Abstract
Background:
Model-based glycemic control relies on sufficiency of underlying models to describe underlying patient physiology. In particular, very preterm infant glucose-insulin metabolism can differ significantly from adults, and is relatively unstudied. In this study, C-peptide concentrations are used to develop insulin-secretion models for the purposes of glycemic control in neonatal intensive care.
Methods:
Plasma C-peptide, insulin, and blood glucose concentrations (BGC) were retrospectively analyzed from a cohort of 41 hyperglycemic very preterm (median age 27.2 [26.2-28.7] weeks) and very low birth-weight infants (median birth weight 839 [735-1000] g). A 2-compartment model of C-peptide kinetics was used to estimate insulin secretion. Insulin secretion was examined with respect to nutritional intake, exogenous and plasma insulin concentration, and BGC.
Results:
Insulin secretion was found to be highly variable between patients and over time, and could not be modeled with respect to age, weight, or protein or dextrose intake. In 13 of 54 samples exogenous insulin was being administered, and insulin secretion was lower. However, low data numbers make this result inconclusive. Insulin secretion was found to increase with BG, with a stronger association in female infants than males (R2 = .51 vs R2 = .13, and R2 = .26 for the combined cohort).
Conclusions:
A sex-based insulin secretion model was created and incorporated into a model-based glycemic control framework. Nutritional intake did not predict insulin secretion, indicating that insulin secretion is a complex function of a number of metabolic factors.
Keywords: glycemic control, insulin therapy, insulin secretion, physiological modeling, very preterm infants
Hyperglycemia (elevated blood glucose concentrations; BGCs) is a common complication of prematurity in very preterm (gestational age [GA] < 32 weeks) infants,1,2 where stress and illness are compounded by immaturity of glucose-insulin physiology.3-5 Hyperglycemia in very preterm babies has been associated with increased risk of morbidity and mortality,1,6,7 while hypoglycemia (low BGC) in preterm babies has also been associated with adverse neurodevelopment outcomes.8
There is currently no best practice method or target for glycemic control in preterm babies and BGC is often controlled by varying nutritional input.9 Insulin therapy has been well established to improve glucose tolerance and increases postnatal weight gain (eg, Ostertag et al,10 Thabet et al,11 Kanarek et al,12 Pollak et al13). However, insulin treatment in preterm babies often results in excessive, iatrogenic, or protocol induced hypoglycemia.14,15 Metabolic variability is a leading cause of this problem.16
STAR (stochastic targeted glycemic control) is a decision support tool that uses a physiological model-based estimate of insulin sensitivity to describe a patient’s metabolic state with respect to insulin-glucose dynamics. STAR has proven safe and effective in adult intensive care,17,18 and a first iteration in the neonatal intensive care unit (NICU) has also shown promising results with tighter control and lower hypoglycemic incidence than other studies.19
STAR is a model-based system and thus relies, in part, on an accurate model of the glucose-insulin regulatory system to facilitate safe and accurate glycemic control. A key aspect of this modeling is endogenous secretion of insulin by the pancreas. Interpatient variability of endogenous insulin secretion can be a major cause of metabolic variability and difficulty in glycemic control. In very low birth weight (VLBW) preterm infants, clinical limitations mean that pancreatic insulin secretion cannot be quantified directly, and most studies indirectly assess insulin secretion through peak plasma insulin concentration.
The aim of this study was to develop a model of insulin secretion in preterm neonates using a C-peptide kinetic model. A moreaccurate model of neonatal insulin secretion will then be used to improve the current glucose-insulin physiological models used by STAR in the NICU. These results build on previous results examining the effect of discrete (eg, sex, ethnicity, singleton vs multiple births) and clinical (eg, CRIB2 score) factors on insulin secretion in preterm neonates.20
Methods
Patient Cohort
The cohort and C-peptide analysis have been described elsewhere in full.15,20 In brief, retrospective analysis was carried out on plasma samples collected during a randomized control trial of glycemic control (The HINT trial,15 Australian Clinical Trials Registry 12606000270516, ethics approval from Northern X ethics committee). Hyperglycemic (2 BGC measures > 153 mg/dL more than 4 hours apart) very preterm (GA < 32 weeks, or birth weight < 1500 g) neonates were assigned to tight glycemic control (TGC, target BGC range 72-108 mg/dL) or standard care at National Women’s Health NICU, Auckland City Hospital, New Zealand.15
BGC, insulin infusions, and daily nutritional intake were recorded. Blood samples were taken to determine plasma insulin (Azsym system auto-analyzer, Abbott Laboratories, Abbott Park, IL) and BGC (glucose oxidise method, ABL 700, Radiometer Ltd, Copenhagen, Denmark) for each infant at randomization, 7 and 14 days after randomization, and at GA = 36 weeks. Remaining plasma samples were frozen.
Retrospective C-peptide analysis (immunometric assays, Elecsys 2010, Roche Diagnostics, Germany) was carried out on some of the frozen samples if there was sufficient remaining blood from samples taken 0-15 days after randomization, and if infant had GA < 32 weeks. Cohort characteristics are given in Table 1.
Table 1.
Sample Cohort Patient Characteristics.
| Characteristic | Value |
|---|---|
| Total patients | 41 |
| Control group | 21 |
| Tight glycemic control group | 20 |
| Male | 20 (49%) |
| Multiple birth | 11 (27%) |
| Antenatal steroid exposure | 39 (95%) |
| Maternal diabetes | 1 (2%) |
| Age | |
| Gestational, weeks | 27.2 [26.2-28.7] |
| Postnatal age, days | 9.5 [4 -17] |
| Birth weight | |
| grams | 839 [735-1000] |
| z score | –0.19 [–1.03-0.14] |
| Small for gestational age | 6 (15%) |
| CRIB 2 score | 12 [10-14] |
| Ethnicity | |
| Asian | 9 (22%) |
| Caucasian | 11 (27%) |
| Maori | 17 (41%) |
| Pacific Islander | 4 (10%) |
| Sample data | |
| Number of samples | 54 |
| Day after randomization | 7 [0-14] |
| BGC, mg/dL | 135 [92-189] |
| Plasma insulin concentration, mU/L | 59.0 [99.3-181.9] |
| Plasma C-peptide concentration,nmol/L | 2.3 [1.1-4.2] |
| Cortisol at randomization, μg/dL | 10.1 [9.1-15.1] |
Numbers are presented as median [IQR] or number (% of total). CRIB 2, Clinical Risk Index for Babies.
Model Equations of C-Peptide Kinetics
C-peptide is secreted in equimolar quantities with insulin, and is predominantly cleared by the kidney. In comparison, insulin is cleared by liver and peripheral tissues in a highly variable manner, as well as through the kidneys. Therefore, the relatively simple kinetics of C-peptide provide a better means to estimate insulin secretion.21 A 2 compartment kinetics model21 is used to describe the concentration of C-peptide in the central compartment of plasma, C, and peripheral extra vascular compartment, Y:
| (1) |
| (2) |
The rate of C-peptide (and insulin) secretion is S, and transport of C-peptide from the central to the peripheral compartment, and vice versa, is described by k1 and k2. The parameter k3 describes the irreversible renal clearance of C-peptide from the central compartment via the kidney.21
Sampling constraints due to limited blood volume in this cohort (~50 mL/kg)22 mean frequent, serial measurements of C-peptide were not physically or ethically possible. Assuming steady-state, it follows from equation 2 that the rate of C-peptide entering and leaving the peripheral compartment must be equal. Hence, substituting this equality into equation 1 and rearranging yields:
| (3) |
Since insulin is secreted in equimolar quantities with C-peptide, under steady-state conditions the rate of secretion of insulin is directly proportional to the measured concentration of C-peptide in the central compartment. In infants fed via constant IV infusion, this steady-state assumption is reasonable.
Since no studies have been performed in preterm or term neonates to determine C-peptide kinetics, adult data and methodology21,23 were used as an approximation given that the functionality is also no different. A short half life of 4.95 minutes and a fraction, F, of 0.96 was used based on nonobese or diabetic adult data.21 The long half life thus was calculated using:21
| (4) |
To give an estimated long half-life of 29.2 minutes for newborns.23 The kinetic parameters were then individually calculated using these cohort specific values, as per:21
| (5) |
| (6) |
| (7) |
Where a = log(2)/(short half life), and b = log(2)/(long half life). The resulting calculated value for for all neonates was 0.0644 min-1, which is within the reported normal clearance rates in Table 2.
Table 2.
| Patient cohort | k1 [1/min] | k2 [1/min] | k3 [1/min] | |
|---|---|---|---|---|
| Eaton et al, 198024 | Normal n = 20 | 0.047 ± 0.002 | 0.035 ± 0.002 | 0.049 ± 0.001 |
| Van Cauter et al, 199221 | Normal n = 111 | 0.053 ± 0.002 | 0.051 ± 0.001 | 0.062 ± 0.001 |
| Obese n = 53 | 0.067 ± 0.003 | 0.051 ± 0.002 | 0.065 ± 0.013 | |
| Polonsky et al, 198623 | Normal n = 10 | 0.057 ± 0.006 | 0.054 ± 0.006 | 0.06 ± 0.002 |
| Diabetic n = 7 | 0.037 ± 0.004 | 0.031 ± 0.004 | 0.057 ± 0.002 |
Values are mean ± SEM.
Trend Analysis
Endogenous insulin secretion was calculated using equations 3-7. Results were analyzed with respect to patient birth weight, GA, dextrose and protein intake, nutritional delivery method, plasma insulin and BGC, and patient sex, to determine strong predictors of insulin secretion within this cohort.
Statistical Analysis
Results are presented as median with interquartile range (IQR). Nonparametric data were analyzed by the Mann–Whitney U test, and the Kruskal–Wallis test, which extends the Mann–Whitney U test to more than 2 samples. Correlations were calculated using a linear least squares regression analysis, and P values are given with respect to the null hypothesis that the slope of the linear regression is 0. Statistical power of subgroup results analysis is calculated using the method of Whitley and Ball26 applied to log-normalized insulin secretion values. Multiple linear regression across a range of variables, such as GA, weight, postnatal age, and BGC, was used to generate more complex models.
Results
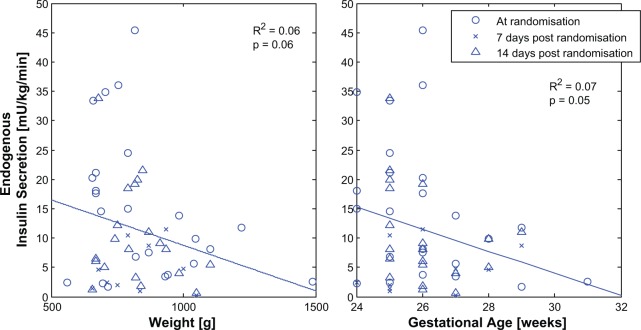
Birth weight or GA and insulin secretion were not strongly correlated (Figure 1; R2 ≤ .07), but endogenous insulin secretion did decrease with increasing weight and/or GA (P ≤ .06). However, trends with postnatal age are confounded by the fact that there was significantly higher BGC at randomization than 7-14 days postrandomization (99 [81-125] vs 191 [164-229] mg/dL, P < .005).
Figure 1.
Insulin secretion is highly variable with birth weight.
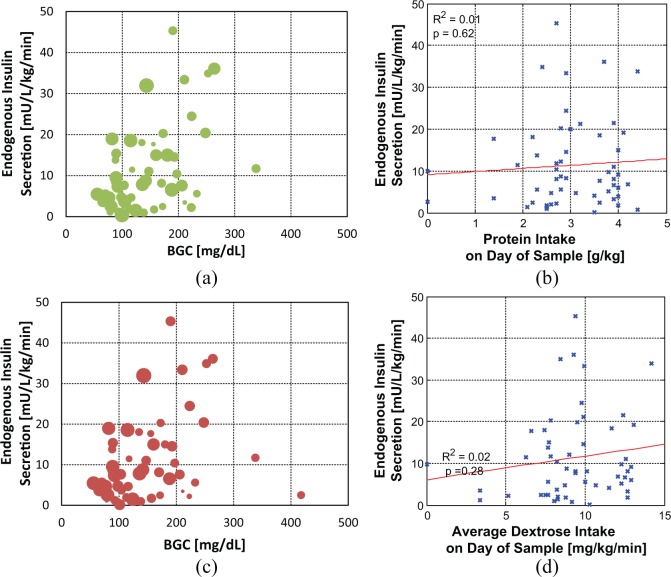
Neither daily protein nor dextrose intake was significantly correlated with insulin secretion (Figure 2). Adjusting for BGC at the time of the sample did not affect this result, with high and low protein intakes being equally scattered with respect to blood glucose and insulin secretion rate. Insulin secretion could not be modeled based on nutritional intake.
Figure 2.
Endogenous insulin secretion and blood glucose concentration (BGC) with respect to protein (a and b) and total dextrose intake (c and d) on the day the sample was taken. In (a) and (c) data points are scaled in size by the magnitude of nutritional intake, with data points from infants with a larger mass of intake being larger in size.
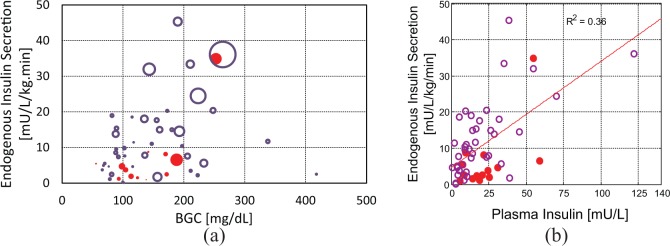
In 13 babies there was an exogenous insulin infusion at the time of the C-peptide sample. Figure 3 shows lower insulin secretion in the presence of exogenous insulin (3.7 [1.8-6.9] vs 9.8 [4.7-17.8] mU.kg-1.min-1, P = .02, statistical power 90%). There was a positive relationship between plasma insulin concentration and insulin secretion, as shown in Figure 3b, but this is heavily influenced by a relative few measures toward the upper end of the data range. There was no clear relationship between both BGC and plasma insulin with insulin secretion (Figure 2a). While there is evidence of suppression of insulin secretion with exogenous insulin, data are insufficient to build further models.
Figure 3.
Endogenous insulin secretion with (a) blood glucose concentration (BGC) and plasma insulin concentration, and (b) plasma insulin. In (a) data points are scaled in size by the magnitude of plasma insulin, with larger data points representing samples with higher plasma insulin concentration. Open (o) and closed (●) circles denote results from infants not receiving and receiving exogenous insulin at the time of sampling, respectively.
There was a weak relationship between insulin secretion and BGC (Figure 4), which was stronger in females than males. It has been previously reported that the difference between the sexes in insulin secretion was true over the entire BGC range (P < .005, statistical power > 95%) with no statistically significant difference between clinical characteristics (P ≥ .17), plasma insulin concentration (P = .30), or nutrition regimes (P ≥ .34).20 Figure 4 also shows separate male and female models for BGC dependant secretion in these cohorts, as well as an overall cohort method. Insulin secretion as a function of sex and BGC is defined:
Figure 4.
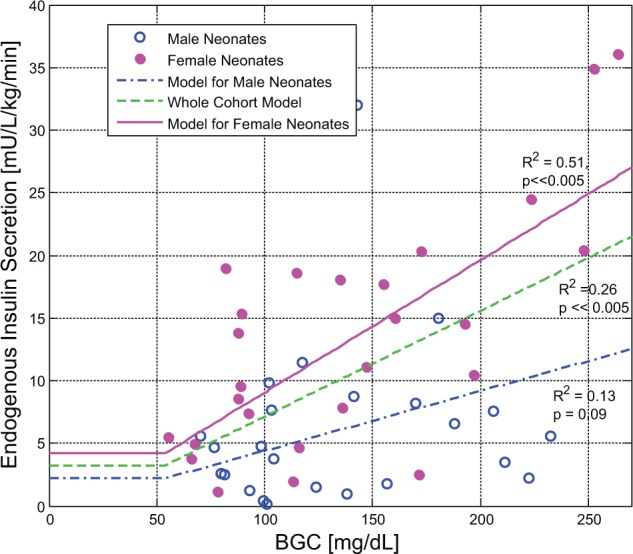
Models of endogenous insulin secretion as a function of blood glucose concentration (BGC) over the whole cohort and male and female subcohorts. A total of 5 data points from 54 total (9%) were excluded from the analysis as outliers based on a 2- to 3-fold difference with other data points of similar BGC. Of these data points, 3/5 were from heavier and older patients of GA > 29 weeks. However, 2 were from male and female babies with a GA of 26 weeks.
| (8) |
The 95% confidence interval for the intercept and slope were (–7.9, 4.9) and (0.061, 0.150), respectively for the female subcohort, and (–8.0, 8.0) and (–0.150, 0.206), respectively for the male subcohort. The whole cohort model in Figure 4 is defined:
| (9) |
The 95% confidence interval for the intercept and slope of the whole cohort model was (–7.2, 4.6) and (-0.046, 0.132).
Multiple linear regression models that accounted for combinations of GA, weight, postnatal age, BGC, dextrose intake, and exogenous insulin were not able to predict insulin secretion (R2 ≤ .2).
Discussion
In the past insulin secretion in preterm infants has been indirectly analyzed using peak plasma insulin concentration. In contrast, this study assesses insulin secretion using C-peptide concentrations, which is a more accurate predictor insulin secretion due to its simpler and less variable clearance kinetics. From these data, models of insulin secretion were built.
Insulin secretion was found to increase with increasing BGC, a result reported previously in infant and adult dogs.27 In preterm infants, a reduction in plasma glucose concentration in hyperglycemic preterm infants has been observed to be accompanied by a reduction in insulin secretion.5 This result matches expected physiology, where GLUT2 transporters in pancreatic beta cells enable sensitivity to changes in BGC.28
Sensitivity of insulin secretion to BGC was higher in females. This result is consistent with previous work showing overall higher insulin secretion in female preterm infants, independent of a number of clinical factors.20 Insulin secretion was similar between males and females at the lower end of the basal blood glucose range, but the linear model fitted to the female subcohort had a larger slope, indicating heightened pancreatic response to changes in BGC. This result perhaps suggests that the males were sicker or had higher C-peptide clearance. Higher insulin secretion in the females at comparable plasma insulin concentrations could also indicate higher insulin clearances. However, glomerular filtration rate has not been observed to differ with sex previously.29 Insulin secretion and glycemic differences between the sexes in later life is more fully discussed elsewhere.20
No differentiation of insulin secretion rates was seen between differing levels of protein or glucose intake in the neonates. This result is unexpected as an increase in plasma insulin concentrations in response to a glucose stimulus,30-34 amino acids such as theophylline,32,35-38 and glucose priming30 has been previously been observed in preterm infants. This discrepancy could be a result of differing study methodology and the more direct approach to estimating insulin secretion taken here. It could also be a result of nutrition records reflecting daily totals only, or reflect the differing physiological stress and degree of prematurity. The latter is regarded as more likely, as nutrition infusions or feeds are usually kept relatively constant throughout the day. Previous work analyzed differences between feeding methods, and found no difference in insulin secretion with method or bias by infant sex distribution between groups.20 In contrast to previous studies, this cohort is hyperglycemic, and thus more likely to have an underlying condition. In this study, some neonates showed high insulin secretion rates with relatively higher protein intakes (3-5 g/kg/day), as would be expected. In those that did not behave as expected, it is possible that if a pancreas is compromised due to prematurity, or for any reason, then the presence of protein is unlikely to affect endogenous insulin secretion.
There was a positive correlation between endogenous insulin secretion and plasma insulin. Infants in this post hoc analysis were evenly distributed between control and TGC cohorts in the original HINT study.15 Male and female infants were evenly spread between the exogenous insulin and no exogenous insulin groups, indicating sex was not responsible for this difference, and vice versa. These data points are tightly clustered with respect to BGC, and are thus heavily influenced by 1 or 2 points at extremes in BGC. In addition, endogenous insulin secretion was not further suppressed in the presence of increasing exogenous insulin infusion, as might be expected.
The regulation of blood glucose is complex and involves both the liver and the pancreas. Unfortunately, it was not possible to describe the contribution of the liver to glycemic regulation in this study. In adults, insulin secretion by the pancreas is regulated by plasma insulin and BGC, as well as hormones such as glucagon, cortisol, and adrenaline.39-41 Stress can also affect secretion,42 generally through these aforementioned hormones. Previous work did not find trends between insulin secretion and GA, randomization, singleton versus multiple birth, ethnicity, plasma cortisol, prenatal steroid exposure, or nutrition delivery type.20 Multiple linear regression using GA, weight, postnatal age, nutritional intake, exogenous insulin, and BGC as predictor variables did not significantly alter insulin secretion predictability. The individual effect of each of these factors is impossible to isolate, and very complicated models that could not be specified easily at the bedside, if at all, would be required to successfully model insulin secretion to a high degree of accuracy. Much of the variability observed in these data can probably be attributed to stress, differing patient conditions, and the effect of hormone signaling on the steady-state assumption. In addition, data available were insufficient to give an indication of insulin secretion between morbidity groups in the cohort.
The major assumption of this research is that of steady-state C-peptide kinetics. This assumption was driven by necessity, as the very low blood volume of very premature neonates (~50 mL/kg)22 means serial sampling of blood over time periods necessary to capture metabolic dynamics is not ethically and practically possible,43 particularly given the large percentage of total blood volume that would be required. However, while this assumption may be necessary, it does not inevitably follow that it is entirely inaccurate.
C-peptide dynamics are predominantly a function of insulin (and C-peptide) secretion and kidney clearance. Insulin secretion is known to be affected by a number of factors, which are predominantly nutritional. Steady state is therefore a reasonable assumption in premature infants who receive their nutrition intravenously. While this assumption is theoretically less reasonable in enterally fed infants, no significant difference in insulin secretion was found between enterally and parenterally fed infants (P = .59), and the sex-based difference in insulin secretion held if only parenterally fed infants were considered.20 This perhaps suggests that enteral nutrition administration times (~5-30 minutes depending on delivery method and patient condition) are long enough, and/or feeds are administered frequently enough, to approximate steady-state administration, or that there was little effect on insulin secretion due to immaturity in gut function, hormonal signaling, or metabolism.
Renal clearance rates are affected by GA and weight,44 medication, and illness and injury such as sepsis and intraventricular hemorrhage.45 Differences in renal clearance of C-peptide may account for some of the interpatient variability in insulin secretion results, but is unlikely to affect the assumption of steady state, particularly given constant nutrition inputs. The insulin secretion model developed is a population model and reflects a generalized response across the cohort. As previously mentioned, glomerular filtration rate has not been previously observed to differ with sex.29
Finally, while there is the potential that some glucose-insulin flux was occurring in any given infant, across the cohorts used there are enough subjects to assure that the central tendency holds. Thus, the snapshot of data obtained would be random in regard to a given net tendency or flux. Hence, since the model is based on cohort trends, the central tendency should hold around this assumption.
Adult C-peptide kinetic parameters, adjusted for age, were used because of the inability to comprehensively derive parameters for the neonatal cohort. If the resulting insulin secretion is also calculated using k3 kinetic values that are approximately 2 standard deviations (k3 = [0.05, 0.07]) from the normal kinetics reported in Table 2, then the insulin secretion could be in error by up to ~20%. In addition, it is not possible in this cohort to determine patient specific C-peptide kinetic parameter values. For the purposes of model-based control, the k3 parameter used is thus sufficient, and changes to this parameters does not change any of the results or trends observed, but only shift these trends. In addition, in terms of outputs from the control protocol, any scale inaccuracies are absorbed and scale the time varying patient-specific insulin sensitivity parameter.46 In model-based control, it is the insulin secretion dynamic shape, more than the value (assuming it is within a reasonable rang of the true value), that is important, so scale inaccuracies will not significantly affect control outputs.
This study has been carried out in the context of model-based control. While samples were taken from both arms of a glycemic control trial and reflect a range of different clinical intervention histories, it is believed that this cohort adequately describes likely candidates for glycemic control and thus the results provide new insights from data that are only rarely available. A total of 54 samples is greater than the 51 samples required for a regression analysis with a single independent variable.47 As a result of this study, a sex- and BGC-based model for insulin secretion in preterm infants was created.
Conclusions
Insulin secretion was estimated from C-peptide concentrations and used to generate a model for use in model-based glycemic control. BGC and sex were found to be the strongest predictors for insulin secretion, with females having higher insulin secretion and a more consistent increase with BGC. Insulin secretion was observed to be lower in the presence of exogenous insulin, but data were insufficient to be conclusive. Insulin secretion was not found to be highly correlated with glucose or protein intake. Insulin secretion in preterm neonates is a complex function of a number of factors, and high variability is seen between patients of a similar GA and weight.
Footnotes
Abbreviations: BGC, blood glucose concentration; BW, birth weight; CRIB 2, Clinical Risk Index for Babies; DOR, day of randomization; GA, gestational age; NICU, neonatal intensive care unit; STAR, stochastic targeted glycemic control; TGC, tight glucose control; VLBW, very low birth weight.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Jennifer Dickson was funded by a Canterbury PhD Scholarship. The original HINT study from which the blood samples were obtained was funded by the Auckland Medical Research Foundation.
References
- 1. Hey E. Hyperglycaemia and the very preterm baby. Semin Fetal Neonatal Med. 2005;10(4):377-387. [DOI] [PubMed] [Google Scholar]
- 2. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE study. J Pediatr. 2010;157(5):715-719. [DOI] [PubMed] [Google Scholar]
- 3. Farrag HM, Nawrath LM, Healey JE, et al. Persistent glucose production and greater peripheral sensitivity to insulin in the neonate vs. the adult. Am J Physiol. 1997;272(1 pt 1):E86-E93. [DOI] [PubMed] [Google Scholar]
- 4. Cowett RM, Oh W, Schwartz R. Persistent glucose production during glucose infusion in the neonate. J Clin Invest. 1983;71(3):467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitanchez-Mokhtari D, Lahlou N, Kieffer F, Magny J-F, Roger M, Voyer M. Both relative insulin resistance and defective islet {beta}-cell processing of proinsulin are responsible for transient hyperglycemia in extremely preterm infants. Pediatrics. 2004;113(3):537-541. [DOI] [PubMed] [Google Scholar]
- 6. Hall NJ, Peters M, Eaton S, Pierro A. Hyperglycemia is associated with increased morbidity and mortality rates in neonates with necrotizing enterocolitis. J Pediatr Surg. 2004;39(6):898-901; discussion 898-901. [DOI] [PubMed] [Google Scholar]
- 7. Alaedeen DI, Walsh MC, Chwals WJ. Total parenteral nutrition-associated hyperglycemia correlates with prolonged mechanical ventilation and hospital stay in septic infants. J Pediatr Surg. 2006;41(1):239-244. [DOI] [PubMed] [Google Scholar]
- 8. Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. Brit Med J. 1988;297(6659):1304-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alsweiler JM, Kuschel CA, Bloomfield FH. Survey of the management of neonatal hyperglycaemia in Australasia. J Paediatr Child Health. 2007;43(9):632-635. [DOI] [PubMed] [Google Scholar]
- 10. Ostertag S, Jovanovic L, Lewis B, Auld P. Insulin pump therapy in the very low birth weight infant. Pediatrics. 1986;78(4):625-630. [PubMed] [Google Scholar]
- 11. Thabet F, Bourgeois J, Guy B, Putet G. Continuous insulin infusion in hyperglycaemic very-low-birth-weight infants receiving parenteral nutrition. Clin Nutr. 2003;22(6):545-547. [DOI] [PubMed] [Google Scholar]
- 12. Kanarek KS, Santeiro ML, Malone JI. Continuous infusion of insulin in hyperglycemic low-birth weight infants receiving parenteral nutrition with and without lipid emulsion. J Parenter Enteral Nutr. 1991;15(4):417-420. [DOI] [PubMed] [Google Scholar]
- 13. Pollak A, Cowett RM, Schwartz R, Oh W. Glucose disposal in low-birth-weight infants during steady state hyperglycemia: effects of exogenous insulin administration. Pediatrics. 1978;61(4):546. [PubMed] [Google Scholar]
- 14. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. 2008;359(18):1873-1884. [DOI] [PubMed] [Google Scholar]
- 15. Alsweiler JM, Harding JE, Bloomfield FH. Tight glycemic control with insulin in hyperglycemic preterm babies: a randomized controlled trial. Pediatrics. 2012;129(4):639-647. [DOI] [PubMed] [Google Scholar]
- 16. Chase JG, Le Compte AJ, Suhaimi F, et al. Tight glycemic control in critical care—the leading role of insulin sensitivity and patient variability: a review and model-based analysis. Comput Methods Programs Biomed. 2011;102(2):156-171. [DOI] [PubMed] [Google Scholar]
- 17. Fisk L, Lecompte A, Penning S, Desaive T, Shaw G, Chase G. STAR development and protocol comparison. IEEE Trans Biomed Eng. 2012;59(12):3357-3364. [DOI] [PubMed] [Google Scholar]
- 18. Evans A, Shaw GM, Le Compte A, et al. Pilot proof of concept clinical trials of Stochastic Targeted (STAR) glycemic control. Ann Intensive Care. 2011;1(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le Compte AJ, Lynn AM, Lin J, Pretty CG, Shaw GM, Chase JG. Pilot study of a model-based approach to blood glucose control in very-low-birthweight neonates. BMC Pediatr. 2012;12(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dickson JL, Chase JG, Pretty CG, Gunn CA, Alsweiler JM. Hyperglycaemic preterm babies have sex differences in insulin secretion. Neonatology. 2015;108(2):93-98. [DOI] [PubMed] [Google Scholar]
- 21. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368-377. [DOI] [PubMed] [Google Scholar]
- 22. Usher R, Lind J. Blood volume of the newborn premature infant. Acta Paediatr Scand. 1965;54:419-431. [DOI] [PubMed] [Google Scholar]
- 23. Polonsky KS, Licinio-Paixao J, Given BD, et al. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest. 1986;77(1):98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eaton RP, Allen RC, Schade DS, Erickson KM, Standefer J. Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab. 1980;51(3):520-528. [DOI] [PubMed] [Google Scholar]
- 25. Polonsky KS, Given BD, Pugh W, et al. Calculation of the systemic delivery rate of insulin in normal man. J Clin Endocrinol Metab. 1986;63(1):113-118. [DOI] [PubMed] [Google Scholar]
- 26. Whitley E, Ball J. Statistics review 4: sample size calculations. Crit Care. 2002;6(4):335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adam PA, Kornhauser D, Link D, Schwartz R. Relationship of insulin secretion rate to blood glucose concentration in newborn and adult dogs. Biol Neonat. 1969;14(3):194-202. [DOI] [PubMed] [Google Scholar]
- 28. Tirone TA, Brunicardi FC. Overview of glucose regulation. World J Surg. 2001;25(4):461-467. [DOI] [PubMed] [Google Scholar]
- 29. Rhodin MM, Anderson BJ, Peters AM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67-76. [DOI] [PubMed] [Google Scholar]
- 30. Grasso S, Distefano G, Messina A, Vigo R, Reitano G. Effect of glucose priming on insulin response in the premature infant. Diabetes. 1975;24(3):291-294. [DOI] [PubMed] [Google Scholar]
- 31. Grasso S, Fallucca F, Romeo MG, Distefano G, Sciullo E, Reitano G. Glucagon and insulin secretion in low birthweight preterm infants. The effect of glucose infusion. Acta Paediatrica Scandinavica. 1990;79(3):280-285. [DOI] [PubMed] [Google Scholar]
- 32. Grasso S, Messina A, Distefano G, Vigo R, Reitano G. Insulin secretion in the premature infant. Response to glucose and amino acids. Diabetes. 1973;22(5):349-353. [DOI] [PubMed] [Google Scholar]
- 33. Andronikou S, Hanning I. Parenteral nutrition effect on serum insulin in the preterm infant. Pediatrics. 1987;80(5):693. [PubMed] [Google Scholar]
- 34. Nakai T, Hayashi M, Kanazawa Y, Kosaka K, Kigawa T. Alterations of insulin-secreting response to glucose in human infants during the early postnatal period. Endocrinol Jpn. 1976;23(1):61-64. [DOI] [PubMed] [Google Scholar]
- 35. Grasso S, Messina A, Saporito N, Reitano G. Serum-insulin response to glucose and aminoacids in the premature infant. Lancet. 1968;2(7571):755-756. [DOI] [PubMed] [Google Scholar]
- 36. Grasso S, Messina A, Saporito N, Reitano G. Effect of theophylline, glucagon and theophylline plus glucagon on insulin secretion in the premature infant. Diabetes. 1970;19(11):837-841. [DOI] [PubMed] [Google Scholar]
- 37. Salle BL, Ruiton-Ugliengo A. Effects of oral glucose and protein load on plasma glucagon and insulin concentrations in small for gestational age infants. Pediatr Res. 1977;11(2):108-112. [DOI] [PubMed] [Google Scholar]
- 38. Cser A, Milner RD. Glucose tolerance and insulin secretion in very small babies. Acta Paediatrica Scandinavica. 1975;64(3):457-463. [DOI] [PubMed] [Google Scholar]
- 39. Gelfand RA, Matthews DE, Bier DM, Sherwin RS. Role of counterregulatory hormones in the catabolic response to stress. J Clin Invest. 1984;74(6):2238-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bessey PQ, Lowe KA. Early hormonal changes affect the catabolic response to trauma. Ann Surg. 1993;218(4):476-489; discussion 489-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980;65(3):717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Black PR, Brooks DC, Bessey PQ, Wolfe RR, Wilmore DW. Mechanisms of insulin resistance following injury. Ann Surg. 1982;196(4):420-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Farrag HM, Cowett RM. Glucose homeostasis in the micropremie. Clin Perinatol. 2000;27(1):1-22. [DOI] [PubMed] [Google Scholar]
- 44. Coulthard MG. Maturation of glomerular filtration in preterm and mature babies. Early Hum Dev. 1985;11(3-4):281-292. [DOI] [PubMed] [Google Scholar]
- 45. Gubhaju L, Sutherland MR, Horne RSC, et al. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Renal Physiol. 2014;307(2):F149-F158. [DOI] [PubMed] [Google Scholar]
- 46. Le Compte AJ, Chase JG, Russell G, et al. Modeling the glucose regulatory system in extreme preterm infants. Comput Methods Programs Biomed. 2011;102(3):253-266. [DOI] [PubMed] [Google Scholar]
- 47. Green SB. How many subjects does it take to do a regression-analysis. Multivariate Behav Res. 1991;26(3):499-510. [DOI] [PubMed] [Google Scholar]