Abstract
During embryonic development, tissues undergo major rearrangements that lead to germ layer positioning, patterning, and organ morphogenesis. Often these morphogenetic movements are accomplished by the coordinated and cooperative migration of the constituent cells, referred to as collective cell migration. The molecular and biomechanical mechanisms underlying collective migration of developing tissues have been investigated in a variety of models, including border cell migration, tracheal branching, blood vessel sprouting, and the migration of the lateral line primordium, neural crest cells, or head mesendoderm. Here we review recent advances in understanding collective migration in these developmental models, focusing on the interaction between cells and guidance cues presented by the microenvironment and on the role of cell–cell adhesion in mechanical and behavioral coupling of cells within the collective.
Introduction
The ability of cells to migrate is essential for physiological functions such as immunosurveillance, wound healing, and tissue morphogenesis during development. Pathological processes such as cancer invasion and metastasis also rely on the ability of malignant cells to acquire invasive and migratory capabilities (Friedl and Gilmour, 2009). The molecular mechanisms through which individual cells move have been extensively studied (Ridley et al., 2003; Petrie et al., 2009; Bear and Haugh, 2014). In recent years, the importance of collective cell migration in orchestrating complex morphogenetic events during embryo development has been increasingly recognized. Collective migration is defined as the ability of groups of cells to move together and simultaneously affect the behavior of one another, for example through stable or transient cell–cell connections (Rørth, 2012; Theveneau and Mayor, 2012). It is important to distinguish collective migration from a global ordering of cell migration, such as long-range chemotaxis, where the overall movement is largely independent of the interaction of the individuals and is rather governed by the interaction of each individual cell with the global external stimulus (Friedl et al., 2012). Thus, collective cell migration requires coordination and cooperation between migrating cells.
Collective cell migration has been extensively studied in vivo in both vertebrate and invertebrate models. Archetypal examples of epithelial collective migration include Drosophila melanogaster border cells, Zebrafish lateral line and branching and sprouting morphogenesis of Drosophila trachea and mouse retina. Collectively migrating mesenchymal cohorts include neural crest and mesendoderm from Xenopus laevis and zebrafish. They deploy a variety of strategies to effectively achieve collective migration (Table 1). Nevertheless, the core mechanisms required for group migration, which emerged from the study of these models, are conserved.
Table 1. Comparing collective cell migration across different models.
| Model | Chemoattractant | Leader/ follower | Rac activation at leader cell | Traction substrate | Cadherin subtype | CIL/contact- dependent polarity | Gradient of chemoattractant |
|---|---|---|---|---|---|---|---|
| Border cell | PVF/EGF (1–4) Gurken(2) | Yes (5) Dynamically rearranged (5,6) | Yes (7–10) | E-cadherin (7,11) | E-cadherin (7,11) | Yes Observations of contact-dependent cell polarity (5) Active suppression of internal protrusions (12) and Rac1 polarization (7) | Not yet elucidated PVF-1 protein is expressed in the oocyte (2), and Krn and Spi mRNAs are also detected in the oocyte (3) |
| Lateral line | CXCL12/SDF-1 (13–15) | Yes (14) Dynamic rearrangements not yet elucidated | Not yet elucidated | Not yet elucidated | E-cadherin (16) N-cadherin (17) | Yes Observations of contact-dependent cell polarity (14,18) | Yes Self-generated SDF-1 gradient (13) Moving source of FGF: anterior lateral line (19) |
| Branching morphogenesis | Drosophila Trachea: Branchless (20–22) Mouse retina: VEGF (23) | Yes Specified by Btl/VEGF signaling levels (22–25), dynamic rearrangements may occur (26–29) | Yes Drosophila trachea (24,30) Mouse retina: not yet elucidated | Mouse retina: FN ECM (31) | Drosophila trachea: E-cadherin (32,33) Mouse retina: VE-cadherin (29) | Yes Observations of contact-dependent cell polarity and Rac1 polarization (24) | Yes Drosophila trachea: O-sulfotransferases sulfateless and sugarless genetically interact with branchless (34), although gradient not yet elucidated Mouse hindbrain: VEGF isoforms binding to ECM create a gradient of VEGF protein (35) |
| Neural crest | CXCL12/SDF-1 (36–39) VEGF (55) | Yes (40,41) Dynamically rearranged (42) | Yes (36,41,43,44) | Fibronectin ECM (45–47) | N-cadherin (36, 37,41,42) | Yes Mediated by N-cadherin and Wnt/PCP (36,37,40) Rac1 polarization and suppression of protrusions at internal contacts (36,40,41) | Yes Moving source of SDF-1: epibranchial placodes (37) VEGF gradient suggested (55) |
| Mesendoderm | PDGF (48–50) | No All cells in the collective form oriented unipolar protrusions (48,51) | Yes Rac required for protrusion formation in zebrafish (52) | Xenopus: FN ECM (51,53) Zebrafish: E-cadherin (52,54) | E-cadherin (52,54), C-cadherin (56) | Yes Mediated by E-cadherin and Wnt/PCP via Rac1 (52) Tension-dependent polarization mediated by C-cadherin (56) | Not yet elucidated. PDGF mRNA expressed in roof plate but protein localization not yet investigated (49,50) |
(1) Duchek and Rørth, 2001; (2) Duchek et al., 2001; (3) McDonald et al., 2006; (4) McDonald et al., 2003; (5) Prasad and Montell, 2007; (6) Bianco et al., 2007; (7) Cai et al., 2014; (8) Ramel et al., 2013; (9) Wang et al., 2010; (10) Fernández-Espartero et al., 2013; (11) Niewiadomska et al., 1999; (12) Lucas et al., 2013; (13) Donà et al., 2013; (14) Haas and Gilmour, 2006; (15) Valentin et al., 2007; (16) Matsuda and Chitnis, 2010; (17) Revenu et al., 2014; (18) Lecaudey et al., 2008; (19) Dalle Nogare et al., 2014; (20) Sutherland et al., 1996; (21) Klämbt et al., 1992; (22) Ghabrial and Krasnow, 2006; (23) Gerhardt et al., 2003; (24) Lebreton and Casanova, 2014; (25) Hellström et al., 2007; (26) Arima et al., 2011; (27) Jakobsson et al., 2010; (28) Caussinus et al., 2008; (29) Bentley et al., 2014; (30) Chihara et al., 2003; (31) Stenzel et al., 2011b; (32) Cela and Llimargas, 2006; (33) Shaye et al., 2008; (34) Lin et al., 1999; (35) Ruhrberg et al., 2002; (36) Theveneau et al., 2010; (37) Theveneau et al., 2013; (38) Belmadani et al., 2005; (39) Olesnicky Killian et al., 2009; (40) Carmona-Fontaine et al., 2008; (41) Scarpa et al., 2015; (42) Kuriyama et al., 2014; (43) Carmona-Fontaine et al., 2011; (44) Moore et al., 2013; (45) Alfandari et al., 2003; (46) Kil et al., 1996; (47) Lallier et al., 1992; (48) Montero et al., 2003; (49) Damm and Winklbauer, 2011; (50) Nagel et al., 2004; (51) Davidson et al., 2002; (52) Dumortier et al., 2012; (53) Boucaut and Darribere, 1983; (54) Montero et al., 2005; (55) McLennan and Kulesa, 2010; (56) Weber et al., 2012.
Epithelial and mesenchymal collective migration
Embryonic tissues undergo during development major rearrangements required for morphogenesis. These collective migration events may involve either the collective movement of epithelial sheets with cells retaining stable adherens junctions and apicobasal polarity markers (Fig. 1 a), or the cooperative interaction between looser mesenchymal cohorts mediated by transient adherens junctions (Fig. 1 b; Theveneau et al., 2010; Scarpa et al., 2015). Despite their different characteristics, cell–cell interactions in both epithelial and mesenchymal collectives are required to mechanically link one cell to the other as well as to influence each other’s motile and protrusive behavior (Theveneau et al., 2010; Weber et al., 2012; Cai et al., 2014; Davis et al., 2015). In addition to cell–cell interactions, both epithelial and mesenchymal cell collectives interact with their extracellular environment during migration. In particular, interactions with the ECM, with other tissues, and responses to chemotactic cues produced in the surrounding environment are essential for collective tissue guidance during embryonic development.
Figure 1.
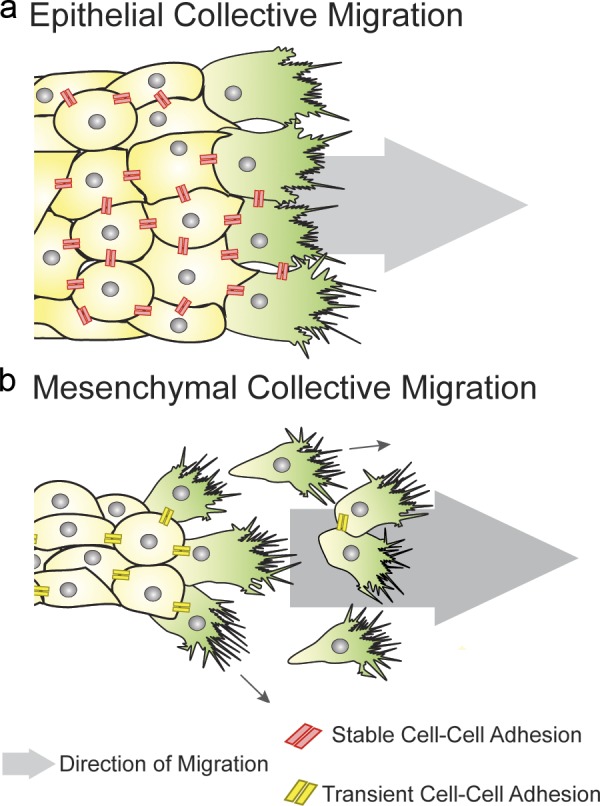
Epithelial and mesenchymal collective migration. (a) Epithelial cells move as cohesive groups, maintaining cell–cell adhesions. Leader cells form protrusions oriented in the direction of migration, whereas followers form smaller cryptic protrusions (not depicted). (b) Mesenchymal cells migrate directionally as a collective, but they form transient cell–cell connections, which may redirect protrusion formation contributing to the overall directionality.
Polarization of the collective: leaders and followers
The migration of a collective of cells can, to a certain extent, be compared with an isolated migrating cell. To displace toward the target a cohort of cells may require the front of the group to form protrusions that exert tractions on the extracellular environment and sense any environmental cues that may help guidance, equivalent to the protrusions produced during single-cell migration. In addition, the front and the back of the cluster will have to be mechanically linked to achieve successful migration and displacement of the cells in the posterior rows. Studies in developmental models have highlighted how collectively migrating groups exploit different strategies to achieve such polarization. Seminal work stemmed from an invertebrate model for branching morphogenesis, Drosophila trachea (Fig. 2 a). Epithelial tube branching is essential for the morphogenesis of several organs, including lungs, blood vessels, mammary gland, and nephric ducts, and requires collective migration. In Drosophila, tracheal branching occurs through collective migration and cell intercalation in the absence of cell division. Tracheal cells invaginate from numerous epithelial placodes to form tracheal sacs and respond via the receptor Btl (breathless) to the ligand Bnl (branchless) secreted in the adjacent tissue (Klämbt et al., 1992; Sutherland et al., 1996). Cells with the highest levels of Btl signaling will take leader positions at the terminal end of the branch, where they produce large protrusions and drive collective migration (tip cells; Fig. 2 b; Caussinus et al., 2008; Lebreton and Casanova, 2014), whereas the others behave as follower stalk cells, which only form small, cryptic protrusions (Fig. 2 b; Lebreton and Casanova, 2014). In this context, establishment of leader cells is highly stereotyped and requires Delta-Notch–dependent lateral inhibition: cells with high levels of Btl produce Delta, which activates Notch in neighboring cells (Ghabrial and Krasnow, 2006). Interestingly, individual Btl+ cells are able to reach the tip position and rescue branching morphogenesis in a Btl mutant background (Lebreton and Casanova, 2014), thus highlighting how tip positions are taken by cells with the highest levels of Btl signaling. As a consequence of tip cell migration, stalk cells undergo passive intercalation, which further contributes to the elongation of the branch: laser ablation of tip cells results in impaired stalk cell intercalation, suggesting that tensile forces exerted by the migrating tip cell drive intercalation (Caussinus et al., 2008). Similar mechanisms orchestrate sprouting angiogenesis of vertebrate blood vessels in the postnatal mouse retina (Fig. 2 a′). In this context, however, stalk elongation is obtained via mitotic division of follower cells and active stalk cell rearrangements (Fig. 2 b′; Gerhardt et al., 2003). The tip cell state is specified by high levels of VEGF-A signaling, which in turn induces expression of Dll-4 (Delta-like 4) and Notch1-dependent lateral inhibition of tip cell state in the neighbors, compelling them to become stalk cells. Indeed, Dll4 haploinsufficiency or endothelial-specific deletion of Notch1 results in supernumerary tip cells (Hellström et al., 2007; Suchting et al., 2007). Another well-studied model of branching morphogenesis is mammary gland development. In contrast with tracheal and vessel branching, here elongation of the mammary duct network during puberty requires a variety of rearrangements of the epithelial tissue, in which stalk cell elongation is obtained via asymmetric division of luminal cells to produce a transiently stratified terminal end bud of the mammary gland (Huebner et al., 2014). Collective migration of cells in the stratified epithelium then occurs concomitantly to cell proliferation in absence of outward directed protrusions (Ewald et al., 2008), with individual cells actively forming protrusions in the bulk of the epithelial bud (Ewald et al., 2012). Although mammary gland collective migration shares some features with tracheal branching, such as requirement of FGF (Branchless in Drosophila) signaling (Lu et al., 2008; Zhang et al., 2014), morphogenesis of the mammary gland greatly involves transitions between epithelial states, which have been comprehensively discussed in recent reviews (Andrew and Ewald, 2010; Huebner and Ewald, 2014).
Figure 2.
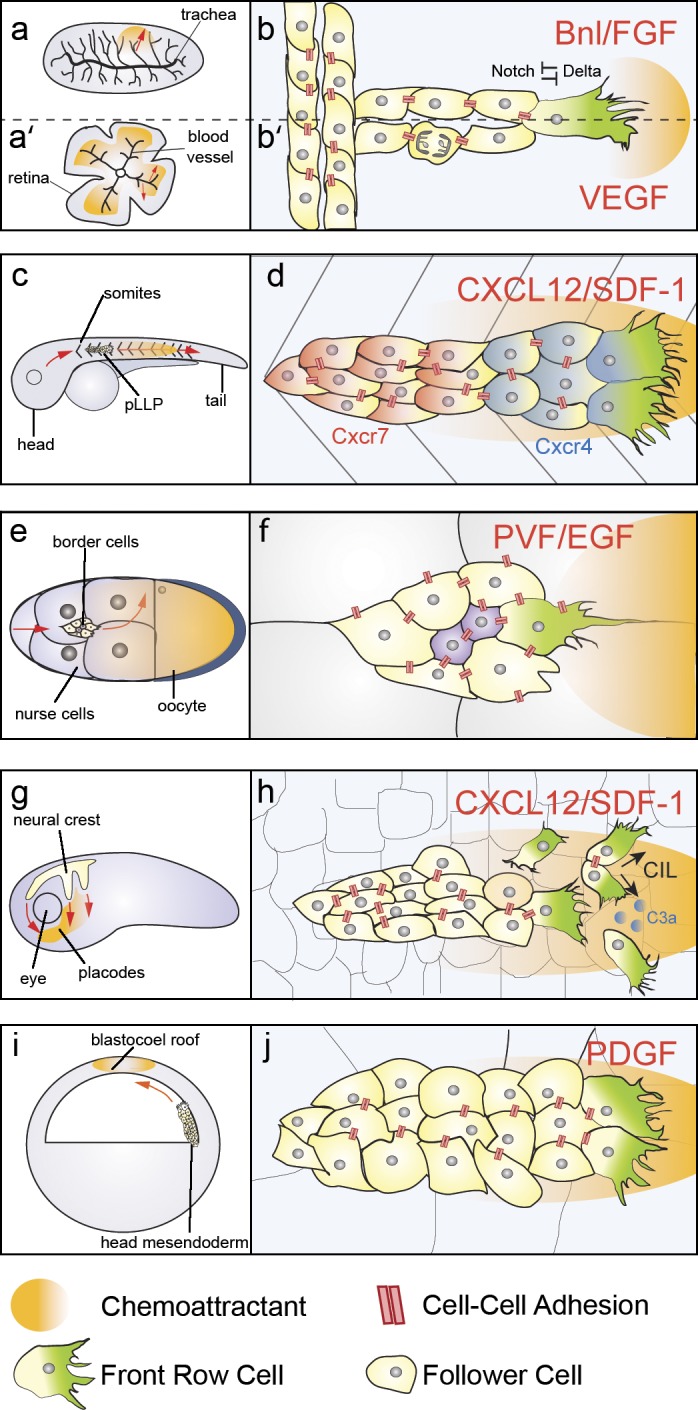
Overview of models of collective migration in development. (a) Branching morphogenesis of Drosophila trachea. (a′) Sprouting morphogenesis of mouse retina, red arrows indicate the direction of migration. Orange shadow represents the source of the chemoattractants Bnl (b) or VEGF (b′). (b) Bnl/FGF signaling induces tip cell state in the drosophila trachea via Delta/Notch lateral inhibition. Stalk cells intercalate passively. (b′) VEGF signaling induces tip cell state in endothelial cells via Delta/Notch lateral inhibition. Follower cells contribute to stalk elongation via proliferation. (c) The lateral line primordium migrates caudally along the horizontal myoseptum of the zebrafish embryo, which is a source of CXCL12/SDF-1 (orange); red arrows indicate direction of migration. (d) CXCL12/SDF-1 acts as a chemoattractant for the primordium. Back cells express the Cxcr7 (red) and Cxcr4 (not depicted) receptors, whereas front cells express Cxcr4 (blue) receptor. Front (leader) cells form large protrusions, cell–cell contacts are maintained throughout the primordium. (e) Border cell migration. Border cells delaminate from the anterior pole of the egg chamber to migrate posteriorly (red arrow) and then turn dorsally (red curved arrow) toward the end of their path. Orange shadow represents the gradient of chemoattractants PVR/EGF and Gurken. (f) The border cell cluster migrates in between the nurse cells. Cell–cell adhesions are present at the border cell–border cell (yellow) interface, at the border cell–polar cell (purple) interface, and at the border cell–nurse cell (white) interface. PVF-1 and EGF guide border cell migration by polarizing the protrusions of the cell with the highest RTK signaling levels. (g) Neural crest delaminates from the neural plate border and migrates dorsoventrally across the head of the embryo, where SDF-1 (orange) acts as a chemoattractant; red arrows indicate direction of migration. (h) Neural crest migration requires transient cell–cell contacts, which polarize the front cells via contact inhibition of locomotion, local attraction via C3a/C3aR, and chemotaxis toward SDF-1. (i) Head mesendoderm migrates collectively toward the BCR, which is a source of PDGF in Xenopus; red arrows indicate direction of migration. (j) Mesendodermal cells orient their protrusions in a PDGF and cell–cell contact–dependent manner.
In branching morphogenesis of the trachea and of blood vessels, the tip cell in leader position actively migrates, whereas follower cells contribute to stalk elongation via other mechanisms such as intercalation of proliferation. On the other hand, in zebrafish lateral line, Drosophila border cells, and in models of mesenchymal collective migration such as neural crest and head mesendoderm, the whole group of cells is migratory. Here, polarization of the cohort is achieved through different strategies. The zebrafish posterior lateral line primordium (pLLP) originates as an epithelial placode just posterior of the otic vesicle and translocates anteroposteriorly along the horizontal myoseptum of the zebrafish embryo (Fig. 2 c). In this context, collective migration is coupled with organogenesis, as the pLLP cluster deposits sensory organs called proneuromasts while migrating (Haas and Gilmour, 2006). pLLP cells migrate as a polarized cohesive collective: the front cells are organized in a mesenchymal fashion, displaying a front-rear polarity and exhibiting large lamellipodial and filopodial protrusions (Fig. 2 d), whereas the trailing cells assemble epithelial rosettes that are deposited as proneuromasts as the leading cells advance (Haas and Gilmour, 2006). The polarization of the pLLP collective does not only occur at the morphological level: the receptors Cxcr4b and Cxcr7, which are required in the pLLP for chemoattraction to the chemokine CXCL12/SDF-1, are differentially expressed between the front and rear cells. Cxcr4b is located in the anterior and posterior cells, whereas the Cxcr7 is found in the trailing cells (Valentin et al., 2007).
Another well-studied model for epithelial collective migration in which leader and follower cells exhibit cell motility is that of the border cells of the Drosophila egg chamber. This is composed of one oocyte and 15 nurse cells, which provide cytoplasmic contents for the developing oocyte. They are surrounded by a monolayer of somatic follicle cells which form a supporting structure, the egg chamber (Fig. 2 e). Specialized follicle cells called anterior polar cells (Wu et al., 2008) recruit six to eight adjacent follicle cells to form the migratory border cell cluster (Montell et al., 1992), which detaches from the follicular epithelium and migrates in between the nurse cells to eventually reach the anterior dorsal border of the oocyte (Fig. 2 e; Rørth, 2002; Montell, 2003), where they will form a structure necessary for sperm entry, the micropyle. Border cells therefore migrate as an isolated epithelial cluster through the surrounding tissue. Live imaging of border cell migration has revealed that the border cell cluster is highly dynamic: at any given time point, a single cell is found in leader position, extending and retracting protrusions in between the nurse cells. The position of leader is stochastically acquired and leader stability varies in time, with leader and followers exchanging positions during the course of migration (Prasad and Montell, 2007). Importantly, perturbations that lead to formation of multiple leaders, such as knockdown of the guidance receptor PVR (Prasad and Montell, 2007) or expression of a dominant-negative form of the small GTPase Rac1 (Wang et al., 2010), lead to impaired migration, stressing how the group needs to globally adopt a leading edge/trailing edge polarity (Fig. 2 f; Prasad and Montell, 2007). In mesenchymal models of collective migration, such as the neural crest, establishment of protrusive activity at the front of the collective relies on yet other processes. Neural crest cells are a highly migratory population, which arises in vertebrates at late gastrula stages at the neural plate border. They delaminate from the neural tube, undergo a epithelial-to-mesenchymal transition (EMT), and migrate dorsoventrally to reach their target locations (Fig. 2 g), where they will differentiate into a plethora of derivatives (Le Douarin and Kalcheim, 1999). Similarly to border cells, neural crest cells rapidly exchange positions within the group, and the position of the leader is only transient (Kuriyama et al., 2014). Migrating neural crest cells form large lamellipodial protrusions oriented in the direction of migration both in vivo and in vitro (Matthews et al., 2008). Importantly, cells at the free edge of the cluster form larger protrusions than cells in the follower rows. Follower cells maintain these smaller and more transient protrusions because of contact inhibition of locomotion with neighbor cells (Carmona-Fontaine et al., 2008; Theveneau et al., 2010). A similar mechanism has been suggested to contribute to protrusion polarization in another example of mesenchymal collective migration, the head mesendoderm. During gastrulation, mesoderm and endoderm cells are internalized to form the three germ layers: ectoderm, mesoderm, and endoderm (Stern, 1992). In Xenopus, mesendodermal cells internalize as a cohesive sheet (Fig. 2 i; Bouwmeester et al., 1996), whereas in zebrafish, they undergo EMT and internalize as single mesenchymal cells but migrate collectively as a cohesive cluster afterward (Warga and Kimmel, 1990). In both models, migrating mesendoderm cells orient their protrusions toward the direction of migration (Fig. 2 j; Davidson et al., 2002; Montero et al., 2003; Diz-Muñoz et al., 2010; Damm and Winklbauer, 2011; Dumortier et al., 2012; Weber et al., 2012). Similar to the neural crest, the Wnt/planar cell polarity (PCP) pathway has been suggested to contribute to orientation of protrusions and coordination of migration of mesendodermal cells (Ulrich et al., 2003; Dumortier et al., 2012).
The role of chemotactic cues in guiding the collective
A common feature in developmental collective migration is the presence of guidance cues in the environment surrounding the migrating cohort, which direct the cells to their target and promote their motile behavior. As mentioned in the previous section, Drosophila tracheal cells are attracted to the FGF family ligand Bnl, expressed in cells adjacent to each tracheal sac (Sutherland et al., 1996), which the tracheal cells sense through the Btl receptor. Mutation of either Bnl or Btl results in absence of tracheal branching (Klämbt et al., 1992; Sutherland et al., 1996). Genetic mosaic analysis has shown that Bnl signaling induces competition between tracheal cells for their position in the migrating collective: cells with the highest levels of Btl signaling will take leader positions at the terminal end of the branch (Ghabrial and Krasnow, 2006). A similar function is covered by VEGF in mammalian angiogenesis (Gerhardt et al., 2003). In the mouse retina, endothelial cells sense VEGF-A, secreted by the underlying astrocytes, via the receptor VEGFR2 (Gerhardt et al., 2003). Endothelial tip cells respond to VEGF-A gradients by undergoing guided migration, whereas stalk cells respond to the global VEGF-A concentration rather than to its gradient by proliferating (Gerhardt et al., 2003). The zebrafish lateral line migrates directionally from the anterior trunk to the tail of the fish larva (Fig. 2 e) and is guided by the chemokine CXCL12/SDF-1, which is expressed along the fish horizontal myoseptum (Fig. 2 e). Experiments using mutant fish, which express CXCL12 in the most anterior part of the trunk but have lost its expression in the posterior myoseptum, have shown that the lateral line is able to undergo a U-turn, thus migrating back toward the head, once it reaches the area depleted of CXCL12 (Haas and Gilmour, 2006). This observation suggests that the polarity and migration direction of the lateral line is intrinsic to the cluster, and it is not determined by a preexisting CXCL12 gradient. Knockdown of the CXCL12 receptor Cxcr4b impairs migration and reduces protrusion formation, and transplant experiments demonstrated that few Cxcr4b-expressing cells in leader positions in a Cxcr4 mutant are sufficient to drive migration of the whole primordium (Haas and Gilmour, 2006). Conversely, knockdown of the CXCL12 receptor Cxcr7 results in the stretching of the lateral line and impaired migration, which can be rescued by transplanting Cxcr7-expressing cells at the trailing end of the primordium (Valentin et al., 2007). These and other experiments indicate that Cxcr7 works as a sink for CXCL12, generating a gradient of chemoattractant within the primordium and determining its directional migration (Donà et al., 2013). CXCL-12 (also called SDF-1) also acts as a chemoattractant for neural crest cells. In this context, collective chemotaxis is driven by the expression of the chemokine along the path of cranial and trunk neural crest migration, whereas its receptor, Cxcr4, is expressed in the neural crest cells (Fig. 2 g; Belmadani et al., 2005; Olesnicky Killian et al., 2009; Theveneau et al., 2010). Interestingly, the SDF-1/Cxcr4 axis promotes neural crest chemotaxis in a cell–cell contact–dependent manner (Theveneau et al., 2010): single neural crest cells exhibit poor chemotaxis toward SDF-1, whereas cell collectives display highly effective chemotaxis. In addition, chick cranial neural crest (CNC) are able to migrate toward a source of VEGF or of VEGF-expressing tissue in vitro (McLennan and Kulesa, 2010). In vivo, the ectoderm of the second brachial arch endogenously produces VEGF, and blocking of neuropilin-VEGF signaling by injection of the soluble form of VEGFR1 reduces the migration of neural crest cells into the second brachial arch (McLennan and Kulesa, 2010). However, the function of VEGF as a chemoattractant is still discussed as introduction of an ectopic VEGF source in the tissue in vivo slightly redirects cells toward the source but does not produce ectopic migration (McLennan and Kulesa, 2010). For Drosophila border cells, directional migration across the egg chamber is controlled by the expression of multiple chemoattractants in the oocyte and nurse cells. Initially, the border cells migrate posteriorly toward the oocyte (Fig. 2 b; Duchek and Rørth, 2001; Duchek et al., 2001; McDonald et al., 2003; McDonald and Montell, 2005). The tyrosine kinase receptor ligands PVF1 (Duchek et al., 2001) and EGF (Duchek and Rørth, 2001) act redundantly to guide border cells at this stage. Importantly, ectopic expression of either ligand redirects migration of border cells (McDonald et al., 2003, 2006). In addition, EGFR and another ligand, Gurken, which is localized dorsally, are required to guide migration of border cells toward the dorsal end of the egg chamber, after they reach the posterior half of the structure (Duchek and Rørth, 2001). In the head mesendoderm, PDGF has been suggested as a potential chemoattractant (Fig. 2 j). In zebrafish, PDGF receptor signals via PI3K and PKB to promote protrusion formation (Montero et al., 2003). In Xenopus, PDGF is expressed in the blastocoel roof (BCR; Fig. 2 j; Nagel et al., 2004; Damm and Winklbauer, 2011) in two isoforms. The longer one has been suggested to interact with the ECM of the BCR (Fig. 2 i) and to regulate directional migration of leading edge precursors in a PDGFR/PI3K-dependent manner (Nagel et al., 2004). A shorter, soluble one instead regulates protrusion orientation and directionality of migration of deep mesoderm prechordal cells (Damm and Winklbauer, 2011).
The role of extracellular substrate in guiding the collective
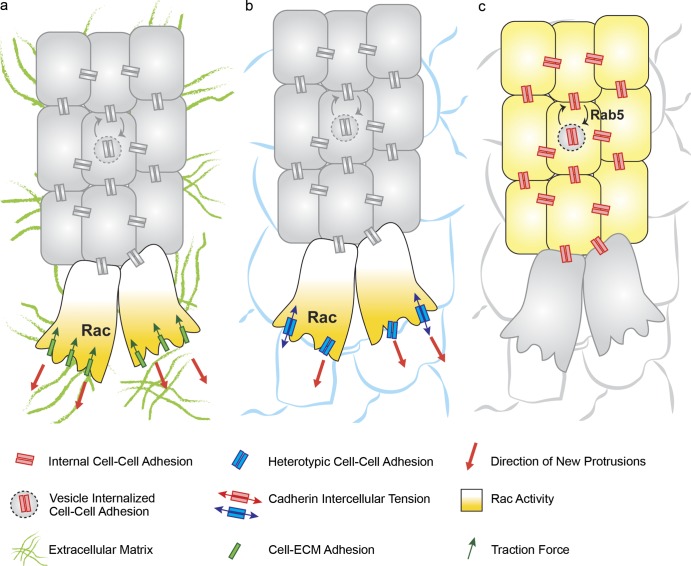
To form polarized protrusions that allow collective movement, migrating collectives have to adhere and exert traction on extracellular substrates. It is well established that collectively migrating CNC cells require interaction with a fibronectin (FN) matrix in the extracellular environment for migration. Chick and Xenopus neural crest cells are able to migrate on FN substrates in vitro (Lallier et al., 1992; Kil et al., 1996). In Xenopus CNC, FN is deposited around the neural crest streams in vivo and interaction of neural crest cells with FN requires integrin α5β1 (Alfandari et al., 2003). Furthermore, additional components of the ECM regulate neural crest migration: the proteoglycan Syndecan-4 is required for CNC migration in Xenopus and zebrafish and controls protrusion polarity as well as directionality of migration by inhibiting the activity of the small GTPase Rac1 (Matthews et al., 2008). Collective migration of Xenopus mesendoderm also requires integrin–FN interactions. Xenopus mesendoderm produces unipolar actin-rich protrusions both in vivo, where it migrates on the FN-rich BCR (Boucaut and Darribere, 1983) and in vitro when cultured on FN substrates. Importantly, functional blockade of α5β1 integrin impairs mesendoderm migration and protrusion formation (Davidson et al., 2002). In further support of the requirement for cell–ECM interactions in Xenopus mesendoderm, knockdown of the integrin-associated kinase FAK leads to impaired collective migration caused by misoriented protrusions, defective actin organization, and to a decrease in traction forces (Bjerke et al., 2014). In parallel to providing tractional adhesion to the migrating collective, protrusive activity of mesendodermal cells also contributes to the assembly of FN fibrils on the BCR, by exerting traction forces that strain the FN dimers and thus promoting their polymerization (Davidson et al., 2008). Altogether, studies in neural crest cells and in Xenopus mesendoderm highlight a role for FN ECM as an integrin substrate permissive for protrusion formation and migration (Fig. 3 a). In sprouting morphogenesis of blood vessels, integrin-dependent contacts between endothelial cells and ECM and the interaction of VEGF ligands with ECM components play important and diversified regulatory functions. VEGF-A is expressed as an alternatively spliced isoform, VEGF188, which encodes two heparin binding domains and is tightly associated with a heparan-sulfate proteoglycan (HSPG) in the ECM: VEGF120, which lacks these domains and is freely diffusible, and VEGF164, which possesses only one of the HSPG binding domains and has intermediate properties (Park et al., 1993). Mutant mice engineered to express exclusively the VEGF120 isoform display a reduction in vessel branching complexity, a reduction in VEGF gradient formation in the developing hindbrain, and impaired tip cell filopodia formation (Ruhrberg et al., 2002). On the other hand, mice expressing exclusively the VEGF188 isoform have excessive branching and ectopic filopodia, thus highlighting how appropriate VEGF binding to the ECM is essential to regulate its bioavailability to achieve correct angiogenesis. Similarly, in Drosophila trachea, mutations in the sugarless and sulfateless genes, which encode enzymes for the synthesis of heparan-sulfate glycosamminoglycans, lead to tracheal branching defects, which can be rescued by bnl overexpression, highlighting a conserved function for HSPGs in regulating growth factor distribution (Lin et al., 1999). In addition, in sprouting angiogenesis of the mouse retina, FN secreted by the underlying astrocytes regulates vessel migration by controlling both growth factor signaling and tip cell filopodia: tissue-specific deletion of FN in astrocytes leads to a decrease in the migration of the vascular front because of a decrease in VEGFR2 activity. Furthermore, knockin mutation of the integrin-binding RGD domain of FN in astrocytes or loss of function of integrin α5 leads to loss of alignment of tip cell filopodia to FN fibrils (Stenzel et al., 2011b). Other ECM components such as laminins, expressed by the endothelial cells, actively contribute to the tip cell/stalk cell selection via integrin dependent regulation of Dll4/Notch signaling (Estrach et al., 2011; Stenzel et al., 2011a). Altogether, these reports highlight multiple roles for the ECM in branching morphogenesis: it may control availability and distribution of growth factors, regulates growth factor signaling, mediates tip cell filopodia attachment, and may contribute to tip cell selection.
Figure 3.
Cell–ECM and cell–cell interactions in collective migration. (a) Integrin-dependent adhesions between collectively migrating cells and underlying ECM allow leader cells to form protrusions and exert traction forces on the ECM. Cell–ECM interactions may promote directionality of migration by enhancing Rac-dependent protrusion formation. (b) Heterotypic adhesions between the leaders of the migrating cluster and surrounding cells occur in border cell and mesendoderm migration. Cell–cell adhesion is required for protrusion formation via activation of the small GTPase Rac. Such adhesions can be under tension. (c) Cell–cell adhesion between inner cells is required to maintain mechanical integrity of the collectively migrating group. Adhesion needs to be relatively dynamic to allow rearrangements between cells; this is achieved in a variety of systems via Rab5-dependent internalization of cell–cell adhesions. NC, neural crest.
In contrast, in other systems, ECM-mediated interactions are dispensable for collective migration, and cells directly exploit the surfaces of other cells for migration. In particular, cadherin-dependent adhesion can be deployed as a substrate, allowing cell protrusion and forward movement (Fig. 3 b). For example, Drosophila border cells do not migrate on an ECM but move across the egg chamber by adhering to nurse cells via E-cadherin. Mutation of E-cadherin specifically in nurse cells impairs their migration (Niewiadomska et al., 1999; Cai et al., 2014). Importantly, E-cadherin molecules are under tension at the front of the group (Fig. 3 b); they contribute to protrusion formation at the front of the border cell cluster and act cooperatively with guidance receptors (Cai et al., 2014). Similarly, zebrafish prechordal mesendoderm does not migrate on a layer of ECM but deploys E-cadherin to form protrusions on the underlying epiblast, and E-cadherin morpholino knockdown results in impaired migration as well as impaired formation of unipolar protrusions (Montero et al., 2005). Collectively, these findings highlight how collectively migrating cells exploit extracellular substrates via a variety of strategies to ensure protrusion formation and traction, detection of chemotactic cues, and leader cell selection.
Cell–cell interactions during collective cell migration
In collective migration, cell–cell interactions are necessary to maintain adhesion of the migrating group, but at the same time they are required to be dynamically remodeled for cell rearrangements to occur. The importance of cell–cell adhesion for maintenance of mechanical integrity of the group has been demonstrated in a variety of epithelial models. E-cadherin is expressed at cell–cell contacts between border cells and at the junction between border cells and polar cells (Fig. 2 f). A recent study used cell type–specific promoters to knock down E-cadherin expression, which revealed different functions for E-cadherin junctions. E-cadherin contacts between border cells mechanically couple the cells to one another, whereas adhesion between polar cells and border cells is required to maintain integrity of the cluster (Cai et al., 2014). In addition to E-cadherin, apicobasal polarity factors such as Par3, Par6, and Cdc42 also contribute to the cohesion of the migrating cluster (Pinheiro and Montell, 2004; Llense and Martín-Blanco, 2008). E-cadherin–mediated cell–cell adhesion is also required for the epithelial integrity of the migrating Drosophila trachea (Cela and Llimargas, 2006). Here, EGFR signaling maintains tissue integrity by posttranscriptionally modulating E-cadherin levels. Downregulation of E-cadherin, as a consequence of overexpression of the negative EGF signaling regulator Mkp3, leads to loss of tube integrity. On the other hand, upregulation of the pathway leads to increased tissue stiffness caused by excessive junctional E-cadherin, leading to defects in branch extension and cell rearrangements (Cela and Llimargas, 2006). The requirement for dynamic cell–cell adhesion during tracheal migration is further supported by the observation that dynamic remodeling of junctions via Rab5-dependent internalization, as well as Rab11-dependent recycling of E-cadherin, is essential for intercalation of tracheal cells in dorsal branches (Fig. 3 c; Shaye et al., 2008). Other migrating epithelia, such as the lateral line, also require maintenance of cell–cell contacts throughout its development for efficient migration; interfering with Notch signaling leads to downregulation of E-cadherin expression and fragmentation of the primordium (Matsuda and Chitnis, 2010). In mesenchymal collective migration, cell–cell contacts have to be dynamically reorganized to allow movement. For example, in neural crest cells, LPA receptor and Rab5-dependent N-cadherin recycling is required for neural crest migration in vivo, as it allows the collective to acquire enough tissue plasticity to pass through narrow spaces (Kuriyama et al., 2014). Similarly, in zebrafish prechordal plate, Wnt11 controls Rab5-dependent E-cadherin endocytosis to ensure a correct level of cohesiveness of the tissue (Ulrich et al., 2005). Collectively, these findings emphasize the concept that cadherin junctional levels need to be tightly regulated to prevent loss of tissue integrity but still allow cell movements during collective migration.
Leaders respond to guidance cues and are dynamically established by cell interactions
How do guidance cues promote directional migration? In border cells, which migrate as an isolated group, cells dynamically exchange positions and take turns in forming protrusions (Bianco et al., 2007; Prasad and Montell, 2007). In vivo imaging reveals that upon mutation of guidance receptors PVR and EGFR, the migration of border cells is impaired because of uncontrolled extension of multiple, nondirectional protrusions (Prasad and Montell, 2007). Genetic mosaic experiments manipulating RTK signaling in individual cells of the cluster led to the “collective guidance” hypothesis, according to which leaders are dynamically selected as the cells with the highest RTK/MAPK signaling levels at a given time (Bianco et al., 2007). This concept is further reinforced by observations in models of branching morphogenesis. Indeed, despite significant differences in gene expression between tip cells and stalk cells in blood vessel sprouting (Gerhardt et al., 2003; Siekmann and Lawson, 2007; Tammela et al., 2008), recent studies highlighted the plasticity of the tip and stalk cell states. Live imaging revealed that sprouting endothelial cells are highly dynamic and that stalk cells can actively rearrange to acquire leader cell positions (Jakobsson et al., 2010; Arima et al., 2011). Notch-regulated differential VE-cadherin dynamics are associated with dynamic rearrangements of tip and stalk cells (Bentley et al., 2014). In addition, ablation of tip cells in Drosophila trachea results in stalk cells assuming leader positions (Caussinus et al., 2008), thus reinforcing the concept that the leader cell state is the result of dynamic social interactions between cells.
How do guidance cues promote motility? Early studies suggested the small GTPase Rac1, which is essential for lamellipodial protrusion (Ridley et al., 2003), and its GEF, Mbc/DOCK180, act downstream of PVF-1 (Duchek et al., 2001). Furthermore, Rac activity (Murphy and Montell, 1996) and the Rac GEF Vav2 (Fernández-Espartero et al., 2013) are essential for border cell migration. Recent studies have reported Rac activity to be polarized at the front of the border cell cluster (Fig. 2 f; Ramel et al., 2013; Fernández-Espartero et al., 2013; Cai et al., 2014). Importantly, photoactivation of Rac1 in an individual cell of the cluster in vivo is sufficient to reroute migration and inhibit protrusion formation in the surrounding cells, whereas photoactivation of a dominant-negative Rac1 mutant induces multiple protrusions in the clusters, phenocopying loss of RTK function (Wang et al., 2010). Similarly, in Drosophila trachea, Rac genetically interacts with guidance signaling: mutation of Rac1 and Rac2 strongly enhance the Bnl mutant phenotype, and live imaging suggests Rac activity to be required for branch elongation (Chihara et al., 2003). Importantly, Förster resonance energy transfer analysis revealed that Rac is active in leader tip cells (Lebreton and Casanova, 2014). Further studies exploiting photoactivatable Rac analogs may help clarify the role of Rac in tracheal tip cell migration. Activity of the small GTPase Rac1 is also polarized in cells located at the edge of neural crest cell groups, where it promotes lamellipodia formation (Theveneau et al., 2010; Scarpa et al., 2015). Neural crest cells frequently exchange neighbors because of rapid N-cadherin endocytosis (Kuriyama et al., 2014). Importantly, N-cadherin–dependent cell–cell interactions are essential for efficient chemotaxis toward the chemokine CXCL12/SDF-1, as SDF-1 is unable to modify Rac1 activity in nonpolarized cells, but it does increase Rac1 activity and stability of protrusions in cells that are already polarized in an N-cadherin contact–dependent manner (Theveneau et al., 2010). This highlights how different molecular mechanisms such as contact-dependent polarity and chemotaxis are integrated at the cell collective level to achieve collective migration (Theveneau et al., 2010).
Self-generated gradient of chemoattractants
We previously discussed how chemotactic cues are essential in guiding collective migrations during development. Chemokines are thought to drive a cellular response via spatial changes in their concentration (Majumdar et al., 2014). However, during the migration of the zebrafish lateral line and Xenopus neural crest, where chemotaxis plays an important role, the chemokine CXCL12/SDF-1 appears to be uniformly expressed in the tissue. Recently, self-generated gradients have been suggested as a likely mechanism driving migration in these tissues. The discovery of polarized expression of chemokine receptors Cxcr4b and Cxcr7 in lateral line cells led to the formulation of a model in which Cxcr7 may act as a scavenger for CXCL12 by locally depleting the availability of the chemoattractant at the trailing end of the collective (Streichan et al., 2011). By using a reporter transgenic line that allows the detection of Cxcr4b ligand–induced receptor turnover, Donà et al. (2013) demonstrated that the polarized distribution of Cxcr7 and Cxcr4b across the lateral line primordium results in a self-generated gradient of the uniformly expressed CXCL12/SDF-1 (Fig. 4 a). In addition, generation of an ectopic Cxcr7 source by expressing the receptor in the posterior lateral line nerve, which extends en route under the primordium, is sufficient to perturb the migration and morphology of wild-type primordia (Donà et al., 2013). Although this work elucidates a mechanism that accounts for chemokine gradient formation across the primordium, how do trailing cells, which express the decoy receptor Cxcr7 (Luker et al., 2010; Rajagopal et al., 2010) leading to minimal levels of chemokine signaling, manage to follow leader cells? One possible answer is mechanical coupling trough cadherin-dependent cell–cell interactions (Matsuda and Chitnis, 2010). Alternatively, trailing cells might be actively attracted to the leading end of the primordium via chemotaxis, as front cells secrete FGFs whereas trailing cells express FGF receptors (Dalle Nogare et al., 2014). Laser ablation of front cells or pharmacological inhibition of FGF receptor both result in the inability of trailing cells to migrate directionally, whereas inhibitor washout restores protrusive ability of the trailing fragment, which is then able to make contact with the leading end of the primordium and resume coordinated migration (Fig. 4 b; Dalle Nogare et al., 2014). Similar to the zebrafish lateral line, SDF-1 is expressed in a uniform manner in tissues surrounding the neural crest (Theveneau et al., 2010). A recent study highlights another mechanism through which SDF-1 may promote chemotaxis in Xenopus neural crest cells (Theveneau et al., 2013). Indeed, SDF-1 is expressed in the epibranchial placodes, which lay adjacent to Xenopus CNC (Theveneau et al., 2013). Neural crest cells exhibit chemotaxis in vitro toward placodal explants, which express SDF-1 (Theveneau et al., 2013). At the onset of migration, neural crest cells form protrusions and engage in heterotypic adhesions with the adjacent placodal cells. Placodal cells have low motility, but when contacted by neural crest cells, they produce a repulsive response, collapsing their focal adhesions at the site of contact and moving away from the neural crest cells (Theveneau et al., 2013). Such behavior, named “chase and run,” is required for guiding the comigration of neural crest and placode collectives: neural crest cells are chemoattracted to placodes, but as soon as they reach them, the placodes move away because of an N-cadherin–dependent contact inhibition of locomotion (CIL) response, thus luring the neural crest further ventrally across the migratory path (Fig. 4 c; Theveneau et al., 2013). Collectively, these works highlight how cell collectives use different strategies to respond to cues located in their surroundings and how differential expression of surface molecules within the collective itself is exploited to promote a local response to chemoattractants. Interestingly, self-generated chemokine gradients have recently been found to promote migration of invasive melanoma cells (Muinonen-Martin et al., 2014), which highlights how collectively migrating cancer cells might deploy similar tactics.
Figure 4.
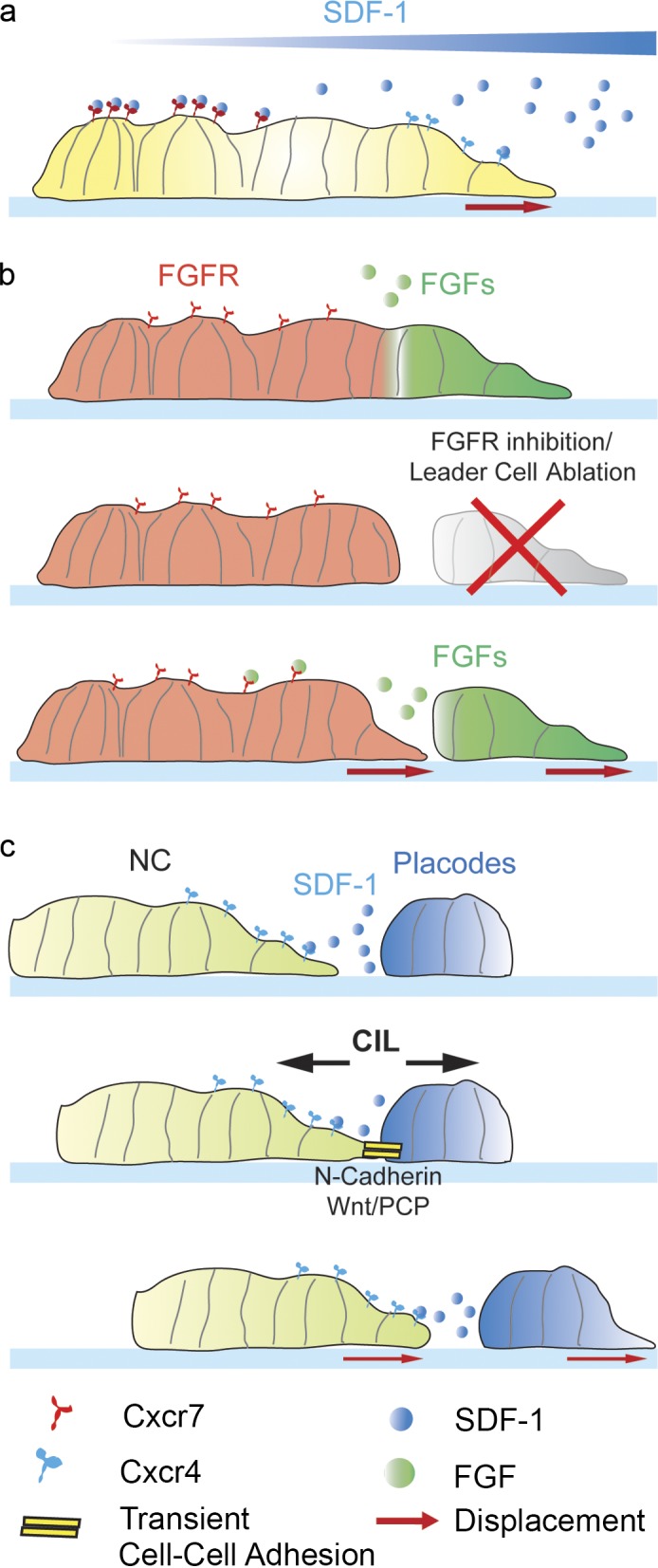
Strategies to create chemoattractant gradients. (a) Lateral view of the lateral line primordium. The SDF-1 receptor Cxcr7 is expressed at the back of the group, whereas Cxcr4 is expressed at the front and the back. Cxcr7 works as a scavenger of SDF-1, leading to a self-generated gradient caused by binding of SDF-1 to the Cxcr7 receptor at the back of the cluster, so that the local concentration of SDF-1 is higher at the front, where it promotes protrusion formation. (b) Lateral line front cells express FGF ligands, whereas FGF receptors (FGFR) are expressed in the posterior part of the cluster. Upon laser microsectioning of the primordium, the posterior end cannot migrate if the front end is ablated or if FGF signaling is inhibited. The back of the primordium is attracted toward the FGF produced by the primordium front. (c) Chase and run drives neural crest–placode comigration. Neural crest cells are chemoattracted toward the adjacent placodes, which secrete the chemokine SDF-1, and migrate toward them (chase). Then neural crest forms a transient adhesion with placodal cells, engaging in a heterotypic CIL response, which leads to displacement of the placodes away from the neural crest (run), thus displacing away the source of the chemoattractant.
Contact inhibition of locomotion: Polarizing the collective
The case of neural crest
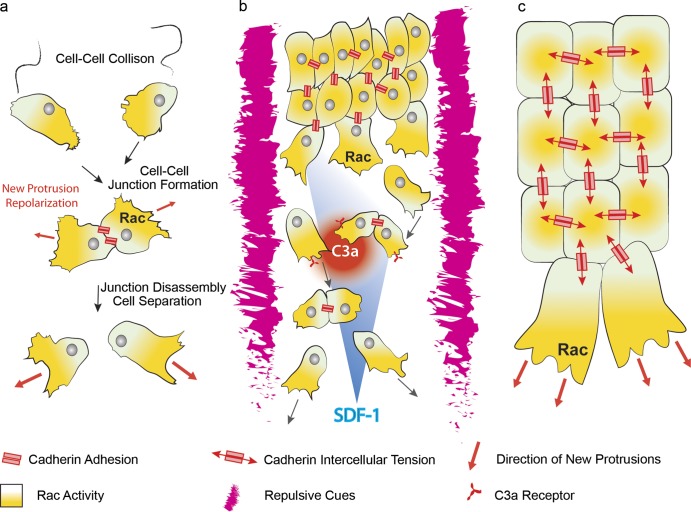
CIL is defined as the ability of a migrating cell to halt its movement and change the direction of its motion after contact with another cell (Fig. 5 a; Abercrombie and Heaysman, 1953). Its fundamental relevance in guiding migratory processes during embryonic development has been demonstrated in vivo for dispersion of macrophages (Stramer et al., 2010) and neurons (Villar-Cerviño et al., 2013) and for collective migration of neural crest cells (Carmona-Fontaine et al., 2008). When two neural crest cells interact, they assemble a transient cell–cell junction, which contains the classical cadherin complex (Scarpa et al., 2015), but they eventually repolarize their protrusions in opposite directions and move away from one another (Fig. 5 a; Carmona-Fontaine et al., 2008). The newly formed protrusion exerts large traction forces on the substrate, which eventually lead to the disassembly of the cell–cell junction (Scarpa et al., 2015). In neural crest cells, CIL depends on Wnt/PCP, which induces activation of the small GTPase RhoA at the cell–cell contacts (Carmona-Fontaine et al., 2008) as well as on N-cadherin and Par3 signaling (Theveneau et al., 2010; Moore et al., 2013). Perturbation of CIL leads to misoriented protrusions and impaired directional migration in vivo (Carmona-Fontaine et al., 2008). This occurs because CIL is required to confer a contact-dependent cell polarity to the collective: cells at the free edge of the cohort are able to form large protrusions, whereas cells in the center of the group, in contact with other cells, will form smaller, cryptic protrusions (Fig. 5 c). As a consequence of this behavior, cells exhibiting CIL do not crawl over their neighbors leading to monolayer formation in groups and eventually, as cell density decreases as a consequence of monolayering, to scattering to single cells (Fig. 5 b; Mayor and Carmona-Fontaine, 2010). CIL appears to be the driving force for polarized protrusion formation in neural crest, but at the same time it promotes dispersion of the collective (Carmona-Fontaine et al., 2008; Moore et al., 2013). Importantly, additional mechanisms operating during neural crest migration oppose CIL and ensure the maintenance of collectiveness. First of all, neural crest cell migration is spatially restricted into streams because of repulsive cues, such as semaphorins and ephrins (Robinson et al., 1997; Smith et al., 1997; Eickholt et al., 1999; Yu and Moens, 2005; Gammill et al., 2007; Koestner et al., 2008) expressed in the adjacent tissues, thus providing a confined environment permissive for migration (Fig. 5 b). In addition, SDF-1–mediated chemotaxis acts as a positive cue promoting displacement of the group in the direction of its source (Fig. 5 b), the placodal cells adjacent to the neural crest (Theveneau et al., 2010, 2013). A third mechanism of autocrine chemotaxis, named coattraction, directly opposes CIL-mediated cell scattering: neural crest cells secrete the complement factor C3a and express its receptor C3aR (Carmona-Fontaine et al., 2011). The C3a/C3aR system acts in an autocrine fashion to attract neural crest cells together, thus counterbalancing the dispersion promoted by CIL (Fig. 5 b). C3a signaling promotes cell–cell interactions, thus contributing to cohesion of the neural crest cell collective, and is necessary for efficient chemotaxis toward SDF-1 (Carmona-Fontaine et al., 2011). In summary, a variety of mechanisms cooperate in migration of CNC cells. Repulsive cues confine neural crest streams to the appropriate territories, contact inhibition of locomotion polarizes the group and promotes scattering and monolayering, and coattraction partially contrasts CIL by promoting cohesion within the group. The combination of these mechanisms maintains collectiveness in a mesenchymal cohort, enabling the neural crest cells to respond efficiently to chemotactic cues such as SDF-1 (Fig. 5 b).
Figure 5.
Contact inhibition of locomotion determines contact-dependent polarity of cell collectives. (a) Overview of CIL in single migrating mesenchymal cells. Collision between two cells leads to assembly of a transient cell–cell adhesion. Upon junction formation, the small GTPase Rac1 is active away from the cell–cell contact, leading to repolarization of newly formed protrusions (red arrows). Persistent protrusive activity eventually leads to junction disassembly and cell separation. (b) Collective migration of mesenchymal neural crest cells. CIL, cell confinement, and chemotaxis cooperate in collective migration of neural crest cells. CIL promotes formation of large protrusions in cells at the front of the group because of polarized Rac1 activity, leading to monolayering and cell dispersion. The presence of repulsive cues such as semaphorins and ephrins around neural crest streams restricts migration in a confined space, therefore favoring cell–cell interactions. In addition, neural crest cells secrete the chemokine C3a and express its receptor C3aR, which contribute to opposing cell dispersion by attracting neural crest cells toward one another. Finally, a gradient of the chemokine SDF-1 confers directionality to the migrating collective. (c) Contact-dependent cell polarity in cohesive cell collectives. Directional information is conveyed at cell–cell contacts, leading to small GTPase Rac activity and protrusion formation at the free edge of the group and to smaller cryptic protrusions in the center. Such information may rely on tension-dependent signaling at the cell–cell contacts.
Contact-dependent cell polarity in epithelial collective migration
Directional information can be conveyed at cell–cell contacts via CIL. Although the role of CIL in controlling directional migration of the neural crest is well established (Carmona-Fontaine et al., 2008; Theveneau et al., 2010, 2013), accumulating evidence suggests that CIL is present in other collective migration systems as well. Recent work has shown that Wnt/PCP-mediated contact-dependent polarity drives collective migration of zebrafish prechordal plate cells (Dumortier et al., 2012) by controlling E-cadherin/Rac–dependent protrusion formation. Transplant experiments show that isolated prechordal plate cells are unable to migrate directionally until they make contact with the rest of the collective (Dumortier et al., 2012). Importantly, this work suggested that intrinsic directional information might arise from cell–cell interactions independently of guidance cues (Dumortier et al., 2012). Does contact-dependent polarity also occur in migratory epithelial collectives? Observations of Drosophila border cells suggest that this might be the case, as cells extend protrusions at the nurse cell–border cell interface, but not at the border cell–border cell or border cell–polar cell interface (Prasad and Montell, 2007). Similar to observations in neural crest cells, Rac1 activity is highly polarized toward the nurse cell–border cell interface (Cai et al., 2014). In addition, recent work suggested that protrusion formation at internal border cell boundaries is actively suppressed by the Hippo pathway by control of Ena/VASP-dependent actin polymerization (Lucas et al., 2013). Also in migrating tracheal branches, stalk cells accumulate cell–cell junction components at the apical membrane and form small, cryptic protrusions at the cell–cell contacts (Fig. 2 b; Lebreton and Casanova, 2014). On the other hand, tip cells acquire a front–rear polarity reminiscent of single migrating cells, with their protrusions oriented in the direction of migration. Here, active Rac1 is polarized toward the leading edge (Fig. 2 b) and away from the cell–cell contact (Lebreton and Casanova, 2014), consistent with CIL-dependent polarity. Similarly, in the lateral line primordium, large protrusions orient toward the free space at the leading edge and at the sides of the migrating cluster (Haas and Gilmour, 2006). On the other hand, cells in the center of the group also form cryptic protrusions at cell–cell contacts as revealed by clonal analysis of transplanted cells (Haas and Gilmour, 2006; Lecaudey et al., 2008). Interestingly, interfering with FGF signaling leads to formation of large misoriented protrusions in the center of the cluster and to impaired directional migration, a behavior reminiscent of loss of CIL (Carmona-Fontaine et al., 2008; Lecaudey et al., 2008). How do cell–cell contacts communicate polarity? Increasing evidence supports a role for cell-adhesion–dependent mechanical coupling (Fig. 5 c). In Xenopus head mesendoderm, a C-cadherin–dependent mechanosensitive pathway directs migration in response to tension across cell–cell contacts. Application of tension by pulling on C-cadherin–coated magnetic beads that are in contact with single mesodermal cells is sufficient to polarize cell protrusions in a direction opposite to the applied tension (Weber et al., 2012). In addition, work in cultured epithelial cells has shown that Merlin, a Hippo pathway component localized to cell–cell junctions in nonmigrating cells, is redistributed from the cell–cell contacts to the cytoplasm in a tension- and myosin II–dependent manner upon wound healing and induces polarized Rac1 activation. This finding provides an intriguing mechanism for tension induced planar polarization of Rac1 activity (Das et al., 2015). Another example is the developmental dispersal of hemocytes in Drosophila, driven by an intercellular actin clutch. Tracking of actin flow and laser ablation experiments demonstrated that an actin cable forms between colliding cells and via a clutch-like mechanism builds up tension at transient cell–cell junctions formed by the interacting hemocytes and guides CIL (Davis et al., 2015). These studies highlight how directional information at cell–cell contacts may rely on mechanical tension transmitted across cell–cell junctions. Although the study of mechanical properties of cell–cell adhesions in vivo has long remained elusive, new techniques such as laser ablation (Davis et al., 2015) and tension-sensitive Förster resonance energy transfer probes (Grashoff et al., 2010; Cai et al., 2014) will allow further elucidation of the relationship between mechanosensing and signaling in developmental models of collective migration. Importantly, sensing of mechanical properties of the environment (Levental et al., 2009; Calvo et al., 2013) and contact-dependent repulsion (Astin et al., 2010) have been suggested to promote cancer invasion in vitro and in vivo. Deciphering the molecular signals underlying epithelial contact–dependent polarity and mechanosensing at cell–cell adhesions in development may contribute to our understanding of collective invasion of cancer cells in vivo.
Concluding remarks
During embryonic development, collective cell migration is adopted by a variety of cell types to reach distant sites or achieve complex organ shapes during morphogenesis. Although the modalities through which collective migration is performed by epithelial or mesenchymal cells in model organisms are diverse, common features emerge, such as the ability to form organized cohorts characterized by leader and follower cell with different protrusive and tractional potential, the plasticity of leader and follower cells, the ability to sense and respond to chemotactic cues, and the mechanical coordination of cells in a collective through transient or stable cell–cell adhesions. Importantly, it is becoming increasingly clear that many metastatic cancer cells often invade tissues not as single individuals but as organized collectives, whose morphology is reminiscent of collective migrations observed during embryo development (Friedl and Gilmour, 2009; Friedl et al., 2012). Recent advances in embryonic collective migration have highlighted the importance of interactions between the migrating collective and the chemical or mechanical properties of the surrounding environment; gradients of chemokines such as SDF-1 may emerge from differential receptor activity in the migrating zebrafish lateral line primordium (Donà et al., 2013) or from CIL-mediated chase and run of neural crest and placodal cells in the Xenopus embryo (Theveneau et al., 2013). Although the detection of gradients in other models still remains unaddressed, the development of genetically encoded fluorescently tagged probes sensitive to receptor–ligand complex lifetime (Donà et al., 2013) may help discovery of new gradients in vivo. In addition, dynamic cell–cell interactions are paramount in supporting protrusions on cellular substrates, maintaining mechanical integrity of the collective, and providing directional information via polarization of protrusions of the migrating cohort. Although recent work has highlighted a role for cadherin-dependent mechanical feedback in promoting polarized protrusion formation in diverse models such as border cells (Cai et al., 2014), Xenopus mesendoderm (Weber et al., 2012), and neural crest (Scarpa et al., 2015), the molecular mechanisms linking stress-sensing at the cell–cell adhesion to control of distant lamellipodial activity are not yet completely clear. In vivo observations suggest that similar mechanisms may be hijacked in tumor collective invasion (Astin et al., 2010; Muinonen-Martin et al., 2014), and developmental models of collective migration are likely to provide useful insights.
Acknowledgments
We apologize all authors whose work could not be cited due to space constraints. We thank Brian Stramer and András Szabó for comments on the manuscript.
Work in R. Mayor’s laboratory is supported by grants from the Medical Research Council (M010465 and J000655), Biotechnology and Biological Sciences Research Council (M008517), and Wellcome Trust. E. Scarpa is a recipient of a Wellcome Trust PhD fellowship.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- BCR
- blastocoel roof
- CIL
- contact inhibition of locomotion
- CNC
- cranial neural crest
- EMT
- epithelial-to-mesenchymal transition
- FN
- fibronectin
- HSPG
- heparan-sulfate proteoglycan
- PCP
- planar cell polarity
- pLLP
- posterior lateral line primordium
References
- Abercrombie M., and Heaysman J.E.. 1953. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp. Cell Res. 5:111–131. [DOI] [PubMed] [Google Scholar]
- Alfandari D., Cousin H., Gaultier A., Hoffstrom B.G., and DeSimone D.W.. 2003. Integrin alpha5beta1 supports the migration of Xenopus cranial neural crest on fibronectin. Dev. Biol. 260:449–464. [DOI] [PubMed] [Google Scholar]
- Andrew D.J., and Ewald A.J.. 2010. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev. Biol. 341:34–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima S., Nishiyama K., Ko T., Arima Y., Hakozaki Y., Sugihara K., Koseki H., Uchijima Y., Kurihara Y., and Kurihara H.. 2011. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development. 138:4763–4776. [DOI] [PubMed] [Google Scholar]
- Astin J.W., Batson J., Kadir S., Charlet J., Persad R.A., Gillatt D., Oxley J.D., and Nobes C.D.. 2010. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat. Cell Biol. 12:1194–1204. [DOI] [PubMed] [Google Scholar]
- Bear J.E., and Haugh J.M.. 2014. Directed migration of mesenchymal cells: where signaling and the cytoskeleton meet. Curr. Opin. Cell Biol. 30:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani A., Tran P.B., Ren D., Assimacopoulos S., Grove E.A., and Miller R.J.. 2005. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J. Neurosci. 25:3995–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley K., Franco C.A., Philippides A., Blanco R., Dierkes M., Gebala V., Stanchi F., Jones M., Aspalter I.M., Cagna G., et al. . 2014. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat. Cell Biol. 16:309–321. [DOI] [PubMed] [Google Scholar]
- Bianco A., Poukkula M., Cliffe A., Mathieu J., Luque C.M., Fulga T.A., and Rørth P.. 2007. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 448:362–365. [DOI] [PubMed] [Google Scholar]
- Bjerke M.A., Dzamba B.J., Wang C., and DeSimone D.W.. 2014. FAK is required for tension-dependent organization of collective cell movements in Xenopus mesendoderm. Dev. Biol. 394:340–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucaut J.C., and Darribere T.. 1983. Fibronectin in early amphibian embryos. Migrating mesodermal cells contact fibronectin established prior to gastrulation. Cell Tissue Res. 234:135–145. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T., Kim S., Sasai Y., Lu B., and De Robertis E.M.. 1996. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 382:595–601. [DOI] [PubMed] [Google Scholar]
- Cai D., Chen S.C., Prasad M., He L., Wang X., Choesmel-Cadamuro V., Sawyer J.K., Danuser G., and Montell D.J.. 2014. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 157:1146–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I., Harrington K., Williamson P., Moeendarbary E., Charras G., and Sahai E.. 2013. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C., Matthews H.K., Kuriyama S., Moreno M., Dunn G.A., Parsons M., Stern C.D., and Mayor R.. 2008. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 456:957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C., Theveneau E., Tzekou A., Tada M., Woods M., Page K.M., Parsons M., Lambris J.D., and Mayor R.. 2011. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev. Cell. 21:1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussinus E., Colombelli J., and Affolter M.. 2008. Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. Curr. Biol. 18:1727–1734. [DOI] [PubMed] [Google Scholar]
- Cela C., and Llimargas M.. 2006. Egfr is essential for maintaining epithelial integrity during tracheal remodelling in Drosophila. Development. 133:3115–3125. [DOI] [PubMed] [Google Scholar]
- Chihara T., Kato K., Taniguchi M., Ng J., and Hayashi S.. 2003. Rac promotes epithelial cell rearrangement during tracheal tubulogenesis in Drosophila. Development. 130:1419–1428. [DOI] [PubMed] [Google Scholar]
- Dalle Nogare D., Somers K., Rao S., Matsuda M., Reichman-Fried M., Raz E., and Chitnis A.B.. 2014. Leading and trailing cells cooperate in collective migration of the zebrafish posterior lateral line primordium. Development. 141:3188–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm E.W., and Winklbauer R.. 2011. PDGF-A controls mesoderm cell orientation and radial intercalation during Xenopus gastrulation. Development. 138:565–575. [DOI] [PubMed] [Google Scholar]
- Das T., Safferling K., Rausch S., Grabe N., Boehm H., and Spatz J.P.. 2015. A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat. Cell Biol. 17:276–287. [DOI] [PubMed] [Google Scholar]
- Davidson L.A., Hoffstrom B.G., Keller R., and DeSimone D.W.. 2002. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin alpha(5)beta(1), fibronectin, and tissue geometry. Dev. Biol. 242:109–129. [DOI] [PubMed] [Google Scholar]
- Davidson L.A., Dzamba B.D., Keller R., and Desimone D.W.. 2008. Live imaging of cell protrusive activity, and extracellular matrix assembly and remodeling during morphogenesis in the frog, Xenopus laevis. Dev. Dyn. 237:2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.R., Luchici A., Mosis F., Thackery J., Salazar J.A., Mao Y., Dunn G.A., Betz T., Miodownik M., and Stramer B.M.. 2015. Inter-cellular forces orchestrate contact inhibition of locomotion. Cell. 161:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A., Krieg M., Bergert M., Ibarlucea-Benitez I., Muller D.J., Paluch E., and Heisenberg C.P.. 2010. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 8:e1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donà E., Barry J.D., Valentin G., Quirin C., Khmelinskii A., Kunze A., Durdu S., Newton L.R., Fernandez-Minan A., Huber W., et al. . 2013. Directional tissue migration through a self-generated chemokine gradient. Nature. 503:285–289. [DOI] [PubMed] [Google Scholar]
- Duchek P., and Rørth P.. 2001. Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science. 291:131–133. [DOI] [PubMed] [Google Scholar]
- Duchek P., Somogyi K., Jékely G., Beccari S., and Rørth P.. 2001. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 107:17–26. [DOI] [PubMed] [Google Scholar]
- Dumortier J.G., Martin S., Meyer D., Rosa F.M., and David N.B.. 2012. Collective mesendoderm migration relies on an intrinsic directionality signal transmitted through cell contacts. Proc. Natl. Acad. Sci. USA. 109:16945–16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickholt B.J., Mackenzie S.L., Graham A., Walsh F.S., and Doherty P.. 1999. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrain regions. Development. 126:2181–2189. [DOI] [PubMed] [Google Scholar]
- Estrach S., Cailleteau L., Franco C.A., Gerhardt H., Stefani C., Lemichez E., Gagnoux-Palacios L., Meneguzzi G., and Mettouchi A.. 2011. Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ. Res. 109:172–182. [DOI] [PubMed] [Google Scholar]
- Ewald A.J., Brenot A., Duong M., Chan B.S., and Werb Z.. 2008. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell. 14:570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald A.J., Huebner R.J., Palsdottir H., Lee J.K., Perez M.J., Jorgens D.M., Tauscher A.N., Cheung K.J., Werb Z., and Auer M.. 2012. Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. J. Cell Sci. 125:2638–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Espartero C.H., Ramel D., Farago M., Malartre M., Luque C.M., Limanovich S., Katzav S., Emery G., and Martín-Bermudo M.D.. 2013. GTP exchange factor Vav regulates guided cell migration by coupling guidance receptor signalling to local Rac activation. J. Cell Sci. 126:2285–2293. [DOI] [PubMed] [Google Scholar]
- Friedl P., and Gilmour D.. 2009. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10:445–457. [DOI] [PubMed] [Google Scholar]
- Friedl P., Locker J., Sahai E., and Segall J.E.. 2012. Classifying collective cancer cell invasion. Nat. Cell Biol. 14:777–783. [DOI] [PubMed] [Google Scholar]
- Gammill L.S., Gonzalez C., and Bronner-Fraser M.. 2007. Neuropilin 2/semaphorin 3F signaling is essential for cranial neural crest migration and trigeminal ganglion condensation. Dev. Neurobiol. 67:47–56. [DOI] [PubMed] [Google Scholar]
- Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., and Betsholtz C.. 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial A.S., and Krasnow M.A.. 2006. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 441:746–749. [DOI] [PubMed] [Google Scholar]
- Grashoff C., Hoffman B.D., Brenner M.D., Zhou R., Parsons M., Yang M.T., McLean M.A., Sligar S.G., Chen C.S., Ha T., and Schwartz M.A.. 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 466:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas P., and Gilmour D.. 2006. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev. Cell. 10:673–680. [DOI] [PubMed] [Google Scholar]
- Hellström M., Phng L.K., Hofmann J.J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A.K., Karlsson L., Gaiano N., et al. . 2007. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 445:776–780. [DOI] [PubMed] [Google Scholar]
- Huebner R.J., and Ewald A.J.. 2014. Cellular foundations of mammary tubulogenesis. Semin. Cell Dev. Biol. 31:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R.J., Lechler T., and Ewald A.J.. 2014. Developmental stratification of the mammary epithelium occurs through symmetry-breaking vertical divisions of apically positioned luminal cells. Development. 141:1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L., Franco C.A., Bentley K., Collins R.T., Ponsioen B., Aspalter I.M., Rosewell I., Busse M., Thurston G., Medvinsky A., et al. . 2010. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12:943–953. [DOI] [PubMed] [Google Scholar]
- Kil S.H., Lallier T., and Bronner-Fraser M.. 1996. Inhibition of cranial neural crest adhesion in vitro and migration in vivo using integrin antisense oligonucleotides. Dev. Biol. 179:91–101. 10.1006/dbio.1996.0243 [DOI] [PubMed] [Google Scholar]
- Klämbt C., Glazer L., and Shilo B.Z.. 1992. breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 6:1668–1678. 10.1101/gad.6.9.1668 [DOI] [PubMed] [Google Scholar]
- Koestner U., Shnitsar I., Linnemannstöns K., Hufton A.L., and Borchers A.. 2008. Semaphorin and neuropilin expression during early morphogenesis of Xenopus laevis. Dev. Dyn. 237:3853–3863. [DOI] [PubMed] [Google Scholar]
- Kuriyama S., Theveneau E., Benedetto A., Parsons M., Tanaka M., Charras G., Kabla A., and Mayor R.. 2014. In vivo collective cell migration requires an LPAR2-dependent increase in tissue fluidity. J. Cell Biol. 206:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallier T., Leblanc G., Artinger K.B., and Bronner-Fraser M.. 1992. Cranial and trunk neural crest cells use different mechanisms for attachment to extracellular matrices. Development. 116:531–541. [DOI] [PubMed] [Google Scholar]
- Lebreton G., and Casanova J.. 2014. Specification of leading and trailing cell features during collective migration in the Drosophila trachea. J. Cell Sci. 127:465–474. [DOI] [PubMed] [Google Scholar]
- Lecaudey V., Cakan-Akdogan G., Norton W.H., and Gilmour D.. 2008. Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development. 135:2695–2705. [DOI] [PubMed] [Google Scholar]
- Le Douarin N.M., and Kalcheim C.. 1999. The Neural Crest. 2nd ed Cambridge University Press, New York: 445 pp. [Google Scholar]
- Levental K.R., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J.T., Fong S.F., Csiszar K., Giaccia A., Weninger W., et al. . 2009. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 139:891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Buff E.M., Perrimon N., and Michelson A.M.. 1999. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development. 126:3715–3723. [DOI] [PubMed] [Google Scholar]
- Llense F., and Martín-Blanco E.. 2008. JNK signaling controls border cell cluster integrity and collective cell migration. Curr. Biol. 18:538–544. [DOI] [PubMed] [Google Scholar]
- Lu P., Ewald A.J., Martin G.R., and Werb Z.. 2008. Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis. Dev. Biol. 321:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E.P., Khanal I., Gaspar P., Fletcher G.C., Polesello C., Tapon N., and Thompson B.J.. 2013. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J. Cell Biol. 201:875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luker K.E., Steele J.M., Mihalko L.A., Ray P., and Luker G.D.. 2010. Constitutive and chemokine-dependent internalization and recycling of CXCR7 in breast cancer cells to degrade chemokine ligands. Oncogene. 29:4599–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar R., Sixt M., and Parent C.A.. 2014. New paradigms in the establishment and maintenance of gradients during directed cell migration. Curr. Opin. Cell Biol. 30:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., and Chitnis A.B.. 2010. Atoh1a expression must be restricted by Notch signaling for effective morphogenesis of the posterior lateral line primordium in zebrafish. Development. 137:3477–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.K., Marchant L., Carmona-Fontaine C., Kuriyama S., Larraín J., Holt M.R., Parsons M., and Mayor R.. 2008. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 135:1771–1780. [DOI] [PubMed] [Google Scholar]
- Mayor R., and Carmona-Fontaine C.. 2010. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 20:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J.A., and Montell D.J.. 2005. Analysis of cell migration using Drosophila as a model system. Methods Mol. Biol. 294:175–202. [DOI] [PubMed] [Google Scholar]
- McDonald J.A., Pinheiro E.M., and Montell D.J.. 2003. PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development. 130:3469–3478. [DOI] [PubMed] [Google Scholar]
- McDonald J.A., Pinheiro E.M., Kadlec L., Schupbach T., and Montell D.J.. 2006. Multiple EGFR ligands participate in guiding migrating border cells. Dev. Biol. 296:94–103. [DOI] [PubMed] [Google Scholar]
- McLennan R., and Kulesa P.M.. 2010. Neuropilin-1 interacts with the second branchial arch microenvironment to mediate chick neural crest cell dynamics. Dev. Dyn. 239:1664–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell D.J. 2003. Border-cell migration: the race is on. Nat. Rev. Mol. Cell Biol. 4:13–24. [DOI] [PubMed] [Google Scholar]
- Montell D.J., Rorth P., and Spradling A.C.. 1992. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell. 71:51–62. [DOI] [PubMed] [Google Scholar]
- Montero J.A., Kilian B., Chan J., Bayliss P.E., and Heisenberg C.P.. 2003. Phosphoinositide 3-kinase is required for process outgrowth and cell polarization of gastrulating mesendodermal cells. Curr. Biol. 13:1279–1289. [DOI] [PubMed] [Google Scholar]
- Montero J.A., Carvalho L., Wilsch-Bräuninger M., Kilian B., Mustafa C., and Heisenberg C.P.. 2005. Shield formation at the onset of zebrafish gastrulation. Development. 132:1187–1198. [DOI] [PubMed] [Google Scholar]
- Moore R., Theveneau E., Pozzi S., Alexandre P., Richardson J., Merks A., Parsons M., Kashef J., Linker C., and Mayor R.. 2013. Par3 controls neural crest migration by promoting microtubule catastrophe during contact inhibition of locomotion. Development. 140:4763–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muinonen-Martin A.J., Susanto O., Zhang Q., Smethurst E., Faller W.J., Veltman D.M., Kalna G., Lindsay C., Bennett D.C., Sansom O.J., et al. . 2014. Melanoma cells break down LPA to establish local gradients that drive chemotactic dispersal. PLoS Biol. 12:e1001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A.M., and Montell D.J.. 1996. Cell type-specific roles for Cdc42, Rac, and RhoL in Drosophila oogenesis. J. Cell Biol. 133:617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M., Tahinci E., Symes K., and Winklbauer R.. 2004. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 131:2727–2736. [DOI] [PubMed] [Google Scholar]
- Niewiadomska P., Godt D., and Tepass U.. 1999. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J. Cell Biol. 144:533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesnicky Killian E.C., Birkholz D.A., and Artinger K.B.. 2009. A role for chemokine signaling in neural crest cell migration and craniofacial development. Dev. Biol. 333:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E., Keller G.A., and Ferrara N.. 1993. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell. 4:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie R.J., Doyle A.D., and Yamada K.M.. 2009. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 10:538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro E.M., and Montell D.J.. 2004. Requirement for Par-6 and Bazooka in Drosophila border cell migration. Development. 131:5243–5251. [DOI] [PubMed] [Google Scholar]
- Prasad M., and Montell D.J.. 2007. Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev. Cell. 12:997–1005. [DOI] [PubMed] [Google Scholar]
- Rajagopal S., Kim J., Ahn S., Craig S., Lam C.M., Gerard N.P., Gerard C., and Lefkowitz R.J.. 2010. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc. Natl. Acad. Sci. USA. 107:628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel D., Wang X., Laflamme C., Montell D.J., and Emery G.. 2013. Rab11 regulates cell-cell communication during collective cell movements. Nat. Cell Biol. 15:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel D., Wang X., Laflamme C., Montell D.J., and Emery G.. 2013. Rab11 regulates cell-cell communication during collective cell movements. Nat. Cell Biol. 15:317–324. 10.1038/ncb2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C., Streichan S., Donà E., Lecaudey V., Hufnagel L., and Gilmour D.. 2014. Quantitative cell polarity imaging defines leader-to-follower transitions during collective migration and the key role of microtubule-dependent adherens junction formation. Development. 141:1282–1291. 10.1242/dev.101675 [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Schwartz M.A., Burridge K., Firtel R.A., Ginsberg M.H., Borisy G., Parsons J.T., and Horwitz A.R.. 2003. Cell migration: integrating signals from front to back. Science. 302:1704–1709. [DOI] [PubMed] [Google Scholar]
- Robinson V., Smith A., Flenniken A.M., and Wilkinson D.G.. 1997. Roles of Eph receptors and ephrins in neural crest pathfinding. Cell Tissue Res. 290:265–274. [DOI] [PubMed] [Google Scholar]
- Rørth P. 2002. Initiating and guiding migration: lessons from border cells. Trends Cell Biol. 12:325–331. [DOI] [PubMed] [Google Scholar]
- Rørth P. 2012. Fellow travellers: emergent properties of collective cell migration. EMBO Rep. 13:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C., Gerhardt H., Golding M., Watson R., Ioannidou S., Fujisawa H., Betsholtz C., and Shima D.T.. 2002. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 16:2684–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa E., Szabó A., Bibonne A., Theveneau E., Parsons M., and Mayor R.. 2015. Cadherin Switch during EMT in neural crest cells leads to contact inhibition of locomotion via repolarization of forces. Dev. Cell. 34:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaye D.D., Casanova J., and Llimargas M.. 2008. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat. Cell Biol. 10:964–970. [DOI] [PubMed] [Google Scholar]
- Siekmann A.F., and Lawson N.D.. 2007. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 445:781–784. [DOI] [PubMed] [Google Scholar]
- Smith A., Robinson V., Patel K., and Wilkinson D.G.. 1997. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr. Biol. 7:561–570. [DOI] [PubMed] [Google Scholar]
- Stenzel D., Franco C.A., Estrach S., Mettouchi A., Sauvaget D., Rosewell I., Schertel A., Armer H., Domogatskaya A., Rodin S., et al. . 2011a Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep. 12:1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel D., Lundkvist A., Sauvaget D., Busse M., Graupera M., van der Flier A., Wijelath E.S., Murray J., Sobel M., Costell M., et al. . 2011b Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. Development. 138:4451–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C.D. 1992. Vertebrate gastrulation. Curr. Opin. Genet. Dev. 2:556–561. [DOI] [PubMed] [Google Scholar]
- Stramer B., Moreira S., Millard T., Evans I., Huang C.Y., Sabet O., Milner M., Dunn G., Martin P., and Wood W.. 2010. Clasp-mediated microtubule bundling regulates persistent motility and contact repulsion in Drosophila macrophages in vivo. J. Cell Biol. 189:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streichan S.J., Valentin G., Gilmour D., and Hufnagel L.. 2011. Collective cell migration guided by dynamically maintained gradients. Phys. Biol. 8:045004. [DOI] [PubMed] [Google Scholar]
- Suchting S., Freitas C., le Noble F., Benedito R., Bréant C., Duarte A., and Eichmann A.. 2007. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. USA. 104:3225–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D., Samakovlis C., and Krasnow M.A.. 1996. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 87:1091–1101. [DOI] [PubMed] [Google Scholar]
- Tammela T., Zarkada G., Wallgard E., Murtomäki A., Suchting S., Wirzenius M., Waltari M., Hellström M., Schomber T., Peltonen R., et al. . 2008. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 454:656–660. [DOI] [PubMed] [Google Scholar]
- Theveneau E., and Mayor R.. 2012. Cadherins in collective cell migration of mesenchymal cells. Curr. Opin. Cell Biol. 24:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E., Marchant L., Kuriyama S., Gull M., Moepps B., Parsons M., and Mayor R.. 2010. Collective chemotaxis requires contact-dependent cell polarity. Dev. Cell. 19:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E., Steventon B., Scarpa E., Garcia S., Trepat X., Streit A., and Mayor R.. 2013. Chase-and-run between adjacent cell populations promotes directional collective migration. Nat. Cell Biol. 15:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F., Concha M.L., Heid P.J., Voss E., Witzel S., Roehl H., Tada M., Wilson S.W., Adams R.J., Soll D.R., and Heisenberg C.P.. 2003. Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development. 130:5375–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F., Krieg M., Schötz E.M., Link V., Castanon I., Schnabel V., Taubenberger A., Mueller D., Puech P.H., and Heisenberg C.P.. 2005. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev. Cell. 9:555–564. [DOI] [PubMed] [Google Scholar]
- Valentin G., Haas P., and Gilmour D.. 2007. The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr. Biol. 17:1026–1031. [DOI] [PubMed] [Google Scholar]
- Villar-Cerviño V., Molano-Mazón M., Catchpole T., Valdeolmillos M., Henkemeyer M., Martínez L.M., Borrell V., and Marín O.. 2013. Contact repulsion controls the dispersion and final distribution of Cajal-Retzius cells. Neuron. 77:457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., He L., Wu Y.I., Hahn K.M., and Montell D.J.. 2010. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat. Cell Biol. 12:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warga R.M., and Kimmel C.B.. 1990. Cell movements during epiboly and gastrulation in zebrafish. Development. 108:569–580. [DOI] [PubMed] [Google Scholar]
- Weber G.F., Bjerke M.A., and DeSimone D.W.. 2012. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev. Cell. 22:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Tanwar P.S., and Raftery L.A.. 2008. Drosophila follicle cells: morphogenesis in an eggshell. Semin. Cell Dev. Biol. 19:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.H., and Moens C.B.. 2005. Semaphorin signaling guides cranial neural crest cell migration in zebrafish. Dev. Biol. 280:373–385. [DOI] [PubMed] [Google Scholar]
- Zhang X., Martinez D., Koledova Z., Qiao G., Streuli C.H., and Lu P.. 2014. FGF ligands of the postnatal mammary stroma regulate distinct aspects of epithelial morphogenesis. Development. 141:3352–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]