.
Materials and Methods
Animals and Experimental Groups
One hundred and three male New Zealand White rabbits (3 months old, 1.5–2 kg) were purchased from the Animal Experimental Center of Zhengzhou University. They were kept at a constant room temperature, with free access to water. All protocols were approved by the Animal Care and Use Committee of Zhengzhou University and were designed to minimize animal suffering. The animals were randomly divided into four groups: naive group (normal diet, n=14), sham-operated group (high-fat diet +sham operation, n = 14), vehicle-treated group (high-fat diet + balloon operation + vehicle, n = 29), and BMMNC-treated group (high-fat diet + balloon operation + BMMNCs, n = 29).
Establishment of Carotid Atherosclerotic Plaque Model
The carotid atherosclerotic plaque model was established in the rabbits by balloon injury [21]. After 1 week of an adaptive phase with a high-fat diet containing 1.5% cholesterol, 6% lard, and 92.5% normal diet (provided by the Animal Experimental Center of Zhengzhou University), 72 animals were anesthetized with an intramuscular injection of Sumianxin II (0.2 mL/kg). Then, an anterior midline incision was made, and the left common carotid artery and the internal and external carotid arteries were isolated. The distal end of the external carotid artery was ligated, and the proximal common carotid artery and internal carotid artery were temporarily occluded with artery clips. The external carotid artery near the ligation was punctured. A 2.5F Fogarty balloon catheter was inserted into the artery up to the bifurcation of the common carotid artery, and 800 µL of air was injected into the balloon. The inflated balloon was pulled back a total of three times in the same arterial segment before the catheter was withdrawn. The artery clips were removed with compression hemostasis, and routine antibiotics were applied to prevent infection. The rabbits received a high-fat diet for 8 weeks after the operation. Plaque formation was assessed by ultrasound (see below). Two randomly selected rabbits from each group were sacrificed and their left common carotid arteries collected for confirmation of plaque formation.
Ultrasound Examination of Carotid Arteries
After rabbits had been fed a high-fat diet for 8 weeks, we examined the carotid arteries in all groups by ultrasound using a Siemens Sequoia 512 ultrasound scanner with a 6–14 MHz linear probe (Siemens, Mountain View, CA, USA). We used a conventional grayscale ultrasound to measure the carotid intima-media thickness and color Doppler flow imaging to further visualize the vascular stenosis. Carotid plaque was identified as a discrete projection, with the intima-media thickness ≥ 50% from the adjacent wall into the vessel lumen [22].
Preparation of BMMNCs
After the 8-week high-fat diet, rabbits in the BMMNC treatment group underwent an autologous BMMNC transplantation. Under aseptic conditions, we anesthetized the rabbits as described above and punctured the lateral tibial tubercle with a No.16 bone marrow puncture needle connected to a 10 mL syringe containing 2 mL of heparin sodium (2500 U/mL). Eight milliliters of bone marrow fluid was obtained from each rabbit. The bone marrow cell suspension was slowly added to an equal volume of Percoll solution (Tianjin TBD Ltd., China). After centrifugation of the suspension at 2500 rpm for 30 min, we extracted the white film in the middle of the mixture and washed it three times with phosphate-buffered saline (PBS). The concentration density of the cells was verified in a Neubauer counting chamber, and viability was determined with Trypan blue staining. To determine their migratory ability, we labeled the BMMNCs with bromodeoxyuridine (BrdU) by incubating them in culture medium containing 12 g/mL BrdU (Sigma, St. Louis, MO, USA) overnight. After they were labeled, the cells were washed with PBS to remove excess unbound BrdU, resuspended in PBS at a concentration of 1×107 cells/mL, and used immediately for implantation. After anesthetizing the rabbits, we shaved the hair over the right marginal ear vein and cleaned the injection site with alcohol. For the transplantation group, we administered 1 mL of PBS containing BMMNCs into the vein by syringe injection. The vehicle-treated group was injected with an equal volume of PBS alone. All rabbits were administered antibiotics for 3 days after the procedure to prevent infection [23].
Assessment of Transplanted BMMNCs
We sacrificed rabbits in the BMMNC- and vehicle-treated groups on day 1 after cell transplantation to assess BMMNC migration (n = 3 per group). The left carotid arteries of these rabbits were removed. Blood vessels were soaked in 4% paraformaldehyde overnight at 4°C and then stored in a 30% sucrose/0.01 mol/L PBS solution until they sank. The vessels were then embedded in Tissue-Tek O.C.T. compound (Sakura Finetechnical, Tokyo, Japan) and serial-sectioned into 5-µm-thick slices on a microtome (RM2235, Leica Microsystems, Wetzlar, Germany). The sections were washed in 0.1 mol/L PBS containing 1% Triton X-100, treated with 1 mol/L HCl for 10 min on ice, washed again, and treated with 2 mol/L HCl for 10 min at room temperature. Then they were incubated with blocking buffer. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 5 min, and nonspecific binding was blocked in 5% normal goat serum/0.3% Triton X in PBS for 1 h at room temperature. The sections were incubated in anti-BrdU sheep polyclonal antibody (1:100, Abcam, Cambridge, MA, USA) overnight at 4°C and then with Alexa Fluor 488-conjugated donkey anti-sheep IgG antibody (1:800, Abcam) for 2 h at room temperature for visualization of antibody binding.
Measurement of Serum Lipid
Fasting blood samples were collected from the ear margin vein before initiation of the high-fat diet (baseline), at the end of the 8-week high-fat diet period (day 0), and on days 7 and 28 after BMMNC transplantation. Blood serum levels of high-density lipoprotein (HDL), LDL, total cholesterol (TC), and triglycerides (TG) were measured with an automated biochemical analyzer (Roche Hitachi 917; Block Scientific, NY, USA).
Histologic Evaluation
Rabbits from BMMNC-treated and vehicle-treated groups were sacrificed, and the left carotid arteries were removed. Vessels were fixed and cut as described above. We stained the tissue slices with hematoxylin and eosin (HE) and Masson trichromatic stain to analyze the plaque size, the percentage of plaque area, and collagen deposition. We randomly chose five cross sections from each rabbit for quantitative measurement and averaged the values [24]. Images were captured on a microcomputer equipped with a digital camera connected to a microscope (Olympus Optical Co., Japan). Two investigators blinded to treatment group performed quantitative assessments using a computer image analysis system (Image-Pro Plus Version 5.0.1).
Western Blot Analysis
We sacrificed six rabbits from each group on day 7 after cell transplantation. Vessels were washed with saline and homogenized in radioimmunoprecipitation assay lysis buffer (Boster Biological Engineering Co. ltd, Wuhan, China). After centrifugation (14,000 rpm, 5 min, 4°C), samples were separated on a 4% stacking gel and 7.5% – 12% separating gel. Protein was transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), which were blocked for 1 h at room temperature or overnight at 4°C with 5% nonfat milk (Sangon Biotech, Shanghai, China). The primary antibodies were mouse anti-rabbit IGF-1 (1:200, Global Biological Technologies Inc., Shanghai, China), rabbit anti-rabbit IGF-1R (1:100, Biosynthesis), rabbit anti-rabbit eNOS (1:400, Boster), mouse anti-rabbit GPx-1 (1:100, Abcam), goat anti-rabbit SOD-1 (1:1000, Abcam), rabbit anti-rabbit caspase-3 (1:1000, Abcam), and mouse anti-rabbit β-actin (1:500, Boster). After three washes, membranes were incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated goat anti-rabbit (1:2000, Boster), goat anti-mouse (1:2000, Sangon Biotech), or donkey anti-goat (1:5000, Sangon Biotech) secondary antibody. Protein bands were visualized by enhanced chemiluminescence with an ECL Plus chemiluminescence detection kit (Sangon Biotech). Optical density of the protein bands was quantified by Gel Analysis V2.02 software (Clinx Science Instruments).
RT-PCR of Carotid Artery
One centimeter of vessel was homogenized for extraction of total RNA by Trizol reagent (ComWin Biotech, Beijing, China) according to the manufacturer’s instructions. First-strand cDNA was synthesized with Super RT kit (ComWin Biotech) and amplified by 35 cycles of PCR with PCR Master Mix (Sangon Biotech). The primers used for amplification were as follows: For IL-1β, the forward primer was 5’-GGTGTTGTCTGGCACGTATG-3’ and the reverse primer was 5’-GGCCACAGGTATCTTGTCGT-3’ (product band, 124 bp, Tm, 52°C). For TNF-α, the forward primer was 5’-ACCCTCACACTCAGATCATCTTCT-3’ and the reverse primer was 5’-CAGATTGACCTCAGCGCTGAGTTG-3’ (product band, 422 bp, Tm, 59°C). For MMP-9, the forward primer was 5’-AAGACGCAGACGGTGGATT-3’ and the reverse primer was 5’-CCAGAACAAACGCCCAGTT-3’ (product band, 314 bp, Tm, 52°C). For glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the forward primer was 5’-CCTGCGACTTCAACAGTGC-3’ and the reverse primer was 5’-GCCTCTCGTCCTCCTCTG-3’ (product band, 210 bp, Tm, 58°C). PCR products were electrophoresed on a 1.5% agarose gel, and the relative amount of each product was calculated by Quantity One software as a ratio of the GAPDH band.
Statistical Analysis
All data were analyzed by SPSS 13.0 statistical software and are presented as mean ± standard deviation (x̄ ±SD). Chi-square test was used to examine the differences in mortality among the BMMNC-treated and vehicle-treated groups. One-way ANOVA and the least significant difference (LSD) test were used to identify group differences of cytokine levels. Repeated measures ANOVA followed by the LSD test was used to determine changes in serum lipid and body weight. Student’s t-test was used to analyze group differences in lesion size and composition. A p value < 0.05 was considered statistically significant.
Results
Confirmation of Model Establishment
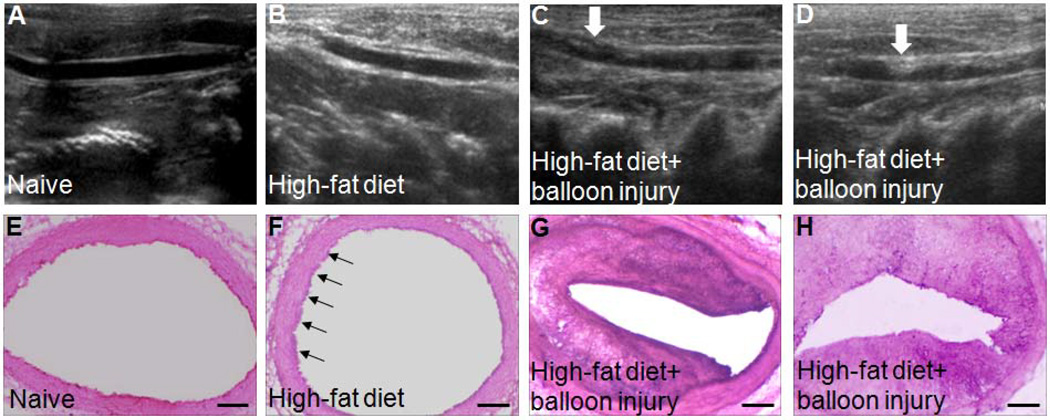
Ultrasound examination showed that all rabbits subjected to balloon injury and an 8-week high-fat diet formed carotid atherosclerotic plaques. No plaques were apparent in naive or sham-operated rabbits (Fig. 1A–D). HE staining revealed marked thickening of the intima and a large lipid core in rabbits that underwent balloon injury with a high-fat diet, indicating that plaques had formed. The intima of the sham-operated, high-fat diet group was somewhat thicker than the adjacent wall. In contrast, vessels in the naive group were smooth, with intact endothelial cells, non-thickened intima, and no fat accumulation (Fig. 1E–H).
Figure 1.
Ultrasound examination and hematoxylin and eosin (HE) staining show that rabbits subjected to balloon injury and a high-fat diet form carotid atherosclerotic plaque. (A–D) Representative ultrasound images of carotid vessels in rabbits. Rabbits fed a high-fat diet and subjected to balloon injury developed carotid atherosclerotic plaques (white arrows). (E–H) Representative HE-stained carotid vessels. The HE staining shows thicker intima in carotid vessels of rabbits after a high-fat diet and balloon injury, indicating the formation of plaques. The intima in carotid vessel of sham rabbits was partly thicker than the adjacent wall. Black arrows point to partly thickened intima. Scale bars = 200 µm. n=2 rabbits per group.
Mortality
Eight of 103 (7.8%) rabbits died of diarrhea, tineapedis, or anesthetic accidents before cell transplantation. The mortality after cell or vehicle treatment was 5/34 (14.7%) in vehicle-treated rabbits and 4/33 (12.1%) in BMMNC-treated rabbits, but did not differ between the two groups (χ2=0.096, p=0.756).
Serum Lipids and Body Weight
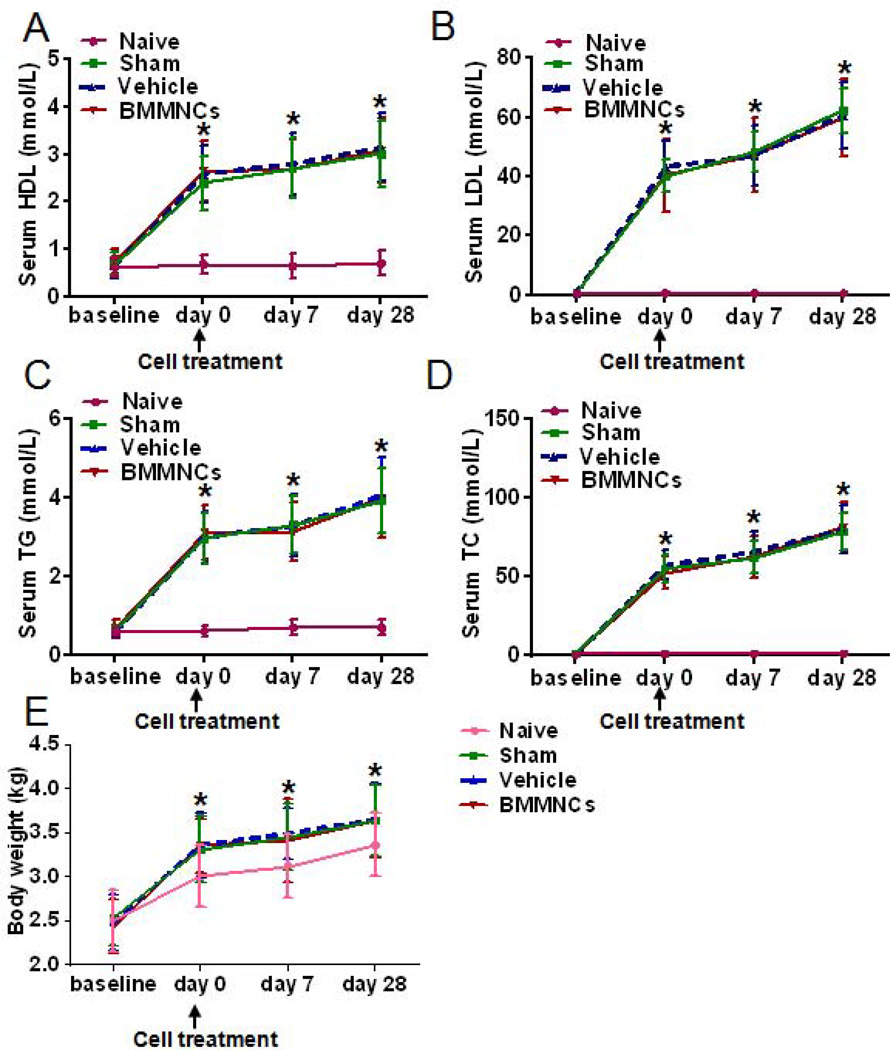
The serum lipid levels (HDL, LDL, TC, and TG) were significantly elevated in rabbits fed the high-fat diet compared to those in the naive group (p<0.05). Levels did not differ significantly between the vehicle-treated group and the sham-operated group (p>0.05). Moreover, BMMNC treatment did not alter serum lipid levels as compared with those of the vehicle group (p>0.05; Fig. 2A–D). Body weights increased significantly from baseline in all groups (p<0.05). However, no significant difference in body weight was present between BMMNC- and vehicle-treated groups (p>0.05; Fig. 2E).
Figure 2.
BMMNC treatment does not alter serum lipid levels or body weight. (A–D) The high-fat diet significantly elevated serum levels of high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), and total cholesterol (TC). *p<0.05 vs naive group. The levels in BMMNC- and vehicle-treated groups did not differ at any time point (p>0.05). Day 0 represents the serum lipid level before initiation of BMMNC (cell) treatment. (E) Body weight changes in each group. Rabbits in the high-fat diet groups were heavier than those in the naive group (*p<0.05 vs. naive group). BMMNC treatment did not affect the body weight of rabbits who had a high-fat diet and balloon injury (p>0.05 vs. vehicle group). n=12 rabbits per group per time point.
Pathology and Morphometry Evaluation of Plaque
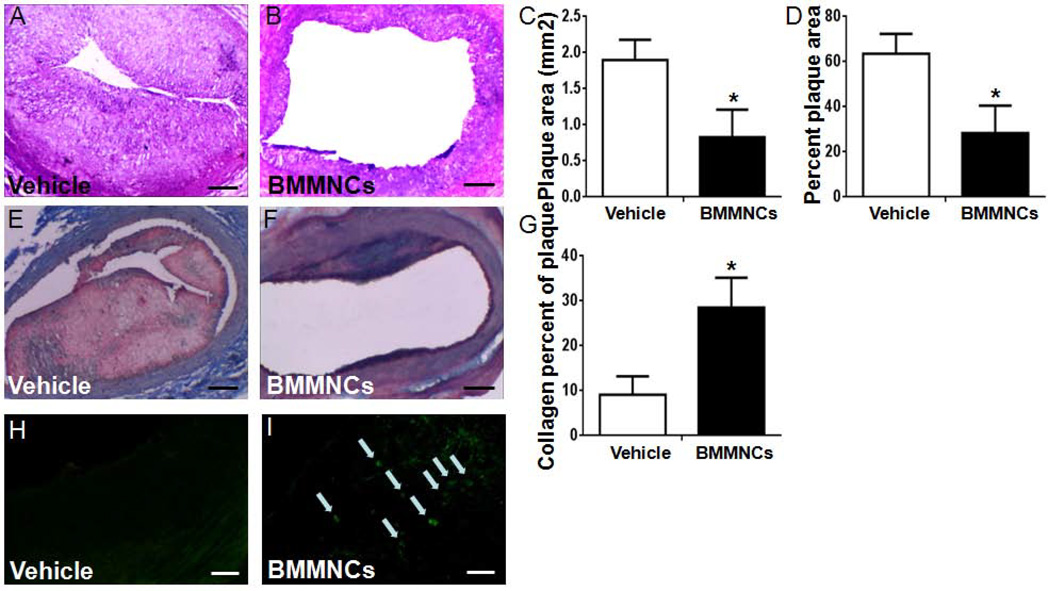
Quantification of HE staining showed that BMMNC transplantation significantly reduced the plaque size (vehicle: 1.972±0.281 µm2; BMMNCs: 0.831 ±0.380 µm2 t=5.901, p<0.001) and percentage of plaque area (vehicle: 63.6±8.7%; BMMNCs: 28.3±12.2%, t=5.768, p<0.001) on day 28 after cell therapy (Fig. 3A–D). At the same time, collagen deposition was dramatically elevated in BMMNC-treated rabbits compared to that in the vehicle-treated group, as assessed by Masson trichromatic staining (Fig. 3E–G).
Figure 3.
Transplanted BMMNCs reduced the plaque size and migrated into the plaque. (A, B) Representative HE-stained carotid vessel sections from vehicle- and BMMNC-treated rabbits on day 28 after cell transplantation. Scale bars = 200 µm. (C, D) Quantitative analysis showed that BMMNC transplantation significantly reduced the atherosclerotic plaque size and the percentage of plaque area in the carotid vessel compared with that of the vehicle-treated group. Percent plaque area= plaque area/vessel area. (E, F) Representative Masson’s trichrome staining on day 28 after cell transplantation. (G) Quantitative analysis showed that BMMNC transplantation significantly increased the percentage of collagen in the carotid vessel compared with that of the vehicle-treated group. Scale bars = 200 µm. *p<0.05 vs. vehicle group. n = 6/group. (H, I) BrdU-labeled BMMNCs (green) migrated to the atherosclerotic plaques. Labeled BMMNCs were observed homing to atherosclerotic plaques on day 1, but no BMMNCs were found in the plaque of vehicle-treated animals. Scale bars = 20 µm. n = 3 rabbits per group.
Migration of BMMNCs
BMMNCs labeled with BrdU were found to have migrated into atherosclerotic plaques on day 1 after cell transplantation (Fig. 3H, I).
BMMNC Treatment Suppresses Oxidative Stress in Plaque
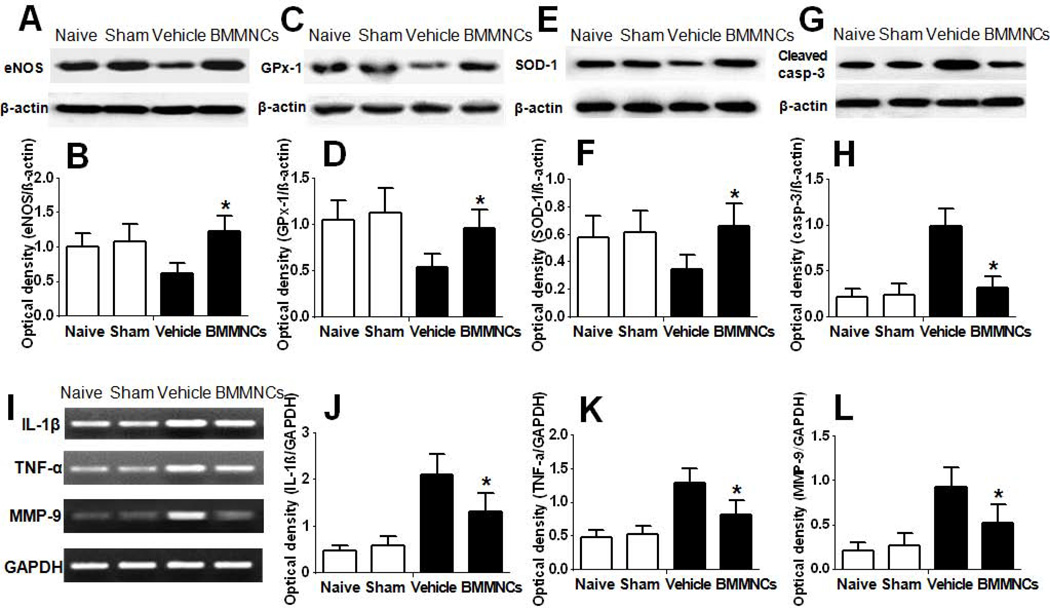
It is well known that oxidative stress contributes to the onset of atherosclerosis and reduces the production of GPx and SOD (antioxidant enzymes) [25] and eNOS (the rate-limiting enzyme of nitric oxide production). The downregulation of these protective proteins leads to acceleration of atherosclerosis progress. Western blotting showed that levels of GPx-1, SOD-1, and eNOS were decreased in the atherosclerotic carotid artery of vehicle-treated rabbits (p<0.05). However, BMMNC treatment ameliorated the reduction in these proteins on day 7 after cell transplantation (p<0.05 versus vehicle treatment; Fig. 4A–F).
Figure 4.
BMMNC transplantation reverses atherosclerosis-induced changes in antioxidant enzymes, eNOS, inflammatory response, and cell apoptosis. (A) Western blot analysis of eNOS in plaques. (B) Quantification analysis showed that plaques from the BMMNC group contained more eNOS than did plaques from the vehicle group. (C–F) BMMNC transplantation upregulated expression of the antioxidant enzymes GPx-1 and SOD-1 in plaque. (C, E) Western blot analysis of GPx-1 and SOD-1 in plaque. (D, F) Quantification of band densities showed that plaques from rabbits treated with BMMNCs had significantly higher GPx-1 and SOD-1 protein expression than did plaques from vehicle-treated rabbits. (G–H) BMMNC transplantation reduced expression of cell-death marker cleaved caspase-3 in plaque. (G) Western blot analysis of cleaved caspase-3. (H) Quantification analysis showed that cleaved caspase-3 was significantly lower in plaques from BMMNC-treated rabbits than in plaques from vehicle-treated rabbits. *p<0.05 vs. vehicle group. (I–L) BMMNC transplantation significantly decreased the mRNA expression of proinflammatory cytokines in plaque. (I) Representative RT-PCR bands showing mRNA expression of IL-1 β, TNF-α, and MMP-9 in plaque. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. (J–L) Analysis of band optical densities showed that BMMNC transplantation significantly decreased the mRNA expression of IL-1 β, TNF-α, and MMP-9 compared with that in vehicle-treated animals. *p<0.05 vs. vehicle group. n=6 rabbits per group.
BMMNC Treatment Prevents Cell Apoptosis in Plaques
The stability of atherosclerotic plaques is related to the balance between cell death and cell survival [26]. Caspase-3 is activated in apoptotic cells and transformed to cleaved caspase-3, which can reflect the extent of cell apoptosis. Western blot analysis showed more cleaved caspase-3 in vehicle-treated rabbits than in naive or sham-operated rabbits (p<0.05). BMMNC transplantation reduced the expression of cleaved caspase-3 compared with the vehicle-treated group (p<0.05 vs. vehicle treatment; Fig. 4G, H).
BMMNC Treatment Attenuates Inflammatory Response in Plaques
To eliminate OxLDL in intima, the immune system recruits a variety of inflammatory cells into the plaque, including monocytes and neutrophils, leading to a series of inflammatory responses [9]. Emerging evidence suggests that autologous BMMNCs have an anti-inflammatory effect in ischemic diseases [27]. Using RT-PCR, we found that production of IL-1β, TNF-α, and MMP-9 was significantly elevated in rabbits of the vehicle-treated group compared with that in the naive and sham-operated groups (p<0.05). MMP-9 is an important proteinase that can degrade extracellular matrix [28,29]; however elevated MMP-9 activity promotes atherosclerotic plaque instability [30]. Levels of the proinflammatory cytokines IL-1β and TNF-α, and the proteinase MMP-9, were significantly reduced by BMMNC treatment (p<0.05 vs. vehicle treatment; Fig. 4I–L).
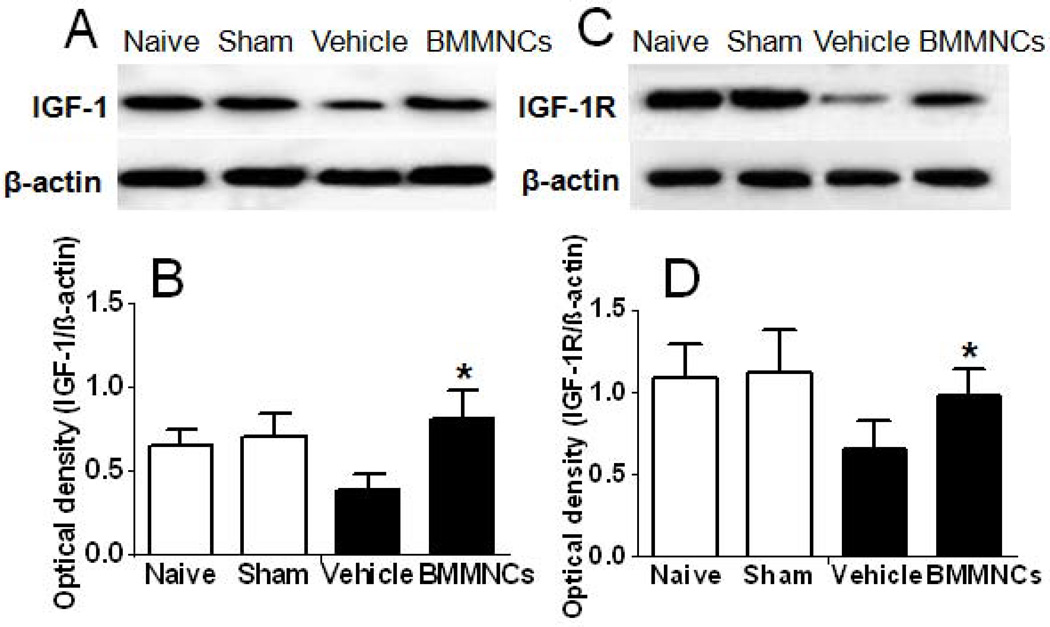
BMMNCs Upregulate the IGF-1/IGF-1R Signaling Pathway
Previous studies have shown that atherosclerosis causes downregulation of IGF-1 and its receptor, IGF-1R [31], which plays a central role in counteracting oxidative effects, inflammatory responses, and cell apoptosis in plaque [32]. In our study, western blot analysis showed that a high-fat diet and balloon injury mediated a significant reduction in the expression of IGF-1 and IGF-1R. BMMNC treatment significantly increased the levels of both IGF-1 and IGF-1R expression in the plaque (p<0.05 vs. vehicle treatment; Fig. 5).
Figure 5.
BMMNCs upregulate the protein expression of IGF-1 and IGF-1R in plaque. (A, C) Western blot analysis of IGF-1 and IGF-1R in plaque. (B, D) Quantification of band densities showed that protein expression of IGF-1 and IGF-1R was significantly higher in plaques of BMMNC-treated rabbits than in plaques of vehicle-treated rabbits. *p<0.05 vs. vehicle group. n=6 rabbits per group.
Discussion
Our results show for the first time that intravenous infusion of autologous BMMNCs reduces atherosclerotic plaque size and increases the collagen content of plaque in a rabbit model of atherosclerosis. The protection provided by the autologous BMMNC transplantation may involve reduction of proinflammatory cytokines (IL-1β, TNF-α), MMP-9 activity, and cleaved caspase-3 expression, and upregulation of eNOS, antioxidant enzymes (GPx-1, SOD-1), IGF-1, and IGF-1R. These novel findings suggest that autologous BMMNC transplantation has benefits for treatment of atherosclerosis.
It has been appreciated that oxidative stress plays a key role in the development and destabilization of arterial plaques [33]. In atherosclerotic pathology, deposition of redundant OxLDL particles in the intima leads to the accumulation and activation of macrophages, T cells, mast cells, and dendritic cells as well as proliferation of SMCs [34,3]. Subsequently, increased macrophages and SMCs form a large number of foam cells after phagocytosis of OxLDL [9]. In response to these changes in arterial wall, innate and adaptive immune responses are instigated, leading to marked elevation of proinflammatory cytokines IL-1β, TNF-α, and MMP-9 [8], which aggravate the instability of plaque [34]. In addition, oxidative stress also lowers NO bioavailability by reducing eNOS [35], the main enzymatic source of NO production [36]. As a defense against oxidative stress, SODs and GPx are the two most important enzymes to neutralize oxidants. However, atherosclerosis leads to persistent downregulation of antioxidant defense systems [6].
IGF-1 is synthesized by most tissues, and IGF-1R is expressed in most cells, including ECs and SMCs [37,38]. IGF-1 binding to IGF-1R leads to a variety of physiological functions [14]. Supplementing IGF-1 can reverse the downregulation of SODs and GPx, indicating that IGF-1 can directly counteract oxidative stress [39]. In addition, IGF-1 can enhance eNOS activity by reducing the inhibitory effect of ROS production on tetrahydrobiopterin. Furthermore, IGF-1 acutely enhances eNOS-dependent NO production by increasing phosphorylation at Ser1177 via a PI3K and Akt pathway [40]. However, OxLDL was shown to significantly and dose-dependently reduce IGF-1 and IGF-1R [31]. Recent evidence has shown that direct injection of IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice [17,32,41], further supporting these findings. Therefore, upregulation of IGF-1/IGF-1R may decelerate plaque formation, reduce the risk of plaque rupture, and lower the probability of cardiovascular diseases, including ischemic stroke and myocardial infarction.
A number of investigators have reported that BMMNCs have protective effects in ischemic diseases, especially myocardial infarction and ischemic stroke [42–44]. Multiple mechanisms have been shown to mediate their therapeutic benefits, including cell differentiation [45], cell fusion, and the secretion of growth factors and cytokines [27]. A recent study showed that BMMNCs could secrete IGF-1 in vitro and vivo and that BMMNC-derived conditioned medium could inhibit H2O2-mediated apoptosis of neonatal cardiomyocytes via IGF-1 [20]. Furthermore, IGF-1 released by BMMNCs facilitated elevation of endogenous IGF-1 in a positive-feedback fashion. In this study, we found that BMMNC transplantation significantly upregulated the expression of GPx-1, SOD-1, eNOS, and IGF-1/IGF-1R and reduced proinflammatory cytokines in plaque, supporting the antioxidative and anti-inflammatory effects of BMMNCs. Previous studies have confirmed that IGF-1 mediates antioxidative and anti-inflammatory effects in plaque. We infer that the antiatherogenic effect of BMMNCs could result from an upregulation of the IGF-1/IGF-1R signaling pathway. Compared with injection of IGF-1 to treat atherosclerosis, our approach is more economical, simple, and convenient.
Previous studies have shown that transplantation of bone marrow cells from naive mice into ApoE-deficient mice protects them from atherosclerosis [46,47]. In clinic, collection of BMMNCs from atherosclerotic patients themselves requires less than 3 h, and the use of autologous BMMNCs can prevent immunological rejection. However, conflicting results have also been reported [48,49]. Researchers who transplanted bone marrow cells from wild-type mice to age-matched apoE−/− mice found that BMMNC treatment increased the size of atherosclerotic plaques. The alteration of components in bone marrow may account for the discrepancy. We and others have demonstrated that stress, such as atherosclerosis and stroke, leads to profound changes in bone marrow [50].
Taken together, our findings indicate that autologous BMMNC transplantation can significantly suppress the process of atherosclerotic plaque development, possibly by attenuating the inflammatory response and oxidative stress and upregulating antioxidant enzymes. Our work provides support for a new treatment option to improve prognosis of atherosclerosis and a foundation for future preclinical studies on therapeutic approaches for atherosclerosis.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81371583) and the National Institutes of Health (R01NS078026, R01AT007317). We thank Michael Hong, Jiarui Wang, and Claire Levine for assistance with this manuscript.
Abbreviations
- BMMNCs
Bone marrow mononuclear cells
- BrdU
Bromodeoxyuridine
- ECs
Endothelial cells
- eNOS
Endothelial nitric oxide synthase
- GPx
Glutathione peroxidase
- IGF-1
Insulin-like growth factor 1
- IGF-1R
Insulin-like growth factor 1 receptor
- IL-1β
Interleukin 1β
- LDL
low-density lipoprotein
- MMP-9
Matrix metalloproteinase-9
- OxLDL
Oxidized low-density lipoprotein
- ROS
Reactive oxygen species
- SMCs
Smooth muscle cells
- SODs
Superoxide dismutases
- TNF-α
Tumor necrosis factor alpha
Footnotes
Compliance with ethical standards
The authors declare that they have no conflict of interest.
References
- 1.Byrnes KR, Ross CB. The current role of carotid duplex ultrasonography in the management of carotid atherosclerosis: foundations and advances. Int J Vasc Med. 2012;2012:187872. doi: 10.1155/2012/187872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Tullio MR, Russo C, Jin Z, Sacco RL, Mohr JP, Homma S. Aortic arch plaques and risk of recurrent stroke and death. Circulation. 2009;119(17):2376–2382. doi: 10.1161/CIRCULATIONAHA.108.811935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld ME, Palinski W, Yla-Herttuala S, Butler S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Duan J, Wang W, Dai S, Wu Y, Sun R, Ren J. Reactive oxygen species mediate oxidized low-density lipoprotein-induced endothelin-1 gene expression via extracellular signal-regulated kinase in vascular endothelial cells. J Hypertens. 2008;26(5):956–963. doi: 10.1097/HJH.0b013e3282f56bb7. [DOI] [PubMed] [Google Scholar]
- 6.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiological reviews. 2004;84(4):1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 7.Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8(3):237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Yurdagul A, Jr, Green J, Albert P, McInnis MC, Mazar AP, Orr AW. alpha5beta1 integrin signaling mediates oxidized low-density lipoprotein-induced inflammation and early atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(7):1362–1373. doi: 10.1161/ATVBAHA.114.303863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, Riksen NP. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol. 2014;34(8):1731–1738. doi: 10.1161/ATVBAHA.114.303887. [DOI] [PubMed] [Google Scholar]
- 10.Succurro E, Andreozzi F, Sciacqua A, Hribal ML, Perticone F, Sesti G. Reciprocal association of plasma IGF-1 and interleukin-6 levels with cardiometabolic risk factors in nondiabetic subjects. Diabetes Care. 2008;31(9):1886–1888. doi: 10.2337/dc08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papatheodorou L, Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal. 2007;9(11):1941–1958. doi: 10.1089/ars.2007.1750. [DOI] [PubMed] [Google Scholar]
- 12.Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F, Lehr HA, Blankenberg S, Forstermann U, Munzel T, Lackner KJ. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27(4):850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 13.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. The Journal of biological chemistry. 1997;272(26):16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 14.Weiss N. Mechanisms of increased vascular oxidant stress in hyperhomocys-teinemia and its impact on endothelial function. Curr Drug Metab. 2005;6(1):27–36. doi: 10.2174/1389200052997357. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Wang M, Zhang S, Zhao L. Oxidative stress in rats with hyperhomo-cysteinemia and intervention effect of lutein. Eur Rev Med Pharmacol Sci. 2014;18(3):359–364. [PubMed] [Google Scholar]
- 16.Lapenna D, de Gioia S, Ciofani G, Mezzetti A, Ucchino S, Calafiore AM, Napolitano AM, Di Ilio C, Cuccurullo F. Glutathione-related antioxidant defenses in human atherosclerotic plaques. Circulation. 1998;97(19):1930–1934. doi: 10.1161/01.cir.97.19.1930. [DOI] [PubMed] [Google Scholar]
- 17.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 18.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24(3):435–444. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 19.Drexler AL, Pietri JE, Pakpour N, Hauck E, Wang B, Glennon EK, Georgis M, Riehle MA, Luckhart S. Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi. PLoS Pathog. 2014;10(6):e1004231. doi: 10.1371/journal.ppat.1004231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iekushi K, Seeger F, Assmus B, Zeiher AM, Dimmeler S. Regulation of cardiac microRNAs by bone marrow mononuclear cell therapy in myocardial infarction. Circulation. 2012;125(14):1765–1773. doi: 10.1161/CIRCULATIONAHA.111.079699. S1761–1767. [DOI] [PubMed] [Google Scholar]
- 21.Zhu F, Li C, Jin XP, Weng SX, Fan LL, Zheng Z, Li WL, Wang F, Wang WF, Hu XF, Lv CL, Liu P. Celastrol may have an anti-atherosclerosis effect in a rabbit experimental carotid atherosclerosis model. Int J Clin Exp Med. 2014;7(7):1684–1691. [PMC free article] [PubMed] [Google Scholar]
- 22.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Jaff M, Kownator S, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS, Zannad F, Zureik M. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23(1):75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 23.Fang SM, Du DY, Li YT, Ge XL, Qin PT, Zhang QH, Liu Y. Allogeneic bone marrow mesenchymal stem cells transplantation for stabilizing and repairing of atherosclerotic ruptured plaque. Thrombosis research. 2013;131(6):e253–e257. doi: 10.1016/j.thromres.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Dong B, Zhang C, Feng JB, Zhao YX, Li SY, Yang YP, Dong QL, Deng BP, Zhu L, Yu QT, Liu CX, Liu B, Pan CM, Song HD, Zhang MX, Zhang Y. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28(7):1270–1276. doi: 10.1161/ATVBAHA.108.164715. [DOI] [PubMed] [Google Scholar]
- 25.Ou HC, Lee WJ, Lee IT, Chiu TH, Tsai KL, Lin CY, Sheu WH. Ginkgo biloba extract attenuates oxLDL-induced oxidative functional damages in endothelial cells. J Appl Physiol (1985) 2009;106(5):1674–1685. doi: 10.1152/japplphysiol.91415.2008. [DOI] [PubMed] [Google Scholar]
- 26.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12(9):1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 27.Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr, Aronowski J, Grotta JC, Savitz SI. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30(1):140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X, Wu T, Chang CF, Wu H, Han X, Li Q, Gao Y, Li Q, Hou Z, Maruyama T, Zhang J, Wang J. Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain Behav Immun. 2015;46:293–310. doi: 10.1016/j.bbi.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Wu T, Hua W, Dong X, Gao Y, Zhao X, Chen W, Cao W, Yang Q, Qi J, Zhou J, Wang J. PGE2 receptor agonist misoprostol protects brain against intracerebral hemorrhage in mice. Neurobiol Aging. 2015;36(3):1439–1450. doi: 10.1016/j.neurobiolaging.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blin J, Ahmad Z, Rampal LR, Mohtarrudin N, Tajudin AK, Adnan RS. Preliminary assessment of differential expression of candidate genes associated with atherosclerosis. Genes & genetic systems. 2013;88(3):199–209. doi: 10.1266/ggs.88.199. [DOI] [PubMed] [Google Scholar]
- 31.Higashi Y, Peng T, Du J, Sukhanov S, Li Y, Itabe H, Parthasarathy S, Delafontaine P. A redox-sensitive pathway mediates oxidized LDL-induced downregulation of insulin-like growth factor-1 receptor. J Lipid Res. 2005;46(6):1266–1277. doi: 10.1194/jlr.M400478-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol Metab. 2010;21(4):245–254. doi: 10.1016/j.tem.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu A, Ming JY, Fiskesund R, Ninio E, Karabina SA, Bergmark C, Frostegard AG, Frostegard J. Induction of Dendritic Cell-Mediated T-Cell Activation by Modified but Not Native Low-Density Lipoprotein in Humans and Inhibition by Annexin A5: Involvement of Heat Shock Proteins. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.304342. [DOI] [PubMed] [Google Scholar]
- 34.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 35.Silambarasan T, Manivannan J, Krishna Priya M, Suganya N, Chatterjee S, Raja B. Sinapic Acid prevents hypertension and cardiovascular remodeling in pharmacological model of nitric oxide inhibited rats. PLoS One. 2014;9(12):e115682. doi: 10.1371/journal.pone.0115682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. 837a-837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chisalita SI, Arnqvist HJ. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab. 2004;286(6):E896–E901. doi: 10.1152/ajpendo.00327.2003. [DOI] [PubMed] [Google Scholar]
- 38.Chisalita SI, Arnqvist HJ. Expression and function of receptors for insulin-like growth factor-I and insulin in human coronary artery smooth muscle cells. Diabetologia. 2005;48(10):2155–2161. doi: 10.1007/s00125-005-1890-4. [DOI] [PubMed] [Google Scholar]
- 39.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295(5):H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67(4):313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forman K, Vara E, Garcia C, Kireev R, Cuesta S, Escames G, Tresguerres JA. Effect of a combined treatment with growth hormone and melatonin in the cardiological aging on male SAMP8 mice. J Gerontol A Biol Sci Med Sci. 2011;66(8):823–834. doi: 10.1093/gerona/glr083. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Hiroi Y, Ngoy S, Okamoto R, Noma K, Wang CY, Wang HW, Zhou Q, Radtke F, Liao R, Liao JK. Notch1 in bone marrow-derived cells mediates cardiac repair after myocardial infarction. Circulation. 2011;123(8):866–876. doi: 10.1161/CIRCULATIONAHA.110.947531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoon CH, Koyanagi M, Iekushi K, Seeger F, Urbich C, Zeiher AM, Dimmeler S. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy: role of cardiovascular lineage commitment. Circulation. 2010;121(18):2001–2011. doi: 10.1161/CIRCULATIONAHA.109.909291. [DOI] [PubMed] [Google Scholar]
- 44.Raval Z, Losordo DW. Cell therapy of peripheral arterial disease: from experimental findings to clinical trials. Circ Res. 2013;112(9):1288–1302. doi: 10.1161/CIRCRESAHA.113.300565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godoy JA, Block DB, Tollefsen DM, Werneck CC, Vicente CP. Dermatan sulfate and bone marrow mononuclear cells used as a new therapeutic strategy after arterial injury in mice. Cytotherapy. 2011;13(6):695–704. doi: 10.3109/14653249.2010.548378. [DOI] [PubMed] [Google Scholar]
- 46.Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, Ninomiya T, Horiguchi H, Endo M, Tabata M, Tazume H, Tian Z, Takahashi O, Terada K, Takeya M, Hao H, Hirose N, Minami T, Suda T, Kiyohara Y, Ogawa H, Kaikita K, Oike Y. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler Thromb Vasc Biol. 2014;34(4):790–800. doi: 10.1161/ATVBAHA.113.303116. [DOI] [PubMed] [Google Scholar]
- 47.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108(4):457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 48.George J, Afek A, Abashidze A, Shmilovich H, Deutsch V, Kopolovich J, Miller H, Keren G. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2005;25(12):2636–2641. doi: 10.1161/01.ATV.0000188554.49745.9e. [DOI] [PubMed] [Google Scholar]
- 49.Silvestre JS, Gojova A, Brun V, Potteaux S, Esposito B, Duriez M, Clergue M, Le Ricousse-Roussanne S, Barateau V, Merval R, Groux H, Tobelem G, Levy B, Tedgui A, Mallat Z. Transplantation of bone marrow-derived mononuclear cells in ischemic apolipoprotein E-knockout mice accelerates atherosclerosis without altering plaque composition. Circulation. 2003;108(23):2839–2842. doi: 10.1161/01.CIR.0000106161.43954.DF. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Yu L, Jiang C, Fu X, Liu X, Wang M, Ou C, Cui X, Zhou C, Wang J. Cerebral ischemia increases bone marrow CD4(+)CD25(+)FoxP3(+) regulatory T cells in mice via signals from sympathetic nervous system. Brain Behav Immun. 2015;43:172–183. doi: 10.1016/j.bbi.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]