Abstract
Chronic administration of selective serotonin reuptake inhibitors (SSRIs), which up-regulates central serotonin (5-HT) system function, enhances adult hippocampal neurogenesis. However, the relationship between central 5-HT system and adult neurogenesis has not fully been understood. Here, we report that lowering 5-HT level in adulthood is also able to enhance adult hippocampal neurogenesis. We used tamoxifen (TM)-induced Cre in Pet1-CreERT2 mice to either deplete central serotonergic (5-HTergic) neurons or inactivate 5-HT synthesis in adulthood and explore the role of central 5-HT in adult hippocampal neurogenesis. A dramatic increase in hippocampal neurogenesis is present in these two central 5-HT-deficient mice and it is largely prevented by administration of agonist for 5-HTR2c receptor. In addition, the survival of new-born neurons in the hippocampus is enhanced. Furthermore, the adult 5-HT-deficient mice showed reduced depression-like behaviors but enhanced contextual fear memory. These findings demonstrate that lowering central 5-HT function in adulthood can also enhance adult hippocampal neurogenesis, thus revealing a new aspect of central 5-HT in regulating adult neurogenesis.
Two major regions of the rodent brain undergo continuous neurogenesis into adulthood: the subventricular zone, from where newly-generated neurons migrate to the olfactory bulb, and the subgranular zone (SGZ), which gives rise to new dentate gyrus granular neurons1,2,3. Adult hippocampal neurogenesis is controlled by many factors, including a number of neurotransmitters3,4. The neurotransmitter serotonin (5-HT) is synthesized in the brain by the enzyme tryptophan hydroxylase 2 (Tph2)5. After its release into the synaptic cleft, 5-HT is removed by the presynaptic 5-HT transporter (5-HTT)6. Monoamine system dysfunction is believed to be a major causative factor of depression7. For this reason, SSRIs are frequently used as first-line antidepressants, in an effort to enhance central 5-HT system function.
Chronic administration of the SSRI fluoxetine leads to increased hippocampal neurogenesis in adult mice8. The role of 5-HT in adult hippocampal neurogenesis has also been investigated in various genetic mouse models, but results were somewhat different than expected. 5-HTT−/− mice showed elevated 5-HT level in the brain, but an increase of proliferation of adult hippocampal stem cells is only observed in aged5-HTT−/− mice (>14.5 months) but not younger adult mice (3 weeks and 3 months)9. Tph2−/− mice that lack 5-HT in the brain, however, present a phenotype similar to that of 5-HTT−/− mice10. In addition to the proliferation of the stem cells, chronic SSRI administration also enhances the survival of adult-born neurons in the hippocampus11. On the other hand, the increased survival of adult-born neurons has been reported recently in the genetic mouse model with less central 5-HT12,13. Thus, the relationship between central 5-HT and adult hippocampal neurogenesis including proliferation of neural stem cells and survival of new-born neurons has not fully been understood.
5-HT is implicated in several aspects of nervous system development, including axonal growth and dendritic spine formation, as well as barrel formation and synaptic plasticity in the somatosensory cortex14,15,16. It is therefore likely that deleting either Tph2, 5-HT receptors (5-HTR) or 5-HTT by means of conventional gene targeting might impair brain development and lead to uncontrolled pleiotropic effects, particularly since most of these genes are already expressed during embryonic stages. To circumvent these potential complications and allow the brain to first develop normally, we used Pet1-CreERT2; Rosa26-DTA (diphtheria fragment A) mice to deplete central 5-HTergic neurons, and Pet1-CreERT2; Tph2flox/flox mice to inactivate central 5-HT synthesis, both in adulthood. We found that adult neurogenesis is significantly increased in the SGZ, thus revealing an unexpected role for central 5-HT in regulating adult neurogenesis.
Results
Depletion of central 5-HTergic neurons in adulthood
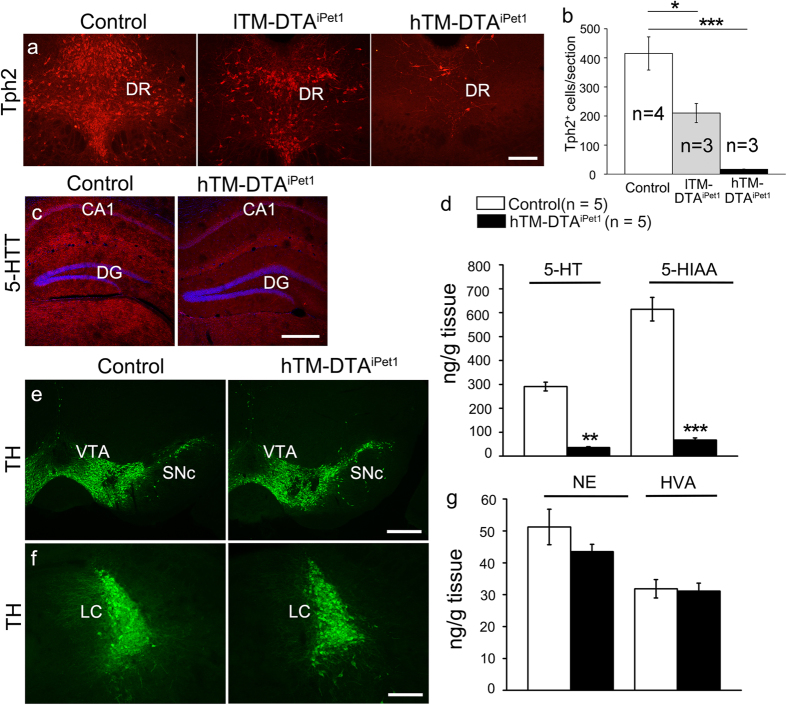
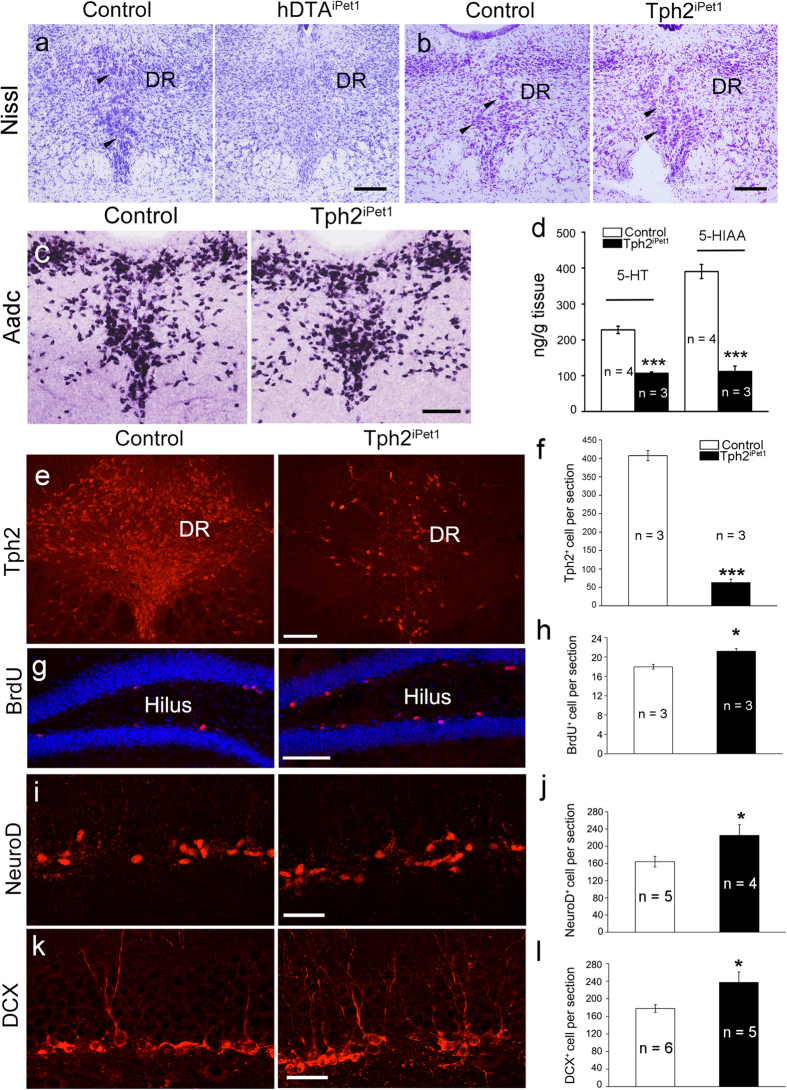
To specifically ablate 5-HTergic neurons in the adult brain, we crossed Pet1-CreERT2 mice, in which inducible Cre recombinase is selectively activated in 5-HTergic neurons17, with Rosa26-DTA mice18 to generate Pet1-CreERT2; Rosa26-DTA (referred to as DTAiPet1) offspring. TM administration led to the selective depletion of 5-HTergic neurons in the brainstem of DTAiPet1 mice, due to the toxic effect of DTA. One month after the final TM treatment, mice were sacrificed for analysis. When a lower dose of TM (80 mg/kg body weight, once daily for 3 consecutive days) was used, approximately 50% of 5-HTergic neurons were depleted, as illustrated by the striking decrease in the number of Tph2+ neurons in the dorsal raphe nucleus (Fig. 1a,b); these mice were referred to as lTM-DTAiPet1 mice. On the other hand, when a higher dose of TM (250 mg/kg body weight, once daily for 4 consecutive days) was used, more than 95% of 5-HTergic neurons were depleted (Fig. 1a,b); these mice were referred to as hTM-DTAiPet1 mice. Correspondingly, only a few 5-HTergic fibers were observed in the hippocampus, as shown by 5-HTT immunostaining (Fig. 1c). HPLC showed that 5-HT and its metabolite 5-HIAA were dramatically decreased in hTM-DTAiPet1 mice (Fig. 1d), which indicated that central 5-HT was dramatically reduced as a result of brainstem 5-HTergic neuronal loss. To determine whether other monoamines were affected or not after loss of 5-HTergic neurons, we examined the expression of tyrosine hydroxylase (TH), which labels dopaminergic neurons in the midbrain (Fig. 1e) and norepinephrinergic neurons in the hindbrain (Fig. 1f), respectively. We found that TH expression in hTM-DTAiPet1 mice was comparable to that in control mice. In consistent with these immunostaining data, HPLC showed that both the concentration of norepinephrine and homovanillic acid, the metabolite of dopamine, was not altered in hTM-DTAiPet1 mice relative to that of control mice (Fig. 1g). Together, 5-HTergic neurons in the brain can be selectively depleted in TM-DTAiPet1 mice in adulthood.
Figure 1. Depletion of central 5-HTergic neurons in DTAiPet1 mice.
(a,b) 5-HTergic neurons are depleted in the dorsal raphe nucleus (DR) after administration of TM in adult DTAiPet1 mice. Tph2 immunostaining reveals that approximately half of 5-HTergic neurons remain in lTM-DTAiPet1 mice (80 mg/kg, once daily for 3 days), but only 5% of 5-HTergic neurons are present in hTM-DTAiPet1 mice (250 mg/kg, once daily for 4 days). The number of Tph2+ neurons in the DR of each of the three treatment groups is quantified in (b), using one-way ANOVA (alpha = 0.05) with post hoc Tukey test (p = 0.025 for comparison of control and lTM-DTAiPet1, p = 0.001 for comparison of control and hTM-DTAiPet1). (c) 5-HTT+ fibers (red) are abundantly distributed throughout the hippocampus of control mice, but only a very few of them are observed in hTM-DTAiPet1 mice. (d) HPLC assay shows hTM-DTAiPet1 mice have extremely low levels of 5-HT and its metabolite 5-HIAA comparing to control mice. Data of 5-HT level were compared using Mann-Whitney rank sum test (p = 0.008), and data of 5-HIAA using Student’s t-test (p = 4.5 × 10−6). (e,f) Immunostaining of TH shows similar expression in midbrain dopaminergic neurons (e) and hindbrain norepinephrinergic neurons (f) between control and hTM-DTAiPet1 mice. (g) HPLC analysis shows comparable levels of HVA, a metabolite of dopamine, and NE in control and hTM-DTAiPet1 mice. Data were compared using Student’s t-test.CA1, field CA1 of hippocampus; DG, dentate gyrus; DR, dorsal raphe; 5-HIAA, 5-Hydroxyindoleacetic acid; HVA, homovanillic acid; LC, locus coeruleus; NE, norepinephrine; SNc, substantia nigra, compact part; VTA, ventral tegmental area. All data are presented as mean ± s.e.m. *p < 0.05; **p < 0.01; ***p < 0.001. Scale bars represent 200 μm (a), 400 μm (c), 500 μm (e) and 200 μm (f).
A drastic increase of immature neurons in hTM-DTAiPet1 mice
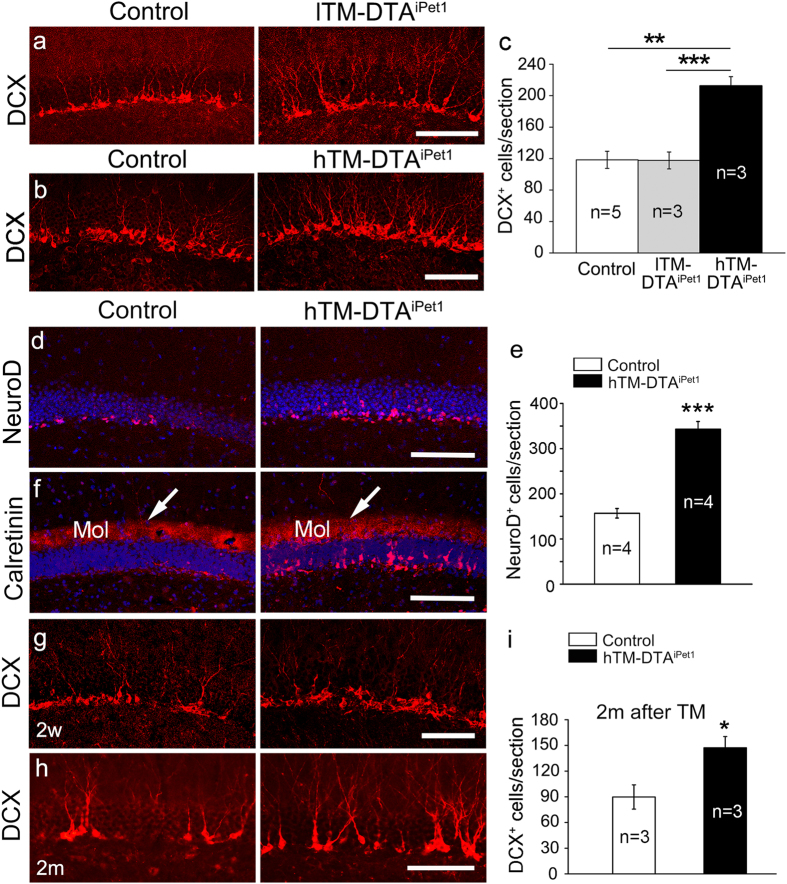
Adult neurogenesis involves proliferation of neural stem cells and differentiation of new-born neurons, two processes that require coordinated expression of a number of different genes3. Firstly, to detect the change of immature neurons after loss of 5-HTergic neurons, DCX immunostaining was performed to label the neuroblasts and immature neurons in the SGZ. We found that the number of DCX-labeled cells in lTM-DTAiPet1 mice was comparable to that in control mice (Fig. 2a,c), indicating that overall neurogenesis level in the hippocampus was unaffected in lTM-DTAiPet1 mice. In contrast to lTM-DTAiPet1 mice, the number of DCX+ cells in the SGZ was dramatically increased in hTM-DTAiPet1 mice (Fig. 2b,c). In addition to DCX+ cells, the number of NeuroD+ neuroblasts was also significantly increased in hTM-DTAiPet1 mice (Fig. 2d,e). Furthermore, the number of CR+, which is expressed in the immature neurons following the initiation of DCX expression1, were increased also in hTM-DTAiPet1 mice (Fig. 2f). There was no change of CR+ fibers in the molecular layer (arrows, Fig. 2f), which are originated from hilar mossy neurons. Finally, the enhanced hippocampal neurogenesis was first detected two weeks after the last TM injection (Fig. 2g) and also observed two months after the last TM injection (Fig. 2h,i), demonstrating that this effect was not a transient one. Together, these results indicate that removing central 5-HTergic neurons during adulthood leads to a significant enhancement in the hippocampal neurogenesis.
Figure 2. Enhanced adult hippocampal neurogenesis in hTM-DTAiPet1 mice.
(a–c) The number of DCX+ cells in the SGZ is dramatically increased in hTM-DTAiPet1 mice (b), but not in lTM-DTAiPet1 mice (a). A quantification of DCX+ cells in the SGZ is shown in (c), using one-way ANOVA (alpha = 0.05) with post hoc Tukey test (p = 0.002 for comparison of control and hTM-DTAiPet1, p = 9.8 × 10−4 for comparison of lTM-DTAiPet1and hTM-DTAiPet1). (d,e) The number of NeuroD+ cells is significantly increased in hTM-DTAiPet1 mice. Data were compared using Student’s t-test (p = 8.8 × 10-5). (f) Immunohistochemical analysis reveals that CR+ neurons are greatly increased in the hippocampal SGZ of hTM-DTAiPet1 mice relative to controls. No changes of CR+ hilar mossy neuron projections in the molecular layer of DG (arrows). (g) Two weeks after the last TM injection, the number of DCX+ cells is increased in the SGZ of hTM-DTAiPet1 mice. (h,i) Two months after the last TM injection, the number of DCX+ cells remains significantly increased in the SGZ of hTM-DTAiPet1 mice. Data were compared using Student’s t-test (p = 0.04). All data are presented as mean ± s.e.m. *p < 0.05; **p < 0.01; ***p < 0.001. Scale bars represent 150 μm (a,d,f) and 100 μm (b,g,h).
Proliferation of neural stem cells is enhanced in hTM-DTAiPet1 mice
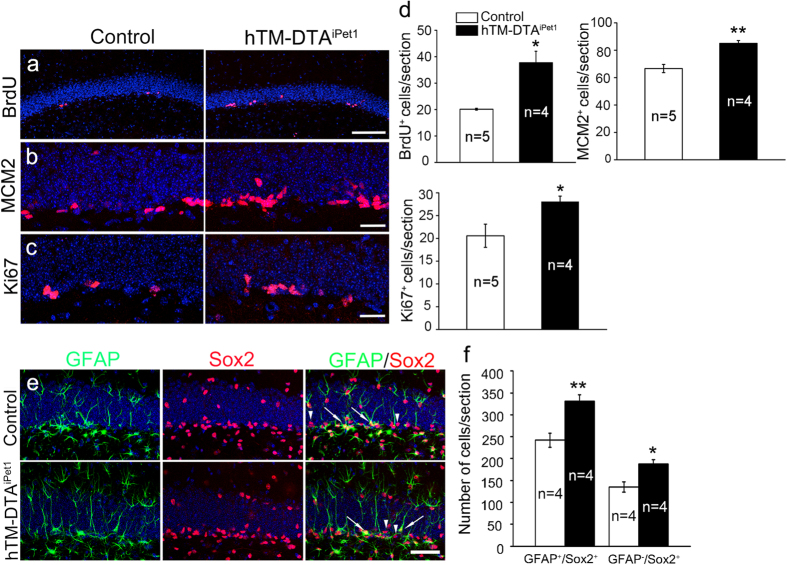
To explore the cause of increase of neuroblasts and immature neurons after depleting 5-HTergic neurons, we checked the proliferation of adult stem cells in the SGZ. First, we examined neural stem cell proliferation by means of BrdU incorporation method, and found that the number of BrdU+ cells was dramatically increased in hTM-DTAiPet1 mice compared with controls (Fig. 3a,d). Besides, we also examined the expression of two other proteins, MCM2 and Ki67, which are expressed in cell cycles of neural stem cells19. Similar to BrdU+ cells, both MCM2+ and Ki67+ cells in hTM-DTAiPet1 mice were significantly increased (Fig. 3b–d). These results indicate that 5-HTergic neuron depletion leads to increased neural stem cell proliferation in the SGZ.
Figure 3. Proliferation of neural stem cells is enhanced in hTM-DTAiPet1 mice.
(a) The number of BrdU+ cells (red) in the SGZ is dramatically increased in hTM-DTAiPet1 mice. Hoechst counterstaining is shown in blue. (b,c) The number of MCM2+ (red in b) and Ki67+ cells (red in c) in the SGZ is significantly increased in hTM-DTAiPet1 mice. Hoechst counterstaining is shown in blue. (d) Quantification of data in (a–c). Data of BrdU+ cell were compared using Mann-Whitney rank sum test (p = 0.016), and others using Student’s t-test (p = 0.002 for MCM2+ cells, p = 0.045 for Ki67+ cells).(e, f) The numbers of GFAP+/Sox2+ cells (QNPs, arrows) and GFAP−/Sox2+ cells (ANPs, arrowheads) are both significantly increased in the SGZ of hTM-DTAiPet1 mice relative to controls. Data were compared using Student’s t-test (p = 0.004 for GFAP+/Sox2+ cells, p = 0.013 for GFAP-/Sox2+ cells). All data are presented as mean ± s.e.m. *p < 0.05; **p < 0.01. Scale bars represent 100 μm (a), 25 μm (b,c) and 50 μm (e).
SSRIs enhance adult hippocampal neurogenesis by increasing the number of amplifying neural progenitor cells (ANPs) rather than quiescent neural progenitor cells (QNPs)20. To determine whether QNPs or ANPs were affected in hTM-DTAiPet1 mice, we performed GFAP and Sox2 co-immunostaining1 and found that the number of GFAP+/Sox2+ QNPs was significantly elevated in the SGZ of hTM-DTAiPet1 mice (Fig. 3e,f). A similar increase was also observed in GFAP-/Sox2+ ANPs (Fig. 3e,f). We therefore conclude that the enhanced adult hippocampal neurogenesis in hTM-DTAiPet1 mice is due to an increase in both QNPs and ANPs in the SGZ.
Increased survival of new-born neurons in hTM-DTAiPet1 mice
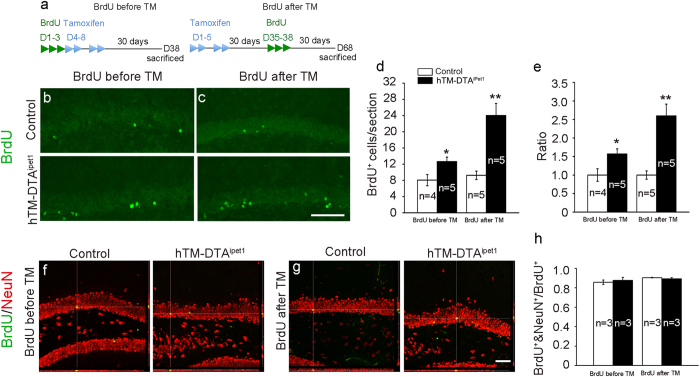
Chronic SSRI administration enhances the survival of adult-born neurons11, and we next set out to explore whether the survival of adult-born neurons was affected or not after adult depletion of 5-HTergic neurons. Firstly, to detect the survival of new-born cells generated before depleting 5-HTergic neurons, BrdU was injected prior to TM administration (Fig. 4a). Because depleting 5-HTergic neurons only occurs after TM administration, BrdU could label similar numbers of proliferating cells in both hTM-DTAiPet1 and control mice, and BrdU+ cells in the hippocampus 30 days after TM administration could be used to reflect changes in the survival of adult-born neurons in hTM-DTAiPet1 mice. We found that BrdU+ cells in the SGZ of hTM-DTAiPet1 mice were increased to about 1.6 fold of that in control mice (Fig. 4b,d,e). Secondly, to determine the survival of new-born neurons generated after depleting 5-HTergic neurons, BrdU were injected 30 days after the TM treatment. BrdU+ cells in the SGZ of hTM-DTAiPet1 mice were increased to about 2.6 fold of that in control mice (Fig. 4c–e). To confirm whether BrdU-incorporated cells eventually become neurons or not, we performed BrdU/NeuN double immunostaining. We found that most of BrdU+cells were NeuN+ in both control and hTM-DTAiPet1 mouse brains without obvious differences (Fig. 4f–g). It is concluded that the survival of new-born neurons in the SGZ is enhanced in hTM-DTAiPet1 mice.
Figure 4. Survival of adult-born neurons is enhanced in hTM-DTAiPet1 mice.
(a) Scheme of survival analysis. For studying the survival of neuron born before depleting 5-HTergic neurons (BrdU before TM), BrdU was injected for 3 consecutive days, followed by 4 times of TM administration. Mice were sacrificed 30 days after last TM administration. For studying the survival of neuron born after depleting 5-HTergic neurons (BrdU after TM), TM was administrated as above and BrdU was injected 30 days after TM administration. Mice were sacrificed 30 days after TM administration. (b,d,e) BrdU was injected before TM administration. More BrdU+ cells are labeled in hTM-DTAiPet1 mice relative to control. (c–e) BrdU was injected 30 days after TM administration. More BrdU+ cells are labeled in hTM-DTAiPet1 mice relative to control. Data of BrdU+ cells in mice with BrdU injected before TM administrationwere compared using Student’s t-test (p = 0.033 in d and e) and data of BrdU+ cells in mice with BrdU injected after TM administrationwere compared using Mann-Whitney rank sum test (p = 0.008 in d and e). (f–h) Double immunostaining of BrdU and NeuN was performed in both “BrdU before TM” and “BrdU after TM” groups. The ratios of BrdU+/NeuN+ to BrdU+ cells are comparable in both control and hTM-DTAiPet1 mice, no matter whether BrdU was incorporated before or after TM administration. Data of percentage were compared using Student’s t-test. All data are presented as mean ± s.e.m. *p < 0.05; **p < 0.01. Scale bars represent 100 μm in (b,c) and 50 μm in (f,g).
Enhanced hippocampal neurogenesis is also present in adult Tph2iPet1 mice
In hTM-DTAiPet1 mice, 5-HTergic neurons are depleted due to toxicity of DTA. To ascertain whether enhanced adult hippocampal neurogenesis is caused by the loss of central 5-HTergic neurons or loss of 5-HT itself, Pet1-CreERT2 mice were crossed with Tph2flox/flox mice21, to create Pet1-CreERT2; Tph2flox/flox (referred to as Tph2iPet1) mice in which 5-HTergic neurons are intact but 5-HT synthesis is inactivated upon TM injection. Nissl staining showed that intensely-stained “5-HTergic-like” neurons were present in the dorsal raphe nucleus of Tph2iPet1 mice (arrowheads, Fig. 5b), but absent from hTM-DTAiPet1 mice (Fig. 5a). The number of Tph2+ neurons remaining in Tph2iPet1 mice was reduced to approximately 15% of controls (Fig. 5e,f), and HPLC data also revealed a drastic reduction of 5-HT and 5-HIAA in Tph2iPet1 mice (Fig. 5d). The maintenance of 5-HTergic neurons in Tph2iPet1 mice was confirmed by in situ hybridization of aromatic L-amino acid decarboxylase (AADC; Fig. 5c), a marker for 5-HTergic neurons. Thus, 5-HTergic neurons remained in Tph2iPet1 brain, despite Tph2 inactivation in most central 5-HTergic neurons. Importantly, BrdU+, NeuroD+ and DCX+ cell numbers were also increased in the SGZ of Tph2iPet1 mice relative to controls (Fig. 5g–l), although to a lesser extent than that observed in hTM-DTAiPet1 mice. Taken together, these findings indicate that reducing central 5-HT levels in adulthood by inactivation of Tph2 is also capable of increasing adult hippocampal neurogenesis.
Figure 5. Enhanced adult hippocampal neurogenesis in Tph2iPet1 mice.
(a) Nissl staining showing that intensely stained 5-HTergic neurons (arrowheads) are present in the DR of control mice, but absent in hTM-DTAiPet1 mice. (b) Nissl staining showing intensely stained 5-HTergic neurons (arrowheads) in the DR of Tph2iPet1 and control mice. (c) In situ hybridization showing comparable numbers of AADC+ cells in the dorsal raphe nucleus (DR) of Tph2iPet1 and control mice (c). (d) HPLC data show that 5-HT and its metabolite 5-HIAA in Tph2iPet1 mice reduce dramatically comparing to control mice. Data were compared using Student’s t-test (p = 2.0 × 10−4 for 5-HT and p = 1.3 × 10−4 for 5-HIAA). (e,f) The number of Tph2+ neurons is dramatically decreased in Tph2iPet1 mice, to approximately 15% of control levels. Data were compared using Student’s t-test (p = 3.0 × 10−5). (g,h) The number of BrdU+ cell is significantly increased in Tph2iPet1 mice relative to controls. Data were compared using Student’s t-test (p = 0.013). (i,j) The number of NeuroD+ neuroblasts is also significantly increased in Tph2iPet1 mice relative to controls. Data were compared using Student’s t-test (p = 0.048). (k,l) The number of DCX+ cells is significantly increased in Tph2iPet1 mice compared with controls. Data were compared using Student’s t-test (p = 0.032). All data are presented as mean ± s.e.m. *p < 0.05; ***p < 0.001. Scale bars represent 200 μm (a–c,e,g) and 30 μm (i,k).
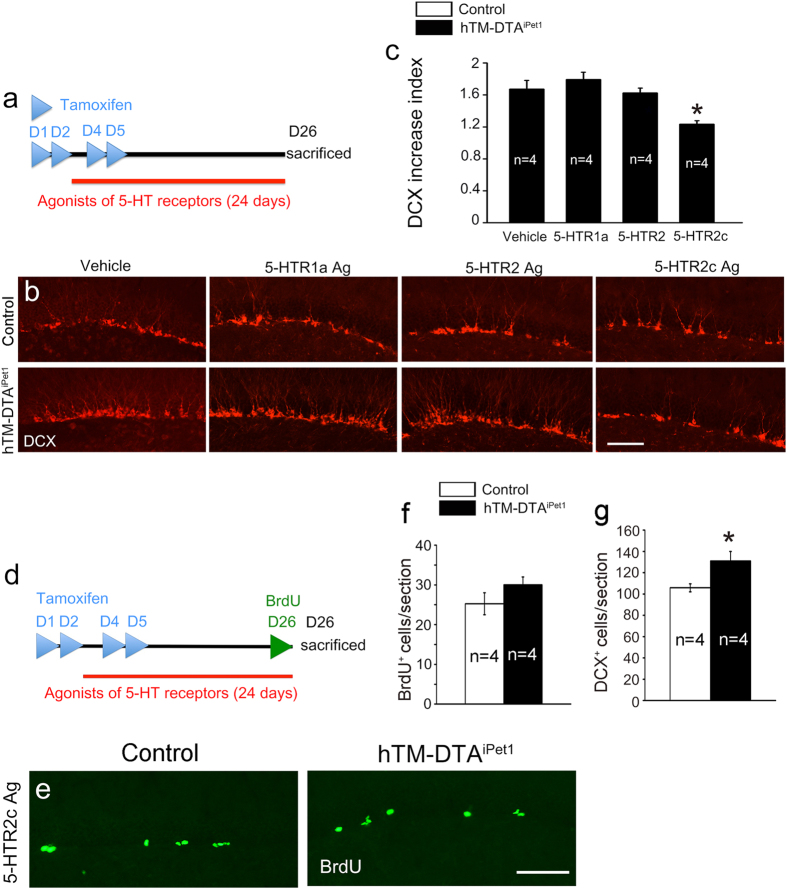
Administration of 5-HTR2c agonist prevents the increased neurogenesis in hTM-DTAiPet1 mice
We speculate that the depletion of 5-HTergic neurons or lowering 5-HT level leads to impairments of activation of some 5-HTRs, which in turn results in the enhanced hippocampal neurogenesis. We therefore set out to explore whether administration of agonists of 5-HTRs in the hTM-DTAiPet1 mice could prevent the increased adult neurogenesis or not. As shown in Fig. 6a, both control and hTM-DTAiPet1 mice were injected with 8-OH DPAT, α-methyl-5-hydroxytryptamine maleate, or WAY161503, which are the agonists for 5-HTR1a, 5-HTR2 family and 5-HTR2c, respectively, for consecutive 24 days and were sacrificed for immunostaining of DCX. To screen out which receptor was involved, as the first step we used DCX increase index (normalizing the numbers of DCX+ cells in agonist-treated hTM-DTAiPet1 mice to that of agonist-treated controls) for comparison in each set of experiment. After administration of HTR1a and HTR2 family agonists, the DCX increase index in hTM-DTAiPet1 mice was not changed relative to vehicle-treated hTM-DTAiPet1 mice (Fig. 6b,c). By contrast, the index was significantly reduced in hTM-DTAiPet1 mice treated with WAY161503, agonist of 5-HTR2c (Fig. 6b,c), suggesting that 5-HTR2c is involved in the enhanced neurogenesis in hTM-DTAiPet1 mice. To know whether the effect was achieved by reducing neural stem cell proliferation, we next examined BrdU-labeled cells in both control and hTM-DTAiPet1 mice after 3-week treatment with WAY161503. BrdU was administrated 4 times with a 2-hour interval before the mice were sacrificed. We found that the number of BrdU+ cells reduced dramatically in number in hTM-DTAiPet1 mice with no significant differences compared with controls (Fig. 6e,f). DCX+ cells were reduced in number as well although it was still higher in hTM-DTAiPet1 mice relative to control (Fig. 6g). These results suggest that impaired activation of 5-HTR2c contributes to the enhanced adult neurogenesis in hTM-DTAiPet1 mice.
Figure 6. HTR2c agonist prevents the increase of adult hippocampal neurogenesis in hTM-DTAiPet1 mice.
(a) Scheme of combinatorial injection of tamoxifen and 5-HTR agonists in mice. Tamoxifen was injected on day 1, 2, 4 and 5, while agonists was from day 3 to day 26. (b) Representative figures of DCX+ cells in control mice and hTM-DTAiPet1 mice treated with different agonists. (c) Quantification of DCX+ cells in control and hTM-DTAiPet1 mice treated with different agonists. Numbers of DCX+ cells in hTM-DTAiPet1 mice were normalized to those of control mice, and expressed as DCX increase index. 5-HTR1a and 5-HTR2 family agonists have no apparent effects on the increase of DCX+ cells, but 5-HTR2c agonist partially blocks this increase in hTM-DTAiPet1 mice. Data were compared using one-way ANOVA (alpha = 0.05) with post hoc Tukey test (p = 0.011 for comparison of vehicle and 5-HTR2c agonist). (d) Scheme of combinatorial injection of tamoxifen, 5-HTR agonists and BrdU in mice. (e) After injection of 5-HTR2c agonist, the increase of BrdU+ cells in hTM-DTAiPet1 mice is prevented. (f,g) Quantification of BrdU+ cells and DCX+ cells. There is no significant differences between control and hTM-DTAiPet1 mice in number of BrdU+ cells while more DCX+ cells are present in hTM-DTAiPet1 mice. Data were compared using Student’s t-test (p = 0.041 in g). All data are presented as mean ± s.e.m. *p < 0.05. Scale bars represent 100 μm (b,e).
Altered anxiety- and depression-like behaviors, and fear memory in hTM-DTAiPet1 mice
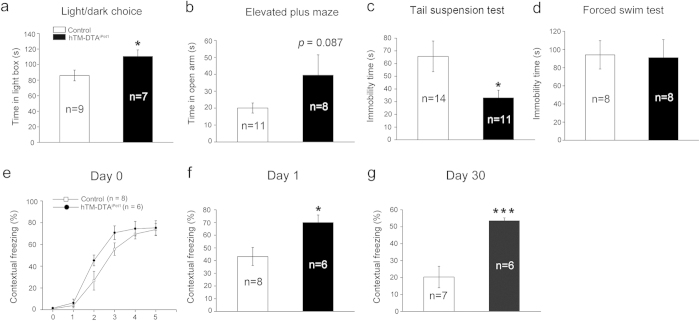
The enhanced adult hippocampal neurogenesis is required for fluoxetine to exert its antidepressant effect22. To date, there have been no published reports describing the behavioral characteristics associated with a genetic mouse model lacking the vast majority of central 5-HTergic neurons from adulthood, but having enhanced hippocampal neurogenesis. We thus set out to characterize the behavioral phenotype of hTM-DTAiPet1 mice, starting with anxiety-like behaviors. In the light/dark choice test, we found that hTM-DTAiPet1 mice spent significantly more time in the lit compartment compared to control mice (Fig. 7a). Consistently, hTM-DTAiPet1 mice spent more time in the open arms of the elevated plus maze than control mice did, although this difference did not reach statistical significance (Fig. 7b; p = 0.086). Basal depression-like behaviors were also assessed with two different tests. No difference was observed in the forced swim test, but immobility time in the tail-suspension test was significantly reduced in hTM-DTAiPet1 mice compared with controls (Fig. 7c,d). These results suggest that the basal anxiety- and depression-like behaviors are altered in hTM-DTAiPet1 mice.
Figure 7. Altered anxiety- and depression-like behaviors, and fear memory in hTM-DTAiPet1 mice.
(a) The time spent in the lit compartment is significantly increased in hTM-DTAiPet1 mice in the light/dark choice test, indicating reduced anxiety-like behaviors. Data were compared using Student’s t-test (p = 0.037). (b) The time spent in the open arms of the elevated plus maze is decreased in hTM-DTAiPet1 mice, but the difference is not statistically significant (p = 0.087). (c–d) Immobility time is significantly decreased in hTM-DTAiPet1 mice in the tail suspension test (c), but comparable between control and hTM-DTAiPet1 mice in the forced swim test (d). Data were compared using Student’s t-test (p = 0.0363 in d). (e–g) Enhanced fear memory in hTM-DTAiPet1 mice. Normal fear freezing is observed in hTM-DTAiPet1 mice on Day 0 (e). Data were compared using Two-way Repeated-Measures ANOVA. On Day 1 (f) and Day 30 (g), however, hTM-DTAiPet1 mice exhibit significantly increased contextual freezing. Data were compared using Student’s t-test (p = 0.017 in (f) and p = 8.2 × 10−4 in g). All data are presented as mean ± s.e.m. *p < 0.05; ***p < 0.001.
Our previous study showed that Pet1-Cre;Lmx1bflox/flox mice, which essentially lack all central 5-HTergic neurons from late embryonic stage, displayed enhanced contextual fear memory23. It has also been reported that mice with decreased adult hippocampal neurogenesis exhibit reduced fear memory24. We were promoted to examine whether contextual fear memory is altered in our hTM-DTAiPet1 mice. On the first day (Day 0), mice were given five foot shocks to acquire fear memory. Both hTM-DTAiPet1 and control mice showed similar increases in freezing time after each foot shock (Fig. 7e). One day (Day 1) and 30 days (Day 30) later, mice were tested to retrieve recent and remote fear memory, respectively. We found that freezing times remained high in hTM-DTAiPet1 mice at both time points, but decreased in control mice (Fig. 7f,g), thus demonstrating that contextual fear memory is enhanced in hTM-DTAiPet1 mice.
Discussion
In the present study, we employed two approaches to genetically lower central 5-HT levels starting from adulthood. Both mouse models with lower adult central 5-HT took advantage of the inducible Cre system, in order to avoid the potential developmental defects associated with depleting central 5-HT during the development. We found that removing 5-HTergic neurons in hTM-DTAiPet1 mice and deleting Tph2 in 5-HTergic neurons in Tph2iPet1 mice both led to significant enhancement of adult neurogenesis in the hippocampus.
Previous studies have examined hippocampal adult neurogenesis in knockout mice constitutively lacking Tph2 or 5-HTT or 5-HT receptors. Normal hippocampal neurogenesis has been reported in adult Tph2−/− and 5-HTT−/− mice9,10. 5-HTR1a antagonists can cause a decrease in adult neurogenesis3, while 5-HTR1a knockout mice appear normal in this regard22. Central 5-HTRs are known to be functional from embryonic stages onwards, and central 5-HT plays important roles in neuronal morphogenesis and neural circuitry formation during embryonic and early postnatal development. Therefore, it is likely that these conventional knockout lines would exhibit developmental defects, and in fact growth retardation has been reported in Tph2−/− mice25,26. Due to known and potential unknown secondary developmental defects, these knockouts may be suboptimal for conclusively characterizing the role of central 5-HT in adult neurogenesis.
It is well known that up-regulating 5-HT system by SSRIs enhances adult hippocampal neurogenesis8. In this study, we revealed that lowering 5-HT system function in adult brain by depleting 5-HTergic neurons or inactivating Tph2 expression could also enhance adult hippocampal neurogenesis. BrdU-labeled, Ki67+ and MCM2+ cells in the SGZ were all increased, and GFAP+/Sox2+ QNPs and GFAP-/Sox2+ ANPs were increased as well. In addition to increase of neural stem cells, we also found that the survival of new-born neurons generated before and after depleting 5-HTergic neurons was also enhanced in hTM-DTAiPet1 mice, which is consistent with the previous findings that the survival of adult-born cells is enhanced in the SGZ of mice with central 5-HT deficiency from embryonic stages including Pet1−/− mice, 5-HTTcre/+; VMAT2f/f and Tph2 KI mice12,13. Based on the data mentioned above, we think that both enhanced new-born survival and increased neural stem cell proliferation contribute to the enhancement of hippocampal neurogenesis in our mouse models.
It has been shown that 5-HTR2c antagonist increases adult hippocampal neurogenesis27. We revealed that chronic administraion of 5-HTR2c agonist lowered the increase of BrdU-labled cells in hTM-DTAiPet1 mice, suggesing that the increased adult neurogenesis in our 5-HT-deficient mice may be if not all at least partially caused by impaired activitaion of 5-HTR2c. However, althrough DCX+ cells were lowered in number after adminstration of 5-HTR2c agonist, it was still higher than control. These results suggest that other 5-HT receptors may be involved, although we did not detect obvouis changes in DCX+ cells after administration of agonist for 5-HTR1a and 5-HTR2 family. Among 5-HT receptors distributed in the dentate gyrus and hippocampal CA1-3 regions, relative expression level of 5-HTR2c is low28. As 5-HT is believed to be an extrinscic factor in regualting adult neurogenesis1,2,3, we speculate that adult 5-HT deficiency may lead to unknown alterations in activities of some neural networks which in turn result in the enhanced adult neurogenesis. Further studies are needed to examine the mechanisms underlying the enhanced hippocampal neurogensis with central 5-HT deficiency exclusively in adulthood.
Here we found that depleting over 95% of central 5-HTergic neurons in hTM-DTAiPet1 mice enhances adult hippocampal neurogenesis, but removing approximately 50% of them has no such effect. In our Tph2iPet1 mice, Tph2 is inactivated in approximately 85% of central 5-HTergic neurons, and increased adult neurogenesis is also observed, although the increasing level is less than that in hTM-DTAiPet1 mice. Based on these data, we can hypothesize that a threshold of central 5-HT level exists, below which hippocampal neurogenesis becomes enhanced. Different 5-HTRs show different roles in regulation of adult neurogenesis29. Different 5-HT receptors are expressed at varying levels within the hippocampus28 (http://mouse.brain-map.org), and their specific affinity to 5-HT is also variable30 (http://www.iuphar-db.org). It is possible that different 5-HT receptor combinations might be activated depending on whether 5-HT levels are high or low, thus affecting adult neurogenesis differentially. Enhancing 5-HT function by SSRIs is also capable of incresesing adult hippocampal neurogenesis22. Thus, the hippocampal neurogenesis is enhanced in the two opposite conditions by 5-HT, in which central 5-HT levels are extremely low (the present study), as well as in SSRIs-treated mice, in which 5-HT levels are high22 through activation different 5-HT receptors in normally-developed brain.
Our hTM-DTAiPet1 mice display reduced anxiety-like behaviors but enhanced contextual fear memory. Our previous results indicated that Pet1-Cre;Lmx1bflox/flox mice lacking central 5-HTergic neurons at embryonic stage show similar alterations in these types of behaviors23 but have normal adult hippocampal neurogenesis. These results suggest that reduced anxiety-like behaviors and enhanced contextual fear memory in hTM-DTAiPet1 mice may be due at least in part to low levels of 5-HT itself. Running wheel training enhances the adult hippocampal neurogenesis in rodents, and physical exercises display the therapeutic effects in major depression and other behavioral deficit31,32,33. Lower hippocampal volume has been reported in patients suffering from depression34, and SSRIs-induced anti-depressant effect requires the enhanced adult hippocampal neurogenesis in mice8,22. Importantly, recent studies from Pet1-Cre;Lmx1bflox/flox and Tph2 KO mice have shown that central 5-HT deficiency is not sufficient to induce depression-like behaviors in mouse35,36. Considering the important role of adult hippocampal neurogenesis in depression, it is likely that the enhanced hippocampal neurogenesis may be a key factor for lowered basal depression-like behaviors in our hTM-DTAiPet1 mice.
In summary, we designed and utilized two novel mouse models, in which the brain develops normally but central 5-HT is depleted in adulthood, in order to investigate the regulation of adult hippocampal neurogenesis. These models uncovered an unexpected new role for central 5-HT, demonstrating that lowering it to a certain level leads to enhanced adult hippocampal neurogenesis and this effect may be partially achieved by inactivation of 5-HTR2c.
Methods
Experimental animals
To deplete 5-HTergic neurons in adulthood, Pet1-CreERT2 mice17 were crossed with Rosa26-DTA mice18, and DTAiPet1 (i.e. Pet1-CreERT2; Rosa26-DTA) were obtained from the progeny. To block 5-HT synthesis in the adult brain, Pet1-CreERT2 mice were crossed with Tph2 floxed mice and Tph2iPet1 (i.e. Pet1-CreERT2; Tph2flox/flox) mice were obtained. In DTAiPet1 mice, tamoxifen (TM) dissolved in corn oil was administered by oral gavage for a total of three times (Day 1, 2, 3) or four times (Day 1, 2, 4, 5) beginning 2.5–3.0 months after birth. Littermates of other genotypes (wild type, Rosa26-DTA or Pet1-CreERT2) received the same TM regimen and were used as controls in each set of experiments. In Tph2iPet1 mice, TM was administered for a total of six times (Day 1, 2, 5, 6, 9, 10) beginning 1.5 months after birth. All experiments were performed in accordance with the Guidelines and Regulation of Laboratory Animals Used for Biomedical Studies of Shanghai, China. Animal care practices and all experiments were reviewed and approved by the Animal Committee of Tongji University School of Medicine, Shanghai, China.
Immunohistochemistry, BrdU labeling analysis, and in situ hybridization
Immunohistochemical staining was performed as described in our previous studies37,38. The following primary antibodies were used: mouse anti-BrdU (1:300; Calbiochem) or rat anti-BrdU (1:1000; Accurate Chemical & Scientific Corporation), goat anti-Calretinin (CR) (1:1000; Chemicon), goat anti-doublecortin (DCX) (1:400; Santa Cruz), goat anti-NeuroD (1:400; Santa Cruz), rabbit anti-Tph2 (1:20,000) (Gutknecht et al., 2008), rabbit anti-GFAP (1:500; Dako), rabbit anti-5-HTT (1:1000; gift from Dr. R.D. Blakely), rabbit anti-Ki67 (1:500; Abcam), mouse anti-MCM2 (1:1000; BD Pharmingen), goat anti-Sox2 (1:400; Santa Cruz), mouse anti-NeuN (1:1000; Chemicon) and rabbit anti-tyrosine hydroxylase (TH) (1:4000; Sigma).
For BrdU pulse labeling experiment to analyze cell proliferation, mice received 4 injections of BrdU at 50 mg/kg body weight at a 2-hour interval, and were sacrificed 2 hours after the last injection. For analysis of new-born cell survival, BrdU was injected before or after TM administration. In BrdU injection before depleting 5-HTergic neurons, BrdU was injected once daily for 3 consecutive days, followed by 4 times of TM administration; mice were sacrificed 30 days after the last TM administration. In BrdU injection after depleting 5-HTergic neurons, BrdU was injected 30 days after the last TM treatment for 3 consecutive days in the same way, and mice were allowed to survive for further 30 days after the BrdU injection. Brain sections were immersed in 0.01 M citrate buffer at 95 °C for 5 min, in 2 N HCl at 37 °C for 20 min and in 0.1 M sodium borate for 10 min, and then washed in PBS. Treated sections were immunostained with anti-BrdU antibody as described above.
AADC in situ hybridization was performed as described previously (Song et al., 2011). Images were captured with an epifluorescence (Eclipse 80i, Nikon) or confocal (TCS SP5, Leica) microscope.
Cell count
We counted labeled cells in every sixth section. For counting BrdU+, NeuroD+, DCX+, MCM2+, Ki67+, GFAP+/Sox2+, and GFAP-/Sox2+ cells in the SGZ, sections at the level of −1.34 mm to −3.52 mm from Bregma were included. For counting Tph2+ cells in the dorsal raphe nucleus, sections at the level of −4.72 mm from Bregma were included17.
High performance liquid chromatography (HPLC)
Samples containing the cerebral cortex and hippocampus were collected and HPLC were performed as described previously23.
Injection of 5-HTR agonists
Control and DTAiPet1 mice from 2.5-3 months old were administrated with different 5-HTR agonists. TM were injected on day 1, day 2, day 4 and day 5, while agonists were injected once daily from day 3 to day 26 on which mice were sacrificed for analysis. All agonists of 5-HTRs were purchased from Tocris and were injected by i.p. The dosage of agonists used was as follows: 8-OH DPAT (8-hydroxy-2-dipropylaminotetralin hydrobromide, agonist of 5-HTR1a), 1 mg/kg body weight; α-Methyl-5-hydroxytryptamine maleate (5-HTR2 family agonist), 0.5 mg/kg body weight; WAY161503 (5-HTR2c agonist), 10 mg/kg body weight.
Behavioral tests
Behavioral observation was performed in adult male mice 1 month after the final TM injection. For forced swim test, mice were placed into glass cylinders (height: 24 cm; diameter: 16 cm) containing 18 cm of 23–25 °C water for 6 min. A mouse was considered to be immobile when it floated in the water, and immobility time was recorded during the last 4 min of the 6-min testing period, after 2 min of habituation.
Tail suspension test. Mice were suspended by the tail with adhesive tape (distance from tip of tail: 2 cm). Their behavior was video recorded for the duration of the 6-min testing period, with immobility time being measured during the last 4 min.
Elevated plus maze test. Mice were initially placed at the far end of one of the closed arms and allowed to freely explore the maze for 5 min. The percentage of time spent in the two open arms was then recorded.
Light/dark choice test. The light/dark choice test apparatus consisted of a small dark chamber (30 cm × 20 cm × 30 cm) connected by an opening (5 cm × 7 cm) to a larger lit chamber (30 cm × 30 cm × 30 cm). A single mouse was initially placed in one corner of the dark chamber and the percentage of time spent in the lit chamber was measured over 5 min.
Contextual fear conditioning. Contextual fear conditioning was performed as previously described23. FreezeFrame and FreezeView software systems were used to record and analyze freezing behaviors. On the first day (Day 0), mice were given five foot shocks (0.7 mA, 2s) at 2 min intervals during which the mice were able to move freely. The percentage of freezing time was measured during each inter-shock interval. On the second day (Day 1) and one month later (Day 30), mice were placed back in the box for 11 min without receiving any foot shocks, and freezing time was measured to test contextual fear memory.
Statistical analysis
All samples passed both the Shapiro-Wilk normality test and equal variancetest except for 5-HT level in Fig. 1d, BrdU+ cell numbers in Fig. 3d and BrdU+ cells in BrdU after TM groups in Fig. 4d–e. Comparisons were performed using the Student’s t-test, one-way ANOVA with post hoc Tukey test or Mann-Whitney rank sum test (for samples which didn’t pass normality or equal variance test). All data are presented as mean ± s.e.m. P values of less than 0.05 were considered statistically significant.
Additional Information
How to cite this article: Song, N.-N. et al. Reducing central serotonin in adulthood promotes hippocampal neurogenesis. Sci. Rep. 6, 20338; doi: 10.1038/srep20338 (2016).
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (2013CB835100, 2012CB966900, 2011CB510000, 2012AA022402), the National Natural Science Foundation of China (81571332, 81221001, 31100788, 81200933, 81200692, 81101026, 91232724), Science and Technology Commission of Shanghai Municipality (12XD1404800), and the Fundamental Research Funds for the Central Universities (2013KJ049).
Footnotes
Author Contributions N.-N.S. and Y.-F.J. conceived and designed the project, performed the experiments, analyzed data, wrote the manuscript. L.Z., Q.Z., Y.H., X.-Z. L., L.H., W.L., L.C. and X.C. collected and analyzed data. K.-P.L. provided Tph2 floxed mice. L.X. and Y.-Q.D. conceived, designed and supervised the project and wrote the manuscript. All authors reviewed, commented on and approved the manuscript.
References
- Duan X., Kang E., Liu C. Y., Ming G. L. & Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol 18, 108–115, 10.1016/j.conb.2008.04.001 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G. L. & Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702, 10.1016/j.neuron.2011.05.001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Deng W. & Gage F. H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660, 10.1016/j.cell.2008.01.033 (2008). [DOI] [PubMed] [Google Scholar]
- Berg D. A., Belnoue L., Song H. & Simon A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 140, 2548–2561, 10.1242/dev.088005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther D. J. et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299, 76, 10.1126/science.1078197 (2003). [DOI] [PubMed] [Google Scholar]
- Canli T. & Lesch K. P. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci 10, 1103–1109, 10.1038/nn1964 (2007). [DOI] [PubMed] [Google Scholar]
- Schildkraut J. J. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry 122, 509–522 (1965). [DOI] [PubMed] [Google Scholar]
- Malberg J. E., Eisch A. J., Nestler E. J. & Duman R. S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20, 9104–9110 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A. et al. Adult neurogenesis in serotonin transporter deficient mice. J Neural Transm 114, 1107–1119, 10.1007/s00702-007-0724-6 (2007). [DOI] [PubMed] [Google Scholar]
- Klempin F. et al. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci 33, 8270–8275, 10.1523/JNEUROSCI.5855-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. W., David D. J., Monckton J. E., Battaglia F. & Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci 28, 1374–1384, 10.1523/JNEUROSCI.3632-07.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz S. L. et al. Paradoxical increase in survival of newborn neurons in the dentate gyrus of mice with constitutive depletion of serotonin. Eur J Neurosci 38, 2650–2658, 10.1111/ejn.12297 (2013). [DOI] [PubMed] [Google Scholar]
- Sachs B. D. et al. The effects of congenital brain serotonin deficiency on responses to chronic fluoxetine. Transl Psychiatry 3, e291, 10.1038/tp.2013.65 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K. P. & Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 76, 175–191, 10.1016/j.neuron.2012.09.013 (2012). [DOI] [PubMed] [Google Scholar]
- Toda T. et al. Birth regulates the initiation of sensory map formation through serotonin signaling. Dev Cell 27, 32–46, 10.1016/j.devcel.2013.09.002 (2013). [DOI] [PubMed] [Google Scholar]
- Gaspar P., Cases O. & Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci 4, 1002–1012, 10.1038/nrn1256 (2003). [DOI] [PubMed] [Google Scholar]
- Song N. N. et al. Adult raphe-specific deletion of Lmx1b leads to central serotonin deficiency. PLoS One 6, e15998, 10.1371/journal.pone.0015998 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D., Liang H. E. & Locksley R. M. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J Immunol 180, 4742–4753 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton S. B., Chan K. K., Anderson J. R., Stoeber K. & Williams G. H. Replicative Mcm2 protein as a novel proliferation marker in oligodendrogliomas and its relationship to Ki67 labelling index, histological grade and prognosis. Neuropathol Appl Neurobiol 27, 305–313 (2001). [DOI] [PubMed] [Google Scholar]
- Encinas J. M., Vaahtokari A. & Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA 103, 8233–8238, 10.1073/pnas.0601992103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht L. et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm 115, 1127–1132, 10.1007/s00702-008-0096-6 (2008). [DOI] [PubMed] [Google Scholar]
- Santarelli L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809, 10.1126/science.1083328 (2003). [DOI] [PubMed] [Google Scholar]
- Dai J. X. et al. Enhanced contextual fear memory in central serotonin-deficient mice. Proc Natl Acad Sci USA 105, 11981–11986, 10.1073/pnas.0801329105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe M. D. et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA 103, 17501–17506, 10.1073/pnas.0607207103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht L. et al. Impacts of Brain Serotonin Deficiency following Tph2 Inactivation on Development and Raphe Neuron Serotonergic Specification. PLoS ONE 7, e43157, 10.1371/journal.pone.0043157 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelieva K. V. et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One 3, e3301, 10.1371/journal.pone.0003301 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M., Soumier A., Hery M., Mocaer E. & Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry 59, 1087–1096, 10.1016/j.biopsych.2005.11.025 (2006). [DOI] [PubMed] [Google Scholar]
- Tanaka K. F., Samuels B. A. & Hen R. Serotonin receptor expression along the dorsal-ventral axis of mouse hippocampus. Philos Trans R Soc Lond B Biol Sci 367, 2395–2401, 10.1098/rstb.2012.0038 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempin F. et al. Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis. Front Mol Neurosci 3, 10.3389/fnmol.2010.00014 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman R. B. et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 102, 2836–2841 (2000). [DOI] [PubMed] [Google Scholar]
- Ernstn C., Olson A., Pinel J., Lam R. & Christie B. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci 31, 84–92 (2006). [PMC free article] [PubMed] [Google Scholar]
- Lucassen P. J. et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol 20, 1–17, 10.1016/j.euroneuro.2009.08.003 (2010). [DOI] [PubMed] [Google Scholar]
- Wolf S. A., Melnik A. & Kempermann G. Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia. Brain Behav Immun 25, 971–980, 10.1016/j.bbi.2010.10.014 (2011). [DOI] [PubMed] [Google Scholar]
- Campbell S., Marriott M., Nahmias C. & MacQueen G. M. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry 161, 598–607 (2004). [DOI] [PubMed] [Google Scholar]
- Angoa-Pérez M. et al. Mice Genetically Depleted of Brain Serotonin Do Not Display a Depression-like Behavioral Phenotype. ACS Chemical Neuroscience 5, 908–919, 10.1021/cn500096g (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y.-F. et al. Abnormal anxiety- and depression-like behaviors in mice lacking both central serotonergic neurons and pancreatic islet cells. Front Behav Neurosci 8, 10.3389/fnbeh.2014.00325 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C. et al. Lmx1b is essential for Fgf8 and Wnt1 expression in the isthmic organizer during tectum and cerebellum development in mice. Development 134, 317–325, 10.1242/dev.02745 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Satb2 is required for dendritic arborization and soma spacing in mouse cerebral cortex. Cereb Cortex 22, 1510–1519, 10.1093/cercor/bhr215 (2012). [DOI] [PubMed] [Google Scholar]