Abstract
Knowledge on domain swapping in vitro is increasing, but domain swapping may not occur regularly in vivo, and its information in cells is limited. Herein, we show that domain-swapped oligomers of a thermostable c-type cytochrome, Hydrogenobacter thermophilus cyt c552, are formed in E. coli which expresses cyt c552. The region containing the N-terminal α-helix and heme was domain-swapped between protomers in the dimer formed in E. coli. The amount of cyt c552 oligomers increased in E. coli as the cyt c552 concentration was increased, whereas that of high-order oligomers decreased in the order of decrease in protein stability, indicating that domain swapping decreases in cells when the protein stability decreases. Apo cyt c552 was detected in the cyt c552 oligomer formed in E. coli, but not in that of the A5F/M11V/Y32F/Y41E/I76V mutant. The cyt c552 oligomer containing its apo protein may form at the periplasm, since the apo protein detected by mass measurements did not contain the signal peptide. These results show that domain-swapped cyt c552 oligomers were formed in E. coli, owing to the stability of the transient oligomer containing the apo protein before heme attachment. This is an indication that exceedingly stable proteins may have disadvantages forming domain-swapped oligomers in cells.
In domain swapping, a protein molecule exchanges its structural region with the corresponding region of another molecule of the same protein. Domain swapping was first reported by Eisenberg and co-workers for diphtheria toxin in 19941. Since then, domain swapping has been reported for many proteins2,3,4. For example, ribonuclease (RNase) A swaps its N-terminal α-helix or C-terminal β-strand, forming two different domain-swapped structures5,6. The domain swapping of RNase A occurs during folding from its partially unfolded state7.
Domain swapping has also been reported for heme proteins8,9,10,11,12,13,14,15,16,17,18. We have shown that horse cytochrome (cyt) c domain swaps its C-terminal α-helix by treatment with ethanol11, or when refolding from its guanidinium ion-induced unfolded state19. The hydrophobic interaction between the N- and C-terminal α-helices at the early stage of folding is important for domain swapping in cyt c19. According to small angle X-ray scattering measurements, a certain amount of cyt c molecules interacted as oligomers in its molten globule state, whereas the domain-swapped dimer of cyt c was obtained by refolding from its molten globule state20. Hydrogenobacter thermophilus (HT) cyt c552 is a thermostable c-type cytochrome. Its high thermostability is achieved by the dense packing of the side chains of hydrophobic amino acids21,22,23,24,25. HT cyt c552 has been shown to form dimers by domain swapping, but the swapping region (N-terminal α-helix and heme) was different from that of horse cyt c12. The domain-swapped dimer of HT cyt c552 also exhibited high thermostability by maintaining the high density of the amino acid side chains as in the monomer, where the dimer did not dissociate to monomers at 70 °C12. For biosynthesis of HT cyt c552 and many gram-negative bacterial c-type cytochromes, the apo precursor protein is synthesized at the cytoplasm and transferred to the periplasm by a secretory protein26,27. After the signal peptide is cleaved at the periplasm, a heme is attached to the apo protein by a cyt c maturation (Ccm) system28. It is presumed that the Ccm system is a chaperone for the apo protein and avoids aggregation of the apo protein during the cyt c maturation process in vivo29.
Similarly, serine protease inhibitors antithrombin and α1-antitrypsin also form oligomers by domain swapping30,31. A disulfide variant of α1-antitrypsin has been shown to form oligomers in two model-cell systems, Pichia pastoris and COS-7 tissue culture cell, by trapping the domain-swapped structure31. When the C-terminal domain of severe acute respiratory syndrome coronavirus main protease (Mpro-C) was expressed in Escherichia coli (E. coli), domain-swapped dimers were observed in addition to monomers32,33. The monomer-to-dimer ratio of Mpro-C was three to two when purified from E. coli, whereas it was nine to one for the equilibrium at 37 °C in vitro34. Domain swapping has also induced two types of splicing when intein was expressed in E. coli35.
However, domain swapping may not occur regularly in cells, and the factors governing domain swapping in vivo remain unrevealed. In this study, we expressed thermostable HT cyt c552 in E. coli cells, and found that HT cyt c552 forms oligomers by domain swapping in E. coli cells. The amount of HT cyt c552 oligomers decreased when the protein stability was decreased. We attribute the decrease in oligomers to the decrease in stability of the transient complex containing the holo and apo proteins, during maturation of the apo protein to the holo protein by heme attachment.
Results
Oligomerization of HT cyt c 552 in E. coli cells
To investigate formation of oligomers in E. coli, we freeze-thawed E. coli cells expressing wild-type (WT) HT cyt c552, and performed size exclusion chromatography for the extracted solution. Peaks were observed at elution volumes of 50–75 and 85 ml in the chromatogram (Fig. 1A). The peak at 85 ml corresponded to that of monomeric HT cyt c552. The ratio of the absorption at 410 nm to that at 280 nm (Abs410/Abs280) for the fractions at 50–75 ml was about 3.6 (Fig. 1A). Heme staining was performed for the sodium dodecylsulfate (SDS)-PAGE gel of the fractions obtained by size exclusion chromatography to investigate the presence of heme c (Fig. 1B). A main band with a mass corresponding to the molecular weight of HT cyt c552 (9.2 kDa) was detected in all of the fractions at 50–75 ml. This value corresponded well to that of HT cyt c552. Therefore, we attributed the peak at 50–75 ml to HT cyt c552 oligomers. However, no oligomers were detected in the chromatogram when HT cyt c552 and cyt c552 free E. coli lysate were freeze-thawed together (Supplementary information, Fig. S1), indicating that HT cyt c552 oligomers were formed in E. coli by non-covalent association of its monomers.
Figure 1. Size exclusion chromatogram of the HT cyt c552 solution obtained from E. coli, and SDS-PAGE analysis of the fractions:
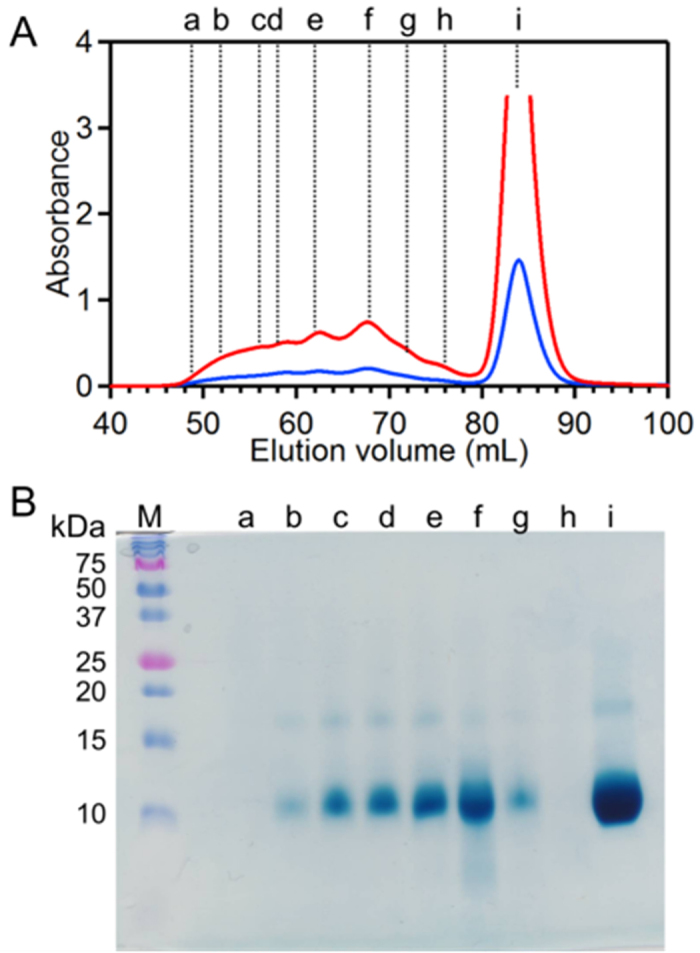
(A) Chromatogram and (B) heme-stained SDS-PAGE gel. Monitoring wavelengths for the chromatogram were 280 nm (blue) and 410 nm (red). Lanes in the SDS-PAGE gel: lane M, protein markers; lane a, 47–49 ml; lane b, 51–53 ml; lane c, 55–57 ml; lane d, 57–59 ml; lane e, 61–63 ml; lane f, 67–69 ml; lane g, 71–73 ml; lane h, 75–77 ml; lane i, 83–85 ml (The volumes represent the fraction positions in the chromatogram).
Structure of dimeric HT cyt c 552 obtained from E. coli
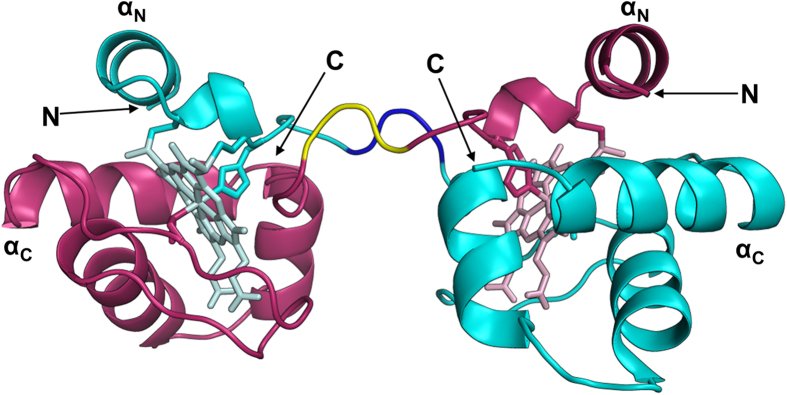
To elucidate the detailed structure of dimeric HT cyt c552 obtained from the E. coli expression system, we purified dimeric HT cyt c552 and solved its X-ray crystallographic structure (PDB code: 4ZID) (Supplementary information, Table S1). There was one HT cyt c552 protomer in the asymmetric unit of the crystal, and a domain-swapped structure of dimeric HT cyt c552 was obtained at 1.8 Å resolution (Fig. 2). The hinge loop was composed of Ala18, Lys19, and Lys20. The N-terminal α-helix from Asn1 to Lys17, together with the heme, was swapped between protomers in the dimer. The dimeric structure was similar to that obtained previously by treatment with ethanol (PDB code: 3VYM)12. The root-mean-square deviation value of Cα carbons between the protomer structure of the dimer obtained from the E. coli expression system and that obtained previously by treatment with ethanol (PDB code: 3VYM) was 0.43 Å, indicating that the protomer structures were similar.
Figure 2. Crystal structure of dimeric HT cyt c552 obtained from E. coli (PDB ID: 4ZID).
Each protomer is shown in magenta and cyan. The hemes and the side-chain atoms of His14 and Met59 are shown as stick models. The hinge loop (Ala18Lys19Lys20) is depicted in yellow and blue. The N- and C-termini and the N- and C-terminal helices are labeled as N, C, αN, and αC, respectively.
Effect of expression amount of HT cyt c 552 on oligomer formation
A His-tag (GSGHHHHHH) was attached to the C-terminus of WT HT cyt c552 to simplify the purification and analyze oligomer formation by Ni affinity chromatography using a His-trap column. The WT holo monomer was detected at ~40 ml in the Ni affinity chromatogram, whereas WT holo dimer and trimer were detected at ~48 and ~53 ml, respectively (Supplementary information, Fig. S2). By performing Ni affinity chromatography for the protein solution obtained from the E. coli cells expressing the WT protein and A5F/M11V/Y32F/Y41E/I76V (quintuple) mutant, red solutions were eluted at 36-75 ml and 32–50 ml, respectively (Fig. 3A,B).
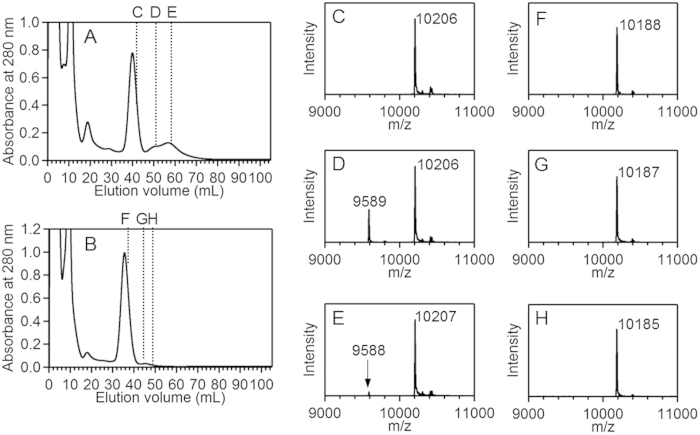
Figure 3. Ni affinity chromatograms of the HT cyt c552 solution extracted from E. coli, and MALDI-TOF mass spectra of the fractions.
Chromatograms of (A) WT and (B) A5F/M11V/Y32F/Y41E/I76V HT cyt c552 solutions, and mass spectra of the fractions at (C) 42-44 ml, (D) 50-52 ml, and (E) 58-60 ml for WT HT cyt c552 and (F) 36-38 ml, (G) 44-46 ml, and (H) 48-50 ml for A5F/M11V/Y32F/Y41E/I76V HT cyt c552 are shown.
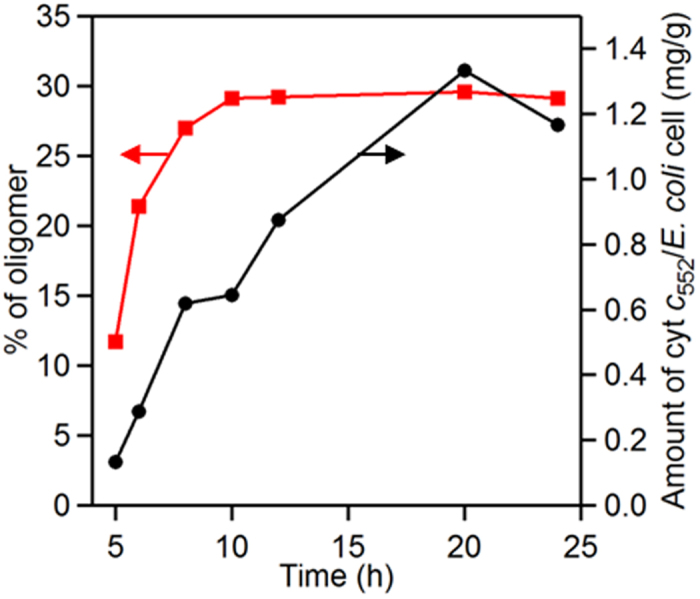
The HT cyt c552 expression amount per 1 g of E. coli cell increased rapidly with longer culturing time from 5 to 12 h, and gradually after 12 h (Fig. 4). The HT cyt c552 oligomer (dimer and higher order oligomers) amount also increased with longer culturing time from 5 to 12 h (Fig. 4). These results showed that HT cyt c552 oligomers increased as the HT cyt c552 concentration increased in E. coli cells.
Figure 4. HT cyt c552 oligomer percentage (red) and HT cyt c552 expression amount per 1 g of E. coli (black) at various culturing times.
Effect of stability of HT cyt c 552 on oligomer formation
To elucidate the effect of protein stability on oligomer formation in E. coli cells, we investigated oligomerization of His-tag-attached WT HT cyt c552 together with I76V, A5F/M11V, Y32F/Y41E, and quintuple mutant HT cyt c552, mutating amino acids important for protein stability. The order of protein stability of WT, I76V, A5F/M11V, Y32F/Y41E, and quintuple mutant HT cyt c552 (WT > I76V > A5F/M11V > Y32F/Y41E > quintuple mutant) has been elucidated by measuring the denaturing temperature and guanidine hydrochloride denaturing concentration (WT, 121.1 °C and 4.46 M; I76V, 117.8 °C and 3.98 M; A5F/M11V, 117.0 °C and 3.70 M; Y32F/Y41E, 108.3 °C and 2.69 M; quintuple mutant, 95.2 °C and 1.67 M)36. The expression amount of the cyt c protein per 1 g of E. coli cell was 1.5 ± 0.2, 1.8 ± 0.2, 0.7 ± 0.1, 1.8 ± 0.3, and 2.0 ± 0.2 mg/g for WT, I76V, A5F/M11V, Y32F/Y41E, and quintuple mutant HT cyt c552, respectively.
A peak was observed at m/z = 10,206 in the MALDI-TOF mass spectrum of the 42-44-ml fraction (shoulder of the 40-ml peak) obtained by Ni affinity chromatography of the WT HT cyt c552 (Fig. 3C). This mass corresponded well to the molecular weight of the WT holo protein (Mw = 10,205). The peak position at 50-60 ml in the Ni affinity chromatogram of the WT protein corresponded well to those of the oligomers larger than the dimer (Fig. 3A and Supplementary information, Fig. S2). A peak with a mass corresponding well to the molecular weight of the WT holo protein was also detected in the mass spectra of the fractions at 50-52 ml and 58-60 ml (Fig. 3D,E), indicating that the peak at 50-60 ml is attributable to oligomers constructed by WT monomeric cyt c552.
In the Ni affinity chromatograms of the protein solution obtained from the E. coli cells expressing WT, I76V, or A5F/M11V HT cyt c552, relatively narrow and broad peaks were observed at ~40 ml and 45-75 ml, respectively, corresponding to the monomer and oligomers, respectively (Supplementary information, Fig. S3). Peaks were observed at ~40 ml and ~48 ml corresponding to the monomer and dimer, respectively, in the Ni affinity chromatograms obtained from the E. coli cells expressing Y32F/Y41E and quintuple mutants (Supplementary information, Fig. S3), although there was no significant difference among growth of E. coli cells expressing WT and mutant proteins. The monomer, dimer, and higher-order oligomer (higher than dimer) amounts were estimated from the peak areas in the chromatograms of WT and mutant proteins (Fig. 5 and Supplementary information, Fig. S3). The amount of high-order oligomers decreased in the order of WT > I76V > A5F/M11V > Y32F/Y41E > quintuple mutant, corresponding to the decrease in protein stability. Taking the results into consideration, we propose that the oligomer amount of HT cyt c552 decreases in E. coli when the protein stability decreases.
Figure 5. Percentages of monomer, dimer, and high-order oligomers (larger than dimer) obtained from E. coli cells expressing WT and mutant HT cyt c552.
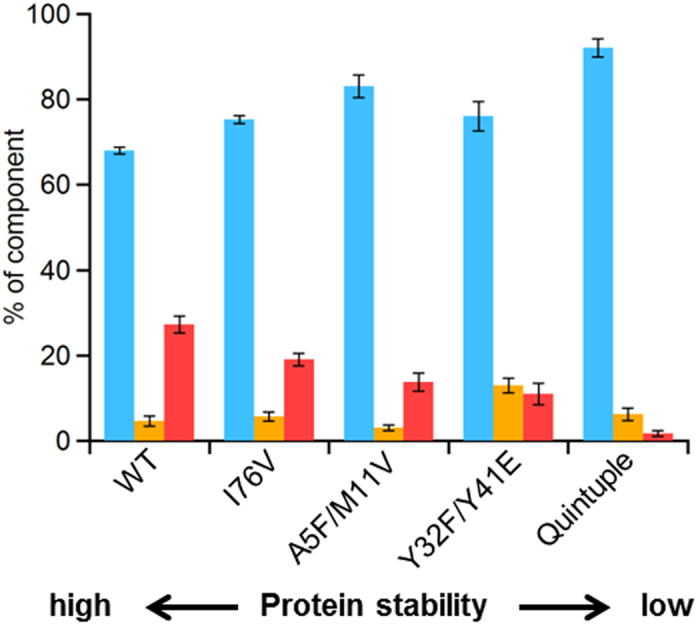
Monomer, dimer, and high-order oligomers are shown in blue, yellow, and red bars, respectively. The order of protein stability is shown at the bottom.
Detection of HT apo cyt c 552 in oligomers
An additional peak from that of the WT holo protein was observed at m/z = 9,588–9,589 in the mass spectra of the fractions at 50-52 ml and 58-60 ml in the Ni affinity chromatogram of the WT protein (Fig. 3D,E). The mass of the additional peak corresponded well to the molecular weight of the protonated WT apo protein with a His-tag attached and the cysteine residues oxidized (Mw = 9,590). These results indicated that HT cyt c552 oligomers containing the apo protein were produced in E. coli. No peak corresponding to the mass of His-tag-attached apo protein was detected in the mass spectrum of the fraction at 42-44 ml (Fig. 3C), although WT apo monomer was eluted at ~41 ml by Ni affinity chromatography (Supplementary information, Fig. S2). No apo cyt c552 was obtained as a monomer by purification from E. coli cells, presumably because the apo monomer decomposed relatively easily in the cells.
The intensity of the oligomer peaks decreased significantly in the Ni affinity chromatogram for the solution obtained from E. coli expressing the His-tag-attached quintuple mutant (Fig. 3B). In the mass spectra of the fractions at 36-38, 44-46, and 48-50 ml obtained by the Ni affinity chromatography of the quintuple mutant, a peak with a mass corresponding well to the molecular weight of the quintuple holo mutant (Mw = 10,189) was detected, whereas no peak corresponding to the mass of its apo protein was detected (Fig. 3F–H). The decrease in the amount of oligomers containing the apo protein may result in a decrease in formation of domain-swapped oligomers. However, no peak corresponding to the mass of the apo protein with the signal peptide was detected in the mass spectra of WT protein and quintuple mutant, giving evidence that apo cyt c552 may co-exist with the holo protein at the periplasm of E. coli.
Interaction of HT holo and apo cyt c 552 during folding
To investigate the effect of the apo protein on the holo protein folding, the His-tag-attached holo protein was unfolded by an addition of guanidinium ion and refolded in vitro in the absence and presence of the apo protein using a desalting column19. After refolding the holo protein, the solution was subjected to Ni affinity chromatography. Peaks were observed at ~44 and 50–62 ml in the Ni affinity chromatograms of the solution obtained by folding in the absence and presence of the apo protein (Supplementary information, Fig. S4A). Precipitation was observed by refolding the holo protein in the presence of the apo protein, but not in the absence of it. The precipitate was colorless, indicating that some amount of the apo protein precipitated during folding. Therefore, the 280-nm absorbance in the Ni affinity chromatogram for the solution containing the apo protein was not twice as high as that without the apo protein. The 410-nm absorption at ~59 ml in the Ni affinity chromatogram increased by performing folding in the presence of the apo protein compared to that in the absence of it, indicating that the oligomers containing the holo protein increased by the addition of the apo protein during folding. Peaks corresponding to the mass of the apo protein were observed at m/z = 9,589 and 9,586 in the MALDI-TOF mass spectra of the fractions at 54-56 and 58-60 ml, respectively (Supplementary information, Fig. S4C,D), showing that oligomers containing the apo protein were produced by refolding the holo protein in the presence of the apo protein. For His-tag-attached quintuple mutant HT holo cyt c552, peaks were observed at about ~43 and ~54 ml in the Ni affinity chromatograms of the solution refolded from the guanidinium ion-induced unfolded state in the absence and presence of the apo protein (Supplementary information, Fig. S4B). The difference was small between the elution curves obtained by refolding the quintuple mutant in the absence and presence of the apo protein, and no peak corresponding to the mass of the apo protein was observed in the mass spectra of the oligomer fractions at 52-54 and 56-58 ml obtained by the Ni affinity chromatography (Supplementary information, Fig. S4E,F). These results show that no oligomers containing the apo protein were detected by refolding the holo and apo quintuple mutant together in vitro, although oligomers were constructed with only the holo protein.
Discussion
A monomeric protein may oligomerize or its oligomer may dissociate to monomers when extracting the protein from living cells. Therefore, careful treatment is necessary to investigate oligomerization of proteins in cells. HT cyt c552 is a stable protein36,37, and it hardly denatures and forms oligomers after it folds in a native structure. Oligomers of HT cyt c552 were detected when it was expressed in E. coli (Fig. 1), and the amount of oligomers increased when the amount of HT cyt c552 expressed was increased (Fig. 4). These results clearly showed that HT cyt c552 oligomerizes by intermolecular interaction in E. coli cells.
Intermolecular interaction at the initial stage of folding is important for formation of domain-swapped oligomers in horse cyt c and horse myoglobin19,38. However, HT cyt c552 molecules fold simultaneously in vitro, and thus folding in vitro and that in E. coli cells are different. A heme is attached to apo HT cyt c552 at the periplasm by the Ccm system consisted of eight proteins (CcmA–CcmH)39. The lifetime of Bradyrhizobium japonicum apo c-type protein without a signal peptide is estimated to be 2–5 min in the periplasm of E. coli40. Therefore, an apo c-type protein may co-exist with its holo c-type protein during maturation in cells.
Oligomers containing the apo protein were detected when His-tag-attached WT HT cyt c552 was purified from E. coli (Fig. 3). The amount of high-order oligomers against monomers in E. coli decreased as the protein stability was decreased (Fig. 5). In addition, the apo protein was not detected in the mass spectra of the fractions of the oligomers in the Ni affinity chromatogram of the quintuple mutant (Fig. 3F–H). For refolding in vitro, the WT holo protein formed oligomers with its apo protein, whereas the quintuple mutant did not (Supplementary information, Fig. S4). The α-helical content of the apo protein of the WT protein and quintuple mutant was estimated at 19% and 11%, respectively, according to the circular dichroism (CD) spectra, whereas that of the holo protein was estimated at 49% and 42%, respectively (Supplementary information, Fig. S5). These results showed that the holo and apo proteins of the quintuple mutant were unfolded more compared to the corresponding forms of the WT protein. The stability of the mutant oligomer containing the apo form may decrease compared to that of the WT protein oligomer due to decrease in protein stability, causing decrease in formation of the domain-swapped oligomers in E. coli.
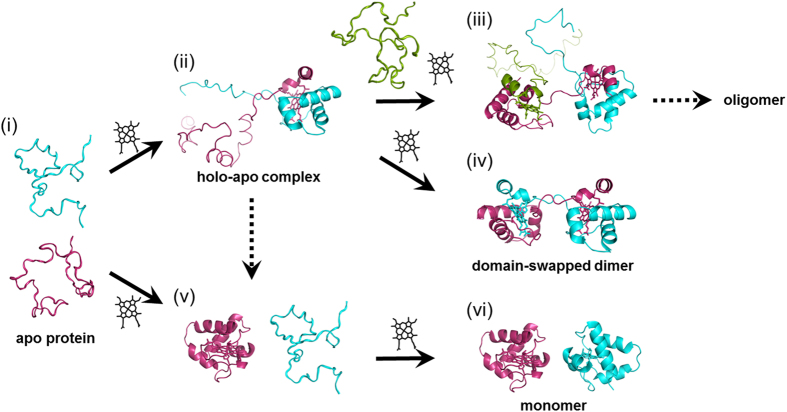
Based on the present results, we propose an oligomerization process for HT cyt c552 in cells (Fig. 6). When a holo protein is formed, it creates a transient oligomer with an apo protein. A heme is inserted to the apo protein of the transient holo-apo complex, refolding to a domain-swapped dimer. Higher order oligomers may form when an apo protein interacts with two holo proteins. For less stable proteins, the holo-apo complex dissociates to holo and apo monomers, eventually forming only holo monomers. Although apo cyt c has been shown to decompose by DegP protease at the periplasm of E. coli41, the apo protein of thermostable HT cyt c552 survives from decomposition in the cells by forming a transient complex with its holo proteins. Our results reveal that more domain-swapped oligomers are formed for more stable proteins, and exceedingly stable proteins may have disadvantages by forming oligomer by-products in cells.
Figure 6. Schematic view of oligomer formation of HT cyt c552 in E. coli.
(i) Model of unfolded apo protein. (ii) Model of transient holo-apo complex. (iii) Model of high order holo-apo complex. (iv) Dimeric holo protein (PDB ID: 4ZID). (v) Monomeric holo protein (PDB ID: 1YNR) and model of apo protein. (vi) Monomeric holo proteins (PDB ID: 1YNR). Different HT cyt c552 molecules are shown in magenta, cyan, and green. Hemes are shown as black stick models.
Methods
Construction of HT cyt c 552 expression system
The pKO2 plasmid DNA coding WT HT cyt c552, its modified plasmid DNA coding I76V, A5F/M11V, Y32F/Y41E, or A5F/M11V/Y32F/Y41E/I76V HT cyt c552, and pEC86 plasmid DNA coding the Ccm system with CcmA–CcmH were provided by Prof. Yoshihiro Sambongi (Hiroshima Univeristy)36. Insertion of a His-tag (GSGHHHHHH) to HT cyt c552 was performed by PCR-based in vitro mutagenesis. A more complete description of the procedures can be found in supplementary information.
Culture of E. coli and purification of HT cyt c 552
HT cyt c552 was expressed in E. coli JCB387. All growths were performed aerobically in LB medium at 37 °C. For analysis of HT cyt c552 expression, a partial amount of E. coli cells was collected by centrifugation (8,000 g, 5 min, 4 °C) after culturing the cells for 5, 6, 8, 10, 12, 20, and 24 h, and subsequently weighed. The SDS-PAGE gel was heme-stained for detection of heme c42. WT HT cyt c552 without a His-tag was purified as reported previously12. His-tag-attached WT, I76V, A5F/M11V, Y32F/Y41E, and quintuple mutant HT cyt c552 were extracted from E. coli by freeze-thaw and sonication, and purified with a Ni affinity column (HisTrap HP, GE Healthcare). A more complete description of the procedures can be found in supplementary information.
Preparation of HT apo cyt c 552
WT and quintuple mutant HT apo cyt c552 were prepared according to the published method43,44. A detailed description of the procedures can be found in supplementary information.
X-ray crystallographic analysis
Dimeric HT cyt c552 obtained from E. coli by freeze-thaw was purified as reported12. Crystallization of dimeric HT cyt c552 without a His-tag was performed at room temperature using the sitting-drop vapor-diffusion method with a crystal plate (CrystalClear D Strips, Douglas Instruments, Hampton Research, CA). The diffraction data were collected at the BL38B1 beamline at SPring-8, Japan, using a Quantum315 detector (ADSC). Additional details on the experimental procedures are provided in supplementary information.
Spectroscopic measurements
CD spectra of WT and A5F/M11V/Y32F/Y41E/I76V HT holo and apo cyt c552 without a His-tag were measured with a J-725 CD spectropolarimeter (Jasco, Japan) using a 0.1-cm-path-length quartz cell at 25 °C. MALDI-TOF mass spectra of HT cyt c552 were obtained with an Autoflex II mass spectrometer (Bruker Daltonics) using sinapinic acid as a matrix in linear mode. Additional details on the experimental procedures are provided in supplementary information.
Refolding of HT cyt c 552
Refolding of His-tag-attached HT cyt c552 was performed using a desalting gel column (PD SpinTrap G-25, GE Healthcare) at 4 °C as reported previously19. Additional details on the experimental procedures are provided in supplementary information.
Additional Information
How to cite this article: Hayashi, Y. et al. Domain swapping oligomerization of thermostable c-type cytochrome in E. coli cells. Sci. Rep. 6, 19334; doi: 10.1038/srep19334 (2016).
Supplementary Material
Acknowledgments
We thank Mr. Leigh McDowell, Nara Institute of Science and Technology, for his advice on manuscript preparation. We also thank the staff at beamline BL38B1, SPring-8, JAPAN (Proposal No. 2013A1851). We are also grateful to Prof. Yoshihiro Sambongi, Hiroshima University, for a kind gift of pKO2 and pEC86 plasmid DNAs carrying the genes of HT cyt c552 and Ccm proteins, respectively. This work was partially supported by Grants-in-Aid from JSPS for Scientific Research (Category B, No. 26288080; S.H.), Challenging Exploratory Research (No. 15K13744; S.H.), and JSPS Fellows (No. 26·10305; Y.H.).
Footnotes
Author Contributions Y.H. and S.H. conceived and designed the research. Y.H., M.Y. and S.N. performed the experiments. Y.H., M.Y., S.N., H.K., Y.H. and S.H. analyzed the results. S.H. wrote the paper with help of Y.H. All authors reviewed the paper.
References
- Bennett M. J., Choe S. & Eisenberg D. Domain swapping - entangling alliances between proteins. Proc. Natl. Acad. Sci. USA 91, 3127–3131 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. & Eisenberg D. 3D domain swapping: as domains continue to swap. Protein Sci. 11, 1285–1299 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F., Schymkowitz J. W. H. & Itzhaki L. S. The unfolding story of three-dimensional domain swapping. Structure 11, 243–251 (2003). [DOI] [PubMed] [Google Scholar]
- Gronenborn A. M. Protein acrobatics in pairs - dimerization via domain swapping. Curr. Opin. Struct. Biol. 19, 39–49 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. S., Hart P. J., Schlunegger M. P. & Eisenberg D. The crystal structure of a 3D domain-swapped dimer of RNase A at a 2.1-Å resolution. Proc. Natl. Acad. Sci. USA 95, 3437–3442 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. S., Gotte G., Libonati M. & Eisenberg D. A domain-swapped RNase A dimer with implications for amyloid formation. Nat. Struct. Biol. 8, 211–214 (2001). [DOI] [PubMed] [Google Scholar]
- López-Alonso J. P. et al. NMR spectroscopy reveals that RNase A is chiefly denatured in 40% acetic acid: implications for oligomer formation by 3D domain swapping. J. Am. Chem. Soc. 132, 1621–1630 (2010). [DOI] [PubMed] [Google Scholar]
- Nurizzo D. et al. N-terminal arm exchange is observed in the 2.15 Å crystal structure of oxidized nitrite reductase from Pseudomonas aeruginosa. Structure 5, 1157–1171 (1997). [DOI] [PubMed] [Google Scholar]
- Crane B. R. et al. N-terminal domain swapping and metal ion binding in nitric oxide synthase dimerization. EMBO J. 18, 6271–6281 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czjzek M. et al. The crystal structure of the secreted dimeric form of the hemophore HasA reveals a domain swapping with an exchanged heme ligand. J. Mol. Biol. 365, 1176–1186 (2007). [DOI] [PubMed] [Google Scholar]
- Hirota S. et al. Cytochrome c polymerization by successive domain swapping at the C-terminal helix. Proc. Natl. Acad. Sci. USA 107, 12854–12859 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y. et al. Domain swapping of the heme and N-terminal α-helix in Hydrogenobacter thermophilus cytochrome c552 dimer. Biochemistry 51, 8608–8616 (2012). [DOI] [PubMed] [Google Scholar]
- Nagao S. et al. Structural and oxygen binding properties of dimeric horse myoglobin. Dalton Trans. 41, 11378–11385 (2012). [DOI] [PubMed] [Google Scholar]
- Silva M. A., Lucas T. G., Salgueiro C. A. & Gomes C. M. Protein folding modulates the swapped dimerization mechanism of methyl-accepting chemotaxis heme sensors. PLoS One 7, e46328 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-W. et al. Rational design of heterodimeric protein using domain swapping for myoglobin. Angew. Chem. Int. Ed. 54, 511–515 (2015). [DOI] [PubMed] [Google Scholar]
- Yamanaka M., Nagao S., Komori H., Higuchi Y. & Hirota S. Change in structure and ligand binding properties of hyperstable cytochrome c555 from Aquifex aeolicus by domain swapping. Protein Sci. 24, 366–375 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao S. et al. Domain-swapped dimer of Pseudomonas aeruginosa cytochrome c551: structural insights into domain swapping of cytochrome c family proteins. PLoS One 10, e0123653 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T. et al. Domain-swapped cytochrome cb562 dimer and its nanocage encapsulating a Zn-SO4 cluster in the internal cavity. Chem. Sci. 6, 7336–7342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parui P. P. et al. Formation of oligomeric cytochrome c during folding by intermolecular hydrophobic interaction between N- and C-terminal alpha-helices. Biochemistry 52, 8732–8744 (2013). [DOI] [PubMed] [Google Scholar]
- Deshpande M. S. et al. Formation of domain-swapped oligomer of cytochrome c from its molten globule state oligomer. Biochemistry 53, 4696–4703 (2014). [DOI] [PubMed] [Google Scholar]
- Hasegawa J. et al. Stabilization of Pseudomonas aeruginosa cytochrome c551 by systematic amino acid substitutions based on the structure of thermophilic Hydrogenobacter thermophilus cytochrome c552. J. Biol. Chem. 274, 37533–37537 (1999). [DOI] [PubMed] [Google Scholar]
- Hasegawa J. et al. Selected mutations in a mesophilic cytochrome c confer the stability of a thermophilic counterpart. J. Biol. Chem. 275, 37824–37828 (2000). [DOI] [PubMed] [Google Scholar]
- Uchiyama S. et al. Thermodynamic characterization of variants of mesophilic cytochrome c and its thermophilic counterpart. Protein Eng. 15, 455–461 (2002). [DOI] [PubMed] [Google Scholar]
- Takahashi Y. T. et al. Further enhancement of the thermostability of Hydrogenobacter thermophilus cytochrome c552. Biochemistry 45, 11005–11011 (2006). [DOI] [PubMed] [Google Scholar]
- Tai H. L., Irie K., Mikami S. & Yamamoto Y. Enhancement of the thermostability of Hydrogenobacter thermophilus cytochrome c552 through introduction of an extra methylene group into its hydrophobic protein interior. Biochemistry 50, 3161–3169 (2011). [DOI] [PubMed] [Google Scholar]
- Natale P., Bruser T. & Driessen A. J. M. Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane - Distinct translocases and mechanisms. Biochim. Biophys. Acta 1778, 1735–1756 (2008). [DOI] [PubMed] [Google Scholar]
- Facey S. J. & Kuhn A. Biogenesis of bacterial inner-membrane proteins. Cell. Mol. Life Sci. 67, 2343–2362 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. M. et al. Cytochrome c biogenesis System I. FEBS J. 278, 4170–4178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Silvio E., Di Matteo A., Malatesta F. & Travaglini-Allocatelli C. Recognition and binding of apocytochrome c to P. aeruginosa CcmI, a component of cytochrome c maturation machinery. Biochim. Biophys. Acta 1834, 1554–1561 (2013). [DOI] [PubMed] [Google Scholar]
- Yamasaki M., Li W., Johnson D. J. D. & Huntington J. A. Crystal structure of a stable dimer reveals the molecular basis of serpin polymerization. Nature 455, 1255–1258 (2008). [DOI] [PubMed] [Google Scholar]
- Yamasaki M., Sendall T. J., Pearce M. C., Whisstock J. C. & Huntington J. A. Molecular basis of α1-antitrypsin deficiency revealed by the structure of a domain-swapped trimer. EMBO Rep. 12, 1011–1017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N. et al. Without its N-finger, the main protease of severe acute respiratory syndrome coronavirus can form a novel dimer through its C-terminal domain. J. Virol. 82, 4227–4234 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N. et al. C-terminal domain of SARS-CoV main protease can form a 3D domain-swapped dimer. Protein Sci. 18, 839–844 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X. et al. Foldon unfolding mediates the interconversion between Mpro-C monomer and 3D domain-swapped dimer. Proc. Natl. Acad. Sci. USA 109, 14900–14905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranko A. S., Oeemig J. S., Kajander T. & Iwaï H. Intermolecular domain swapping induces intein-mediated protein alternative splicing. Nat. Chem. Biol. 9, 616–622 (2013). [DOI] [PubMed] [Google Scholar]
- Oikawa K. et al. Five amino acid residues responsible for the high stability of Hydrogenobacter thermophilus cytochrome c552: reciprocal mutation analysis. J. Biol. Chem. 280, 5527–5532 (2005). [DOI] [PubMed] [Google Scholar]
- Uchiyama S. et al. Complete thermal-unfolding profiles of oxidized and reduced cytochromes c. J. Am. Chem. Soc. 126, 14684–14685 (2004). [DOI] [PubMed] [Google Scholar]
- Ono K., Ito M., Hirota S. & Takada S. Dimer domain swapping versus monomer folding in apo-myoglobin studied by molecular simulations. Phys. Chem. Chem. Phys. 17, 5006–5013 (2015). [DOI] [PubMed] [Google Scholar]
- Arslan E., Schulz H., Zufferey R., Künzler P. & Thöny-Meyer L. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 251, 744–747 (1998). [DOI] [PubMed] [Google Scholar]
- Thöny-Meyer L., Künzler P. & Hennecke H. Requirements for maturation of Bradyrhizobium japonicum cytochrome c550 in Escherichia coli. Eur. J. Biochem. 235, 754–761 (1996). [DOI] [PubMed] [Google Scholar]
- Gao T. & O’Brian M. R. Control of DegP-dependent degradation of c-type cytochromes by heme and the cytochrome c maturation system in Escherichia coli. J. Bacteriol. 189, 6253–6259 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D. & Levin W. Improved staining procedure for detection of peroxidase-activity of cytochrome P450 on sodium dodecyl-sulfate polyacrylamide gels. Anal. Biochem. 75, 168–176 (1976). [DOI] [PubMed] [Google Scholar]
- Fisher W. R., Taniuchi H. & Anfinsen C. B. On the role of heme in the formation of the structure of cytochrome c. J. Biol Chem. 248, 3188–95 (1973). [PubMed] [Google Scholar]
- Yamanaka M., Masanari M. & Sambongi Y. Conferment of folding ability to a naturally unfolded apocytochrome c through introduction of hydrophobic amino acid residues. Biochemistry 50, 2313–20 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.