The international standard ISO 15197 describes system accuracy requirements of blood glucose monitoring systems (BGMS).1 The implementation of a new version of this standard in 2013 aimed to further improve the quality of available systems by setting stricter accuracy criteria.2
For the integrated Accu-Chek® Mobile blood glucose monitoring system (Roche Diagnostics [Roche Diabetes Care] GmbH, Mannheim, Germany), a test cassette with improved chemistry was developed that can be used with the currently available meters. The system combines the meter, 50 test areas on a tape in a cassette, and an attached lancing device containing 6 lancets in a drum.
This study was conducted to assess conformity of the new test cassettes to system accuracy requirements of ISO 15197:2013.
The study was performed at the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft an der Universität Ulm, Ulm, Germany between February and March 2014. The study was performed in compliance with the Good Clinical Practice (GCP) guidelines and approved by the Ethics Committee and the responsible authority.
Meters and test cassettes were provided by the manufacturer; the test cassettes used for the study were not CE-marked. Systems were set and maintained according to the manufacturer’s instructions and daily control measurements were performed.
Following procedures of ISO 15197:2013, capillary blood samples of at least 100 subjects with a defined distribution of blood glucose (BG) concentrations were measured with 3 different lots of the test cassettes of the system and a hexokinase-based comparison method (Cobas® 6000 c501; Roche Diagnostics GmbH, Mannheim, Germany). Comparison measurements were performed at Roche Diagnostics GmbH, Mannheim, Germany; as required by the new ISO 15197:2013 the method is traceable to a reference of higher order according to ISO 17511.3
Data were analyzed at the study site. Differences between results of the BGMS and the comparison measurement were calculated and the number of values within the limits of ISO 15197:2013 was determined. In addition, the relative bias was calculated according to Bland and Altman.4
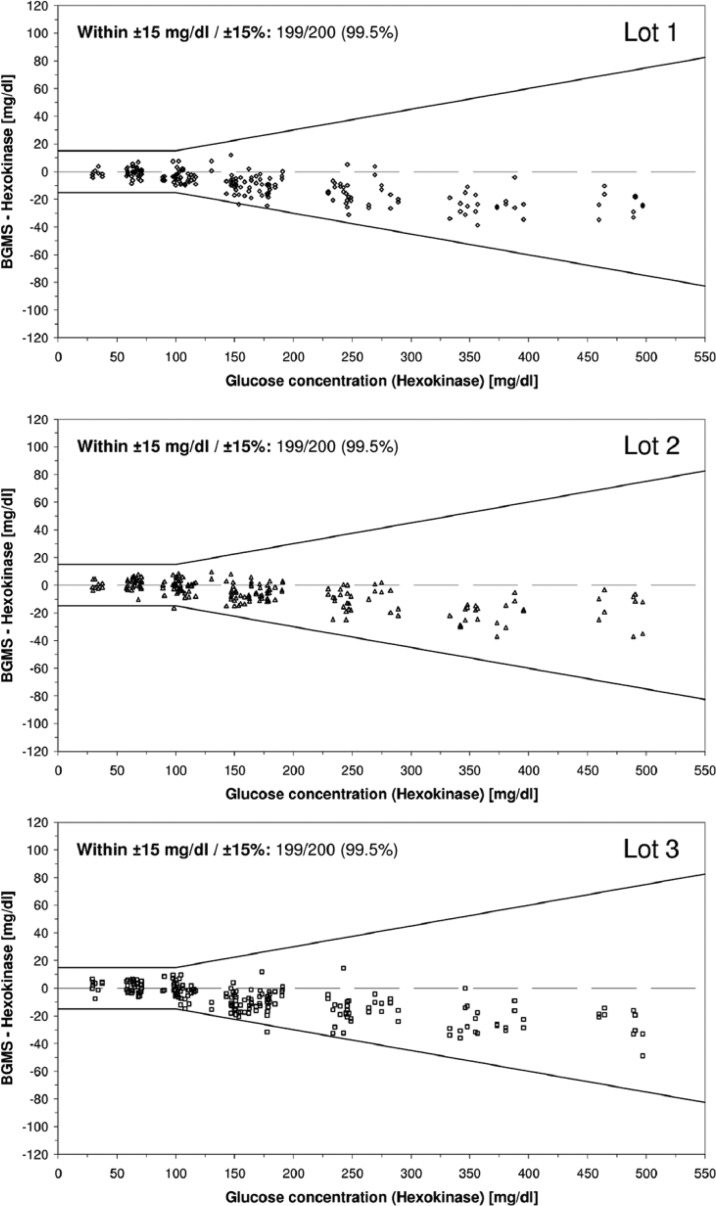
At BG concentrations <100 mg/dL (52 values), 100% (lots 1 and 3) and 98.1% (lot 2) of BGMS results fell within ±15 mg/dL of the comparison values. At BG concentrations ≥100 mg/dL (148 values), 99.3% (lots 1 and 3) and 100% (lot 2) of BGMS results were within ±15%. For all BG concentrations (29 mg/dL-497 mg/dL), 99.5% of the results (lots 1, 2, and 3) were within the respective limits (Figure 1). Furthermore, 100% of the results were in consensus error grid zones A and B. The relative bias was –4.8% (lot 1), –2.3% (lot 2), and –3.6% (lot 3).
Figure 1.
System accuracy for each individual lot: Absolute differences between BGMS results and comparison measurement results. For each lot, 200 data points are shown (100 samples measured in duplicate). Lines indicate limits of ISO 15197:2013, number and percentage of the results within these limits are given in the upper left corner (mg/dL limits for glucose concentrations <100 mg/dL, % limits for glucose concentrations ≥100 mg/dL).
This study confirmed that the system with the evaluated new test cassette chemistry has a high level of accuracy and fulfils the system accuracy requirements of ISO 15197:2013.
Footnotes
Abbreviations: BG, blood glucose; BGMS, blood glucose monitoring system; GCP, Good Clinical Practice; ISO, International Organization for Standardization.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AB and CH are employees of the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany (IDT). MK and AH are employees of Roche Diabetes Care GmbH, Mannheim, Germany. GF is general manager of the IDT, which carries out studies on the evaluation of BG meters and medical devices for diabetes therapy on behalf of various companies. GF/IDT have received speakers’ honoraria or consulting fees from Abbott, Bayer, Berlin-Chemie, Becton-Dickinson, Dexcom, LifeScan, Menarini Diagnostics, Novo Nordisk, Roche Diagnostics, Sanofi, and Ypsomed.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study and writing of the manuscript were funded by Roche Diagnostics (Roche Diabetes Care) GmbH, Mannheim, Germany.
References
- 1. International Organization for Standardization. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. EN ISO 15197:2003 E. [Google Scholar]
- 2. International Organization for Standardization. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. ISO 15197:2013 (E). [Google Scholar]
- 3. International Organization for Standardization. In vitro diagnostic medical devices—measurement of quantities in biological samples—metrological traceability of values assigned to calibrators and control materials. EN ISO 17511:2003 (D). [Google Scholar]
- 4. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310. [PubMed] [Google Scholar]