Abstract
Background:
The Predictive Hypoglycemia Minimizer System (“Hypo Minimizer”), consisting of a zone model predictive controller (the “controller”) and a safety supervision module (the “safety module”), aims to mitigate hypoglycemia by preemptively modulating insulin delivery based on continuous glucose monitor (CGM) measurements. The “aggressiveness factor,” a pivotal variable in the system, governs the speed and magnitude of the controller’s insulin dosing characteristics in response to changes in CGM levels.
Methods:
Twelve adults with type 1 diabetes were studied in closed-loop in a clinical research center for approximately 24 hours. This analysis focused primarily on the effect of the aggressiveness factor on the automated insulin-delivery characteristics of the controller, and secondarily on the glucose control results.
Results:
As aggressiveness increased from “conservative” to “medium” to “aggressive,” the controller recommended less insulin (–3.3% vs –14.4% vs –19.5% relative to basal) with a higher frequency (5.3% vs 14.4% vs 20.3%) during the critical times when the CGM was reading 90-120 mg/dl and decreasing. Blood glucose analyses indicated that the most aggressive setting resulted in the most desirable combination of the least time spent <70 mg/dl and the most time spent 70-180 mg/dl, particularly in the overnight period. Hyperglycemia, diabetic ketoacidosis, or severe hypoglycemia did not occur with any of the aggressiveness values.
Conclusion:
The Hypo Minimizer’s controller took preemptive action to prevent hypoglycemia based on predicted changes in CGM glucose levels. The most aggressive setting was quickest to take action to reduce insulin delivery below basal and achieved the best glucose metrics.
Keywords: Aggressiveness factor, algorithm, artificial pancreas, closed-loop control, model predictive control, type 1 diabetes
Closed-loop insulin delivery systems, which adjust insulin delivery based on data from a continuous glucose monitor (CGM), are designed to limit glucose excursions outside the normoglycemic range, however narrow that range may be. Development of these systems has the potential to improve the lives of individuals with type 1 diabetes by helping them achieve the therapeutic goal of restoring near-normoglycemia. Even well before the 2006 launch of the Artificial Pancreas Project1—a consortium of leading diabetes research institutions in both Academia and Industry—many studies have demonstrated the feasibility of a variety of closed-loop systems in individuals with type 1 diabetes, including insulin-only artificial pancreas systems2-12 and bihormonal systems.13-15
We previously reported that the Hypoglycemia–Hyperglycemia Minimizer (HHM) System was a feasible basis for the development of a closed-loop insulin delivery system.16 The Predictive Hypoglycemia Minimizer System (“Hypo Minimizer”) is a modification of the HHM System, which focuses on preemptively mitigating hypoglycemia by attempting to control glucose to above a specified low glucose threshold (LGT).
The Hypo Minimizer control algorithm utilizes CGM and insulin-infusion data to predict future hypoglycemia, and has been developed to attenuate or suspend insulin infusion to mitigate, if not prevent, this predicted hypoglycemia. In addition, the Hypo Minimizer algorithm generates proactive alerts in advance of predicted hypoglycemia, to better assist the patient in the prevention of hypoglycemia. In contrast to threshold-based low-glucose suspend systems, which abruptly suspend insulin infusion when the CGM reading breaches a LGT (eg, 90 mg/dl), the Hypo Minimizer is designed to mitigate low-glucose excursions in advance by incremental titrations of insulin dose.
One of the pivotal variables in this system is the “aggressiveness factor.”17 The aggressiveness factor in certain model predictive control frameworks is an adjustable tuning parameter that gives relative weights to how “undesirable” it is to change the value of the manipulated variable away from a set point versus how undesirable the resulting predictions of the controlled variable are. In the case of the Hypo Minimizer algorithm, the manipulated variable is the insulin infusion amount, and its set point is the subject’s basal profile, which had been optimized prior to this study in consultation with the subject’s physician; the controlled variable is the glucose as measured by the CGM. Thus, the Hypo Minimizer’s aggressiveness factor is a parameter that affects the speed and magnitude of the insulin-dosing response, as calculated by a controller, to predictions of CGM levels.
The aim of this feasibility study was to evaluate the effect of the Hypo Minimizer’s aggressiveness factor on the automated insulin-delivery characteristics of the controller in response to CGM levels.
Methods
This nonrandomized, uncontrolled, feasibility study enrolled 12 adults with type 1 diabetes at a clinical research center (CRC; Sansum Diabetes Research Institute [SDRI], Santa Barbara, CA) from July 16 to August 27, 2013. Participants were studied in closed-loop for approximately 24 hours, receiving 3 meals of 30-70 g carbohydrates (CHO) each, with matched insulin boluses administered at meal times.
This study abided by the principles of the Declaration of Helsinki, and received approval from the relevant institutional review board (Compass Independent Review Board). All subjects provided written informed consent prior to study initiation.
Study Objectives
The primary objective was to evaluate the effect of the aggressiveness factor on the quantitative insulin-dosing characteristics of the Hypo Minimizer’s controller. The secondary objective was to evaluate the ability of the Hypo Minimizer to safely maintain glucose levels >70 mg/dl.
Participants and Study Design
Inclusion and exclusion criteria for participants and study design were similar to those previously described by Finan and colleagues.16 Briefly, adults with type 1 diabetes using an insulin infusion pump along with rapid-acting insulin and with an A1c <10% were enrolled. Prior to the CRC visit, individual participants’ pump settings, including basal rates and insulin-to-CHO ratios, were assessed and a CGM sensor was inserted. The Hypo Minimizer was assessed during the CRC visit, which lasted approximately 24 hours for each participant. Close clinical supervision and regular Yellow Springs Instruments (YSI) monitoring were performed. The CGM was calibrated in accordance with the manufacturer’s instructions. A follow-up telephone call was conducted 24 hours after discharge.
Treatment Interventions
Treatment interventions included algorithm-initiated and investigator-initiated treatment for hypoglycemia. For algorithm-initiated treatment for hypoglycemia, the Hypo Minimizer’s safety module includes warnings for imminent hypoglycemia, which advise considering ingesting 16 g of supplemental CHO. In this study, per protocol, the investigator administered the supplemental CHO at the time of the warning. For investigator-initiated treatment for hypoglycemia, treatment interventions were given in the form of supplemental CHO in the absence of a Hypo Minimizer System warning for hypoglycemia when the YSI indicated a glucose concentration <60 mg/dl. Due to the short duration of the intervention, no severe hyperglycemia was expected to occur; however, in the case of mild hyperglycemia, in this study defined as hyperglycemia not requiring protocol-mandated safety intervention, treatment consisted of additional insulin at the discretion of the investigator, to better ensure algorithm activity.
Investigational Device
The Hypo Minimizer consisted of an insulin pump (OneTouch® Ping® Glucose Management System, Animas Corporation, West Chester, PA)/CGM (Dexcom G4® Platinum CGM, Dexcom, Inc, San Diego, CA) system, regulated by a proprietary, investigational closed-loop algorithm run on the University of California Santa Barbara (UCSB)/SDRI Artificial Pancreas System (APS©) platform.18 The Hypo Minimizer algorithm included a zone model predictive controller (zMPC; the “controller”)19 to control insulin delivery and a safety supervision module (SSM; the “safety module”) 20,21 with built-in warnings for imminent hypoglycemia.
Based on new glucose data received from the CGM nominally every 5 minutes, glucose and insulin data from the recent past, and internal model states, the Hypo Minimizer algorithm provided a prediction describing the optimal glucose outcome for the near future by manipulating near-future insulin delivery amounts. An insulin micro-bolus consisting of the dose determined to result in the best glucose outcome was delivered immediately. This process was repeated every 5 minutes.
Aggressiveness Factor
The primary parameter evaluated during this study was the aggressiveness factor, which affects how quickly and to what degree the controller responds to changes in glucose. To investigate the effect of insulin-delivery characteristics of the algorithm at the different aggressiveness factor values, 3 aggressiveness factor values were evaluated: a “conservative” value, a “medium” value, and an “aggressive” value. Four participants were assigned to each of the 3 settings. In general, a “conservative” controller will tend to adhere to the basal insulin delivery amount, unless the CGM indicates a severe excursion into hypoglycemia. An “aggressive” controller, on the other hand, will react more quickly to changing CGM levels, readily adjusting insulin delivery away from the basal amount to prevent modest predicted glucose excursions. The controller is designed to deliver only insulin doses that are corrective in nature; it is not designed to dose insulin that is directly related to CHO meals. As such, all “controller doses” reported in this study should be construed as corrective doses.
This Hypo Minimizer is designed to have some redundancy between the controller and the safety module.16 The safety module is not directly affected by the aggressiveness factor; therefore, the current analyses focus on the effect of the aggressiveness factor only on the controller.
Statistical Methods
This early-stage feasibility study was exploratory in nature, and thus never intended to be sufficiently powered to show statistically significant differences in glucose or insulin-delivery metrics. Rather, it was intended to provide directional development guidance as part of our due diligence in designing the algorithm. To this end, the primary objective (to evaluate the effect of the aggressiveness factor on the quantitative insulin-dosing characteristics of the controller) was evaluated for each aggressiveness factor subcohort (4 patients), in aggregate, using the CGM and YSI measurements, insulin infusion records, and logs of CHO meal consumption, as well as any other information collected during these evaluations. A Statistical Analysis Plan was written and finalized prior to locking the final database. All the summaries and analyses were performed using validated original software, commonly used computer programs (eg, Matlab and Microsoft Excel), and SAS Version 9.1 or higher.
Results
Participant Characteristics
A total of 12 adults with type 1 diabetes were enrolled in the study and investigated in closed-loop. Participant characteristics are shown in Table 1.
Table 1.
Participant Characteristics (N = 12).
| Characteristic | |
|---|---|
| Age, years, mean ± SD (range) | 46.0 ± 10.9 (28.4-59.1) |
| Gender, n (%) | |
| Female | 7 (58.3) |
| Male | 5 (41.7) |
| Ethnicity, n (%) | |
| Hispanic | 1 (8.3) |
| Non-Hispanic | 11 (91.7) |
| Race, n (%) | |
| Asian | 1 (8.3) |
| White | 11 (91.7) |
| Weight, lbs | |
| Mean ± SD (range) | 163.7 ± 45.4 (107.8-276.0) |
| Body mass index, kg/m2 | |
| Mean ± SD (range) | 24.3 ± 4.7 (18.5-36.4) |
| Duration of type 1 diabetes, years | |
| Mean ± SD (range) | 28.2 ± 12.2 (8.5-46.6) |
| Duration of pump use, years | |
| Mean ± SD (range) | 10.8 ± 8.7 (1.4-32.6) |
| Hemoglobin A1c, % | |
| Mean ± SD | 7.3 ± 1.0 (5.6-8.7) |
| Range | |
Effect of Aggressiveness Factor on Controller-Calculated Insulin
Overall, a 79.4% reduction in insulin delivery during below-threshold excursions (CGM <90 mg/dl) was observed with the Hypo Minimizer, relative to the basal rate the subject would have received in standard, open-loop conditions (0.21 vs 0.88 U/h). Regardless of the aggressiveness factor, the Hypo Minimizer algorithm took preemptive, insulin-reducing action, prior to every below-threshold excursion; there were a total of 20 such preemptive actions taken (conservative: 6; medium: 6; aggressive: 8).
Table 2 shows the mean insulin dose calculated by the controller relative to the corresponding basal rate, and the frequency of samples for which the controller calculated an insulin delivery amount substantially (≥25%) lower than the corresponding basal amount. As the controller is meant to minimize hypoglycemia, the metrics were calculated only when the CGM reading was decreasing from 1 sample to the next.
Table 2.
Insulin Delivery Calculations by Algorithm Aggressiveness Factor.
| Glucose range and criteria | Insulin delivery metric | Aggressiveness factor value (%) |
||
|---|---|---|---|---|
| Conservative | Medium | Aggressive | ||
| Significantly higher than the LGT, 120 <CGM ≤ 190 mg/dl, decreasing | Average insulin delivery calculated by controller (relative to corresponding basal amount) | −1.7 | −3.0 | −9.8 |
| Frequency of attenuationa | 2.2 | 4.3 | 9.8 | |
| Marginally higher than the LGT, 90 < CGM ≤ 120 mg/dl, decreasing | Average insulin delivery calculated by controller (relative to corresponding basal amount) | −3.3 | −14.4 | −19.5 |
| Frequency of attenuationa | 5.3 | 14.4 | 20.3 | |
| At or marginally lower than the LGT, 70 < CGM ≤ 90 mg/dl, decreasing | Average insulin delivery calculated by controller (relative to corresponding basal amount) | −6.4 | −36.6 | −25.0 |
| Frequency of attenuationa | 11.5 | 43.8 | 31.7 | |
The percentage of controller recommendations that were substantially less than the corresponding basal amount (by at least 25%).
CGM, continuous glucose monitor; LGT, low glucose threshold.
When the CGM was significantly higher than the LGT (defined as 120 <CGM ≤190 mg/dl) and decreasing (defined as the current CGM value < the previous CGM value), the following insulin-dosing characteristics were observed. The conservative controller only minimally attenuated insulin dose, on average calculating 1.7% less insulin than the corresponding basal dose, and calculating substantial attenuation only 2.2% of the time (Table 2). When aggressiveness was increased to “medium” and “aggressive,” both the amount of insulin withheld by the controller and the frequency of doses that were substantially attenuated increased. The aggressive controller calculated on average 9.8% less insulin than basal and attenuated doses substantially 9.8% of the time (Table 2).
When the CGM was only marginally higher than the LGT (defined as 90 <CGM ≤120 mg/dl) and decreasing, indicating a potential near-future breach of the LGT, the trend in dose attenuation among the 3 aggressiveness factors was similar, though each of the controllers was significantly more active. In this region, the average insulin delivery amount calculated by the controller was 3.3%, 14.4%, and 19.5% less than the corresponding basal amount as the aggressiveness factor increased from conservative to medium to aggressive (Table 2). The frequency of controller calculations resulting in substantial attenuation also increased with increasing aggressiveness.
When the CGM was at or marginally lower than the LGT (defined as 70 <CGM ≤90 mg/dl) and decreasing, the degree of activity was further increased for each of the controllers (Table 2). However, the aggressive controller actually withheld less insulin (25.0%) than the medium controller (36.6%) and attenuated the dose less frequently (31.7% of the time vs 43.8% for the medium controller). This counterintuitive result can likely be explained by the paucity of data at the low-glucose range observed in this small feasibility study, resulting in an increased effect of variability on the results.
Effect of Aggressiveness Factor on Glucose Metrics
The secondary objective of the study was related to the ability of the Hypo Minimizer to keep the subject’s glucose levels >70 mg/dl. Table 3 shows the CGM and the YSI glucose results from the 12 subjects studied under closed-loop control. On average, very little closed-loop time was spent at glucose readings <70 mg/dl (0.9% by CGM and 1.5% by YSI). Notably, only 0.4% and 0.3% of the overnight closed-loop time, defined as approximately 11:00 pm-7:00 am, was spent at levels <70 mg/dl, as measured by CGM and YSI, respectively. For the overall period (which included 3 study meals), 68.3% of the time was spent between 70 and180 mg/dl according to CGM readings, while 70.0% of this time was spent in this same range according to YSI readings. For the overnight period, 71.4% and 72.8% of the time was spent between 70 and 180 mg/dl according to CGM and YSI readings, respectively.
Table 3.
Glucose Control Metrics by Algorithm Aggressiveness Factor Based on CGM and YSI.
| Range | Closed-loop glucose control metrics |
|||||||
|---|---|---|---|---|---|---|---|---|
| Overall |
Overnight (approx. 11:00 pm-7:00 am) |
|||||||
| Conservative (n = 4) | Medium (n = 4) | Aggressive (n = 4) | All subjects (n = 12) | Conservative (n = 4) | Medium (n = 4) | Aggressive (n = 4) | All subjects (n = 12) | |
| CGM | ||||||||
| Time spent <70 mg/dl (%) | 1.3 | 1.1 | 0.4 | 0.9 | 1.3 | 0.0 | 0.0 | 0.4 |
| Time spent 70-180 mg/dl (%) | 60.9 | 73.0 | 71.0 | 68.3 | 64.7 | 66.5 | 83.0 | 71.4 |
| Median glucose (mg/dl) | 154 | 149 | 133 | 148 | 136 | 166 | 118 | 149 |
| YSI | ||||||||
| Time spent <70 mg/dl (%) | 0.8 | 3.3 | 0.3 | 1.5 | 0.8 | 0.0 | 0.0 | 0.3 |
| Time spent 70-180 mg/dl (%) | 63.3 | 73.0 | 73.8 | 70.0 | 67.8 | 69.9 | 80.7 | 72.8 |
| Median glucose (mg/dl) | 152 | 144 | 137 | 145 | 129 | 157 | 116 | 141 |
CGM, continuous glucose monitor; YSI, Yellow Springs Instruments.
Compared with the participants using the medium and aggressive settings, those with the conservative setting spent more time <70 mg/dl and less time in the normoglycemic range, both overall and overnight (Table 3). With the aggressive setting, participants spent as much or less time <70 mg/dl, both overall and overnight, as measured by both CGM and YSI; in fact, neither the medium nor aggressive controllers resulted in any time spent <70 mg/dl overnight, as measured by both CGM and YSI. In the overnight period, the aggressive controller resulted in markedly more time spent in the normoglycemic range than the other 2 controllers (Table 3).
The median glucose values (Table 3) indicate that the participants studied at the aggressive setting showed the lowest median glucose both overall and overnight, by CGM and YSI. Overnight, the aggressive setting showed medians of 118 mg/dl by CGM and 116 mg/dl by YSI.
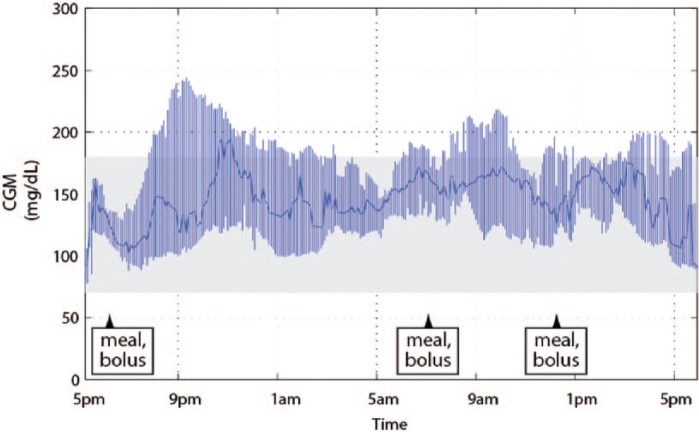
Figure 1 shows the median CGM tracing, with the interquartile range (IQR), for the entire cohort. The median CGM tracing for all participants was almost entirely contained within the desirable 70-180 mg/dl range. Furthermore, the IQR did not breach the lower threshold of 70 mg/dl.
Figure 1.
Median CGM tracing shown with the IQR for the entire cohort (N = 12). The shaded area is the approximately normoglycemic range of 70-180 mg/dl. CGM, continuous glucose monitor; IQR, interquartile range.
Safety
Eight administrations of supplemental CHO were given to mitigate hypoglycemia (conservative: 1; medium: 3; aggressive: 4); 6 were based on algorithm recommendations. Of the other 2 administrations, 1 was in response to a YSI reading <70 mg/dl (YSI 56.2 mg/dl; CGM 76 mg/dl), and the other was at the discretion of the investigator. One algorithm recommendation was ignored at the discretion of the investigator.
There were no protocol-defined glucose-related safety events (severe diabetic ketoacidosis or severe hypoglycemia). There were 16 total “treatments” for mild hyperglycemia (conservative: 2; medium: 7; aggressive: 7), which consisted of small corrective boluses given at the investigator’s discretion to reduce glucose to better ensure algorithm activity.
CGM-Related Issues
Two device malfunctions were reported. In both cases, there was a loss of wireless communication between the CGM and the controller. For 1 subject, the device was restarted within 3 hours. For the other subject, after 2 failed attempts to restart the system, a new computer was used to restart the closed-loop within 2 hours. For both subjects, a correction bolus was administered to compensate for the missing basal insulin during the interruption.
Discussion
Results of this study demonstrated that the Hypo Minimizer was able to take preemptive action to prevent hypoglycemia based on predicted changes in glucose levels as measured by CGM. These predictions of hypoglycemia were evidenced by the controller’s actions, which consisted of reducing insulin delivery rates below the subjects’ corresponding preprogrammed basal rates in the times leading up to low glucose levels.
The primary objective of this study was to evaluate the effect of the aggressiveness factor on the quantitative insulin-dosing characteristics of the Hypo Minimizer’s controller. Differences in insulin-dosing characteristics were observed with the 3 aggressiveness factors. Typically, with increasing aggressiveness, the controller reduced insulin relative to basal more quickly and with greater magnitude and frequency in anticipation of a potential breach of the LGT. By both CGM and YSI, the aggressive setting resulted in the least amount of time spent below the 70 mg/dl threshold overall (0% for overnight) and the most time spent in the 70-180 mg/dl range, particularly overnight. Moreover, the aggressive setting resulted in the lowest median glucose regardless of measurement device and time range. This finding is relevant in that it shows (albeit in this small study) that the relatively aggressive insulin-reducing action did not result in a trade-off of higher glucose values, as might have been expected. Therefore, the most aggressive setting was found to be the most feasible for further investigation.
Preemptive insulin-reducing action was taken prior to every below-threshold excursion for all 3 aggressiveness factors. Overall, the most aggressive controller attenuated doses most frequently, and decreased the delivered dose most compared to the corresponding basal amount; however, in the glucose region just below the LGT, the aggressive controller withheld less insulin (25.0% vs 36.6%) and attenuated the dose less frequently (31.7% vs 43.8% of the time) than the medium controller. These counterintuitive results are probably due to sample sizes that were relatively small and therefore susceptible to effects of variability in individual subject’s in-clinic data, particularly at the low-glucose range.
This study is limited primarily by the small sample size, relatively short observation period, and use of an artificial, sedentary CRC-based environment. In addition, the study was exploratory in nature and was not statistically powered to show reductions in hypoglycemia metrics; however, the insulin-delivery characteristics of the controller were analyzed formally and found to demonstrate feasibility for further product development.
Recent evidence suggests that the psychological and physical benefits of a closed-loop system outweigh practical challenges, when used overnight at home.22 If the efficacy and safety of the current system can be demonstrated in an outpatient setting, it has the potential to substantially improve the lives of individuals with type 1 diabetes. The system could potentially reduce the occurrence of hypoglycemia, particularly nocturnal hypoglycemia (in this study, 0% of overnight time was spent below 70 mg/dl with the medium and aggressive settings). In addition, it could help individuals with type 1 diabetes maintain normoglycemia and achieve their therapeutic goals.
In principle, a closed-loop system such as this one may be made more effective by tailoring controller aggressiveness to the individual, even in a recursive, adaptive framework. The appropriate aggressiveness factor would depend on the accuracy of the model predictions. Model predictions that accurately predict the trajectory of a particular subject’s glucose should beget a very aggressive controller, while model predictions that show some mismatch in its predictions—while still useful—should beget a conservative controller that “hedges its bets” by responding only to large, compelling fluctuations in glucose. Assessing the accuracy of the model predictions in real time is in many cases impossible due to the nature of the controller, which continuously adjusts insulin dose, thereby effectively changing the predicted future from one given sample to the next. However, predictive accuracy may be assessed retrospectively in recent history, providing an indication of how the model predictions may be performing currently. CGM sensor accuracy is another crucial input to the accuracy of the model predictions, which may be similarly evaluated for the recent history via comparisons to, for example, finger-stick readings. More research is warranted to determine the feasibility of this next level of sophistication.
Conclusions
The objectives of this feasibility study were met; these included showing the ability of the Hypo Minimizer to respond by varying insulin infusion rates based on closed-loop CGM glucose levels in an attempt to keep glucose levels above a specified threshold. The aggressive setting of the controller was found to have the most desirable attributes related to its tendency to reduce or suspend insulin within the appropriate CGM context.
Acknowledgments
The authors would like to thank JDRF and the staff of SDRI for their participation in this study. The authors received editorial and writing support from Excerpta Medica for preparation of this manuscript. Clinicaltrials.gov identifier: NCT01919385. Parts of this study were presented at the 74th Scientific Sessions of the American Diabetes Association (ADA), San Francisco, CA, June 13-17, 2014.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DAF, TWM, BLL, and RV are employees of Animas Corporation. ED has received product support from Animas, Dexcom, and Insulet. MDB has received research grants and product support from Animas, Insulet, Dexcom, Roche, Sanofi, Abbott, BD, Lilly, and Tandem Diabetes Care. SDP holds equity interest in TypeZero, LLC. BPK is on the advisory panel for Sanofi Aventis and AstraZeneca; has received research support from Animas, Dexcom, Insulet, Roche Diagnostic, and Tandem Diabetes Care; and receives patent royalties from Sanofi Aventis and J&J. The UCSB technology used in this study developed in part by ED and FJD has been licensed to Animas. The University of Virginia technology used in this study developed in part by BPK, MDB, and SDP has been licensed to Animas.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by JDRF and Animas Corporation.
Abbreviations: APS, artificial pancreas system; CGM, continuous glucose monitor; CHO, carbohydrate; CRC, clinical research center; HHM, Hypoglycemia–Hyperglycemia Minimizer; IQR, interquartile range; LGT, low glucose threshold; SD, standard deviation; SDRI, Sansum Diabetes Research Institute; SSM, safety supervision module; UCSB, University of California, Santa Barbara; YSI, Yellow Springs Instruments; zMPC, zone model predictive controller.
References
- 1. Kowalski AJ. Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther. 2009;11(suppl 1):S113-S119. [DOI] [PubMed] [Google Scholar]
- 2. Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dassau E, Zisser H, Harvey RA, et al. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36(4):801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Del Favero S, Bruttomesso D, Di Palma F, et al. First use of model predictive control in outpatient wearable artificial pancreas. Diabetes Care. 2014;37:1212-1215. [DOI] [PubMed] [Google Scholar]
- 5. Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people living with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37:1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37:1931-1937. [DOI] [PubMed] [Google Scholar]
- 7. Ly TT, Breton MD, Keith-Hynes P, et al. Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care. 2014;37:2310-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kovatchev BP, Renard E, Cobelli C, et al. Feasibility of outpatient fully integrated closed-loop control: first studies of a wearable artificial pancreas. Diabetes Care. 2013;36(7):1851-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37(7):1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824-833. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt S, Boiroux D, Duun-Henriksen AK, et al. Model-based closed-loop glucose control in type 1 diabetes: the DiaCon experience. J Diabetes Sci Technol. 2013;7(5):1255-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thabit H, Lubina-Solomon A, Stadler M, et al. Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol. 2014;2(9):701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El-Khatib FH, Russell SJ, Magyar KL, et al. Autonomous and continuous adaptation of a bihormonal pancreas in adults and adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2014;99(5):1701-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371(4):313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobs PG, El Youssef J, Castle E, et al. Automated control of an adaptive bihormonal, dual-sensor artificial pancreas and evaluation during inpatient studies. IEEE Trans Biomed Eng. 2014;61(10):2569-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finan DA, McCann TW, Jr, Mackowiak L, et al. Closed-loop control performance of the Hypoglycemia-Hyperglycemia Minimizer (HHM) System in a feasibility study. J Diabetes Sci Technol. 2014;8(1):35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finan DA, McCann TW, Jr, Rhein K, et al. Effect of algorithm aggressiveness on the performance of the Hypoglycemia-Hyperglycemia Minimizer (HHM) System. J Diabetes Sci Technol. 2014;8(4):685-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dassau E, Zisser H, Palerm CC, et al. Modular artificial beta-cell system: a proto-type for clinical research. J Diabetes Sci Technol. 2008;2(5):863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doyle FJ, III, Huyett LM, Lee JB, et al. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care. 2014; 37(5):1191-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes CS, Patek SD, Breton MD, Kovatchev BP. Hypoglycemia prevention via pump attenuation and red-yellow-green “traffic” lights using continuous glucose monitoring and insulin pump data. J Diabetes Sci Technol. 2010;4(5):1146-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patek SD, Magni L, Dassau E, et al. Modular closed-loop control of diabetes. IEEE Trans Biomed Eng. 2012;59(11):2986-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barnard KD, Wysocki T, Allen JM, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Res Care. 2014;2(1)e000025. doi: 10.1136/bmjdrc-2014-000025. [DOI] [PMC free article] [PubMed] [Google Scholar]